Numerical Investigation on Coal Combustion in Ultralow CO2 Blast Furnace: Effect of Oxygen Temperature
Abstract
1. Introduction
2. Mathematical Model
2.1. Basic Equations
2.2. Combustion Process
3. Geometry and Operating Conditions
4. Results and Discussion
4.1. Model Validation
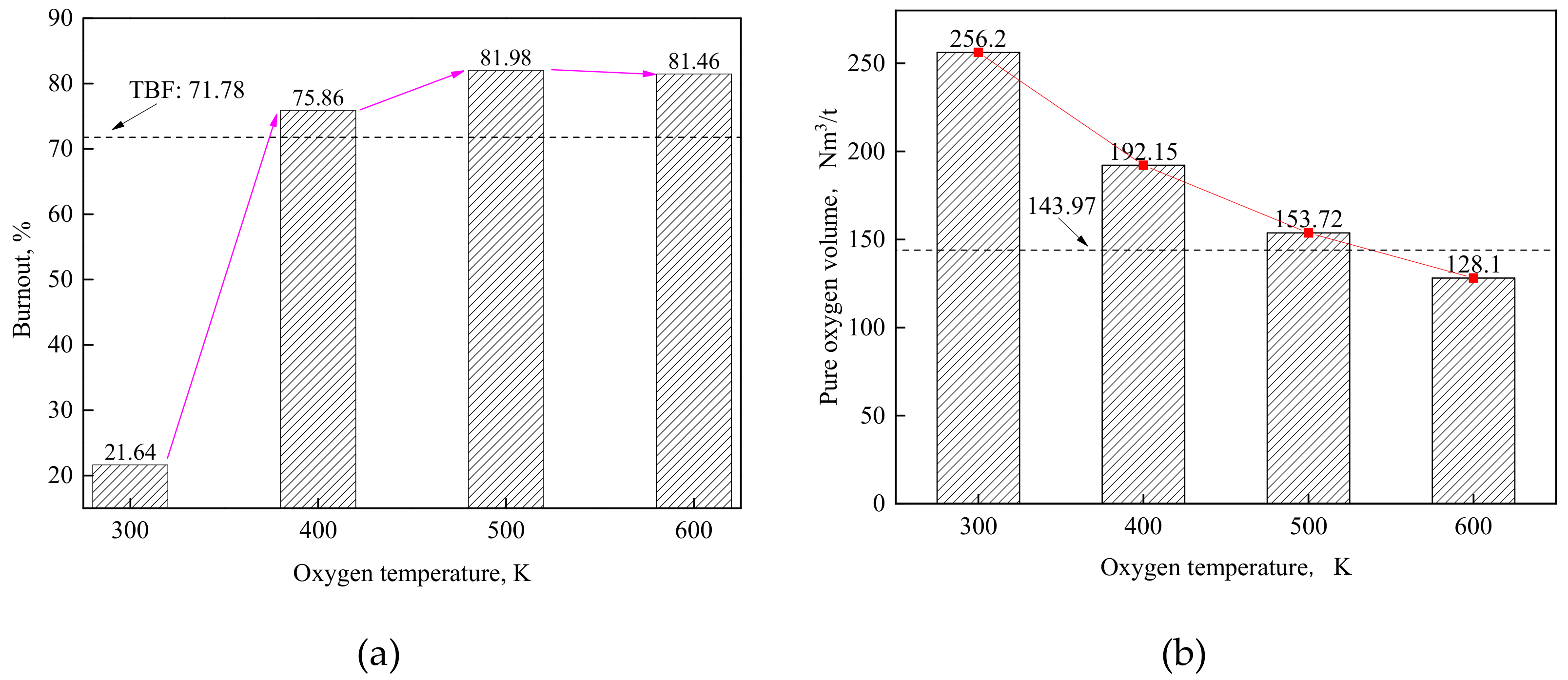
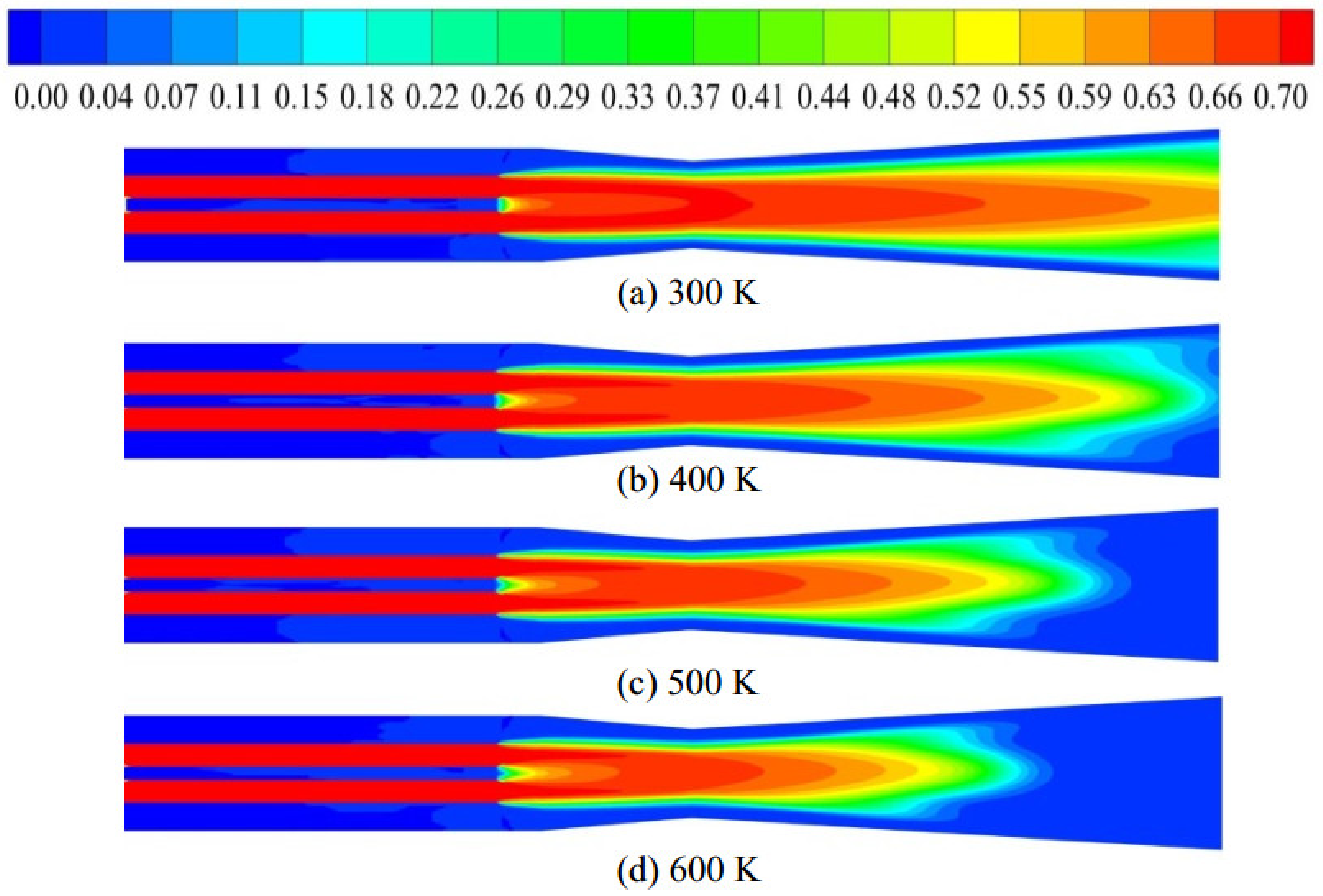
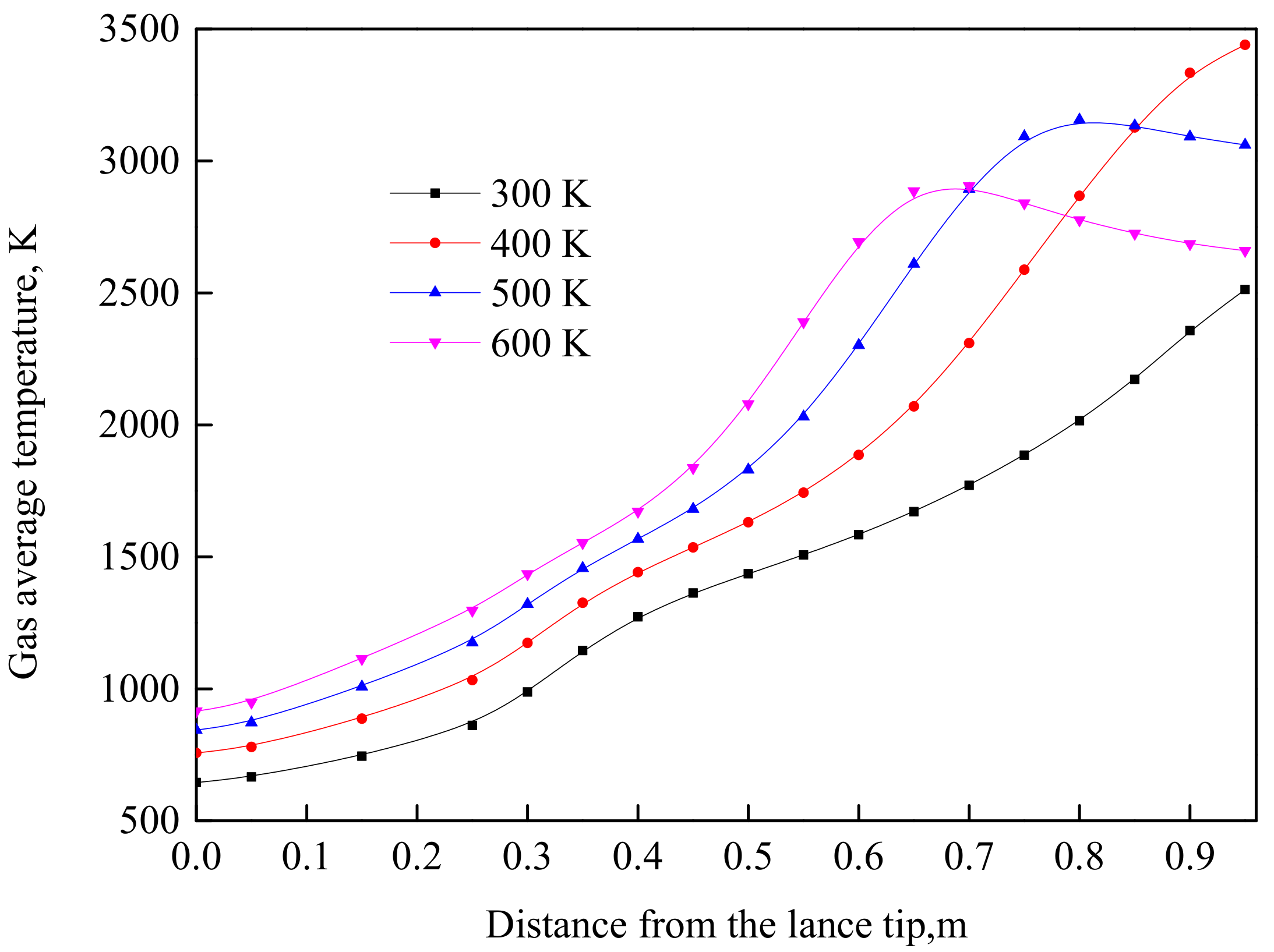
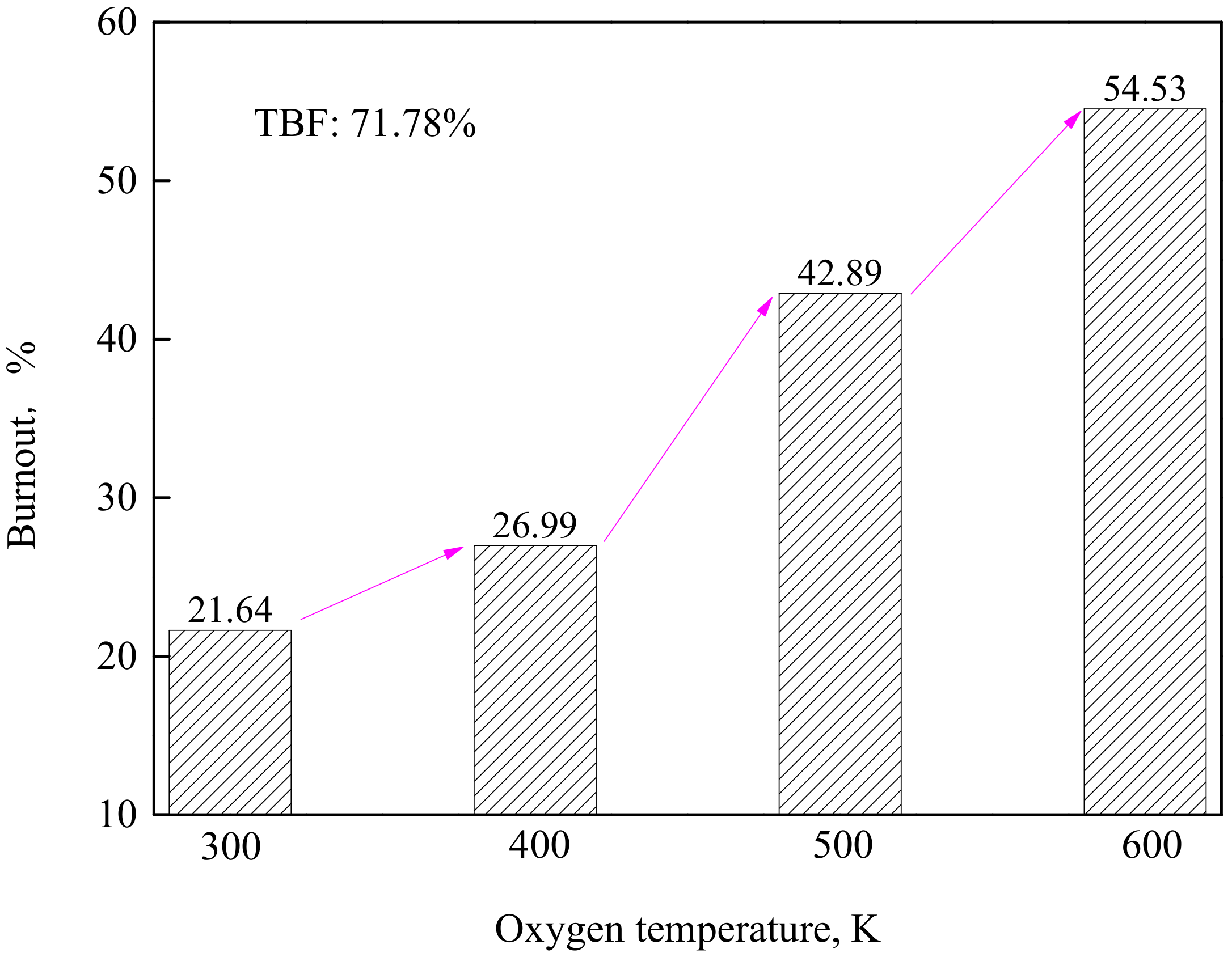
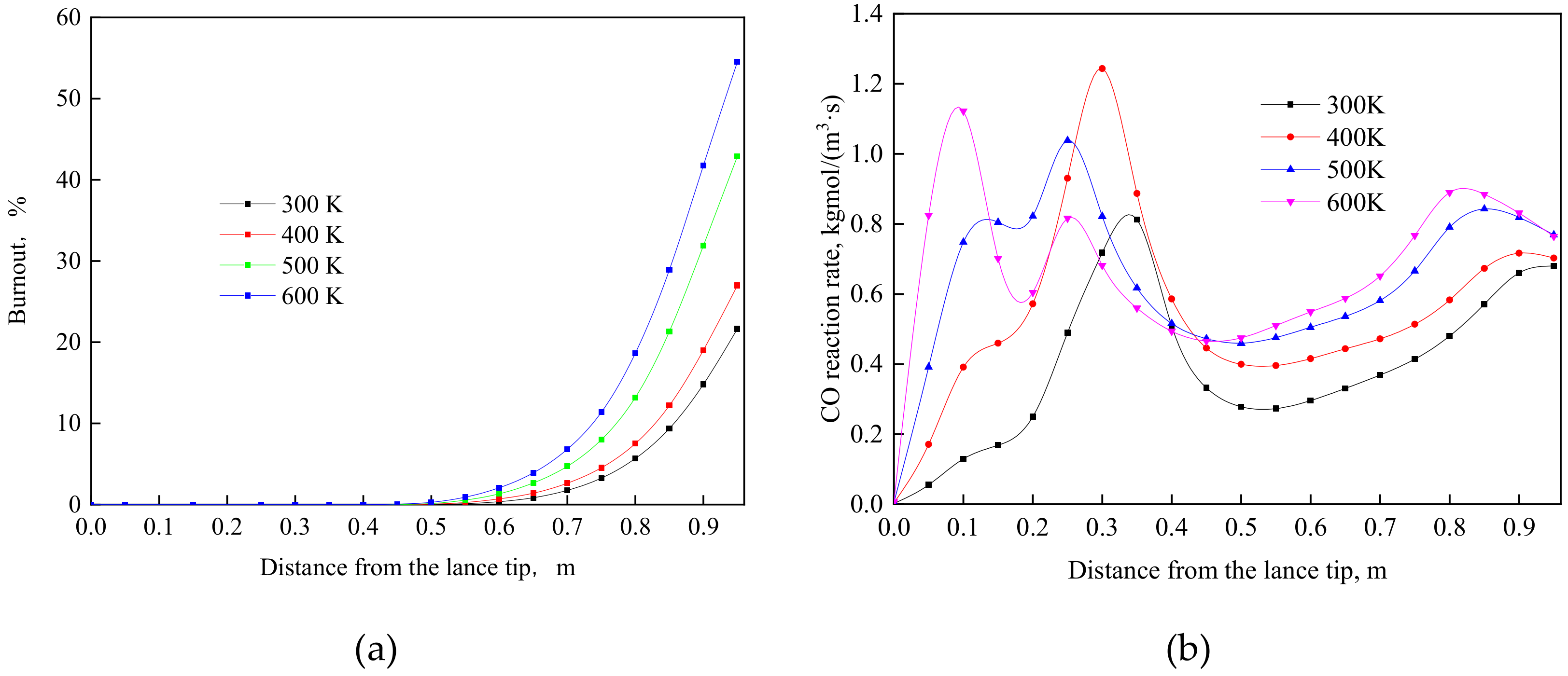
4.2. Effect of Oxygen Temperature at the Same Velocity
4.3. Effect of Oxygen Temperature at the Same Mass Flow
5. Conclusions
- (1)
- The cooling effect of room-temperature oxygen is the main reason causing a lower burnout in TGR-OBF. The coal burnout can be increased significantly by decreasing the cold oxygen volume in direct contact with coal particles. When the oxygen temperature increased from 300 to 500 K at the same velocity, the coal burnout was increased by 60.34%.
- (2)
- The initial heat for coal combustion is from recycling gas combustion. The coal combustion process will be enhanced by improving recycling gas combustion. Increasing the mixing of recycling gas and oxygen will significantly promote the combustion reaction. This is an important method to increase coal burnout of TGR-OBF.
- (3)
- When the oxygen temperature was 500 K at the same velocity, the coal combustion was weakened due to the lack of oxygen at the end of the raceway region. Increasing the oxygen content at the end of the raceway region is also an effective way to further increase coal burnout.
- (4)
- Increasing oxygen temperature is very helpful for coal combustion. The preheating method of oxygen stream will be a very important research direction in future works. Furthermore, the way of injecting the oxygen stream also has a different effect on coal combustion. This is also the focus of future investigation.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Geerdes, M.; van Laar, R.; Vaynshteyn, R. Low-cost hot metal: The future of blast furnace ironmaking. Iron Steel Technol. 2011, 8, 51. [Google Scholar]
- Chen, W.H.; Du, S.W.; Tsai, C.H.; Wang, Z.Y. Torrefied biomasses in a drop tube furnace to evaluate their utility in blast furnaces. Bioresour. Technol. 2012, 111, 433–438. [Google Scholar] [CrossRef]
- Suopajarvi, H.; Pongracz, E.; Fabritius, T. Bioreducer use in Finnish blast furnace ironmaking–Analysis of CO2 emission reduction potential and mitigation cost. Appl. Energy 2014, 124, 82–93. [Google Scholar] [CrossRef]
- Ho, C.K.; Wu, S.M.; Zhu, H.P.; Yu, A.B.; Tsai, S.T. Experimental and numerical investigations of gouge formation related to blast furnace burden distribution. Miner. Eng. 2009, 22, 986–994. [Google Scholar] [CrossRef]
- Arasto, A.; Tsupari, E.; Karki, J.; Lilja, J.; Sihvonen, M. Oxygen blast furnace with CO2 capture and storage at an integrated steel mill—Part I: Technical concept analysis. Int. J. Greenh. Gas Control 2014, 30, 140–147. [Google Scholar] [CrossRef]
- Sato, M.; Takahashi, K.; Nouchi, T.; Ariyama, T. Predictcion of Next-Generation Ironmaking Process Based on Oxygen Blast Furnace Suitable for CO2 Mitigation and Energy Flexibility. ISIJ Int. 2015, 55, 2105–2114. [Google Scholar] [CrossRef]
- Liu, L.; Jiang, Z.; Zhang, X.; Lu, Y.; He, J.; Wang, J.; Zhang, X. Effects of top gas recycling on in-furnace status, productivity, and energy consumption of oxygen blast furnace. Energy 2018, 163, 144–150. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, J.; Xue, Z. Exergy analyses of the oxygen blast furnace with top gas recycling process. Energy 2017, 121, 135–146. [Google Scholar] [CrossRef]
- Han, Y. Fundamental Research on Ironmaking Process of Top Gas Recycling-Oxygen Blast Furnace; University of Science and Technology Beijing: Beijing, China, 2012. [Google Scholar]
- Dasireddy, V.D.; Stefancic, N.S.; Hus, M.; Likozar, B. Effect of alkaline earth metal oxide (MO) Cu/MO/Al2O3 catalysts on methanol synthesis activity and selectivity via CO2 reduction. Fuel 2018, 233, 103–112. [Google Scholar] [CrossRef]
- Marlin, D.S.; Sarron, E.; Sigurbjornsson, O. Process Advantages of Direct CO2 to Methanol Synthesis. Front. Chem. 2018, 6, 446. [Google Scholar] [CrossRef]
- Bobbink, F.D.; Menoud, F.; Dyson, P.J. Synthesis of Methanol and Diols from CO2 via Cyclic Carbonates under Metal-Free, Ambient Pressure, and Solvent-Free Conditions. ACS Sustain. Chem. Eng. 2018, 6, 12119–12123. [Google Scholar] [CrossRef]
- Zhou, Z.F.; Zhang, Y.Y.; Wang, G.; Wang, J.S.; Xue, Q.G. Investigation on Coal Combustion Behaviors in the Oxygen Blast Furnace. In 8th International Symposium on High-Temperature Metallurgical Processing; Springer: San Diego, CA, USA, 2017; pp. 335–346. [Google Scholar]
- Liu, Y.R.; Shen, Y.S. Computational Fluid Dynamics Study of Biomass Combustion in a Simulated Ironmaking Blast Furnace: Effect of the Particle Shape. Energy Fuels 2017, 32, 4372–4381. [Google Scholar] [CrossRef]
- Shen, Y.S.; Yu, A.B. Modelling of injecting a ternary coal blend into a model ironmaking blast furnace. Miner. Eng. 2016, 90, 89–95. [Google Scholar] [CrossRef]
- Shen, Y.S.; Yu, A.B.; Austin, P.R.; Zulli, P. CFD study of in-furnace phenomena of pulverised coal injection in blast furnace: Effects of operating conditions. Powder Technol. 2012, 223, 27–38. [Google Scholar] [CrossRef]
- Du, S.W.; Yeh, C.P.; Chen, W.H.; Tsai, C.H.; Lucas, J.A. Burning characteristics of pulverized coal within blast furnace raceway at various injection operations and ways of oxygen enrichment. Fuel 2015, 143, 98–106. [Google Scholar] [CrossRef]
- Du, S.W.; Chen, W.H.; Lucas, J. Performances of pulverized coal injection in blowpipe and tuyere at various operational conditions. Energy Convers. Manag. 2007, 48, 2069–2076. [Google Scholar] [CrossRef]
- Yeh, C.P.; Du, S.W.; Tsai, C.H.; Yang, R.J. Numerical analysis of flow and combustion behavior in tuyere and raceway of blast furnace fueled with pulverized coal and recycled top gas. Energy 2012, 42, 233–240. [Google Scholar] [CrossRef]
- Sahu, R.K.; Roy, S.K.; Sen, P.K. Applicability of top gas recycle blast furnace with downstream integration and sequestration in an integrated steel plant. Steel Res. Int. 2015, 86, 502–516. [Google Scholar] [CrossRef]
- Helle, M.; Saxen, H. Operation Windows of the Oxygen Blast Furnace with Top Gas Recycling. ISIJ Int. 2015, 55, 2047–2055. [Google Scholar] [CrossRef]
- Xuan, W.W.; Guan, Q.L.; Zhang, J.S. Kinetic model and CFD simulation for an entrained flow coal hydrogasifier and influence of structural parameters. Int. J. Hydrogen Energy 2016, 41, 20023–20035. [Google Scholar] [CrossRef]
- Llbas, M.; Karyeyen, S. A numerical study on combustion behaviours of hydrogen-enriched low calorific value coal gases. Int. J. Hydrogen Energy 2015, 40, 15218–15226. [Google Scholar]
- Zhou, Z.F.; Xue, Q.G.; Li, C.L.; Wang, G.; She, X.F.; Wang, J.S. Coal flow and combustion characteristics under oxygen enrichment way of oxygen-coal double lance. Appl. Therm. Eng. 2017, 123, 1096–1105. [Google Scholar] [CrossRef]
- Zhou, Z.F.; Huo, H.L.; Wang, G.; Xue, Q.G.; She, X.F.; Wang, J.S. Effect of Oxygen-Coal Lance Configurations on Coal Combustion Behavior. Steel Res. Int. 2017, 88, 1600197. [Google Scholar] [CrossRef]
- Zhou, Z.F.; Liu, Y.L.; Wang, G.; She, X.F.; Xue, Q.G.; Wang, J.S. Effect of Local Oxygen-enrichment Ways of Oxygen-coal Double Lance on Coal Combustion. ISIJ Int. 2017, 57, 279–285. [Google Scholar] [CrossRef]
- Shen, Y.S.; Maldonado, D.; Guo, B.Y.; Yu, A.B.; Austin, P.; Zulli, P. Computational fluid dynamics study of pulverized coal combustion in blast furnace raceway. Ind. Eng. Chem. Res. 2009, 48, 10314–10323. [Google Scholar] [CrossRef]
- Wijayanta, A.T.; Alam, M.S.; Nakaso, K.; Fukai, J.; Kunitomo, K.; Shimizu, M. Combustibility of biochar injected into the raceway of a blast furnace. Fuel Process. Technol. 2014, 117, 53–59. [Google Scholar] [CrossRef]
- Ou, Z.S.; Jin, H.; Ren, Z.H.; Zhu, S.X.; Song, M.M.; Guo, L.J. Mathematical model for coal conversion in supercritical water: Reacting multiphase flow with conjugate heat transfer. Int. J. Hydrogen Energy 2018, 44, 15746–15757. [Google Scholar] [CrossRef]
- Jin, H.H.; Xu, B.N.; Li, H.Q.; Ku, X.K.; Fan, J.R. Numerical investigation of coal gasification in supercritical water with the ReaxFF molecular dynamics method. Int. J. Hydrogen Energy 2018, 43, 20513–20524. [Google Scholar] [CrossRef]
- Shen, Y.S.; Yu, A.B. Characterization of Coal Burnout in the Raceway of an Ironmaking Blast Furnace. Steel Res. Int. 2015, 86, 604–611. [Google Scholar] [CrossRef]
- Wang, J.; Lou, H.H.; Yang, F.; Cheng, F. Numerical simulation of a decoupling and Re-burning combinative Low-NOx coal grate boiler. J. Clean. Prod. 2018, 188, 977–988. [Google Scholar] [CrossRef]
- Baum, M.M.; Street, P.J. Predicting the combustion behaviour of coal particles. Combust. Sci. Technol. 1971, 3, 231–243. [Google Scholar] [CrossRef]

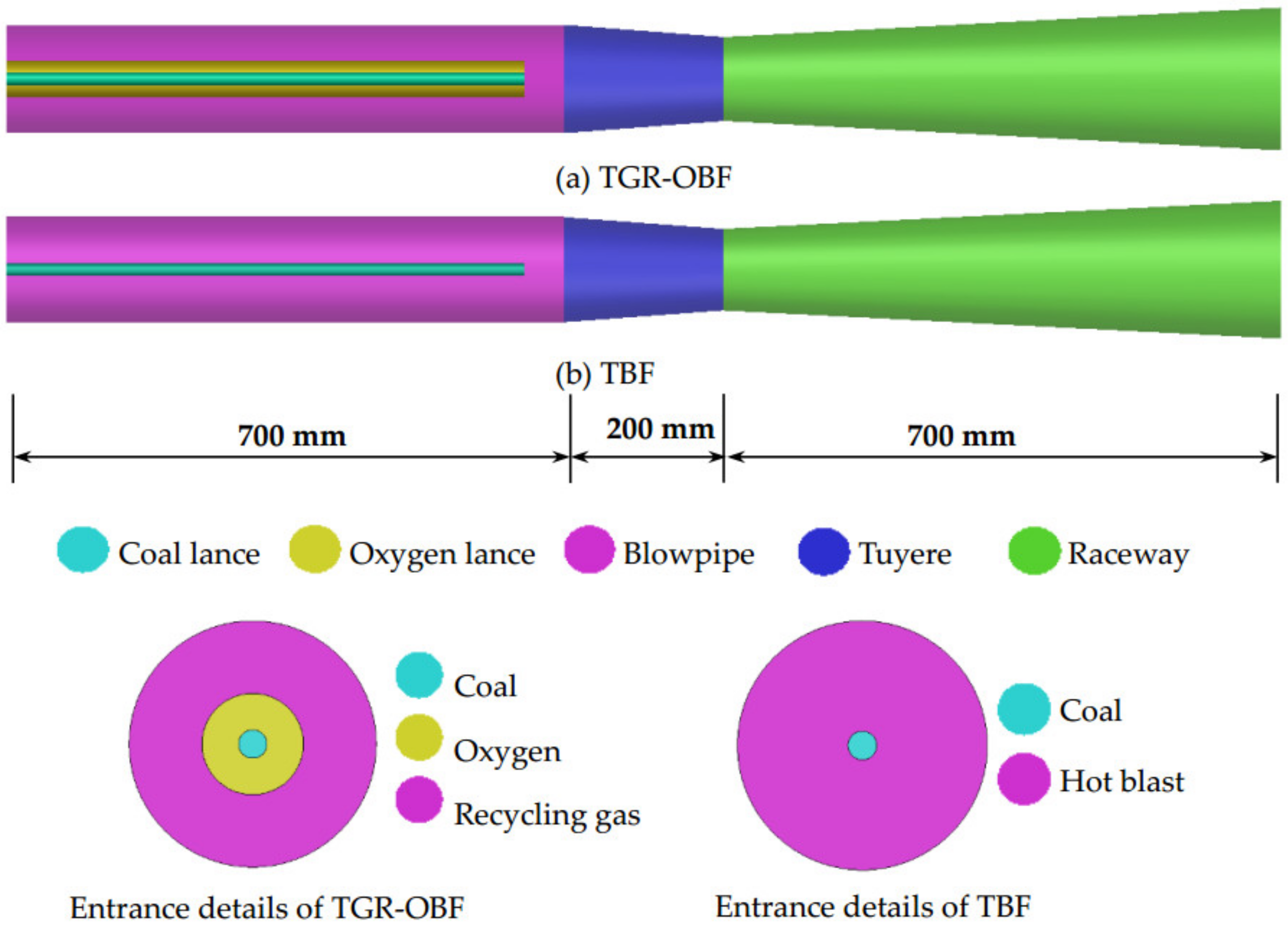




| Blast Volume (Nm3/t) | Blast Temperature (K) | Volume (m3) | Coal Ratio (kg/t) |
|---|---|---|---|
| 1129 | 1473 | 120 | 150 |
| Oxygen | Recycling Top Gas | |||||||
|---|---|---|---|---|---|---|---|---|
| Volume Nm3/t | Content % | Volume Nm3/t | Temperature K | CO % | H2 % | CO2 % | N2 % | H2O % |
| 366 | 70 | 280 | 1173 | 52.58 | 10.81 | 4.5 | 31.49 | 0.62 |
| Proximate Analysis (wt %) | Ultimate Analysis | Size Distribution | ||
|---|---|---|---|---|
| Moisture | 1 | C | 88.78 | 90 μm: 5% |
| Volatiles | 20.02 | H | 4.54 | 63 μm: 25% |
| Ash | 8.32 | O | 4.68 | 45 μm: 55% |
| Fixed carbon | 70.66 | N | 2 | 20 μm: 15% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Z.; Yi, Q.; Wang, R.; Wang, G.; Ma, C. Numerical Investigation on Coal Combustion in Ultralow CO2 Blast Furnace: Effect of Oxygen Temperature. Processes 2020, 8, 877. https://doi.org/10.3390/pr8070877
Zhou Z, Yi Q, Wang R, Wang G, Ma C. Numerical Investigation on Coal Combustion in Ultralow CO2 Blast Furnace: Effect of Oxygen Temperature. Processes. 2020; 8(7):877. https://doi.org/10.3390/pr8070877
Chicago/Turabian StyleZhou, Zhenfeng, Qiujie Yi, Ruihao Wang, Guang Wang, and Chunyuan Ma. 2020. "Numerical Investigation on Coal Combustion in Ultralow CO2 Blast Furnace: Effect of Oxygen Temperature" Processes 8, no. 7: 877. https://doi.org/10.3390/pr8070877
APA StyleZhou, Z., Yi, Q., Wang, R., Wang, G., & Ma, C. (2020). Numerical Investigation on Coal Combustion in Ultralow CO2 Blast Furnace: Effect of Oxygen Temperature. Processes, 8(7), 877. https://doi.org/10.3390/pr8070877




