Recent Insights into Lignocellulosic Biomass Pyrolysis: A Critical Review on Pretreatment, Characterization, and Products Upgrading
Abstract
1. Introduction
2. Lignocellulosic Biomass
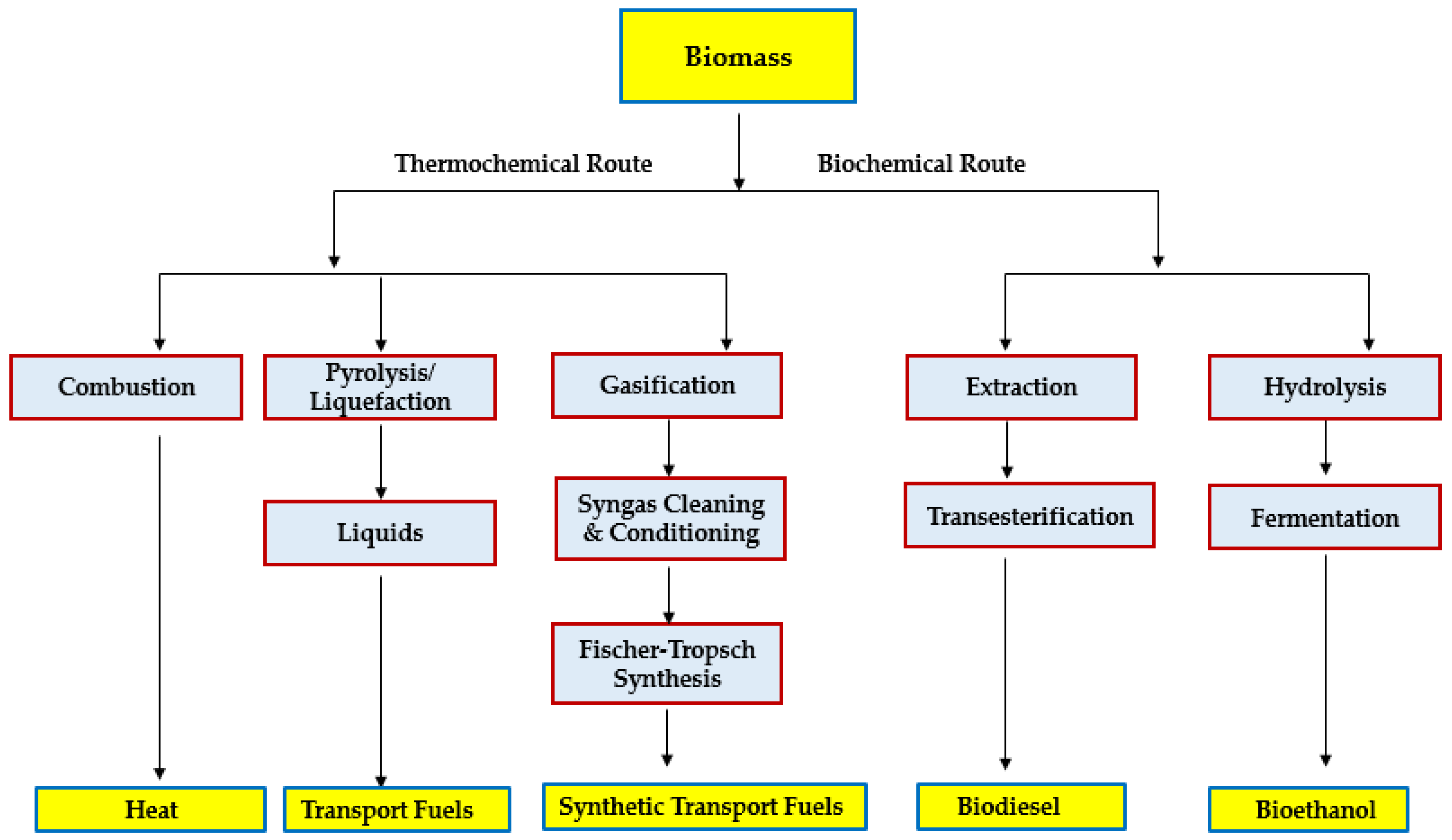
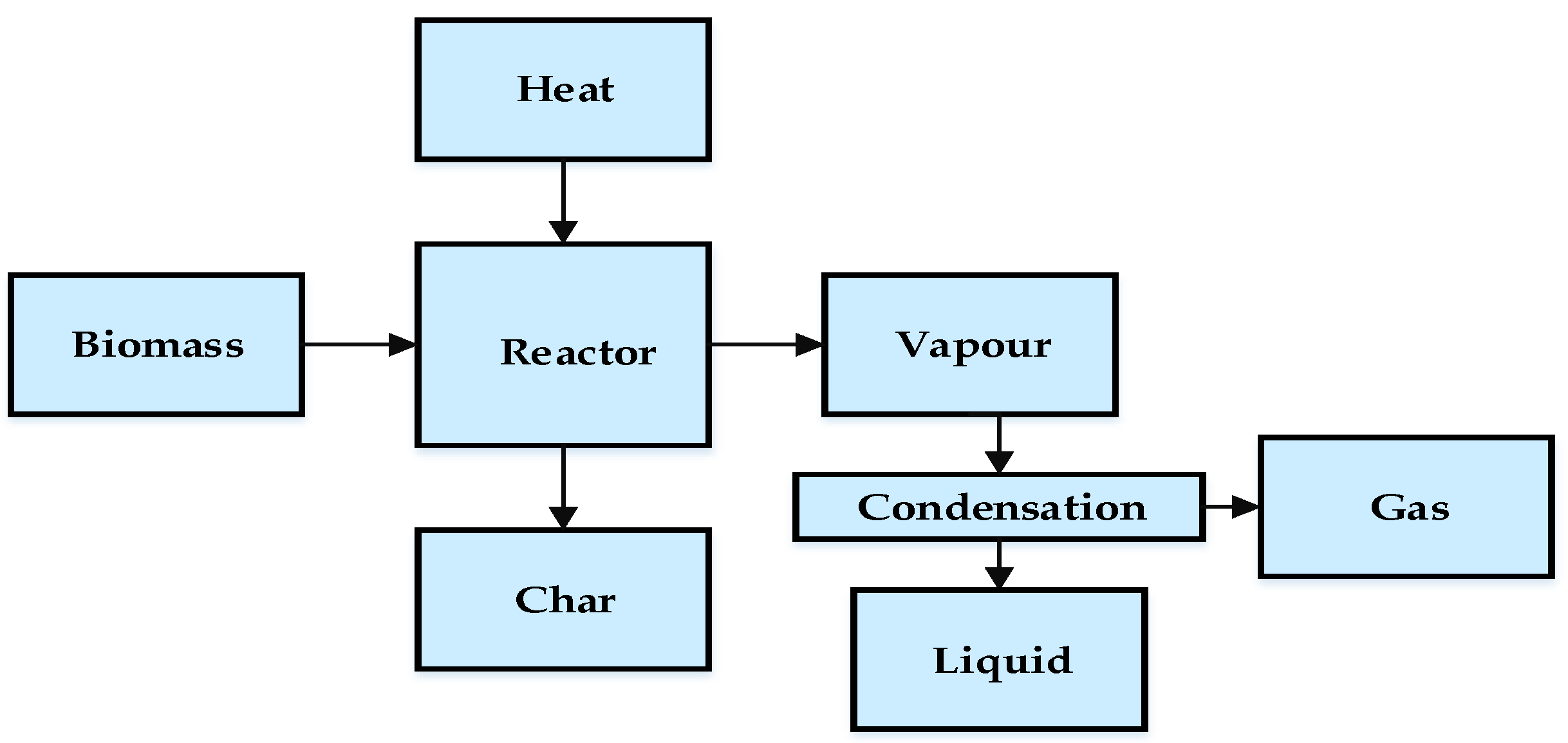
3. Biomass Pyrolysis
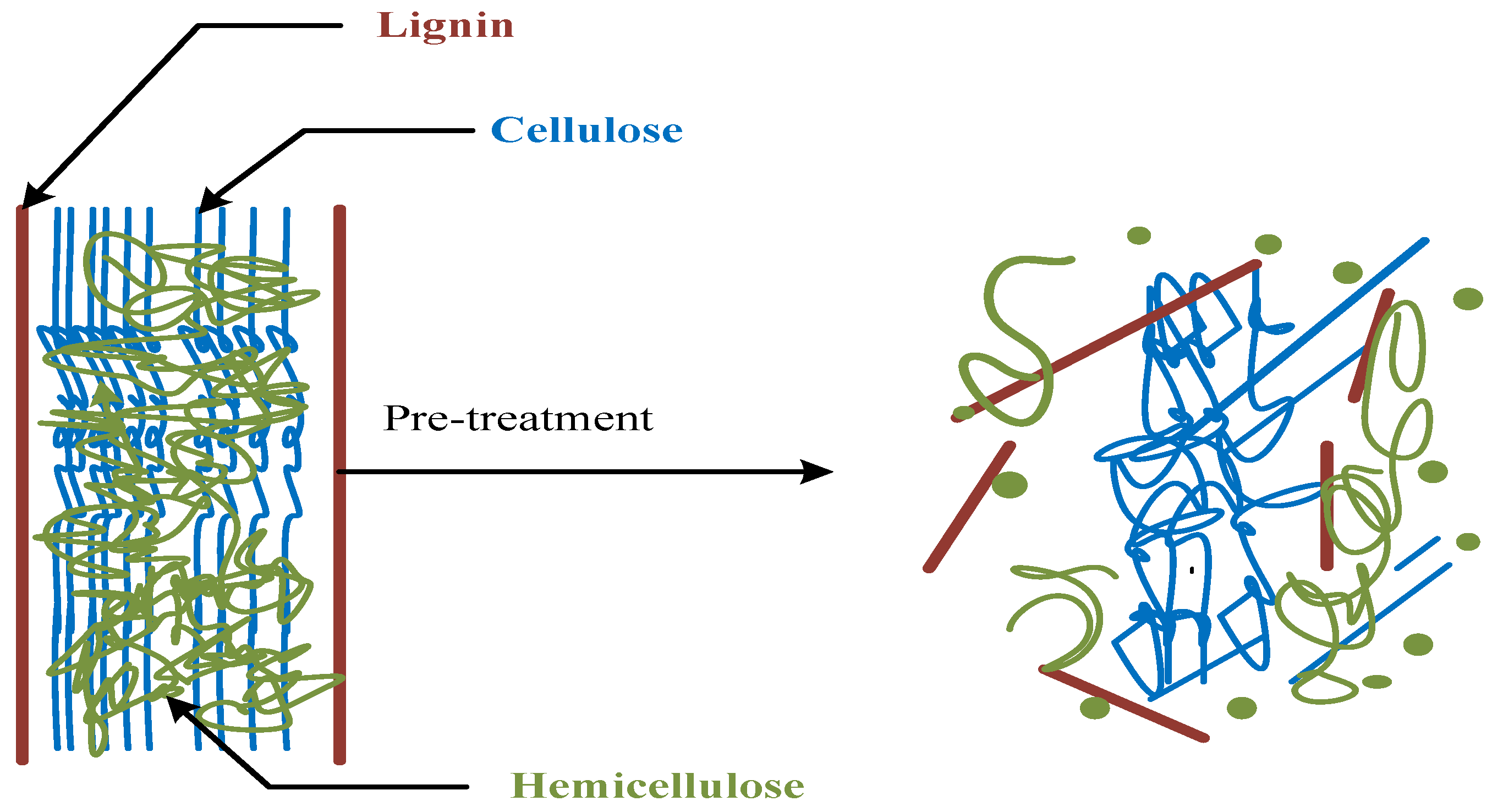
4. Biomass Pretreatment
4.1. Physical Pretreatment
4.2. Chemical Pretreatment
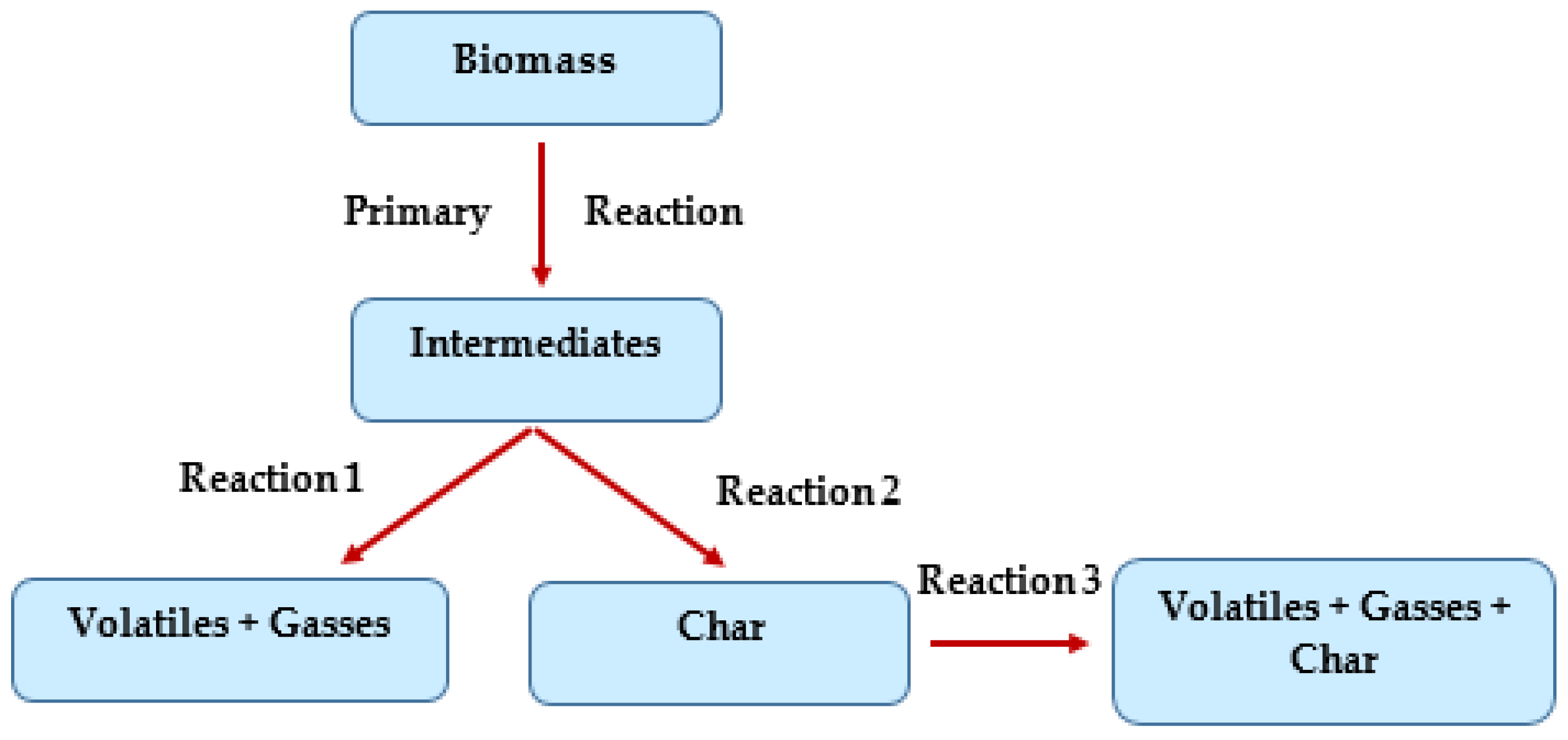
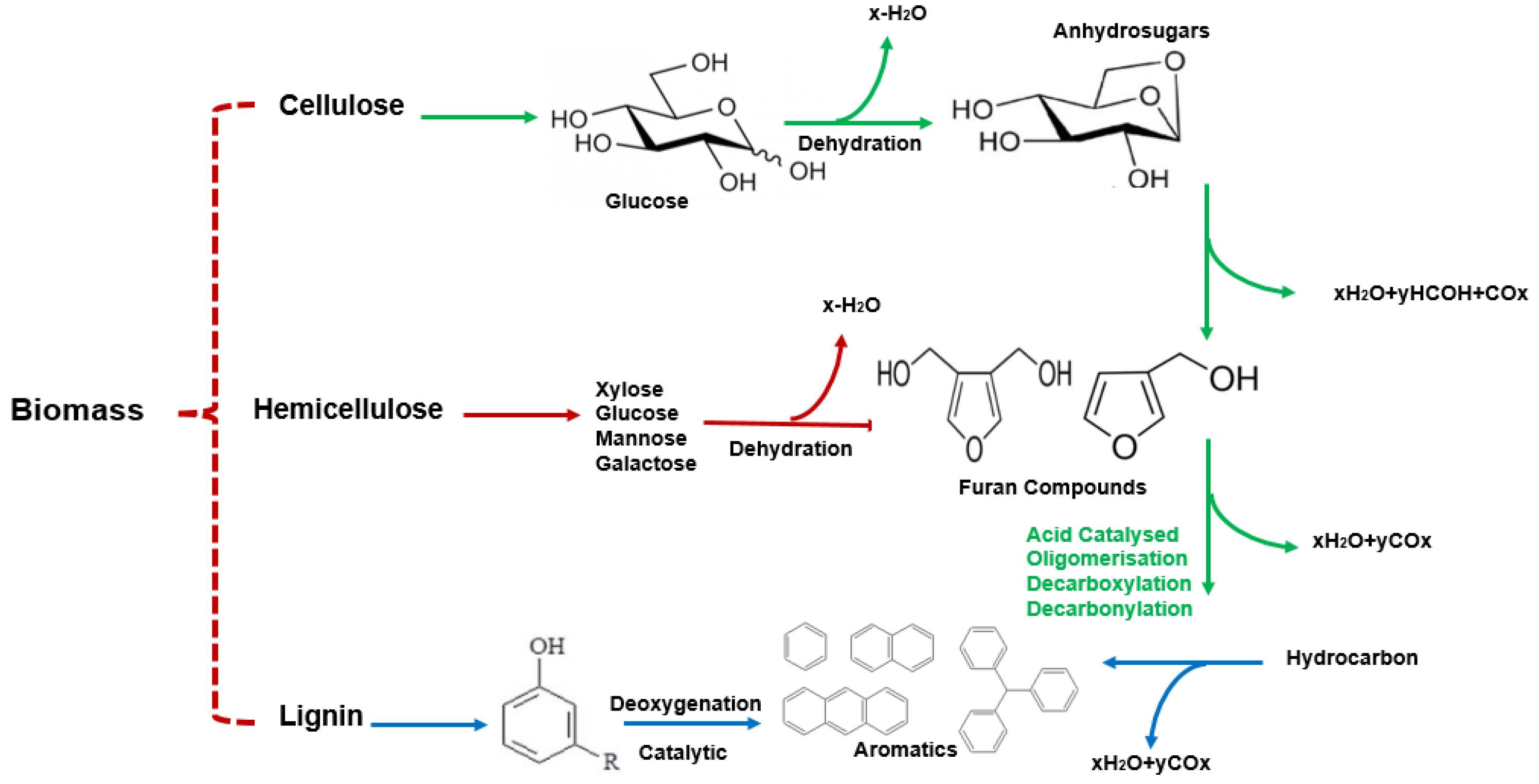
5. Pyrolysis Mechanism
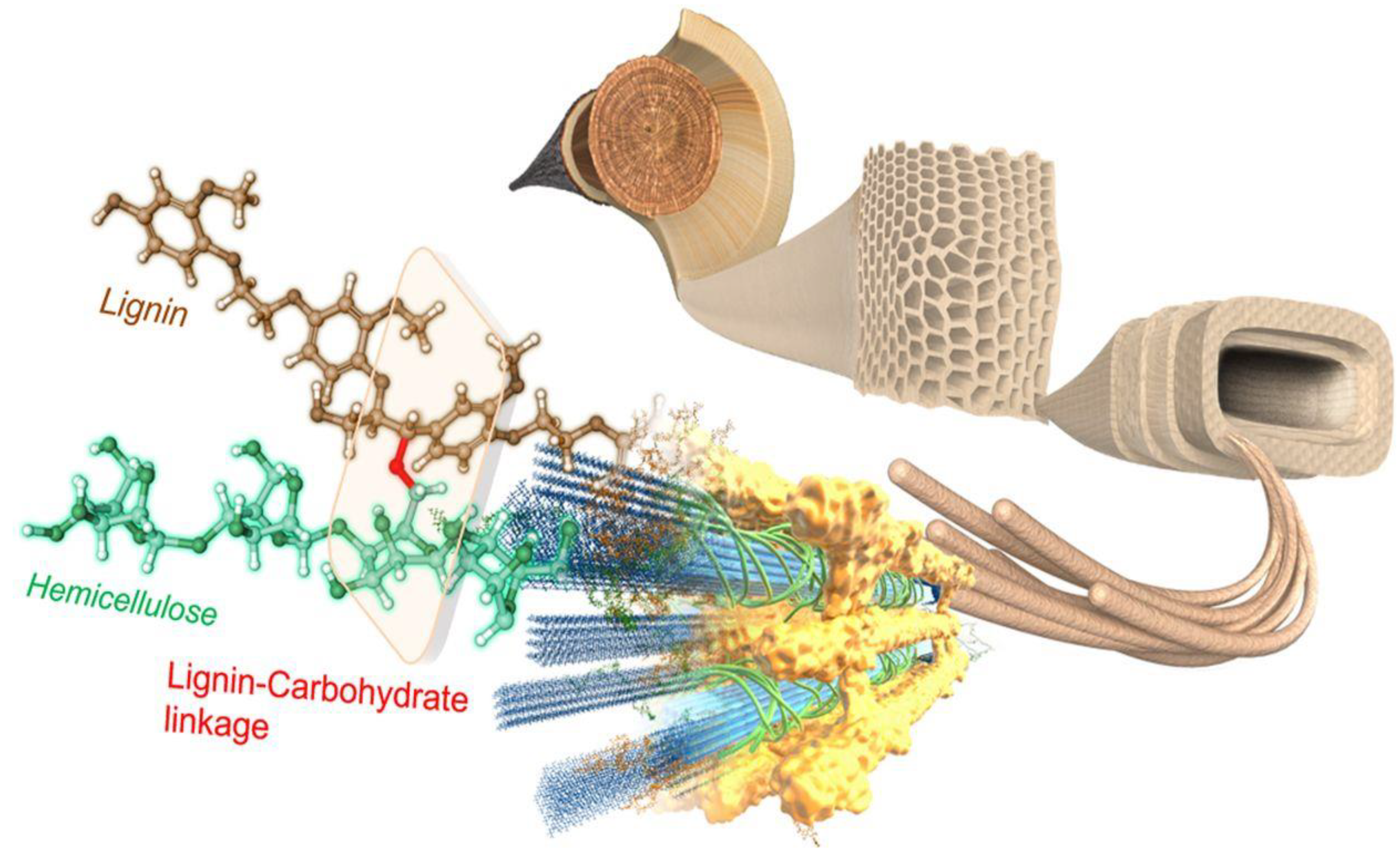
5.1. Composition of Lignocellulosic Biomass
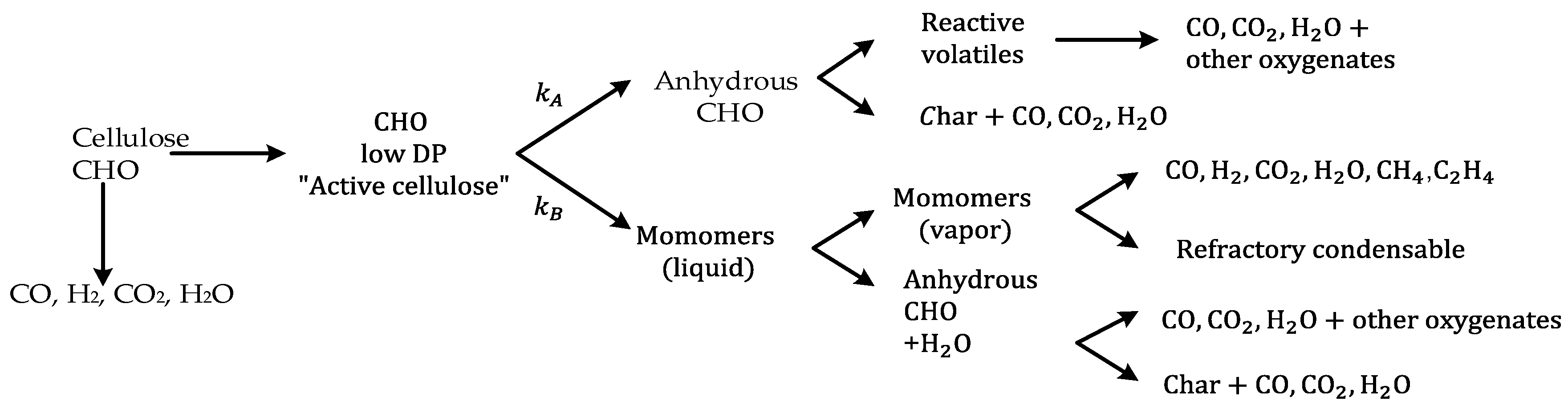
5.2. Cellulose Pyrolysis
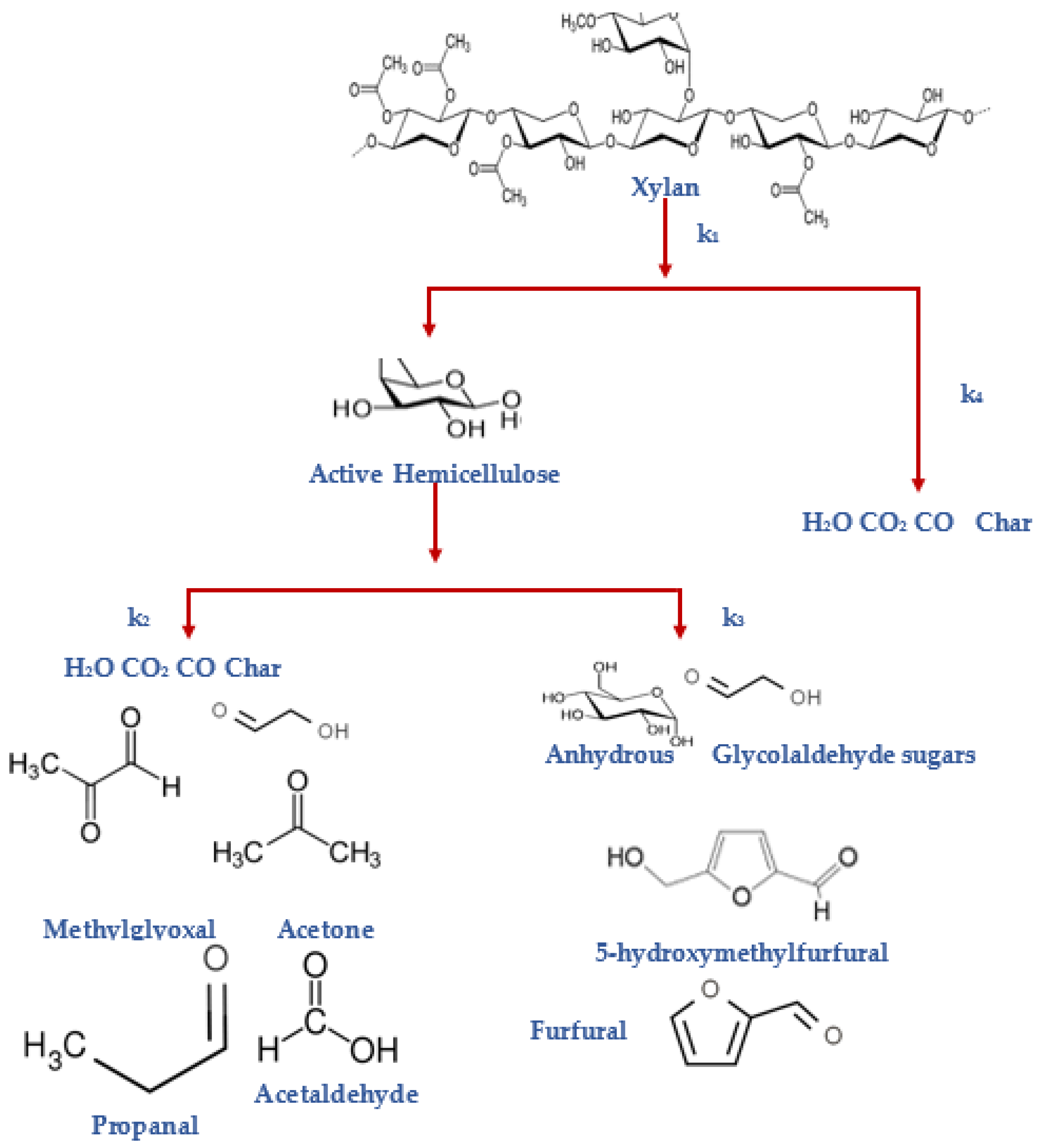
5.3. Hemicellulose Pyrolysis
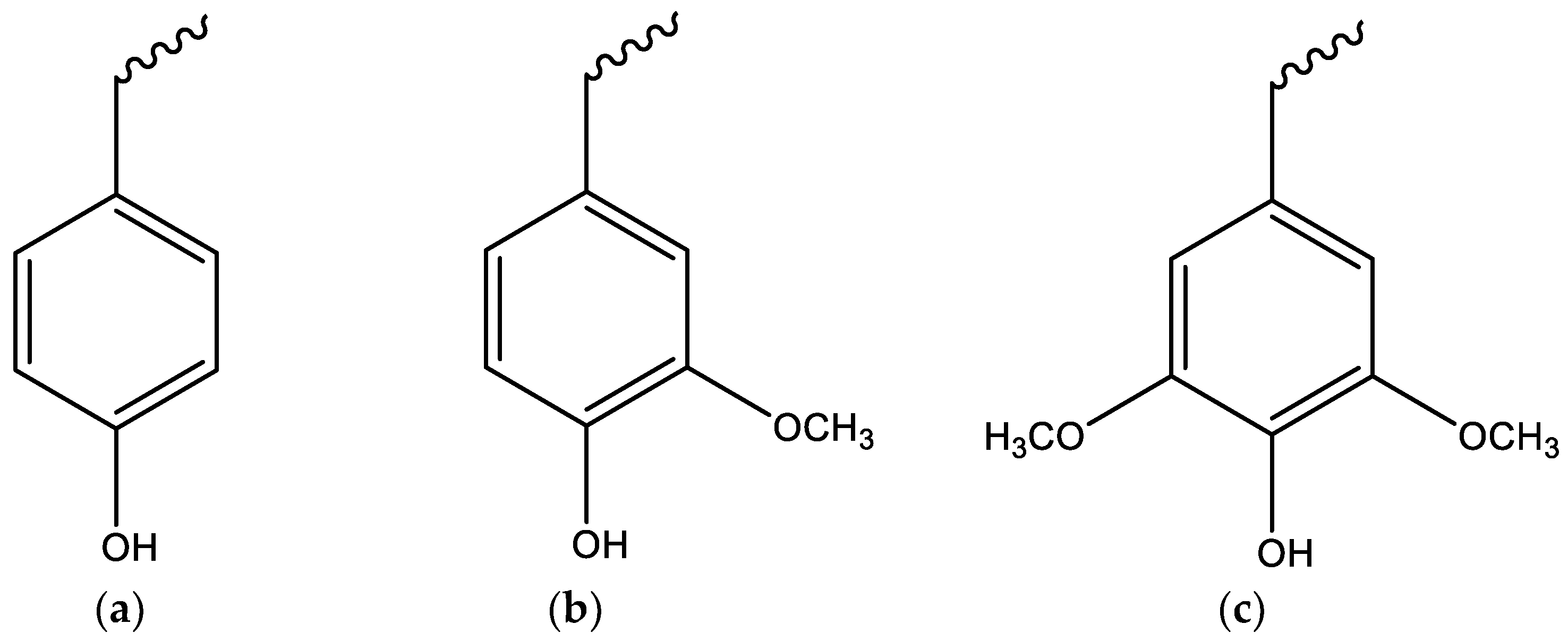
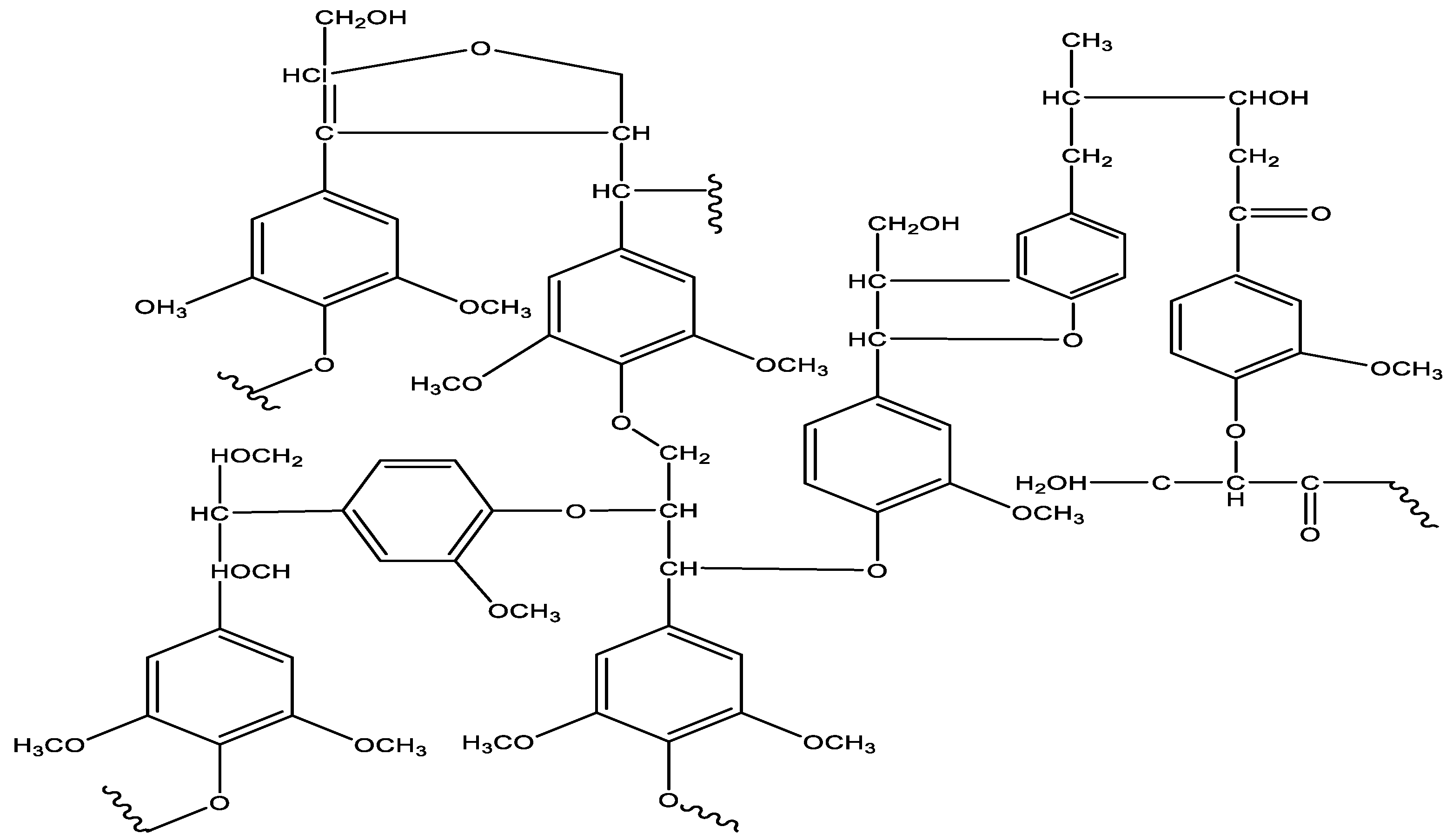
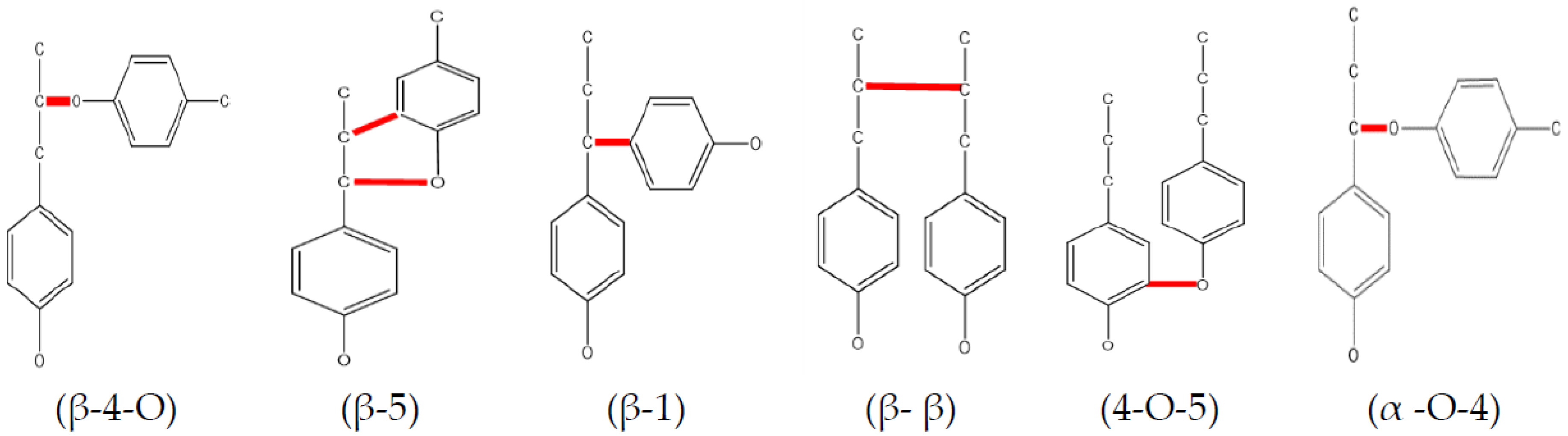
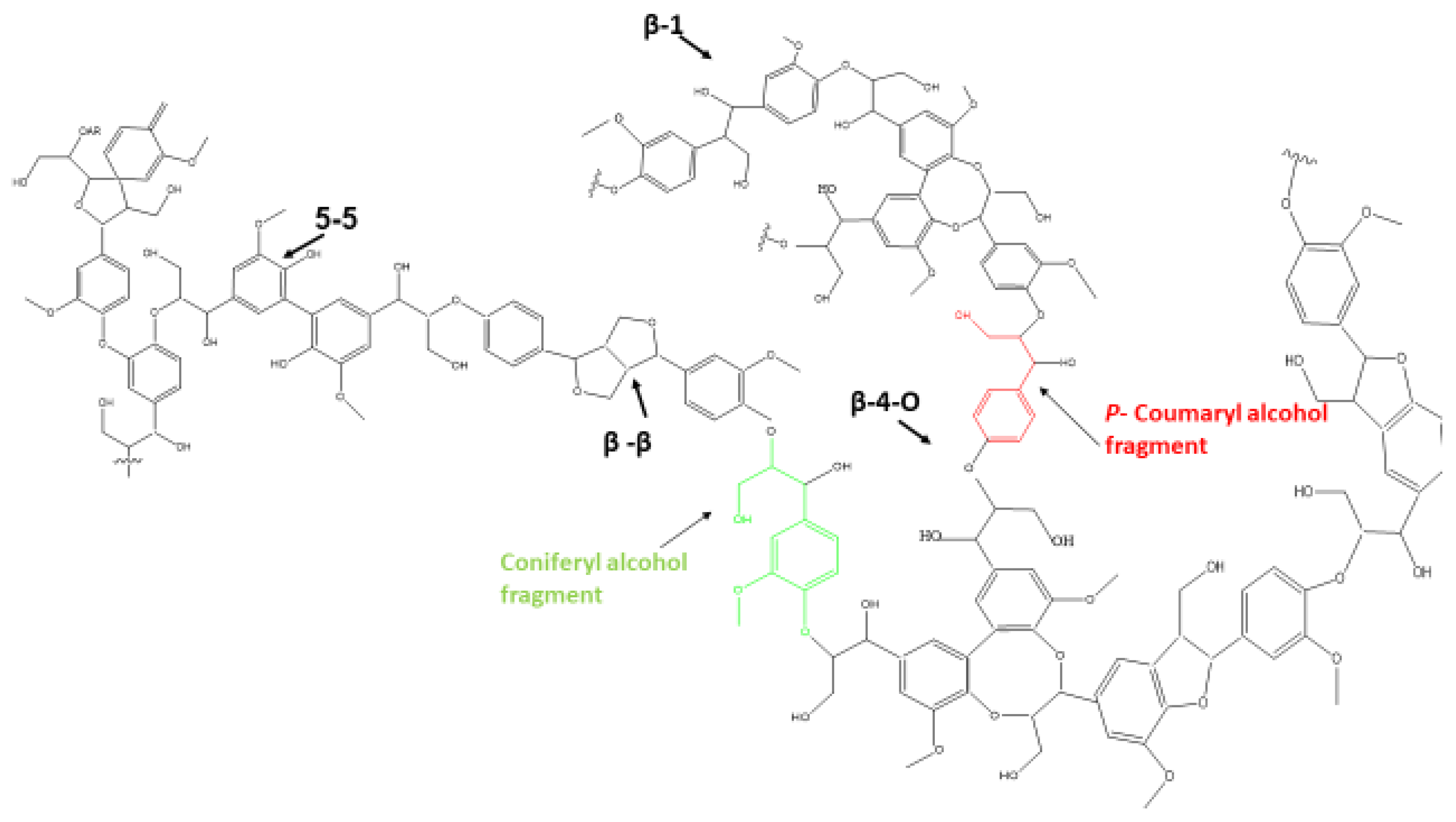
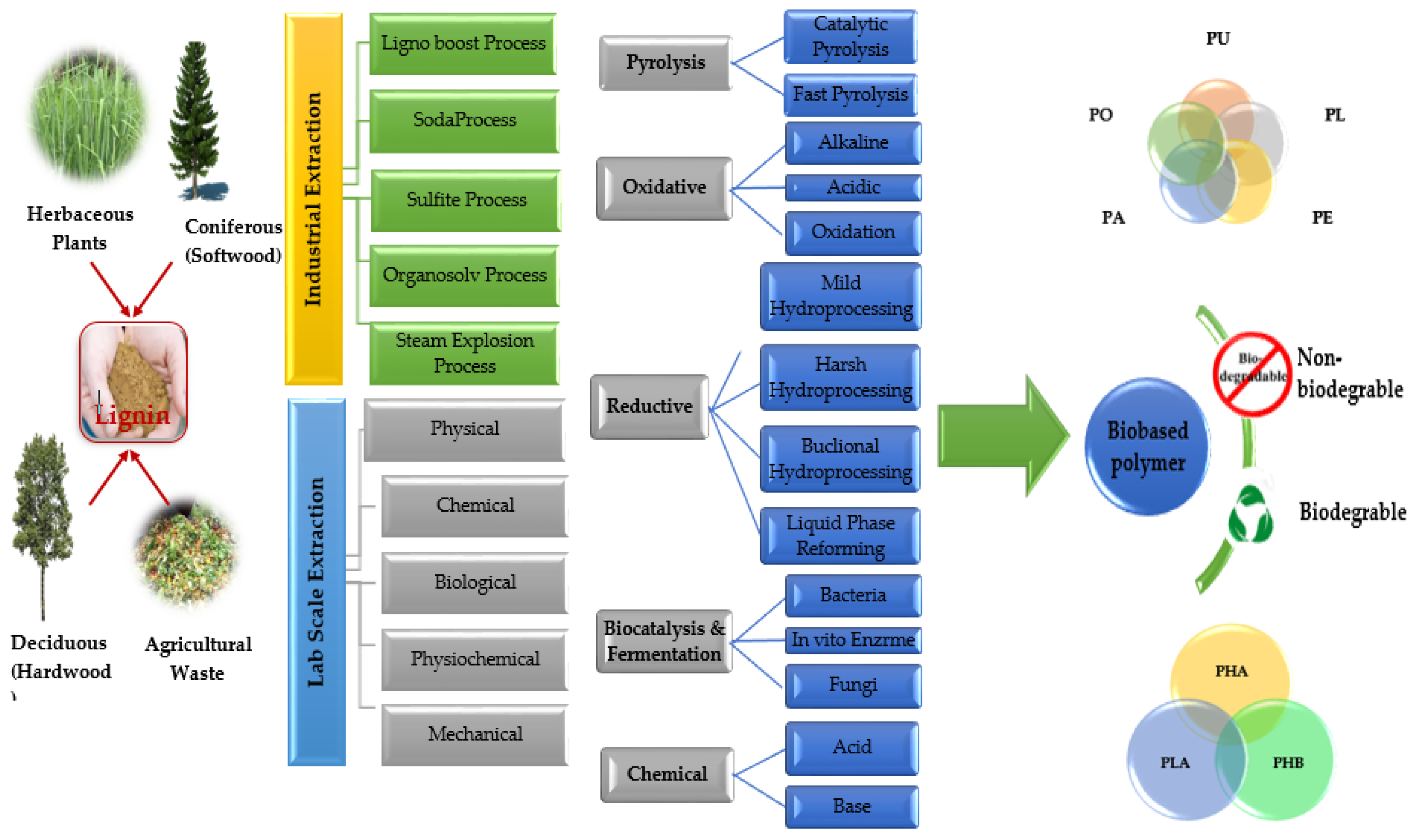
5.4. Lignin Pyrolysis
6. Pyrolysis Products
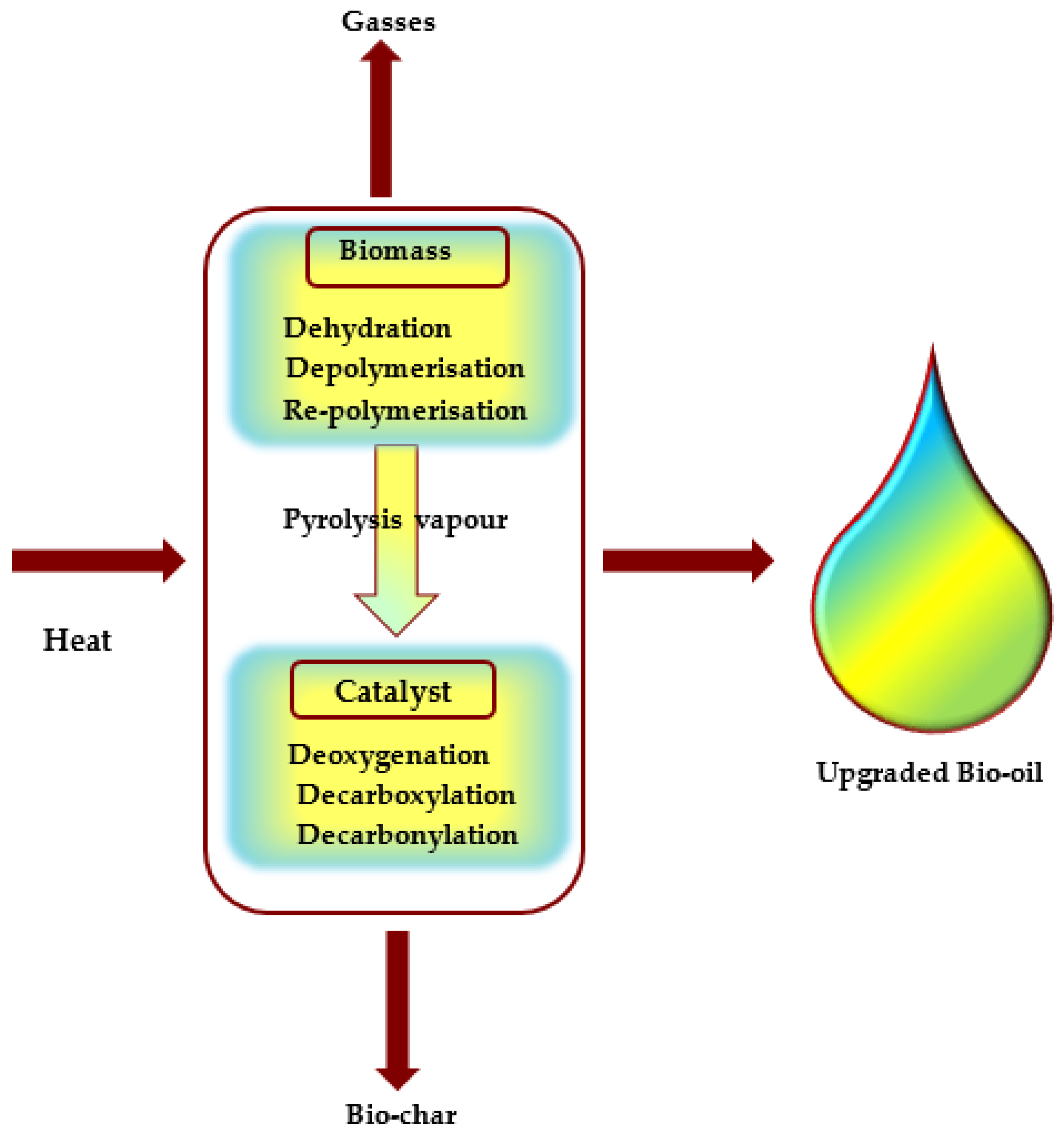
6.1. Bio-Oil
6.2. Biochar
6.3. Pyrolytic Gas
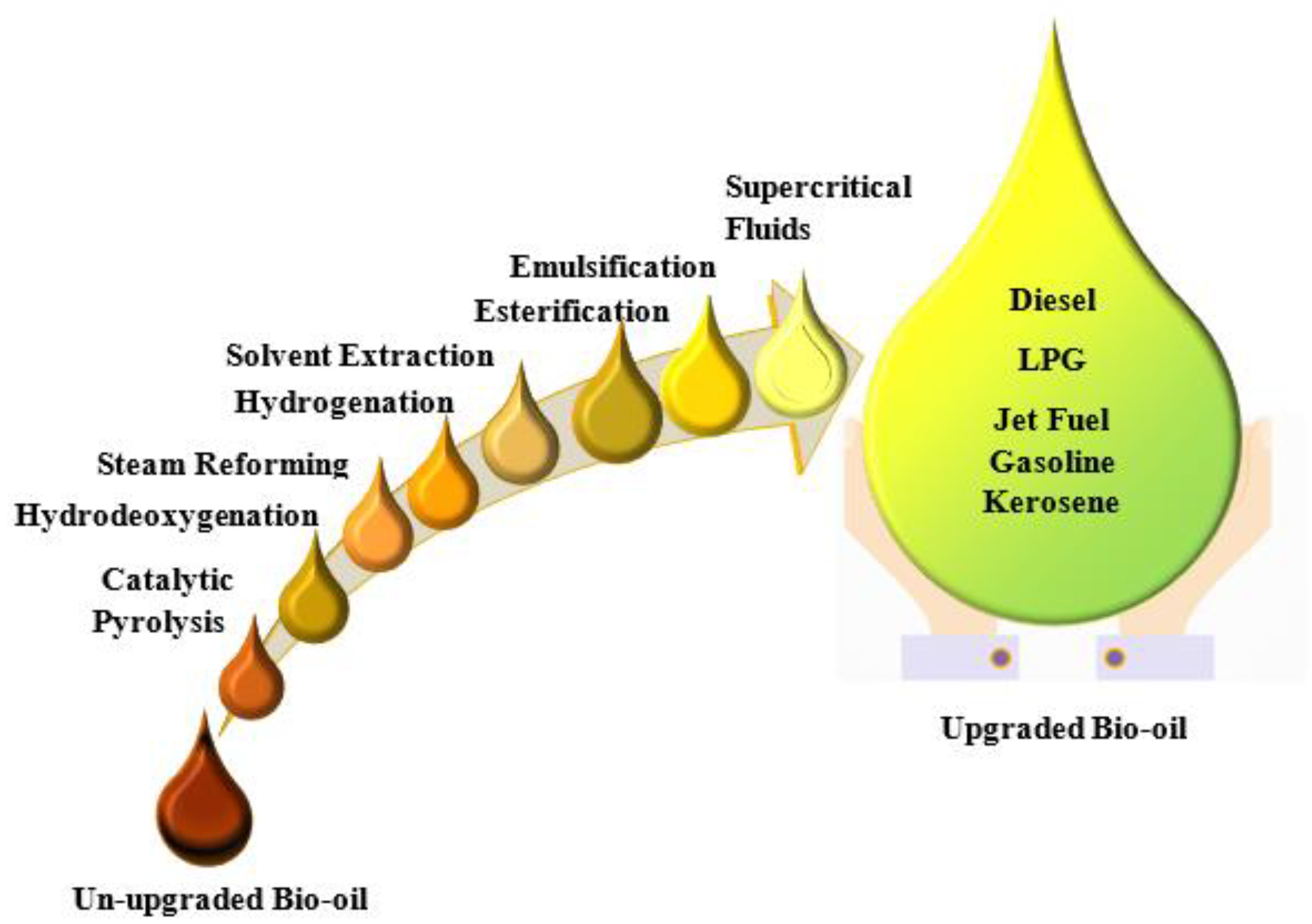
7. Upgrading
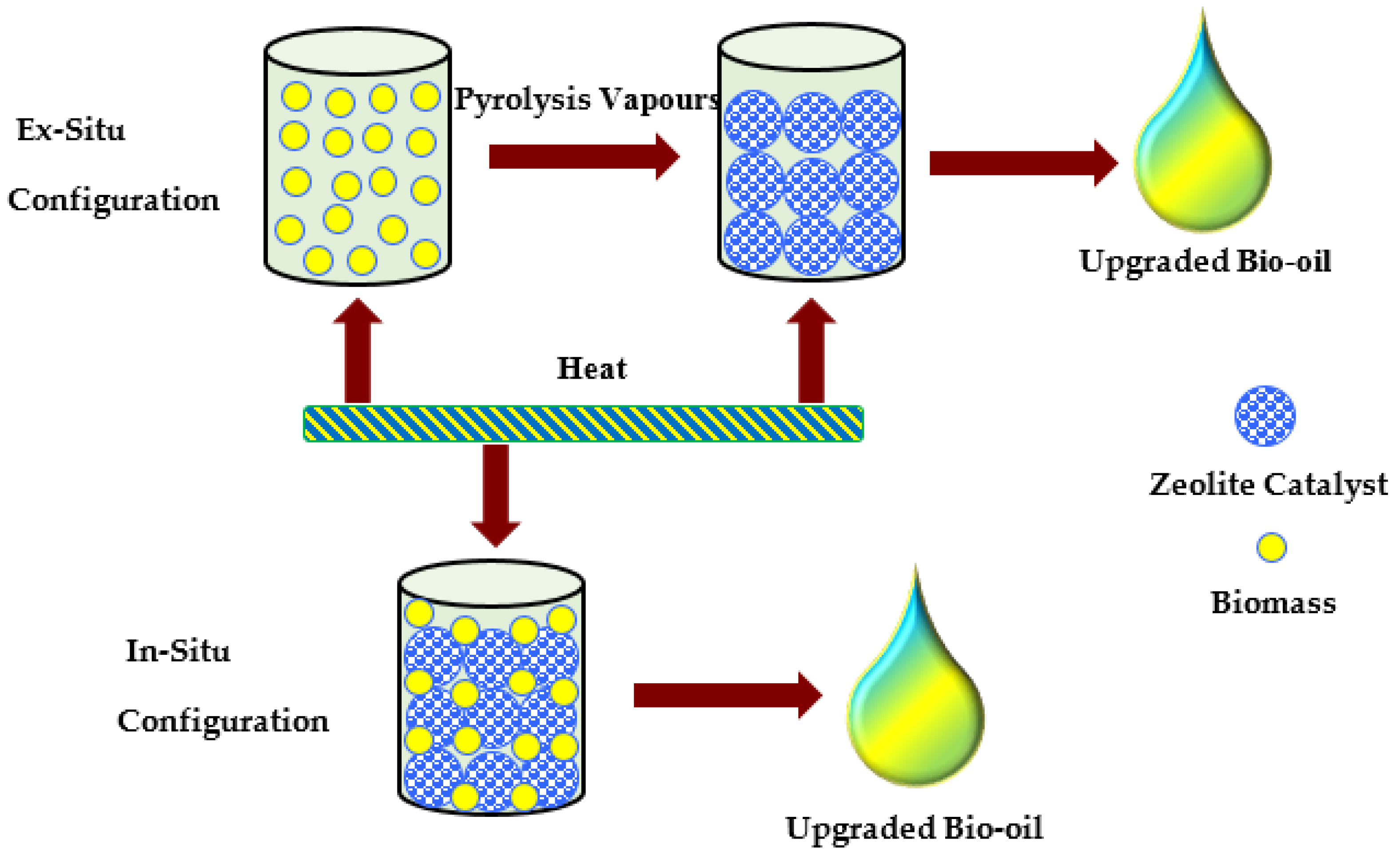
Catalytic Upgrading
8. Conclusions and Perspective
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, X.; Che, Q.; Li, S.; Liu, Z.; Yang, H.; Chen, Y.; Wang, X.; Shao, J.; Chen, H. Recent developments in lignocellulosic biomass catalytic fast pyrolysis: Strategies for the optimization of bio-oil quality and yield. Fuel Process. Technol. 2019, 196, 106180. [Google Scholar] [CrossRef]
- Aboelazayem, O.; Gadalla, M.; Saha, B. Design and simulation of an integrated process for biodiesel production from waste cooking oil using supercritical methanolysis. Energy 2018, 161, 299–307. [Google Scholar] [CrossRef]
- Aboelazayem, O.; Gadalla, M.; Saha, B. Valorisation of high acid value waste cooking oil into biodiesel using supercritical methanolysis: Experimental assessment and statistical optimisation on typical Egyptian feedstock. Energy 2018, 162, 408–420. [Google Scholar] [CrossRef]
- Dai, L.; Wang, Y.; Liu, Y.; Ruan, R.; He, C.; Yu, Z.; Jiang, L.; Zeng, Z.; Tian, X. Integrated process of lignocellulosic biomass torrefaction and pyrolysis for upgrading bio-oil production: A state-of-the-art review. Renew. Sustain. Energy Rev. 2019, 107, 20–36. [Google Scholar] [CrossRef]
- Fakayode, O.A.; Aboagarib, E.A.A.; Zhou, C.; Ma, H. Co-pyrolysis of lignocellulosic and macroalgae biomasses for the production of biochar—A review. Bioresour. Technol. 2020, 297, 122–408. [Google Scholar] [CrossRef]
- Aboelazayem, O.; Gadalla, M.; Saha, B. An experimental-based energy integrated process for Biodiesel production from waste cooking oil using supercritical methanol. Chem. Eng. Trans. 2017, 61, 1645–1650. [Google Scholar]
- Aboelazayem, O.; Gadalla, M.; Saha, B. Derivatisation-free characterisation and supercritical conversion of free fatty acids into biodiesel from high acid value waste cooking oil. Renew. Energy 2019, 143, 77–90. [Google Scholar] [CrossRef]
- Umar, Y.; Aboelazayem, O.; Echresh, Z.; Gadalla, M.; Saha, B. Waste cooking oil valorisation into biodiesel using supercritical methanolysis: Critical assessment on the effect of water content. In Proceedings of the EUBCE 2019—27th European Biomass Conference and Exhibition, Lisbon, Portugal, 26–31 May 2019; pp. 1495–1500. [Google Scholar]
- Ren, S.; Ye, X.P.; Borole, A.P. Separation of chemical groups from bio-oil water-extract via sequential organic solvent extraction. J. Anal. Appl. Pyrolysis 2017, 123, 30–39. [Google Scholar] [CrossRef]
- Mehmood, N.; Alayoubi, R.; Husson, E.; Jacquard, C.; Buchs, J.; Sarazin, C.; Gosselin, I. Kluyveromyces marxianus, an attractive yeast for ethanolic fermentation in presence of ionic liquids. Int. J. Mol. Sci. 2018, 19, 887. [Google Scholar] [CrossRef]
- Rodriguez Correa, C.; Kruse, A. Supercritical water gasification of biomass for hydrogen production—Review. J. Supercrit. Fluids 2018, 133, 573–590. [Google Scholar] [CrossRef]
- Lee, K.T.; Lim, S.; Pang, Y.L.; Ong, H.C.; Chong, W.T. Integration of reactive extraction with supercritical fluids for process intensification of biodiesel production: Prospects and recent advances. Prog. Energy Combust. Sci. 2014, 45, 54–78. [Google Scholar] [CrossRef]
- Alayoubi, R.; Mehmood, N.; Husson, E.; Kouzayha, A.; Tabcheh, M.; Chaveriat, L.; Sarazin, C.; Gosselin, I. Low temperature ionic liquid pretreatment of lignocellulosic biomass to enhance bioethanol yield. Renew. Energy 2020, 145, 1808–1816. [Google Scholar] [CrossRef]
- Alaswad, A.; Dassisti, M.; Prescott, T.; Olabi, A.G. Technologies and developments of third generation biofuel production. Renew. Sustain. Energy Rev. 2015, 51, 1446–1460. [Google Scholar] [CrossRef]
- Damartzis, T.; Zabaniotou, A. Thermochemical conversion of biomass to second generation biofuels through integrated process design-A review. Renew. Sustain. Energy Rev. 2011, 15, 366–378. [Google Scholar] [CrossRef]
- Wang, S.; Dai, G.; Yang, H.; Luo, Z. Lignocellulosic biomass pyrolysis mechanism: A state-of-the-art review. Prog. Energy Combust. Sci. 2017, 62, 33–86. [Google Scholar] [CrossRef]
- Yang, X.; Xie, H.; Du, H.; Zhang, X.; Zou, Z.; Zou, Y.; Liu, W.; Lan, H.; Zhang, X.; Si, C. Facile Extraction of Thermally Stable and Dispersible Cellulose Nanocrystals with High Yield via a Green and Recyclable FeCl3-Catalyzed Deep Eutectic Solvent System. ACS Sustain. Chem. Eng. 2019, 7, 7200–7208. [Google Scholar] [CrossRef]
- Zhou, X.; Li, W.; Mabon, R.; Broadbelt, L.J. A critical review on hemicellulose pyrolysis. Energy Technol. 2017, 5, 52–79. [Google Scholar] [CrossRef]
- Cahyanti, M.N.; Doddapaneni, T.R.K.C.; Kikas, T. Biomass torrefaction: An overview on process parameters, economic and environmental aspects and recent advancements. Bioresour. Technol. 2020, 301, 122737. [Google Scholar] [CrossRef]
- Yang, J.; He, Q.; Yang, L. A review on hydrothermal co-liquefaction of biomass. Appl. Energy 2019, 250, 926–945. [Google Scholar] [CrossRef]
- Mallick, D.; Mahanta, P.; Moholkar, V.S. Co-gasification of coal and biomass blends: Chemistry and engineering. Fuel 2017, 204, 106–128. [Google Scholar] [CrossRef]
- Meyer, P.A.; Snowden-Swan, L.J.; Jones, S.B.; Rappé, K.G.; Hartley, D.S. The effect of feedstock composition on fast pyrolysis and upgrading to transportation fuels: Techno-economic analysis and greenhouse gas life cycle analysis. Fuel 2020, 259, 116218. [Google Scholar] [CrossRef]
- Arni, S. Al Extraction and isolation methods for lignin separation from sugarcane bagasse: A review. Ind. Crops Prod. 2018, 115, 330–339. [Google Scholar] [CrossRef]
- Collard, F.X.; Blin, J. A review on pyrolysis of biomass constituents: Mechanisms and composition of the products obtained from the conversion of cellulose, hemicelluloses and lignin. Renew. Sustain. Energy Rev. 2014, 38, 594–608. [Google Scholar] [CrossRef]
- Yaman, S. Pyrolysis of biomass to produce fuels and chemical feedstocks. Energy Convers. Manag. 2004, 45, 651–671. [Google Scholar] [CrossRef]
- Khan, T.A.; Saud, A.S.; Jamari, S.S.; Rahim, M.H.A.; Park, J.W.; Kim, H.J. Hydrothermal carbonization of lignocellulosic biomass for carbon rich material preparation: A review. Biomass Bioenergy 2019, 130, 105384. [Google Scholar] [CrossRef]
- Undri, A.; Abou-Zaid, M.; Briens, C.; Berruti, F.; Rosi, L.; Bartoli, M.; Frediani, M.; Frediani, P. Bio-oil from pyrolysis of wood pellets using a microwave multimode oven and different microwave absorbers. Fuel 2015, 153, 464–482. [Google Scholar] [CrossRef]
- Geun Yoo, C.; Meng, X.; Pu, Y.; Ragauskas, A.J. The critical role of lignin in lignocellulosic biomass conversion and recent pretreatment strategies: A comprehensive review. Bioresour. Technol. 2020, 301, 122784. [Google Scholar]
- Jiang, G.; Nowakowski, D.J.; Bridgwater, A.V. Effect of the temperature on the composition of lignin pyrolysis products. Energy Fuels 2010, 24, 4470–4475. [Google Scholar] [CrossRef]
- Abdulkhani, A.; Karimi, A.; Mirshokraie, A.; Hamzeh, Y.; Marlin, N.; Mortha, G. Isolation and chemical structure characterization of enzymatic lignin from Populus deltoides wood. J. Appl. Polym. Sci. 2010, 118, 469–479. [Google Scholar] [CrossRef]
- Di Blasi, C. Modeling chemical and physical processes of wood and biomass pyrolysis. Prog. Energy Combust. Sci. 2008, 34, 47–90. [Google Scholar] [CrossRef]
- Lu, Q.; Li, W.Z.; Zhu, X.F. Overview of fuel properties of biomass fast pyrolysis oils. Energy Convers. Manag. 2009, 50, 1376–1383. [Google Scholar] [CrossRef]
- Doumer, M.E.; Arízaga, G.G.C.; Da Silva, D.A.; Yamamoto, C.I.; Novotny, E.H.; Santos, J.M.; Dos Santos, L.O.; Wisniewski, A.; De Andrade, J.B.; Mangrich, A.S. Slow pyrolysis of different Brazilian waste biomasses as sources of soil conditioners and energy, and for environmental protection. J. Anal. Appl. Pyrolysis 2015, 113, 434–443. [Google Scholar] [CrossRef]
- Jeong, Y.W.; Choi, S.K.; Choi, Y.S.; Kim, S.J. Production of biocrude-oil from swine manure by fast pyrolysis and analysis of its characteristics. Renew. Energy 2015, 79, 14–19. [Google Scholar] [CrossRef]
- Suttibak, S.; Sriprateep, K.; Pattiya, A. Production of bio-oil via fast pyrolysis of cassava rhizome in a fluidised-bed reactor. Energy Procedia 2012, 14, 668–673. [Google Scholar] [CrossRef]
- Das, O.; Sarmah, A.K. Mechanism of waste biomass pyrolysis: Effect of physical and chemical pre-treatments. Sci. Total Environ. 2015, 537, 323–334. [Google Scholar] [CrossRef]
- Mosier, N.; Wyman, C.; Dale, B.; Elander, R.; Lee, Y.Y.; Holtzapple, M.; Ladisch, M. Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour. Technol. 2005, 96, 673–686. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Ip, E.; Scott, J.; Foster, P.; Vickers, M.; Baxter, L.L. Effects of particle shape and size on devolatilization of biomass particle. Fuel 2010, 89, 1156–1168. [Google Scholar] [CrossRef]
- Akhtar, J.; Saidina Amin, N. A review on operating parameters for optimum liquid oil yield in biomass pyrolysis. Renew. Sustain. Energy Rev. 2012, 16, 5101–5109. [Google Scholar] [CrossRef]
- Mlonka-Mędrala, A.; Magdziarz, A.; Dziok, T.; Sieradzka, M.; Nowak, W. Laboratory studies on the influence of biomass particle size on pyrolysis and combustion using TG GC/MS. Fuel 2019, 252, 635–645. [Google Scholar] [CrossRef]
- Chang, Q.; Gao, R.; Li, H.; Yu, G.; Liu, X.; Wang, F. Understanding of formation mechanisms of fine particles formed during rapid pyrolysis of biomass. Fuel 2018, 216, 538–547. [Google Scholar] [CrossRef]
- Choi, H.S.; Choi, Y.S.; Park, H.C. Fast pyrolysis characteristics of lignocellulosic biomass with varying reaction conditions. Renew. Energy 2012, 42, 131–135. [Google Scholar] [CrossRef]
- Shen, J.; Wang, X.S.; Garcia-Perez, M.; Mourant, D.; Rhodes, M.J.; Li, C.Z. Effects of particle size on the fast pyrolysis of oil mallee woody biomass. Fuel 2009, 88, 1810–1817. [Google Scholar] [CrossRef]
- Muazu, R.I.; Borrion, A.L.; Stegemann, J.A. Life cycle assessment of biomass densification systems. Biomass Bioenergy 2017, 107, 384–397. [Google Scholar] [CrossRef]
- Di Blasi, C.; Branca, C.; Lombardi, V.; Ciappa, P.; Di Giacomo, C. Effects of particle size and density on the packed-bed pyrolysis of wood. Energy Fuels 2013, 11, 6781–6791. [Google Scholar] [CrossRef]
- Gageanu, I.; Voicu, G.; Bunduchi, G.; Bracacescu, C. Experimental research on the process of pelleting Salix viminalis depending on humidity and granulation. In Proceedings of the 15th Internal Scientific Conference “Engineering for Rural Development”, Jelgava, Latvia, 25–27 May 2016; pp. 624–628. [Google Scholar]
- Mamvura, T.A.; Danha, G. Biomass torrefaction as an emerging technology to aid in energy production. Heliyon 2020, 6, e03531. [Google Scholar] [CrossRef] [PubMed]
- Neupane, S.; Adhikari, S.; Wang, Z.; Ragauskas, A.J.; Pu, Y. Effect of torrefaction on biomass structure and hydrocarbon production from fast pyrolysis. Green Chem. 2015, 122, 95–105. [Google Scholar] [CrossRef]
- Jönsson, L.J.; Martín, C. Pretreatment of lignocellulose: Formation of inhibitory by-products and strategies for minimizing their effects. Bioresour. Technol. 2016, 199, 103–112. [Google Scholar] [CrossRef]
- Giudicianni, P.; Gargiulo, V.; Grottola, C.M.; Alfè, M.; Ragucci, R. Effect of alkali metal ions presence on the products of xylan steam assisted slow pyrolysis. Fuel 2018, 216, 36–43. [Google Scholar] [CrossRef]
- Isahak, W.N.R.W.; Hisham, M.W.M.; Yarmo, M.A.; Yun Hin, T.Y. A review on bio-oil production from biomass by using pyrolysis method. Renew. Sustain. Energy Rev. 2012, 16, 5910–5923. [Google Scholar] [CrossRef]
- Cao, B.; Wang, S.; Hu, Y.; Abomohra, A.E.F.; Qian, L.; He, Z.; Wang, Q.; Uzoejinwa, B.B.; Esakkimuthu, S. Effect of washing with diluted acids on Enteromorpha clathrata pyrolysis products: Towards enhanced bio-oil from seaweeds. Renew. Energy 2019, 138, 29–38. [Google Scholar] [CrossRef]
- Kumar, R.; Strezov, V.; Weldekidan, H.; He, J.; Singh, S.; Kan, T.; Dastjerdi, B. Lignocellulose biomass pyrolysis for bio-oil production: A review of biomass pre-treatment methods for production of drop-in fuels. Renew. Sustain. Energy Rev. 2020, 123, 109763. [Google Scholar] [CrossRef]
- Stirling, R.; Snape, C.E.; Meredith, W. The impact of hydrothermal carbonisation on the char reactivity of biomass. Fuel Process. Technol. 2018, 177, 152–158. [Google Scholar] [CrossRef]
- Reza, M.T.; Lynam, J.G.; Uddin, M.H.; Coronella, C.J. Hydrothermal carbonization: Fate of inorganics. Biomass Bioenergy 2013, 49, 86–94. [Google Scholar] [CrossRef]
- Huzzey, J.M.; Duffield, T.F.; LeBlanc, S.J.; Veira, D.M.; Weary, D.M.; Von Keyserlingk, M.A.G. Short communication: Haptoglobin as an early indicator of metritis. J. Dairy Sci. 2009, 92, 621–625. [Google Scholar] [CrossRef]
- Tabil, L.; Adapa, P.; Kashaninej, M. Biomass Feedstock Pre-Processing—Part 1: Pre-Treatment. In Biofuel’s Engineering Process Technology; IntechOpen: London, UK, 2011. [Google Scholar]
- Sundaram, V.; Muthukumarappan, K.; Kamireddy, S.R. Effect of ammonia fiber expansion (AFEXTM) pretreatment on compression behavior of corn stover, prairie cord grass and switchgrass. Ind. Crops Prod. 2015, 74, 45–54. [Google Scholar] [CrossRef]
- Mathew, A.K.; Parameshwaran, B.; Sukumaran, R.K.; Pandey, A. An evaluation of dilute acid and ammonia fiber explosion pretreatment for cellulosic ethanol production. Bioresour. Technol. 2016, 199, 13–20. [Google Scholar] [CrossRef]
- Vasco-Correa, J.; Ge, X.; Li, Y. Biological Pretreatment of Lignocellulosic Biomass. In Biomass Fractionation Technologies for a Lignocellulosic Feedstock Based Biorefinery; Elsevier Science: Amsterdam, The Netherlands, 2016; ISBN 9780128025611. [Google Scholar]
- Yu, Y.; Zeng, Y.; Zuo, J.; Ma, F.; Yang, X.; Zhang, X.; Wang, Y. Improving the conversion of biomass in catalytic fast pyrolysis via white-rot fungal pretreatment. Bioresour. Technol. 2013, 134, 198–203. [Google Scholar] [CrossRef]
- You, T.; Li, X.; Wang, R.; Zhang, X.; Xu, F. Effects of synergistic fungal pretreatment on structure and thermal properties of lignin from corncob. Bioresour. Technol. 2019, 272, 123–129. [Google Scholar] [CrossRef]
- Huet, G.; Hadad, C.; Husson, E.; Laclef, S.; Lambertyn, V.; Araya Farias, M.; Jamali, A.; Courty, M.; Alayoubi, R.; Gosselin, I.; et al. Straightforward extraction and selective bioconversion of high purity chitin from Bombyx eri larva: Toward an integrated insect biorefinery. Carbohydr. Polym. 2020, 228, 115382. [Google Scholar] [CrossRef]
- Brodeur, G.; Yau, E.; Badal, K.; Collier, J.; Ramachandran, K.B.; Ramakrishnan, S. Chemical and physicochemical pretreatment of lignocellulosic biomass: A review. Enzyme Res. 2011, 787532. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Adjallé, K.; Lai, T.T.; Barnabé, S.; Perrier, M.; Paris, J. Effect of Mechanical Pretreatment for Enzymatic Hydrolysis of Woody Residues, Corn Stover and Alfalfa. Waste Biomass Valorization 2019. [Google Scholar] [CrossRef]
- Putro, J.N.; Soetaredjo, F.E.; Lin, S.Y.; Ju, Y.H.; Ismadji, S. Pretreatment and conversion of lignocellulose biomass into valuable chemicals. RSC Adv. 2016, 6, 46834–46852. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, S.; Zheng, Y.; Luo, Z.; Cen, K. Mechanism study of wood lignin pyrolysis by using TG–FTIR analysis. J. Anal. Appl. Pyrolysis 2008, 82, 170–177. [Google Scholar] [CrossRef]
- Mishra, R.K.; Mohanty, K.; Wang, X. Pyrolysis kinetic behavior and Py-GC–MS analysis of waste dahlia flowers into renewable fuel and value-added chemicals. Fuel 2020, 260, 116338. [Google Scholar] [CrossRef]
- Echresh, Z.; Abdulkhani, A.; Saha, B. Analytical pyrolysis study of different lignin biomass. In Proceedings of the 27th European Biomass Conference and Exhibition Proceedings, Lisbon, Portugal, 26 May 2019; pp. 1241–1245. [Google Scholar]
- Kissinger, H.E. Reaction Kinetics in Differential Thermal Analysis. Anal. Chem. 1957, 11, 1702–1706. [Google Scholar] [CrossRef]
- Hu, X.; Gholizadeh, M. Biomass pyrolysis: A review of the process development and challenges from initial researches up to the commercialisation stage. J. Energy Chem. 2019, 39, 109–143. [Google Scholar] [CrossRef]
- Stefanidis, S.D.; Kalogiannis, K.G.; Iliopoulou, E.F.; Michailof, C.M.; Pilavachi, P.A.; Lappas, A.A. A study of lignocellulosic biomass pyrolysis via the pyrolysis of cellulose, hemicellulose and lignin. J. Anal. Appl. Pyrolysis 2014, 105, 143–150. [Google Scholar] [CrossRef]
- Rocha-Meneses, L.; Raud, M.; Orupõld, K.; Kikas, T. Second-generation bioethanol production: A review of strategies for waste valorisation. Agron. Res. 2017, 3, 830–847. [Google Scholar]
- Anwar, Z.; Gulfraz, M.; Irshad, M. Agro-industrial lignocellulosic biomass a key to unlock the future bio-energy: A brief review. J. Radiat. Res. Appl. Sci. 2014, 7, 163–173. [Google Scholar] [CrossRef]
- Pippo, W.A.; Luengo, C.A.; Alberteris, L.A.M.; Garzone, P.; Cornacchia, G. Energy recovery from sugarcane-trash in the light of 2nd generation biofuels. Part 1: Current situation and environmental aspects. Waste Biomass Valorization 2011, 2, 1–16. [Google Scholar] [CrossRef]
- Abdulkhani, A.; Hojati Marvast, E.; Ashori, A.; Karimi, A.N. Effects of dissolution of some lignocellulosic materials with ionic liquids as green solvents on mechanical and physical properties of composite films. Carbohydr. Polym. 2013, 95, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Greenhalf, C.E.; Nowakowski, D.J.; Harms, A.B.; Titiloye, J.O.; Bridgwater, A.V. Sequential pyrolysis of willow SRC at low and high heating rates—Implications for selective pyrolysis. Fuel 2012, 93, 692–702. [Google Scholar] [CrossRef]
- Ma, Z.; Chen, D.; Gu, J.; Bao, B.; Zhang, Q. Determination of pyrolysis characteristics and kinetics of palm kernel shell using TGA-FTIR and model-free integral methods. Energy Convers. Manag. 2015, 89, 251–259. [Google Scholar] [CrossRef]
- Huang, X.; Cheng, D.-G.; Chen, F.; Zhan, X. Reaction pathways of hemicellulose and mechanism of biomass pyrolysis in hydrogen plasma: A density functional theory study. Renew. Energy 2016, 96, 490–497. [Google Scholar] [CrossRef]
- Westmoreland, P.R. Pyrolysis kinetics for lignocellulosic biomass-to-oil from molecular modeling. Curr. Opin. Chem. Eng. 2019, 23, 123–129. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Lv, G.; Wu, S. Analytical pyrolysis studies of corn stalk and its three main components by TG-MS and Py-GC/MS. J. Anal. Appl. Pyrolysis 2012, 97, 11–18. [Google Scholar] [CrossRef]
- Couhert, C.; Commandre, J.M.; Salvador, S. Is it possible to predict gas yields of any biomass after rapid pyrolysis at high temperature from its composition in cellulose, hemicellulose and lignin? Fuel 2009, 88, 408–417. [Google Scholar] [CrossRef]
- Shen, D.K.; Gu, S.; Bridgwater, A.V. Study on the pyrolytic behaviour of xylan-based hemicellulose using TG-FTIR and Py-GC-FTIR. J. Anal. Appl. Pyrolysis 2010, 87, 199–206. [Google Scholar] [CrossRef]
- Wang, S.; Guo, X.; Liang, T.; Zhou, Y.; Luo, Z. Mechanism research on cellulose pyrolysis by Py-GC/MS and subsequent density functional theory studies. Bioresour. Technol. 2012, 104, 722–728. [Google Scholar] [CrossRef]
- Dussan, K.; Dooley, S.; Monaghan, R. Integrating compositional features in model compounds for a kinetic mechanism of hemicellulose pyrolysis. Chem. Eng. J. 2017, 328, 943–961. [Google Scholar] [CrossRef]
- Jiang, G.; Nowakowski, D.J.; Bridgwater, A.V. A systematic study of the kinetics of lignin pyrolysis. Thermochim. Acta 2010, 498, 61–66. [Google Scholar] [CrossRef]
- Tejado, A.; Peña, C.; Labidi, J.; Echeverria, J.M.; Mondragon, I. Physico-chemical characterization of lignins from different sources for use in phenol-formaldehyde resin synthesis. Bioresour. Technol. 2007, 98, 1655–1663. [Google Scholar] [CrossRef] [PubMed]
- Laurichesse, S.; Avérous, L. Chemical modification of lignins: Towards biobased polymers. Prog. Polym. Sci. 2014, 39, 1266–1290. [Google Scholar] [CrossRef]
- Gómez-Monedero, B.; Ruiz, M.P.; Bimbela, F.; Faria, J. Selective hydrogenolysis of A–O–4, Β–O–4, 4–O–5 C–O bonds of lignin-model compounds and lignin-containing stillage derived from cellulosic bioethanol processing. Appl. Catal. A Gen. 2017, 541, 60–76. [Google Scholar] [CrossRef]
- Zakzeski, J.; Bruijnincx, P.C.A.; Jongerius, A.L.; Weckhuysen, B.M. The catalytic valorization of lignin for the production of renewable chemicals. Chem. Rev. 2010, 110, 3552–3599. [Google Scholar] [CrossRef]
- Moghaddam, L.; Zhang, Z.; Wellard, R.M.; Bartley, J.P.; O’Hara, I.M.; Doherty, W.O.S. Characterisation of lignins isolated from sugarcane bagasse pretreated with acidified ethylene glycol and ionic liquids. Biomass Bioenergy 2014, 70, 498–512. [Google Scholar] [CrossRef]
- Meng, Y.; Lu, J.; Cheng, Y.; Li, Q.; Wang, H. Lignin-based hydrogels: A review of preparation, properties, and application. Int. J. Biol. Macromol. 2019, 135, 1006–1019. [Google Scholar] [CrossRef]
- Cao, Y.; Chen, S.S.; Zhang, S.; Ok, Y.S.; Matsagar, B.M.; Wu, K.C.W.; Tsang, D.C.W. Advances in lignin valorization towards bio-based chemicals and fuels: Lignin biorefinery. Bioresour. Technol. 2019, 291, 121878. [Google Scholar] [CrossRef]
- Custodis, V.B.F.; Bährle, C.; Vogel, F.; Van Bokhoven, J.A. Phenols and aromatics from fast pyrolysis of variously prepared lignins from hard- and softwoods. J. Anal. Appl. Pyrolysis 2015, 115, 214–223. [Google Scholar] [CrossRef]
- Shao, Y.; Sun, K.; Li, Q.; Zhang, L.; Wei, T.; Gao, G.; Zhang, S.; Wang, Y.; Liu, Q.; Hu, X. Dealkaline lignin—The waste from the pulp and paper industry as acid catalyst in biorefinery. Bioresour. Technol. Rep. 2019, 7, 100218. [Google Scholar] [CrossRef]
- Espinoza-Acosta, J.L.; Torres-Chávez, P.I.; Olmedo-Martínez, J.L.; Vega-Rios, A.; Flores-Gallardo, S.; Zaragoza-Contreras, E.A. Lignin in storage and renewable energy applications: A review. J. Energy Chem. 2018, 27, 1422–1438. [Google Scholar] [CrossRef]
- Abdulkhani, A.; Mirshokraie, A.; Karimi, A.; Hamzeh, Y. Chemical structure characterization of lignin from populus deltoides. In Proceedings of the 16th International Symposium on Wood, Fiber and Pulping Chemistry (ISWFPC), Tianjin, China, 8–10 June 2011; Volume 118, pp. 469–479. [Google Scholar]
- Hamzeh, Y.; Ashori, A.; Mirzaei, B.; Abdulkhani, A.; Molaei, M. Current and potential capabilities of biomass for green energy in Iran. Renew. Sustain. Energy Rev. 2011, 15, 4934–4938. [Google Scholar] [CrossRef]
- Szalaty, T.J.; Klapiszewski, Ł.; Jesionowski, T. Recent developments in modification of lignin using ionic liquids for the fabrication of advanced materials—A review. J. Mol. Liq. 2020, 301, 112417. [Google Scholar] [CrossRef]
- Rajesh Banu, J.; Kavitha, S.; Yukesh Kannah, R.; Poornima Devi, T.; Gunasekaran, M.; Kim, S.H.; Kumar, G. A review on biopolymer production via lignin valorization. Bioresour. Technol. 2019, 290, 121790. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, R.; Yin, R.; Mei, Y. Upgrading of bio-oil from biomass fast pyrolysis in China: A review. Renew. Sustain. Energy Rev. 2013, 24, 66–72. [Google Scholar] [CrossRef]
- Bridgwater, A.V. Review of fast pyrolysis of biomass and product upgrading. Biomass Bioenergy 2012, 38, 68–94. [Google Scholar] [CrossRef]
- Bridgwater, A.V. Principles and practice of biomass fast pyrolysis processes for liquids. J. Anal. Appl. Pyrolysis 1999, 51, 3–22. [Google Scholar] [CrossRef]
- Khuenkaeo, N.; Tippayawong, N. Production and characterization of bio-oil and biochar from ablative pyrolysis of lignocellulosic biomass residues. Chem. Eng. Commun. 2019, 207, 153–160. [Google Scholar] [CrossRef]
- Bridgwater, A.V.; Peacocke, G.V.C. Fast pyrolysis processes for biomass. Renew. Sustain. Energy Rev. 2000, 4, 1–73. [Google Scholar] [CrossRef]
- Yang, X.; Lyu, H.; Chen, K.; Zhu, X.; Zhang, S.; Chen, J. Selective Extraction of Bio-oil from Hydrothermal Liquefaction of Salix psammophila by Organic Solvents with Different Polarities through Multistep Extraction Separation. BioResources 2014, 9, 5219–5233. [Google Scholar] [CrossRef]
- Zadeh, Z.E.; Abdulkhani, A.; Saha, B. Characterization of fast pyrolysis bio-oil from hardwood and softwood lignin. Energies 2020, 13, 1–14. [Google Scholar]
- Wang, S. High-Efficiency Separation of Bio-Oil. In Biomass Now—Sustainable Growth and Use; Books on Demand: Norderstedt, Germany, 2013. [Google Scholar]
- Rahman, A.A.; Sulaiman, F.; Abdullah, N. Effect of temperature on pyrolysis product of empty fruit bunches. AIP Conf. Proc. 2015, 1657, 4915150. [Google Scholar]
- Chang, S.H. Rice Husk and Its Pretreatments for Bio-oil Production via Fast Pyrolysis: A Review. Bioenergy Res. 2020, 13, 23–42. [Google Scholar] [CrossRef]
- Qian, Y.; Zhang, J.; Wang, J. Pressurized pyrolysis of rice husk in an inert gas sweeping fixed-bed reactor with a focus on bio-oil deoxygenation. Bioresour. Technol. 2014, 174, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, H.; Wang, X.; Zhang, S.; Chen, H. Biomass-based pyrolytic polygeneration system on cotton stalk pyrolysis: Influence of temperature. Bioresour. Technol. 2012, 107, 411–418. [Google Scholar] [CrossRef]
- Montoya, J.I.; Valdés, C.; Chejne, F.; Gómez, C.A.; Blanco, A.; Marrugo, G.; Osorio, J.; Castillo, E.; Aristóbulo, J.; Acero, J. Bio-oil production from Colombian bagasse by fast pyrolysis in a fluidized bed: An experimental study. J. Anal. Appl. Pyrolysis 2015, 112, 379–387. [Google Scholar] [CrossRef]
- Mutsengerere, S.; Chihobo, C.H.; Musademba, D.; Nhapi, I. A review of operating parameters affecting bio-oil yield in microwave pyrolysis of lignocellulosic biomass. Renew. Sustain. Energy Rev. 2019, 104, 328–336. [Google Scholar] [CrossRef]
- Kumagai, S.; Matsuno, R.; Grause, G.; Kameda, T.; Yoshioka, T. Enhancement of bio-oil production via pyrolysis of wood biomass by pretreatment with H2SO4. Bioresour. Technol. 2015, 178, 76–82. [Google Scholar] [CrossRef]
- Makibar, J.; Fernandez-Akarregi, A.R.; Amutio, M.; Lopez, G.; Olazar, M. Performance of a conical spouted bed pilot plant for bio-oil production by poplar flash pyrolysis. Fuel Process. Technol. 2015, 137, 283–289. [Google Scholar] [CrossRef]
- Alvarez, J.; Lopez, G.; Amutio, M.; Bilbao, J.; Olazar, M. Bio-oil production from rice husk fast pyrolysis in a conical spouted bed reactor. Fuel 2014, 128, 162–169. [Google Scholar] [CrossRef]
- Chan, Y.H.; Yusup, S.; Quitain, A.T.; Uemura, Y.; Sasaki, M. Bio-oil production from oil palm biomass via subcritical and supercritical hydrothermal liquefaction. J. Supercrit. Fluids 2014, 95, 407–412. [Google Scholar] [CrossRef]
- Yin, R.; Liu, R.; Mei, Y.; Fei, W.; Sun, X. Characterization of bio-oil and bio-char obtained from sweet sorghum bagasse fast pyrolysis with fractional condensers. Fuel 2013, 112, 96–104. [Google Scholar] [CrossRef]
- Hu, Z.; Zheng, Y.; Yan, F.; Xiao, B.; Liu, S. Bio-oil production through pyrolysis of blue-green algae blooms (BGAB): Product distribution and bio-oil characterization. Energy 2013, 52, 119–125. [Google Scholar] [CrossRef]
- Zhang, H.; Xiao, R.; Huang, H.; Xiao, G. Comparison of non-catalytic and catalytic fast pyrolysis of corncob in a fluidized bed reactor. Bioresour. Technol. 2009, 100, 1428–1434. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Jin, B.; Zhang, M.; Liu, R. Cotton stalk combustion in a circulating fluidized bed. Chem. Eng. Technol. 2008, 31, 1605–1614. [Google Scholar] [CrossRef]
- Channiwala, S.A.; Parikh, P.P. A unified correlation for estimating HHV of solid, liquid and gaseous fuels. Fuel 2002, 81, 1051–1063. [Google Scholar] [CrossRef]
- Munir, S.; Daood, S.S.; Nimmo, W.; Cunliffe, A.M.; Gibbs, B.M. Thermal analysis and devolatilization kinetics of cotton stalk, sugar cane bagasse and shea meal under nitrogen and air atmospheres. Bioresour. Technol. 2009, 100, 1413–1418. [Google Scholar] [CrossRef]
- Friedl, A.; Padouvas, E.; Rotter, H.; Varmuza, K. Prediction of heating values of biomass fuel from elemental composition. Anal. Chim. Acta 2005, 544, 191–198. [Google Scholar] [CrossRef]
- Demirbaş, A. Calculation of higher heating values of biomass fuels. Fuel 1997, 76, 431–434. [Google Scholar] [CrossRef]
- Qambrani, N.A.; Rahman, M.M.; Won, S.; Shim, S.; Ra, C. Biochar properties and eco-friendly applications for climate change mitigation, waste management, and wastewater treatment: A review. Renew. Sustain. Energy Rev. 2017, 79, 255–273. [Google Scholar] [CrossRef]
- Lee, Y.; Eum, P.R.B.; Ryu, C.; Park, Y.K.; Jung, J.H.; Hyun, S. Characteristics of biochar produced from slow pyrolysis of Geodae-Uksae 1. Bioresour. Technol. 2013, 130, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, M.E.; Lindao, E.; Margaleff, D.; Martínez, O.; Morán, A. Pyrolysis of agricultural residues from rape and sunflowers: Production and characterization of bio-fuels and biochar soil management. J. Anal. Appl. Pyrolysis 2009, 85, 142–144. [Google Scholar] [CrossRef]
- Oliveira, F.R.; Patel, A.K.; Jaisi, D.P.; Adhikari, S.; Lu, H.; Khanal, S.K. Environmental application of biochar: Current status and perspectives. Bioresour. Technol. 2017, 246, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Uchimiya, M.; Wartelle, L.H.; Klasson, K.T.; Fortier, C.A.; Lima, I.M. Influence of pyrolysis temperature on biochar property and function as a heavy metal sorbent in soil. J. Agric. Food Chem. 2011, 59, 2501–2510. [Google Scholar] [CrossRef] [PubMed]
- Uchimiya, M.; Lima, I.M.; Thomas Klasson, K.; Chang, S.; Wartelle, L.H.; Rodgers, J.E. Immobilization of heavy metal ions (CuII, CdII, NiII, and PbII) by broiler litter-derived biochars in water and soil. J. Agric. Food Chem. 2010, 58, 5538–5544. [Google Scholar] [CrossRef] [PubMed]
- Demirbas, A. Production and characterization of bio-chars from biomass via pyrolysis. Energy Sources Part A Recover. Util. Environ. Eff. 2006, 28, 413–422. [Google Scholar] [CrossRef]
- Lehmann, J. Biological carbon sequestration must and can be a win-win approach. Clim. Change 2009, 97, 459–463. [Google Scholar] [CrossRef]
- Ghani, W.A.W.A.K.; Mohd, A.; da Silva, G.; Bachmann, R.T.; Taufiq-Yap, Y.H.; Rashid, U.; Al-Muhtaseb, A.H. Biochar production from waste rubber-wood-sawdust and its potential use in C sequestration: Chemical and physical characterization. Ind. Crops Prod. 2013, 44, 18–24. [Google Scholar] [CrossRef]
- Najib, S.; Erdem, E. Current progress achieved in novel materials for supercapacitor electrodes: Mini review. Nanoscale Adv. 2019, 1, 2817–2827. [Google Scholar] [CrossRef]
- González, A.; Goikolea, E.; Barrena, J.A.; Mysyk, R. Review on supercapacitors: Technologies and materials. Renew. Sustain. Energy Rev. 2016, 58, 1189–1206. [Google Scholar] [CrossRef]
- Lewandowski, W.M.; Januszewicz, K.; Kosakowski, W. Efficiency and proportions of waste tyre pyrolysis products depending on the reactor type—A review. J. Anal. Appl. Pyrolysis 2019, 140, 25–53. [Google Scholar] [CrossRef]
- Lee, J.; Kwon, E.E.; Park, Y.K. Recent advances in the catalytic pyrolysis of microalgae. Catal. Today 2019, 126, 8–18. [Google Scholar] [CrossRef]
- Zhan, H.; Zhuang, X.; Song, Y.; Liu, J.; Li, S.; Chang, G.; Yin, X.; Wu, C.; Wang, X. A review on evolution of nitrogen-containing species during selective pyrolysis of waste wood-based panels. Fuel 2019, 253, 1214–1228. [Google Scholar] [CrossRef]
- Moreno, A.I.; Font, R. Pyrolysis of furniture wood waste: Decomposition and gases evolved. J. Anal. Appl. Pyrolysis 2015, 113, 464–473. [Google Scholar] [CrossRef]
- Fateh, T.; Rogaume, T.; Luche, J.; Richard, F.; Jabouille, F. Modeling of the thermal decomposition of a treated plywood from thermo-gravimetry and Fourier-transformed infrared spectroscopy experimental analysis. J. Anal. Appl. Pyrolysis 2013, 101, 35–44. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Q.; Wang, T.; Li, B.; Xu, Y.; Ma, L. Efficient upgrading process for production of low quality fuel from bio-oil. Fuel 2016, 179, 312–321. [Google Scholar] [CrossRef]
- Gollakota, A.R.K.; Reddy, M.; Subramanyam, M.D.; Kishore, N. A review on the upgradation techniques of pyrolysis oil. Renew. Sustain. Energy Rev. 2016, 58, 1543–1568. [Google Scholar] [CrossRef]
- Ikura, M.; Stanciulescu, M.; Hogan, E. Emulsification of pyrolysis derived bio-oil in diesel fuel. Biomass Bioenergy 2003, 24, 221–232. [Google Scholar] [CrossRef]
- Jiang, X.; Ellis, N. Upgrading bio-oil through emulsification with biodiesel: Thermal stability. Energy Fuels 2010, 24, 2699–2706. [Google Scholar] [CrossRef]
- Qi, G.; Dong, P.; Wang, H.; Tan, H. Study on biomass pyrolysis and emulsions from biomass pyrolysis oils and diesel. In Proceedings of the 2nd International Conference on Bioinformatics and Biomedical Engineering (iCBBE 2008), Shanghai, China, 16–18 May 2008; pp. 4735–4737. [Google Scholar]
- Xiaoxiang, J.; Ellis, N. Upgrading bio-oil through emulsification with biodiesel: Mixture production. Energy Fuels 2010, 24, 1358–1364. [Google Scholar]
- Kummer, J.T. Use of noble metals in automobile exhaust catalysts. J. Phys. Chem. 1986, 90, 4747–4752. [Google Scholar] [CrossRef]
- Saidi, M.; Samimi, F.; Karimipourfard, D.; Nimmanwudipong, T.; Gates, B.C.; Rahimpour, M.R. Upgrading of lignin-derived bio-oils by catalytic hydrodeoxygenation. Energy Environ. Sci. 2014, 7, 103–129. [Google Scholar] [CrossRef]
- Si, Z.; Zhang, X.; Wang, C.; Ma, L.; Dong, R. An overview on catalytic hydrodeoxygenation of pyrolysis oil and its model compounds. Catalysts 2017, 7, 169. [Google Scholar] [CrossRef]
- Rennard, D.; French, R.; Czernik, S.; Josephson, T.; Schmidt, L. Production of synthesis gas by partial oxidation and steam reforming of biomass pyrolysis oils. Int. J. Hydrog. Energy 2010, 35, 4048–4059. [Google Scholar] [CrossRef]
- Rioche, C.; Kulkarni, S.; Meunier, F.C.; Breen, J.P.; Burch, R. Steam reforming of model compounds and fast pyrolysis bio-oil on supported noble metal catalysts. Appl. Catal. B Environ. 2005, 61, 130–139. [Google Scholar] [CrossRef]
- Xiu, S.; Shahbazi, A. Bio-oil production and upgrading research: A review. Renew. Sustain. Energy Rev. 2012, 16, 4406–4414. [Google Scholar] [CrossRef]
- Kabir, G.; Hameed, B.H. Recent progress on catalytic pyrolysis of lignocellulosic biomass to high-grade bio-oil and bio-chemicals. Renew. Sustain. Energy Rev. 2017, 70, 945–967. [Google Scholar] [CrossRef]
- Hu, X.; Gunawan, R.; Mourant, D.; Hasan, M.D.M.; Wu, L.; Song, Y.; Lievens, C.; Li, C.Z. Upgrading of bio-oil via acid-catalyzed reactions in alcohols—A mini review. Fuel Process. Technol. 2017, 55, 2–19. [Google Scholar] [CrossRef]
- Fan, L.; Chen, P.; Zhou, N.; Liu, S.; Zhang, Y.; Liu, Y.; Wang, Y.; Omar, M.M.; Peng, P.; Addy, M.; et al. In-situ and ex-situ catalytic upgrading of vapors from microwave-assisted pyrolysis of lignin. Bioresour. Technol. 2018, 247, 851–858. [Google Scholar] [CrossRef]
- Kalogiannis, K.G.; Stefanidis, S.D.; Lappas, A.A. Catalyst deactivation, ash accumulation and bio-oil deoxygenation during ex situ catalytic fast pyrolysis of biomass in a cascade thermal-catalytic reactor system. Fuel Process. Technol. 2019, 186, 99–109. [Google Scholar] [CrossRef]
- Iisa, K.; French, R.J.; Orton, K.A.; Yung, M.M.; Johnson, D.K.; Ten Dam, J.; Watson, M.J.; Nimlos, M.R. In Situ and ex Situ Catalytic Pyrolysis of Pine in a Bench-Scale Fluidized Bed Reactor System. Energy Fuels 2016, 30, 2144–2157. [Google Scholar] [CrossRef]
- Huo, X.; Xiao, J.; Song, M.; Zhu, L. Comparison between in-situ and ex-situ catalytic pyrolysis of sawdust for gas production. J. Anal. Appl. Pyrolysis 2018, 135, 189–198. [Google Scholar] [CrossRef]
- Saraeian, A.; Nolte, M.W.; Shanks, B.H. Deoxygenation of biomass pyrolysis vapors: Improving clarity on the fate of carbon. Renew. Sustain. Energy Rev. 2019, 262, 262–280. [Google Scholar] [CrossRef]
- Stephanidis, S.; Nitsos, C.; Kalogiannis, K.; Iliopoulou, E.F.; Lappas, A.A.; Triantafyllidis, K.S. Catalytic upgrading of lignocellulosic biomass pyrolysis vapours: Effect of hydrothermal pre-treatment of biomass. Catal. Today 2011, 167, 37–45. [Google Scholar] [CrossRef]
- Nguyen, T.S.; Zabeti, M.; Lefferts, L.; Brem, G.; Seshan, K. Catalytic upgrading of biomass pyrolysis vapours using faujasite zeolite catalysts. Biomass Bioenergy 2013, 48, 100–110. [Google Scholar] [CrossRef]
- Carlson, T.R.; Cheng, Y.T.; Jae, J.; Huber, G.W. Production of green aromatics and olefins by catalytic fast pyrolysis of wood sawdust. Energy Environ. Sci. 2011, 4, 145–161. [Google Scholar] [CrossRef]
- Tan, S.; Zhang, Z.; Sun, J.; Wang, Q. Recent progress of catalytic pyrolysis of biomass by HZSM-5. Cuihua Xuebao Chin. J. Catal. 2013, 34, 641–650. [Google Scholar] [CrossRef]
- Liu, C.; Wang, H.; Karim, A.M.; Sun, J.; Wang, Y. Catalytic fast pyrolysis of lignocellulosic biomass. Chem. Soc. Rev. 2014, 43, 7594–7623. [Google Scholar] [CrossRef]
- Baloch, H.A.; Nizamuddin, S.; Siddiqui, M.T.H.; Riaz, S.; Jatoi, A.S.; Dumbre, D.K.; Mubarak, N.M.; Srinivasan, M.P.; Griffin, G.J. Recent advances in production and upgrading of bio-oil from biomass: A critical overview. J. Environ. Chem. Eng. 2018, 6, 5101–5118. [Google Scholar] [CrossRef]
- Dorado, C.; Mullen, C.A.; Boateng, A.A. H-ZSM5 catalyzed co-pyrolysis of biomass and plastics. ACS Sustain. Chem. Eng. 2014, 2, 301–311. [Google Scholar] [CrossRef]
- Song, M.; Zhong, Z.; Dai, J. Different solid acid catalysts influence on properties and chemical composition change of upgrading bio-oil. J. Anal. Appl. Pyrolysis 2010, 89, 166–170. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, J.H.; Park, J.; Kim, J.K.; An, D.; Song, I.K.; Choi, J.W. Catalytic pyrolysis of lignin over HZSM-5 catalysts: Effect of various parameters on the production of aromatic hydrocarbon. J. Anal. Appl. Pyrolysis 2015, 114, 273–280. [Google Scholar] [CrossRef]
- Castello, D.; He, S.; Ruiz, M.P.; Westerhof, R.J.M.; Heeres, H.J.; Seshan, K.; Kersten, S.R.A. Is it possible to increase the oil yield of catalytic pyrolysis of biomass? A study using commercially-available acid and basic catalysts in ex-situ and in-situ modus. J. Anal. Appl. Pyrolysis 2019, 137, 77–85. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, T.; Ma, L.; Chen, G. Upgrading of fast pyrolysis liquid fuel from biomass over Ru/γ-Al2O3 catalyst. Energy Convers. Manag. 2012, 55, 172–177. [Google Scholar]
- Zheng, Y.; Wang, F.; Yang, X.; Huang, Y.; Liu, C.; Zheng, Z.; Gu, J. Study on aromatics production via the catalytic pyrolysis vapor upgrading of biomass using metal-loaded modified H-ZSM-5. J. Anal. Appl. Pyrolysis 2017, 126, 169–179. [Google Scholar] [CrossRef]
- Kumar, R.; Strezov, V.; Lovell, E.; Kan, T.; Weldekidan, H.; He, J.; Dastjerdi, B.; Scott, J. Bio-oil upgrading with catalytic pyrolysis of biomass using Copper/zeolite-Nickel/zeolite and Copper-Nickel/zeolite catalysts. Bioresour. Technol. 2019, 279, 404–409. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, H.; Liu, X.; Zhu, S.; Hu, L.; Zhang, Q. Upgrading of bio-oil from catalytic pyrolysis of pretreated rice husk over Fe-modified ZSM-5 zeolite catalyst. Fuel Process. Technol. 2018, 8, 322–343. [Google Scholar] [CrossRef]




| Pretreatment | Advantages | Disadvantages |
|---|---|---|
| Mechanical |
|
|
| Dilute acid |
|
|
| AFE |
|
|
| Steam explosion |
|
|
| Biological |
|
|
| Lignocellulosic Material | Lignin (%) | Hemicellulose (%) | Cellulose (%) |
|---|---|---|---|
| Sugar cane bagasse | 20 | 25 | 42 |
| Sweet sorghum | 21 | 27 | 45 |
| Hardwood | 18–25 | 24–40 | 40–55 |
| Softwood | 25–35 | 25–35 | 45–50 |
| Corn cobs | 15 | 35 | 45 |
| Corn Stover | 19 | 26 | 38 |
| Rice straw | 18 | 24 | 32.1 |
| Nut shells | 30–40 | 25–30 | 25–30 |
| Newspaper | 18–30 | 25–40 | 40–55 |
| Grasses | 10–30 | 25–50 | 25–40 |
| Wheat straw | 16–21 | 26–32 | 29–35 |
| Banana waste | 14 | 14.8 | 13.2 |
| Bagasse | 23 | 27 | 46 |
| Physical Property | Typical Value |
|---|---|
| Moisture content | 25% |
| pH | 2.5 |
| Elemental analysis | |
| C | 56% |
| H | 6% |
| O | 38% |
| N | 0–0.1% |
| HHV (Higher heating values) as produced | 17 MJ/kg |
| Viscosity (40 °C and 25% water) | 40–100 mPa s |
| Solids (char) | 0.1% |
| Vacuum distillation residue | up to 50% |
| Biomass Type | Type of Reactor | T (°C) | Bio-Oil Yield wt.% | Reference |
|---|---|---|---|---|
| Sugarcane bagasse | Fluidized bed | 500 | 74.0 | [113] |
| Sawdust | Fluidized bed | 500 | 76.0 | [113] |
| Banana rachis | Fluidized bed | 500 | 28.0 | [113] |
| Corncob | Fluidized bed | 550 | 56.8 | [114] |
| Rice husks | Fluidized bed | 450 | 60.0 | [114] |
| Cedar wood | Quartz glass tube reactor | 550 | 46.8 | [115] |
| Poplar | Spouted bed | 455 | 69.0 | [116] |
| Rice husk | Spouted bed reactor | 450 | 70.0 | [117] |
| Palm kernel shell (PKS) | Iconel batch | 390 | 38.5 | [118] |
| Empty fruit bunch (EFB), | Iconel batch | 390 | 37.4 | [118] |
| Palm mesocarp fiber (PMF) | Iconel batch | 390 | 34.3 | [118] |
| Sweet sorghum bagasse | Fluidized bed | 500 | 43.5 | [119] |
| Blue-green algae blooms | Fixed bed | 500 | 55.0 | [120] |
| Corncob | Fluidized bed | 550 | 56.8 | [121] |
| Cotton Stalk | Fluidized bed | 510 | 55.0 | [122] |
| Biomass Type | C (wt.%) | H (wt.%) | N (wt.%) | S (wt.%) | O (wt.%) | Ash (wt.%) | HHV (MJ/kg) | Reference |
|---|---|---|---|---|---|---|---|---|
| Sugarcane bagasse | 45.5 | 6.0 | 45.2 | - | 0.15 | 3.2 | 18.7 | [123] |
| Coconut shell | 50.2 | 5.7 | 43.4 | - | - | 0.71 | 20.5 | [123] |
| Cotton stalk | 47.1 | 4.6 | 1.2 | - | 42.1 | 5.1 | 17.4 | [124] |
| Sunflower | 50.5 | 5.9 | 1.3 | 0.1 | 34.9 | 6.9 | 20.3 | [125] |
| Energy grass | 48.3 | 5.5 | 0.6 | 0.1 | 41.5 | 3.8 | 19.1 | [125] |
| Wood waste | 49.7 | 6.0 | 1.7 | 0.0 | 41.0 | 1.5 | 18.6 | [125] |
| Corncob | 49.0 | 5.4 | 0.4 | - | 44.6 | 1.0 | 17.0 | [126] |
| Tea waste | 48.6 | 5.5 | 0.5 | - | 39.5 | 1.4 | 17.1 | [126] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zadeh, Z.E.; Abdulkhani, A.; Aboelazayem, O.; Saha, B. Recent Insights into Lignocellulosic Biomass Pyrolysis: A Critical Review on Pretreatment, Characterization, and Products Upgrading. Processes 2020, 8, 799. https://doi.org/10.3390/pr8070799
Zadeh ZE, Abdulkhani A, Aboelazayem O, Saha B. Recent Insights into Lignocellulosic Biomass Pyrolysis: A Critical Review on Pretreatment, Characterization, and Products Upgrading. Processes. 2020; 8(7):799. https://doi.org/10.3390/pr8070799
Chicago/Turabian StyleZadeh, Zahra Echresh, Ali Abdulkhani, Omar Aboelazayem, and Basudeb Saha. 2020. "Recent Insights into Lignocellulosic Biomass Pyrolysis: A Critical Review on Pretreatment, Characterization, and Products Upgrading" Processes 8, no. 7: 799. https://doi.org/10.3390/pr8070799
APA StyleZadeh, Z. E., Abdulkhani, A., Aboelazayem, O., & Saha, B. (2020). Recent Insights into Lignocellulosic Biomass Pyrolysis: A Critical Review on Pretreatment, Characterization, and Products Upgrading. Processes, 8(7), 799. https://doi.org/10.3390/pr8070799






