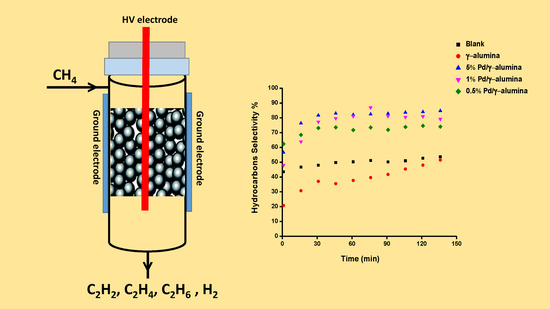
Plasma Catalytic Conversion of CH4 to Alkanes, Olefins and H2 in a Packed Bed DBD Reactor
Abstract
1. Introduction
2. Results and Discussion
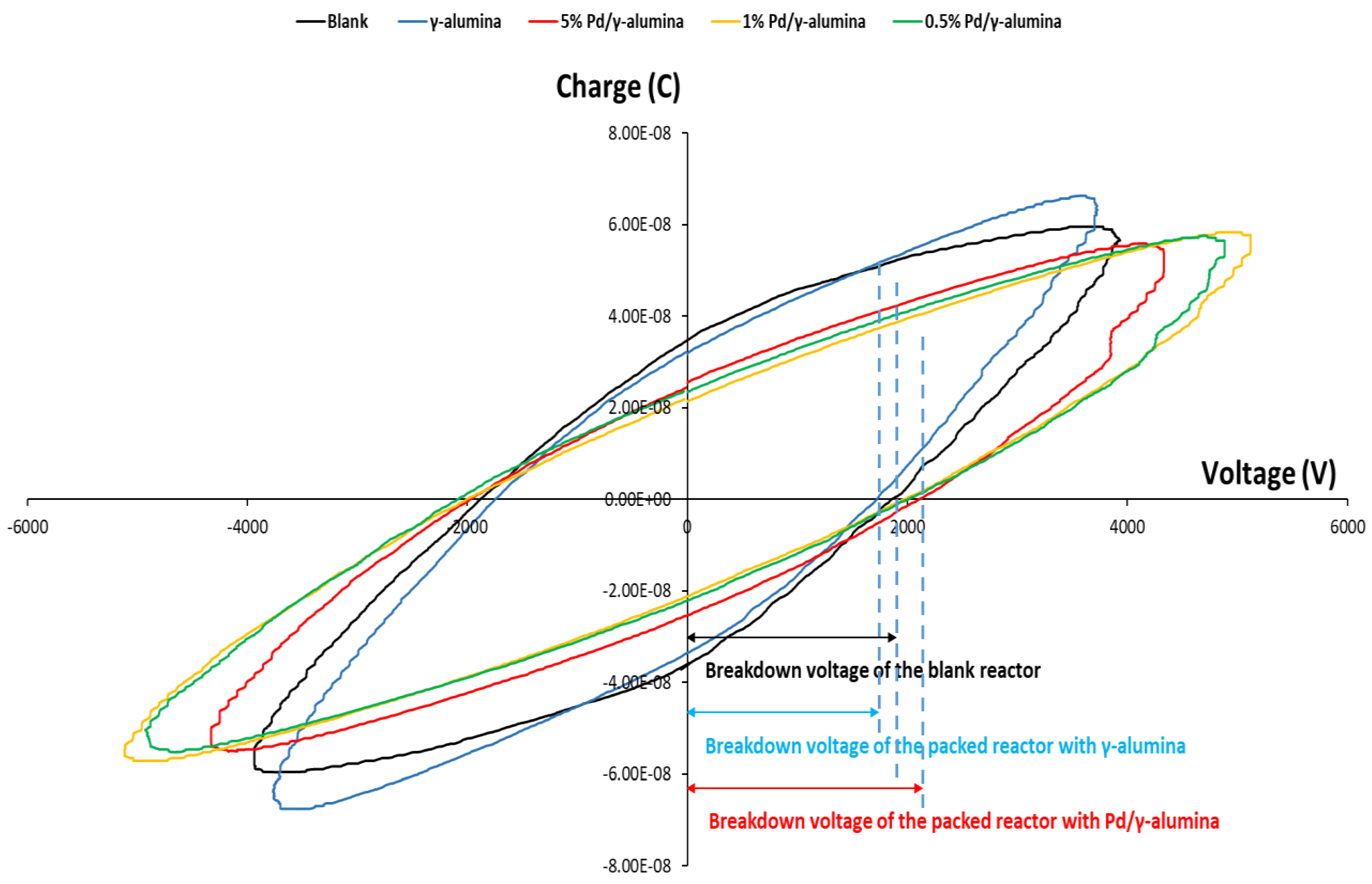
2.1. The Plasma Electrical Parameters
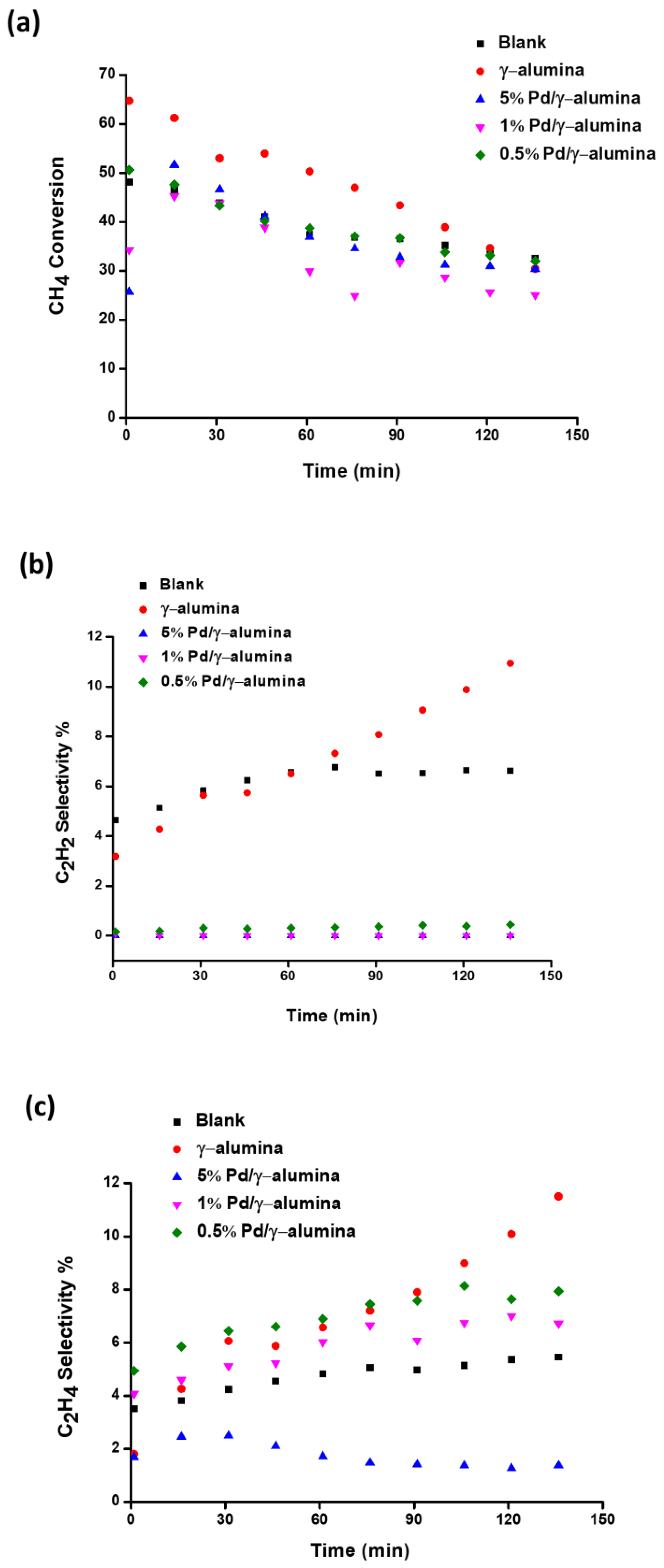
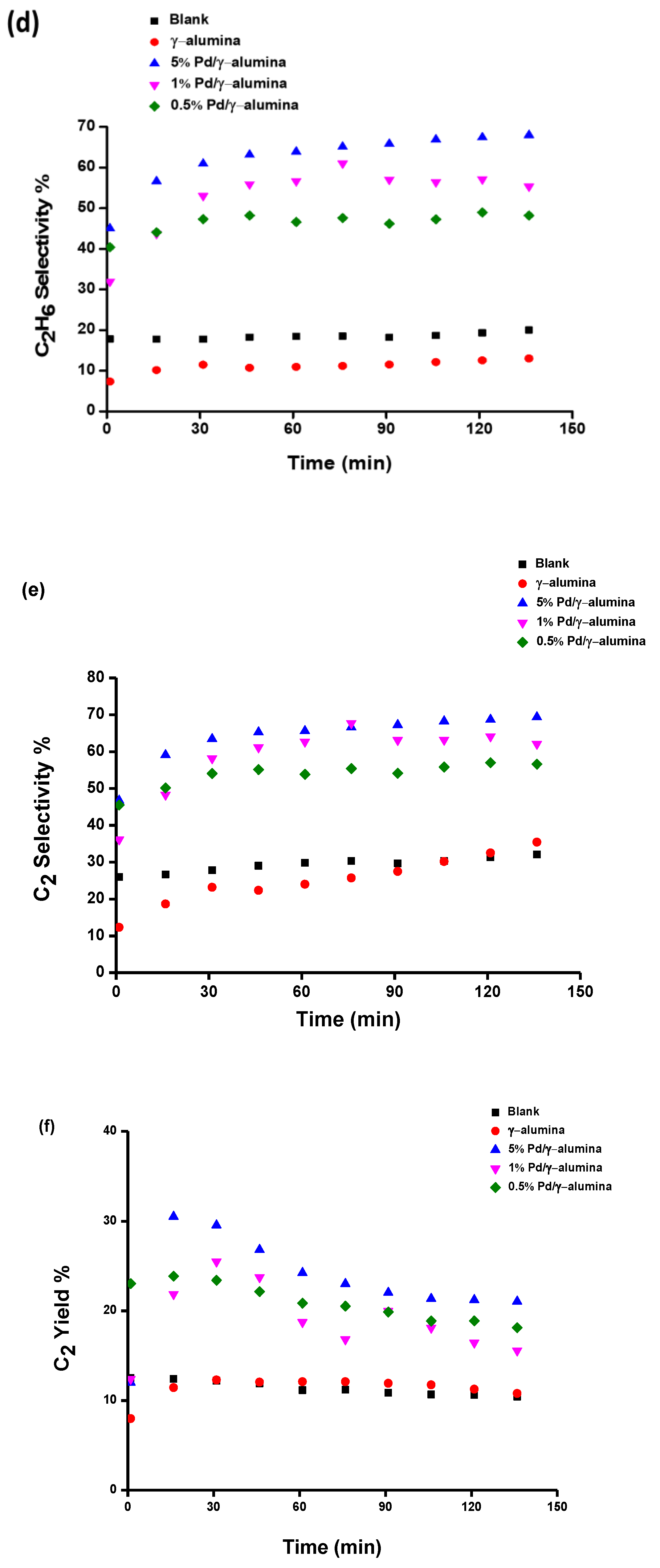
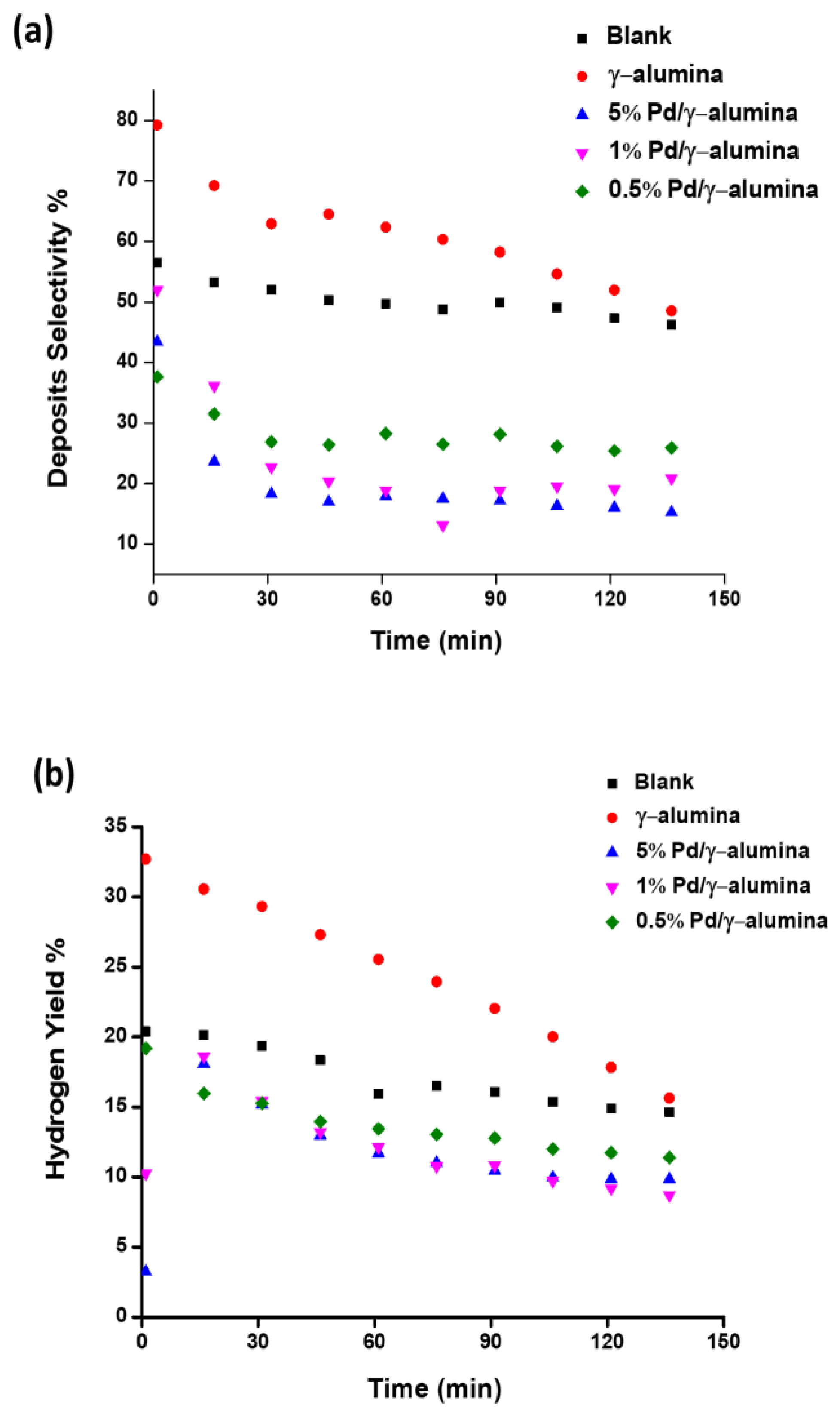
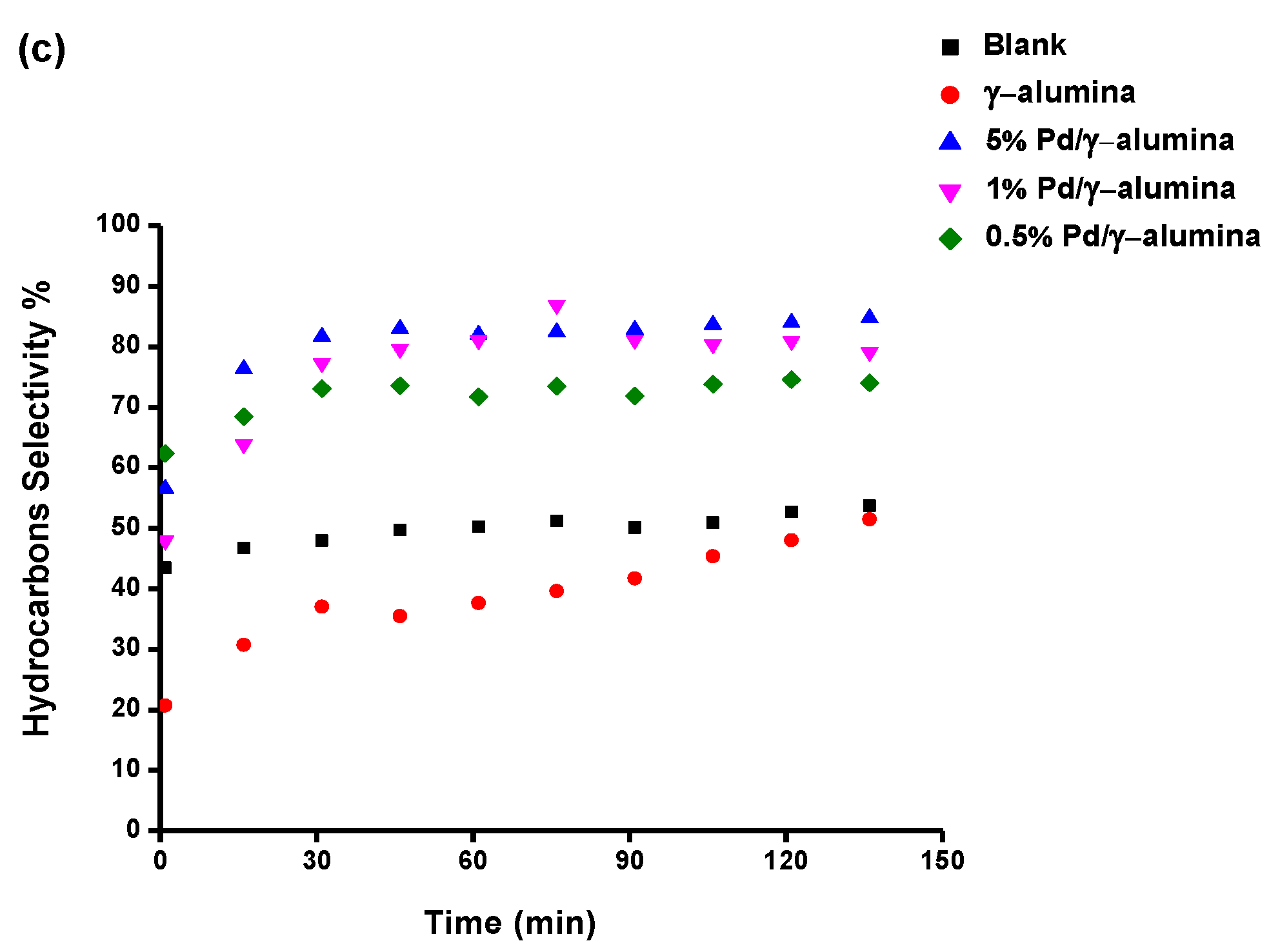
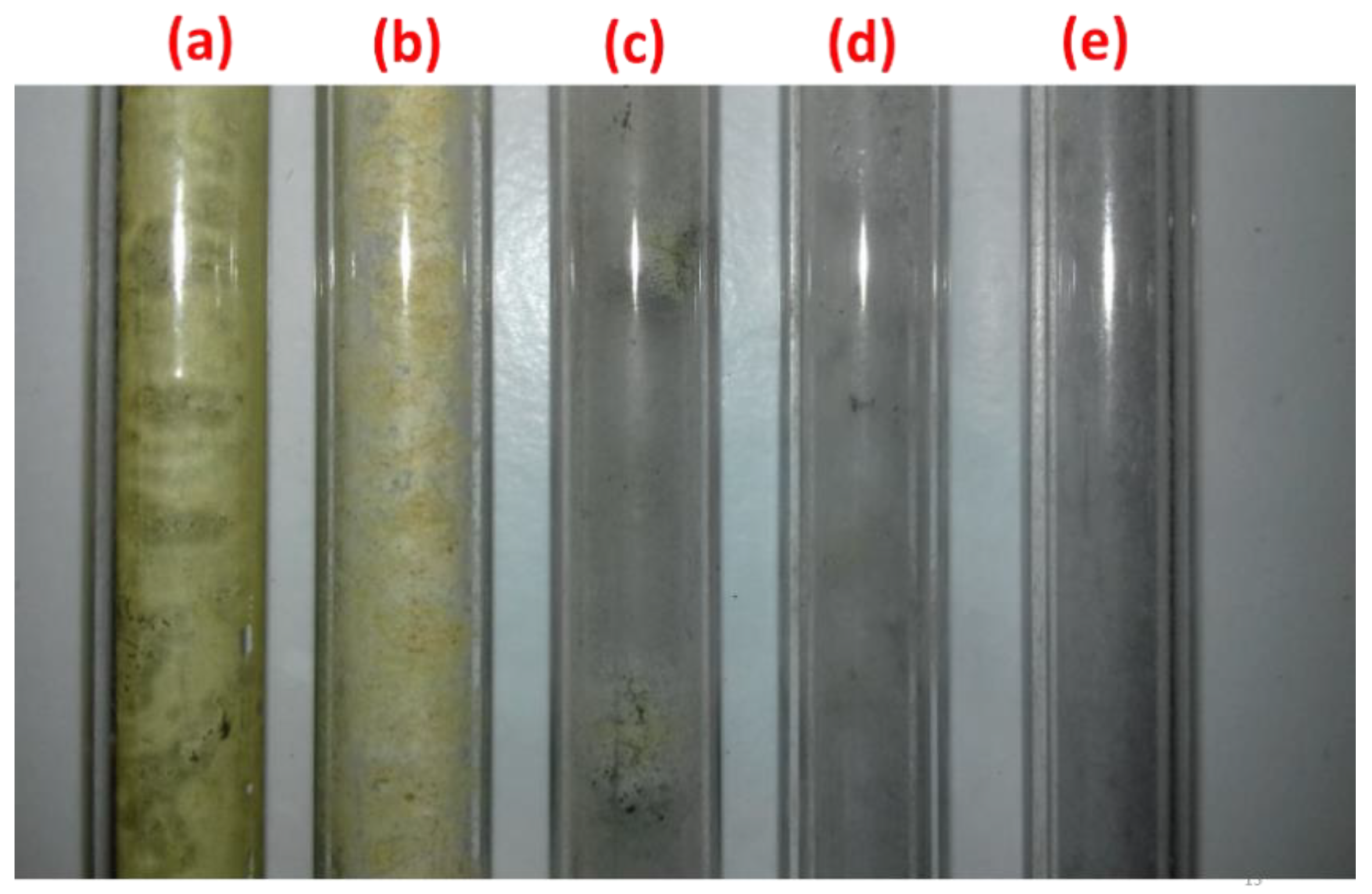
2.2. The Conversion of Methane and the Selectivity of Products
2.3. The Energy Consumption Analysis
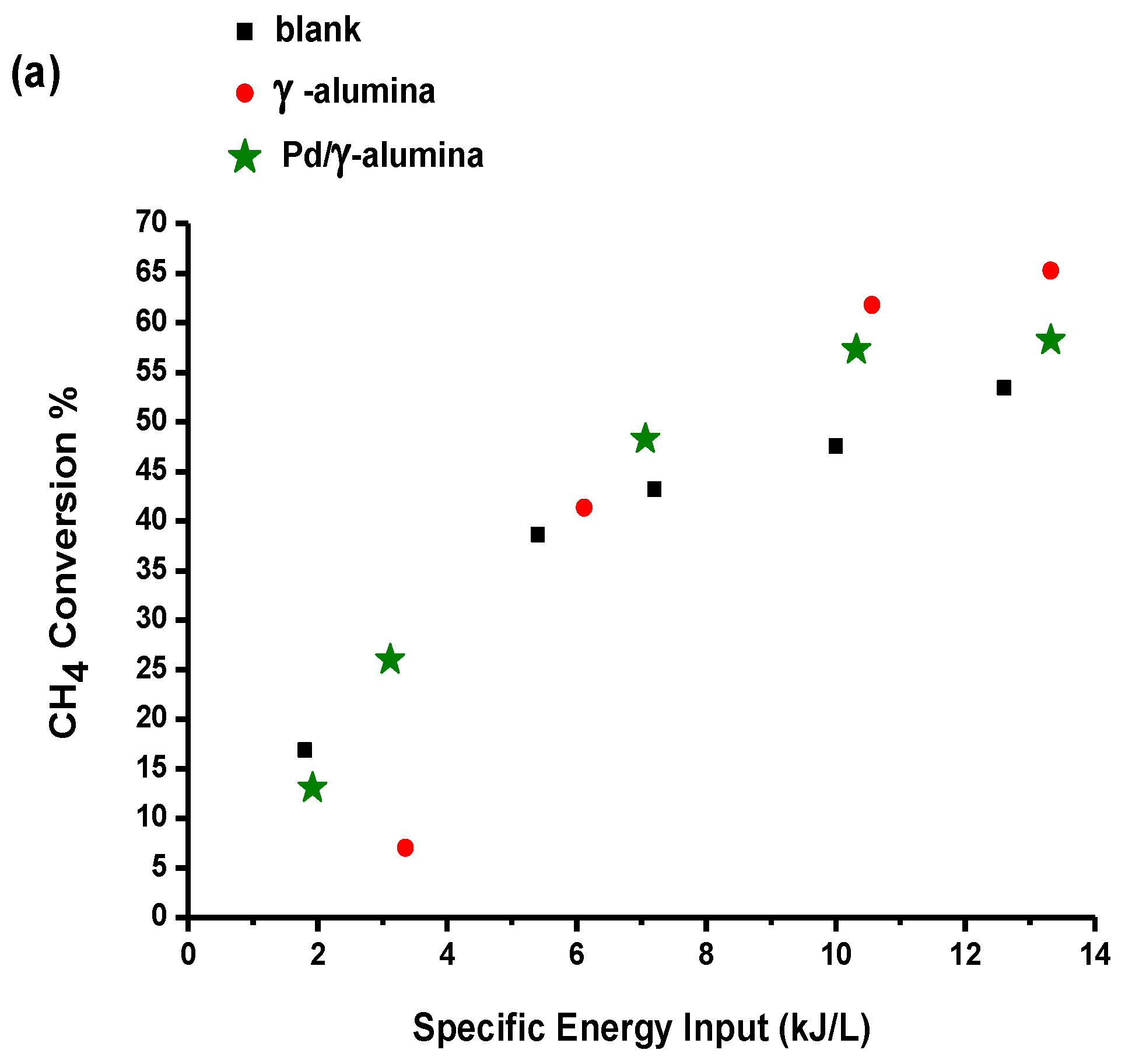
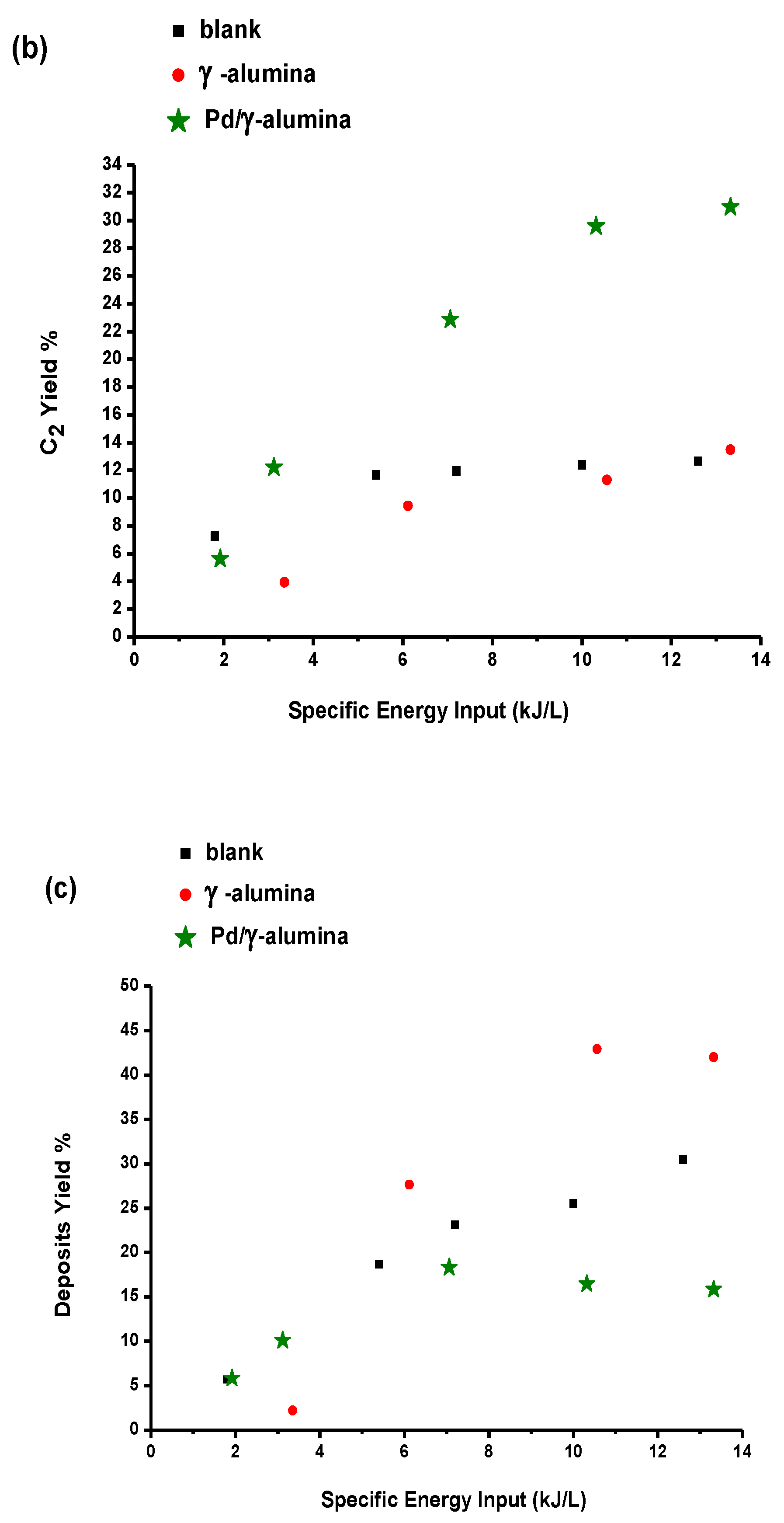
2.3.1. The Effect of Specific Energy Input (SEI)
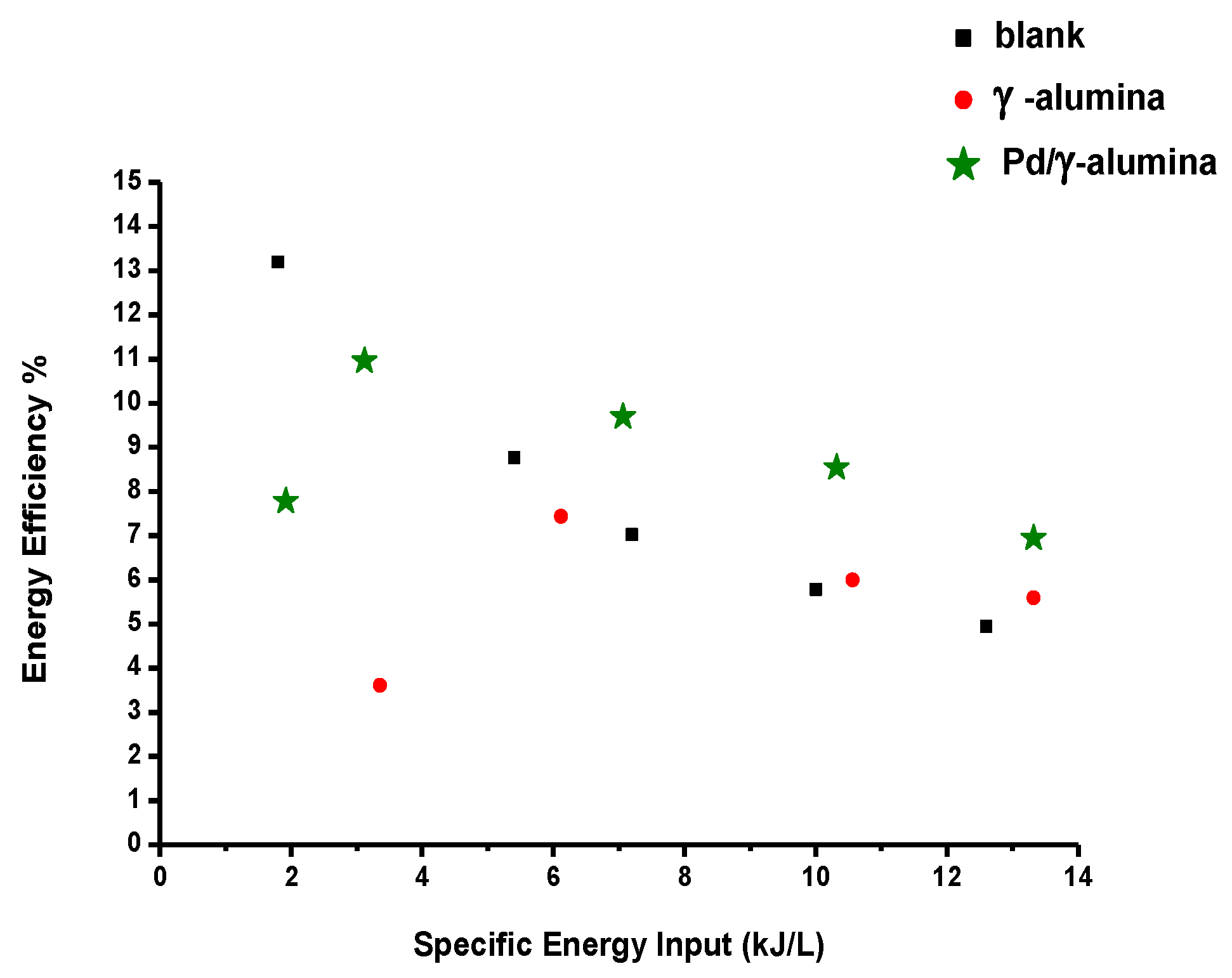
2.3.2. The Energy Efficiency
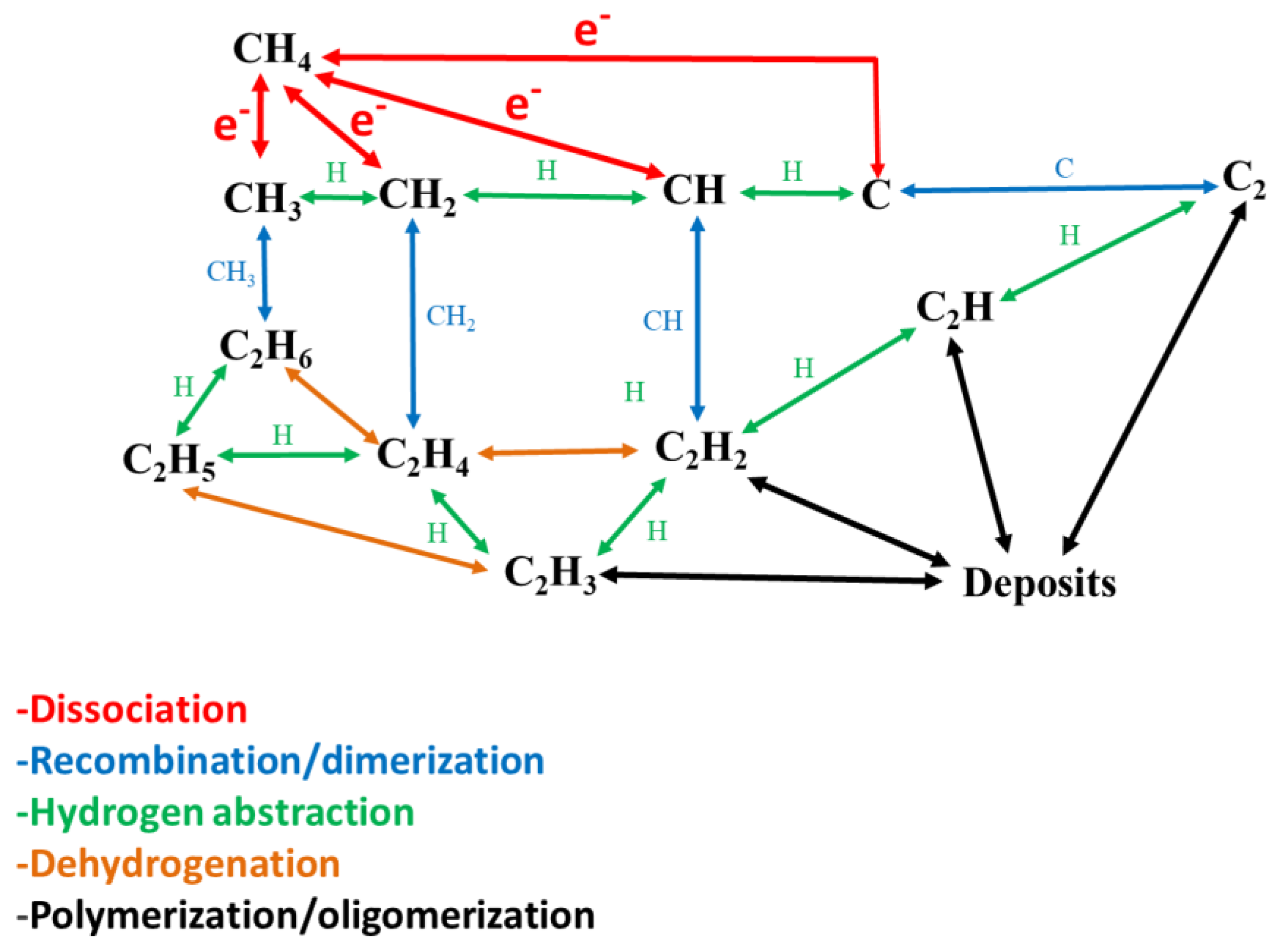
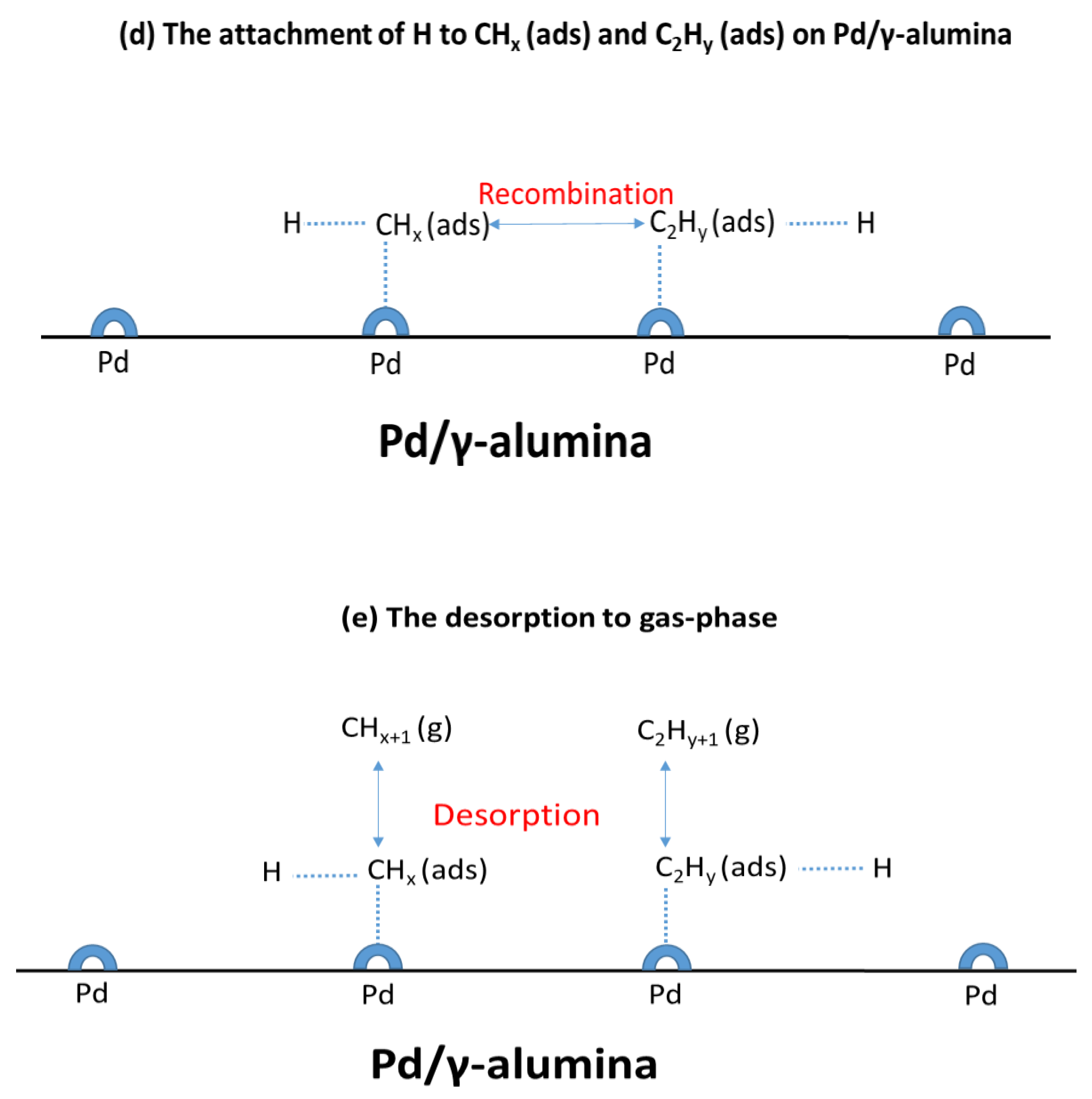
2.4. Reaction Pathways
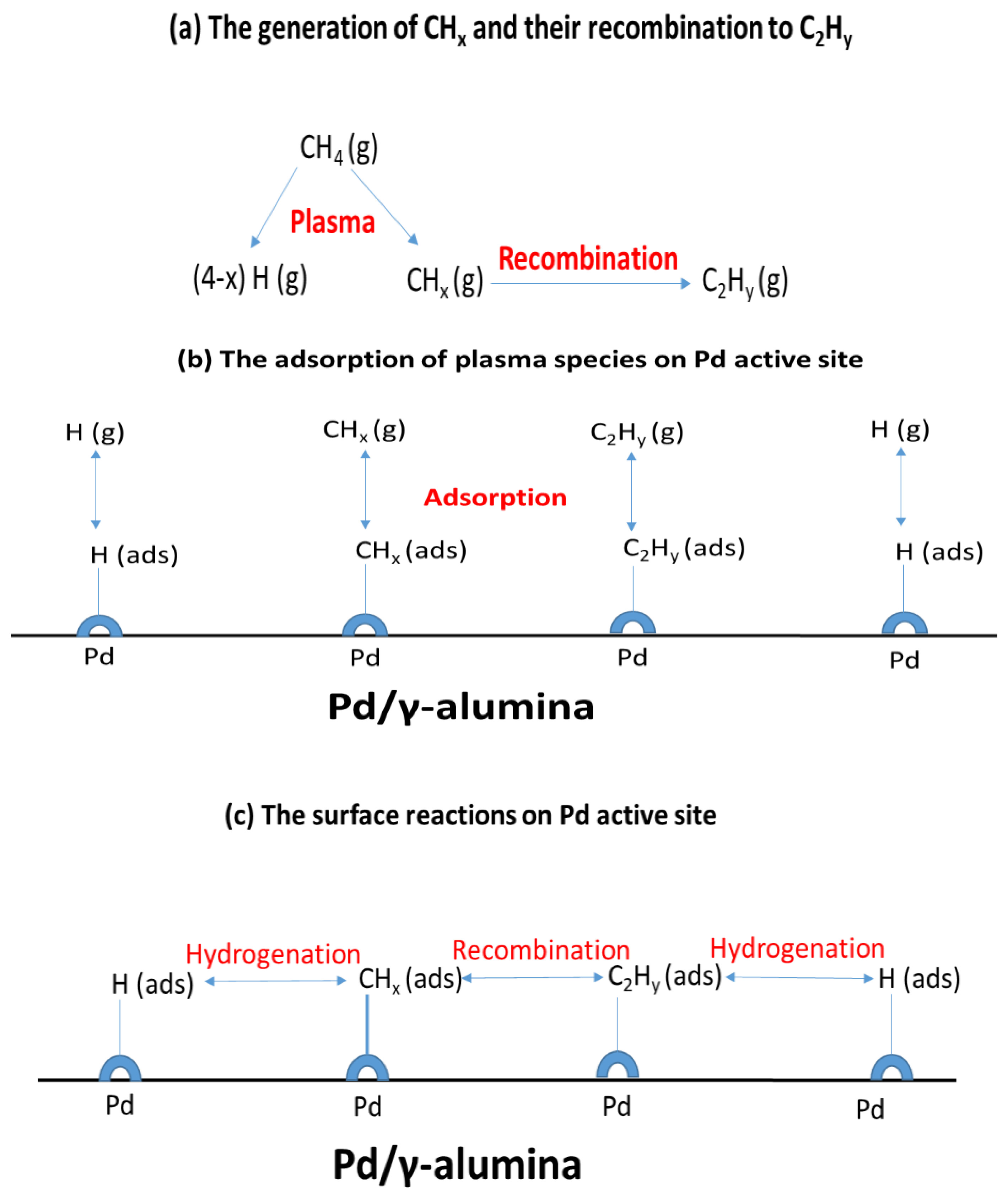
2.5. The Synergy of the Plasma and the Pd/γ-alumina Catalyst
3. Materials and Methods
3.1. Materials and Characterizations
3.2. The Implemented Packed Bed DBD Plasma Reactor and Its Operation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kim, H.H.; Teramoto, Y.; Negishi, N.; Ogata, A. A multidisciplinary approach to understand the interactions of nonthermal plasma and catalyst: A review. Catal. Today 2015, 256, 13–22. [Google Scholar] [CrossRef]
- Jo, S.; Lee, D.H.; Song, Y.H. Product analysis of methane activation using noble gases in a non-thermal plasma. Chem. Eng. Sci. 2015, 130, 101–108. [Google Scholar] [CrossRef]
- Neyts, E.C. Plasma-Surface Interactions in Plasma Catalysis. Plasma Chem. Plasma Process. 2016, 36, 185–212. [Google Scholar] [CrossRef]
- Zeng, Y.X.; Wang, L.; Wu, C.F.; Wang, J.Q.; Shen, B.X.; Tu, X. Low temperature reforming of biogas over K-, Mg- and Ce-promoted Ni/Al2O3 catalysts for the production of hydrogen rich syngas: Understanding the plasma-catalytic synergy. Appl. Catal. B Environ. 2018, 224, 469–478. [Google Scholar] [CrossRef]
- Rueangjitt, N.; Sreethawong, T.; Chavadej, S.; Sekiguchi, H. Non-Oxidative Reforming of Methane in a Mini-Gliding Arc Discharge Reactor: Effects of Feed Methane Concentration, Feed Flow Rate, Electrode Gap Distance, Residence Time, and Catalyst Distance. Plasma Chem. Plasma Proc. 2011, 31, 517–534. [Google Scholar] [CrossRef]
- Wang, B.; Guan, H.M. Highly Efficient Conversion of Methane to Olefins via a Recycle-Plasma-Catalyst Reactor. Catal. Lett. 2016, 146, 2193–2199. [Google Scholar] [CrossRef]
- Kasinathan, P.; Park, S.; Choi, W.; Hwang, Y.; Chang, J.; Park, Y. Plasma-Enhanced Methane Direct Conversion over Particle-Size Adjusted MOx/Al2O3 (M = Ti and Mg) Catalysts. Plasma Chem. Plasma Proc. 2014, 34, 1317–1330. [Google Scholar] [CrossRef]
- Górska, A.; Krawczyk, K.; Jodzis, S.; Schmidt-Szałowski, K. Non-oxidative methane coupling using Cu/ZnO/Al2O3 catalyst in DBD. Fuel 2011, 90, 1946–1952. [Google Scholar] [CrossRef]
- Kim, J.; Abbott, M.S.; Go, D.B.; Hicks, J.C. Enhancing C–H Bond Activation of Methane via Temperature-Controlled, Catalyst–Plasma Interactions. ACS Energy Lett. 2016, 1, 94–99. [Google Scholar] [CrossRef]
- Kim, S.; Kwon, B.; Kim, J. Plasma catalytic methane conversion over sol–gel derived Ru/TiO2 catalyst in a dielectric-barrier discharge reactor. Catal Commun. 2007, 8, 2204–2208. [Google Scholar] [CrossRef]
- Tang, Y.; Zhuo, J.; Cui, W.; Li, S.; Yao, Q. Enhancing ignition and inhibiting extinction of methane diffusion flame by in situ fuel processing using dielectric-barrier-discharge plasma. Fuel Process. Technol. 2019, 194, 106128. [Google Scholar] [CrossRef]
- Van Laer, K.; Bogaerts, A. How bead size and dielectric constant affect the plasma behaviour in a packed bed plasma reactor: A modelling study. Plasma Process Polym. 2017, 14, 1600129. [Google Scholar] [CrossRef]
- Nozakia, T.; Okazaki, K. Non-thermal plasma catalysis of methane: Principles, energy efficiency, and applications. Catal. Today 2013, 211, 29–38. [Google Scholar] [CrossRef]
- Li, X.S.; Zhu, A.M.; Wang, K.J.; Xu, Y.; Song, Z.M. Methane conversion to C2 hydrocarbons and hydrogen in atmospheric non-thermal plasmas generated by different electric discharge techniques. Catal. Today 2004, 98, 617–624. [Google Scholar] [CrossRef]
- Jo, S.; Lee, D.H.; Kang, W.S.; Song, Y.H. Methane activation using noble gases in a dielectric barrier discharge reactor. Phys. Plasmas 2013, 20, 083509. [Google Scholar] [CrossRef]
- Sentek, J.; Krawczyk, K.; Młotek, M.; Kalczewska, M.; Kroker, T.; Kolb, T.; Schenk, A.; Gericke, K.H.; Schmidt-Szałowski, K. Plasma-catalytic methane conversion with carbon dioxide in dielectric barrier discharges. Appl. Catal. B Environ. 2010, 94, 19–26. [Google Scholar] [CrossRef]
- Jo, S.; Kim, T.; Lee, D.H.; Kang, W.S.; Song, Y.H. Effect of the Electric Conductivity of a Catalyst on Methane Activation in a Dielectric Barrier Discharge Reactor. Plasma Chem. Plasma Process. 2014, 34, 175–186. [Google Scholar] [CrossRef]
- Khalifeh, O.; Taghvaei, H.; Mosallanejad, A.; Rahimpour, M.R.; Shariati, A. Extra pure hydrogen production through methane decomposition using nanosecond pulsed plasma and Pt-Re catalyst. Chem. Eng. J. 2016, 294, 132–145. [Google Scholar] [CrossRef]
- Kim, S.; Choi, J.; Kim, J.; Lee, H.; Song, H.K. Plasma catalytic reaction of methane over nanostructured Ru/-Al2O3 catalysts in dielectric-barrier discharge. J. Ind. Eng. Chem. 2005, 11, 533–539. [Google Scholar]
- Lu, J.; Li, Z. Conversion of natural gas to C2 hydrocarbons via cold plasma technology. J. Nat. Gas Chem. 2010, 19, 375–379. [Google Scholar] [CrossRef]
- Taheraslani, M.; Gardeniers, H. Coupling of CH4 to C2 Hydrocarbons in a Packed Bed DBD Plasma Reactor: The Effect of Dielectric Constant and Porosity of the Packing. Energies 2020, 13, 468. [Google Scholar] [CrossRef]
- Mei, D.; Zhu, X.; He, Y.L.; Yan, J.D.; Tu, X. Plasma-assisted conversion of CO2 in a dielectric barrier discharge reactor: Understanding the effect of packing materials. Plasma Sources Sci. Technol. 2015, 24, 015011. [Google Scholar] [CrossRef]
- Butterworth, T.; Allen, R.W.K. Plasma-catalyst interaction studied in a single pellet DBD reactor: Dielectric constant effect on plasma dynamics. Plasma Sources Sci. Technol. 2017, 26, 065008. [Google Scholar] [CrossRef]
- Belov, I.; Paulussen, S.; Bogaerts, A. Appearance of a conductive carbonaceous coating in a CO2 dielectric barrier discharge and its influence on the electrical properties and the conversion efficiency. Plasma Sources Sci. Technol. 2016, 25, 015023. [Google Scholar] [CrossRef]
- Chandrashekaraiah, T.H.; Bogdanowicz, R.; Rühl, E.; Danilov, V.; Meichsner, J.; Thierbach, S.; Hippler, R. Spectroscopic Study of Plasma Polymerized a-C:H Films Deposited by a Dielectric Barrier Discharge. Materials 2016, 9, 594. [Google Scholar] [CrossRef]
- Gadzhieva, N.N. Methane Adsorption and Plasma-Assisted Catalytic Conversion on the Surface of γ-Alumina. High Energy Chem. 2003, 37, 38–43. [Google Scholar] [CrossRef]
- Nozaki, T.; Muto, N.; Kado, S.; Okazaki, K. Dissociation of vibrationally excited methane on Ni catalyst: Part 1. Application to methane steam reforming. Catal. Today 2004, 89, 57–65. [Google Scholar] [CrossRef]
- Puliyalil, H.; Jurković, D.L.; Dasireddy, V.D.B.C.; Likozar, B. A review of plasma-assisted catalytic conversion of gaseous carbon dioxide and methane into value-added platform chemicals and fuels. RSC Adv. 2018, 8, 27481–27508. [Google Scholar] [CrossRef]
- Correale, G.; Winkel, R.; Kotsonis, M. Energy deposition characteristics of nanosecond dielectric barrier discharge plasma actuators: Influence of dielectric material. J. Appl. Phys. 2015, 118, 083301. [Google Scholar] [CrossRef]
- Taheraslani, M.; Gardeniers, H. High-Resolution SEM and EDX Characterization of Deposits Formed by CH4+Ar DBD Plasma Processing in a Packed Bed Reactor. Nanomaterials 2019, 9, 589. [Google Scholar] [CrossRef]
- Cho, W.; Baek, Y.; Chaikim, Y.; Anpo, M. Plasma catalytic reaction of natural gas to C2 product over Pd-NiO/Al2O3 and Pt-Sn/Al2O3 catalysts. Res. Chem. Intermed. 2002, 28, 343–357. [Google Scholar] [CrossRef]
- Tu, X.; Gallon, H.J.; Twigg, M.V.; Gorry, P.A.; Whitehead, J.C. Dry reforming of methane over a Ni/Al2O3 catalyst in a coaxial dielectric barrier discharge reactor. J. Phys. D Appl. Phys. 2011, 44, 274007. [Google Scholar] [CrossRef]
- Tu, X.; Gallon, H.J.; Whitehead, J.C. Plasma-assisted reduction of a NiO/Al2O3 catalyst in atmospheric pressure H2/Ar dielectric barrier discharge. Catal. Today 2013, 211, 120–125. [Google Scholar] [CrossRef]
- De Bie, C.; Verheyde, B.; Martens, T.; van Dijk, J.; Paulussen, S.; Bogaerts, A. Fluid Modelling of the Conversion of Methane into Higher Hydrocarbons in an Atmospheric Pressure Dielectric Barrier Discharge. Plasma Process. Polym. 2011, 8, 1033–1058. [Google Scholar] [CrossRef]
- Kado, S.; Urasaki, K.; Sekine, Y.; Fujimoto, K.; Nozaki, T.; Okazaki, K. Reaction mechanism of methane activation using non-equilibrium pulsed discharge at room temperature. Fuel 2003, 82, 2291–2297. [Google Scholar] [CrossRef]
- Bae, J.; Lee, M.; Park, S.; Hong, M.G.J.D.Y.; Kim, Y.D.; Park, Y.K.; Hwang, Y.K. Investigation of intermediates in non-oxidative coupling of methane by non-thermal RF plasma. Catal. Today 2017, 293, 105–112. [Google Scholar] [CrossRef]
- Tomaszewska, A.; Stępień, Z.M. The influenced of the external electric field on the hydrogen-palladium system. J. Phys. 2007, 79, 012028. [Google Scholar] [CrossRef]

| Experiment | Blank | γ-alumina | 0.5%Pd/ γ-alumina | 1.0%Pd/ γ-alumina | 5.0%Pd/ γ-alumina |
|---|---|---|---|---|---|
| Applied Voltage (kV) | 7.6 | 9.0 | 9.0 | 8.9 | 8.8 |
| Breakdown Voltage (kV) | 1.9 | 1.7 | 1.8 | 1.9 | 2.1 |
| Experiment | CH4 Conversion % | C2 Yield % | Deposits Yield % | Energy Efficiency % |
| The non-packed reactor | 48% | 12.4% | 25.5% | 5.8% |
| The γ-alumina packed reactor | 62% | 11.3% | 42.9% | 6.0% |
| The Pd/γ-alumina packed reactor | 57% | 29.6% | 16.5% | 8.6% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taheraslani, M.; Gardeniers, H. Plasma Catalytic Conversion of CH4 to Alkanes, Olefins and H2 in a Packed Bed DBD Reactor. Processes 2020, 8, 774. https://doi.org/10.3390/pr8070774
Taheraslani M, Gardeniers H. Plasma Catalytic Conversion of CH4 to Alkanes, Olefins and H2 in a Packed Bed DBD Reactor. Processes. 2020; 8(7):774. https://doi.org/10.3390/pr8070774
Chicago/Turabian StyleTaheraslani, Mohammadreza, and Han Gardeniers. 2020. "Plasma Catalytic Conversion of CH4 to Alkanes, Olefins and H2 in a Packed Bed DBD Reactor" Processes 8, no. 7: 774. https://doi.org/10.3390/pr8070774
APA StyleTaheraslani, M., & Gardeniers, H. (2020). Plasma Catalytic Conversion of CH4 to Alkanes, Olefins and H2 in a Packed Bed DBD Reactor. Processes, 8(7), 774. https://doi.org/10.3390/pr8070774