Linking CFD and Kinetic Models in Anaerobic Digestion Using a Compartmental Model Approach
Abstract
:1. Introduction
2. Methods
2.1. CFD Modelling of Anaerobic Digester Mixing
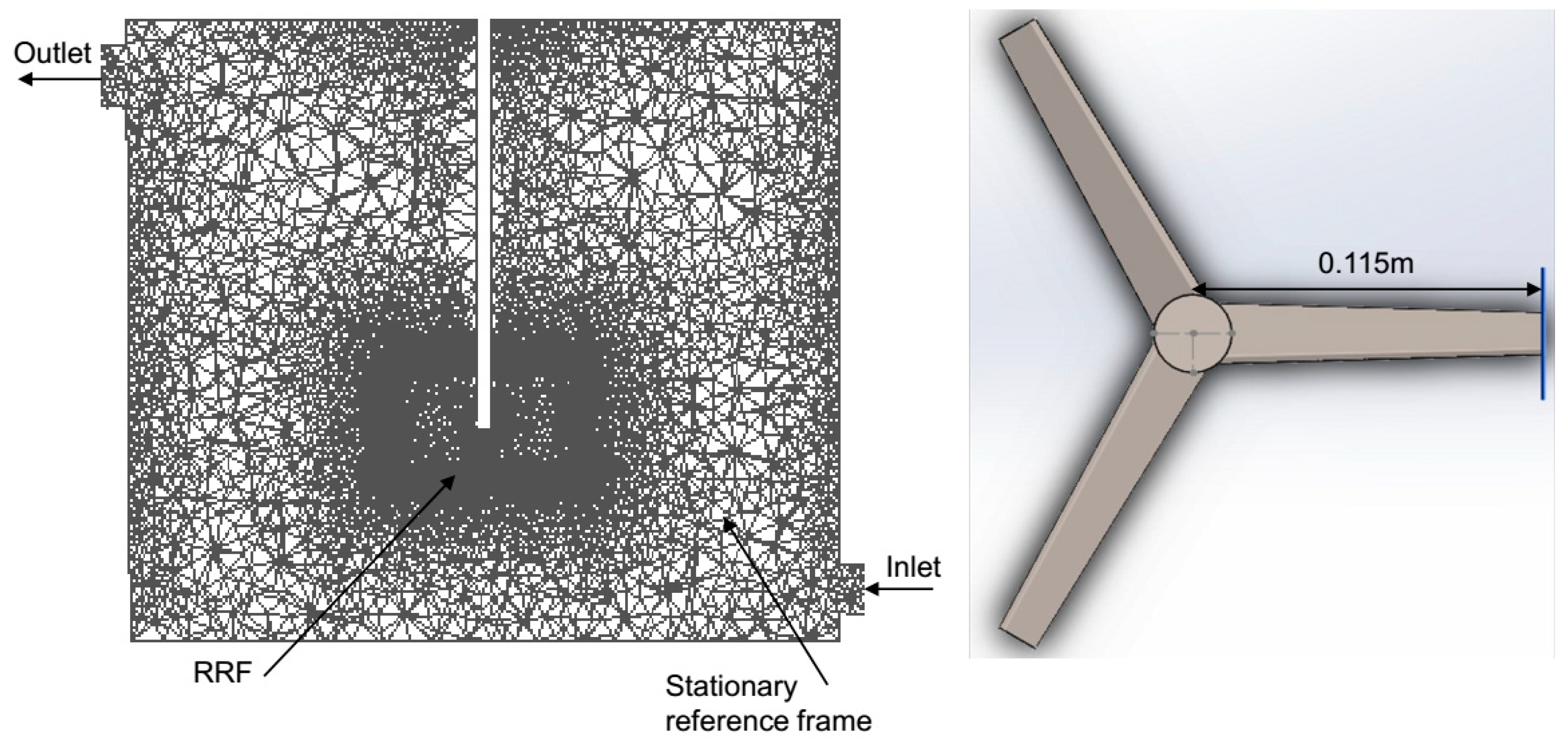
2.1.1. AD Geometry Development and Meshing
2.1.2. Discretization Method
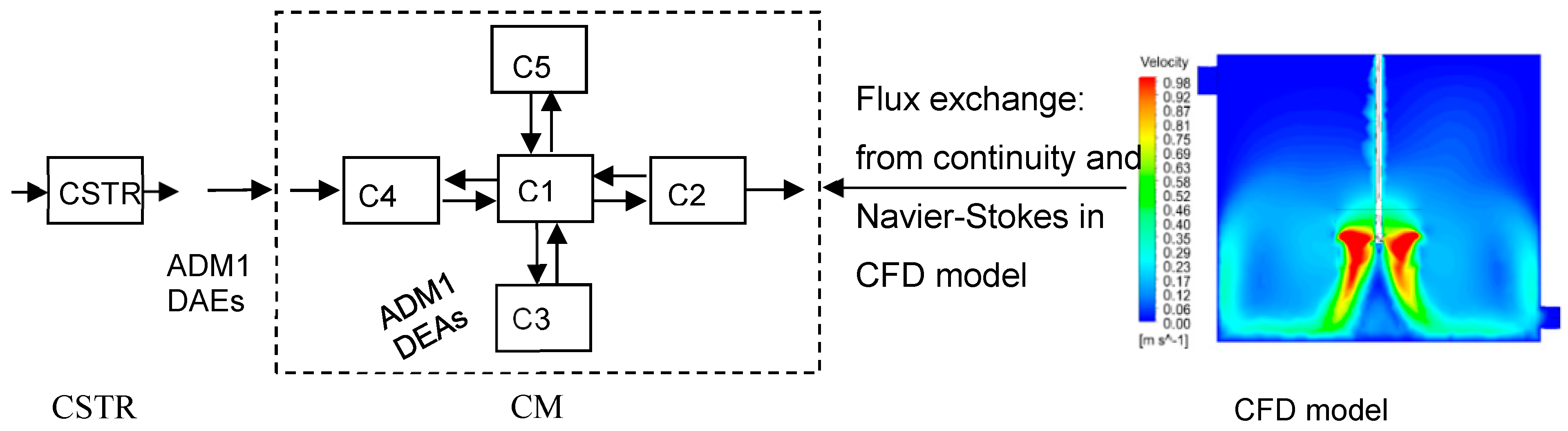
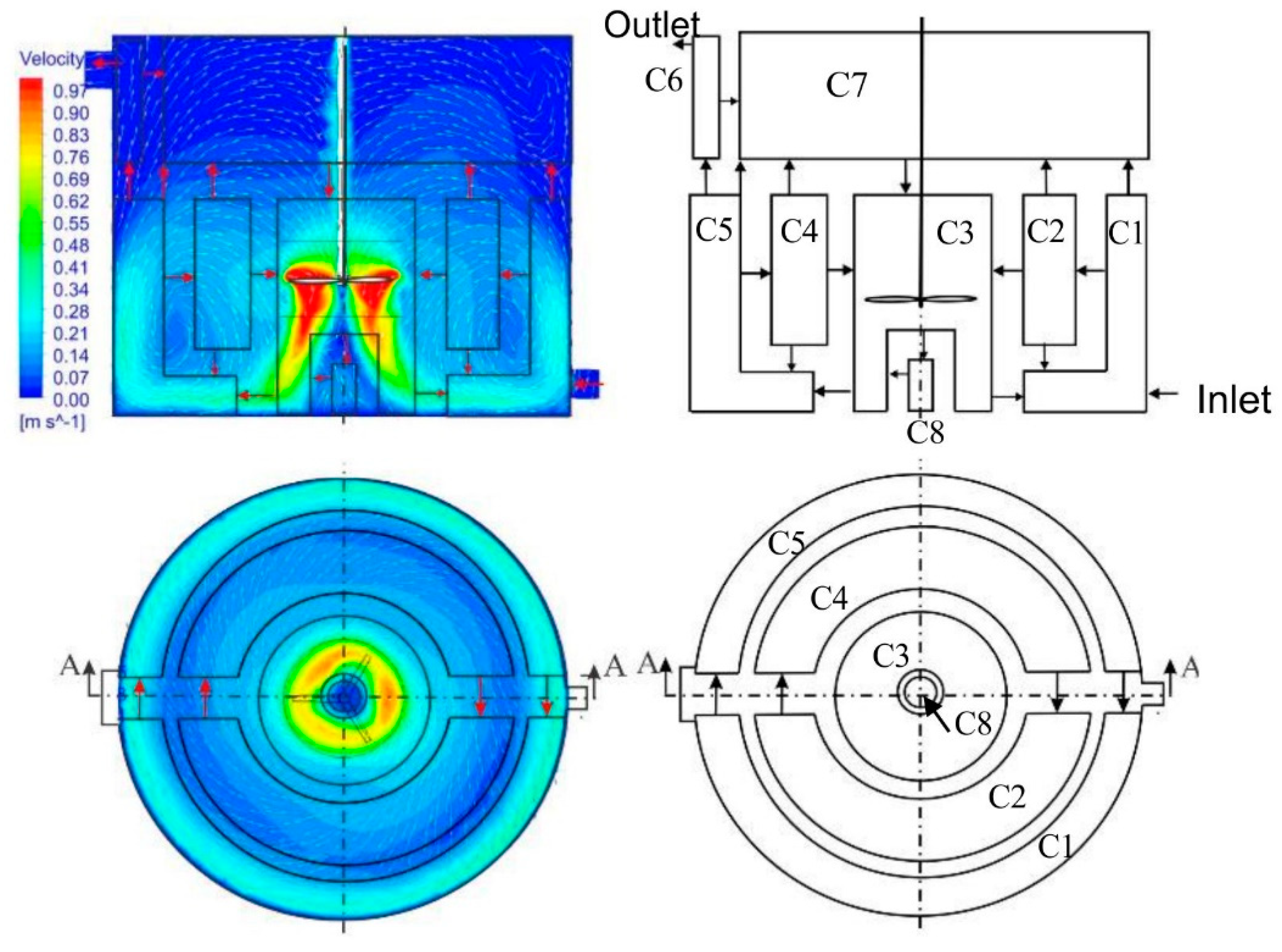
2.2. Compartmentalization of AD from the CFD Model
2.3. Implementation of ADM1 into the CM
3. Results and Discussion
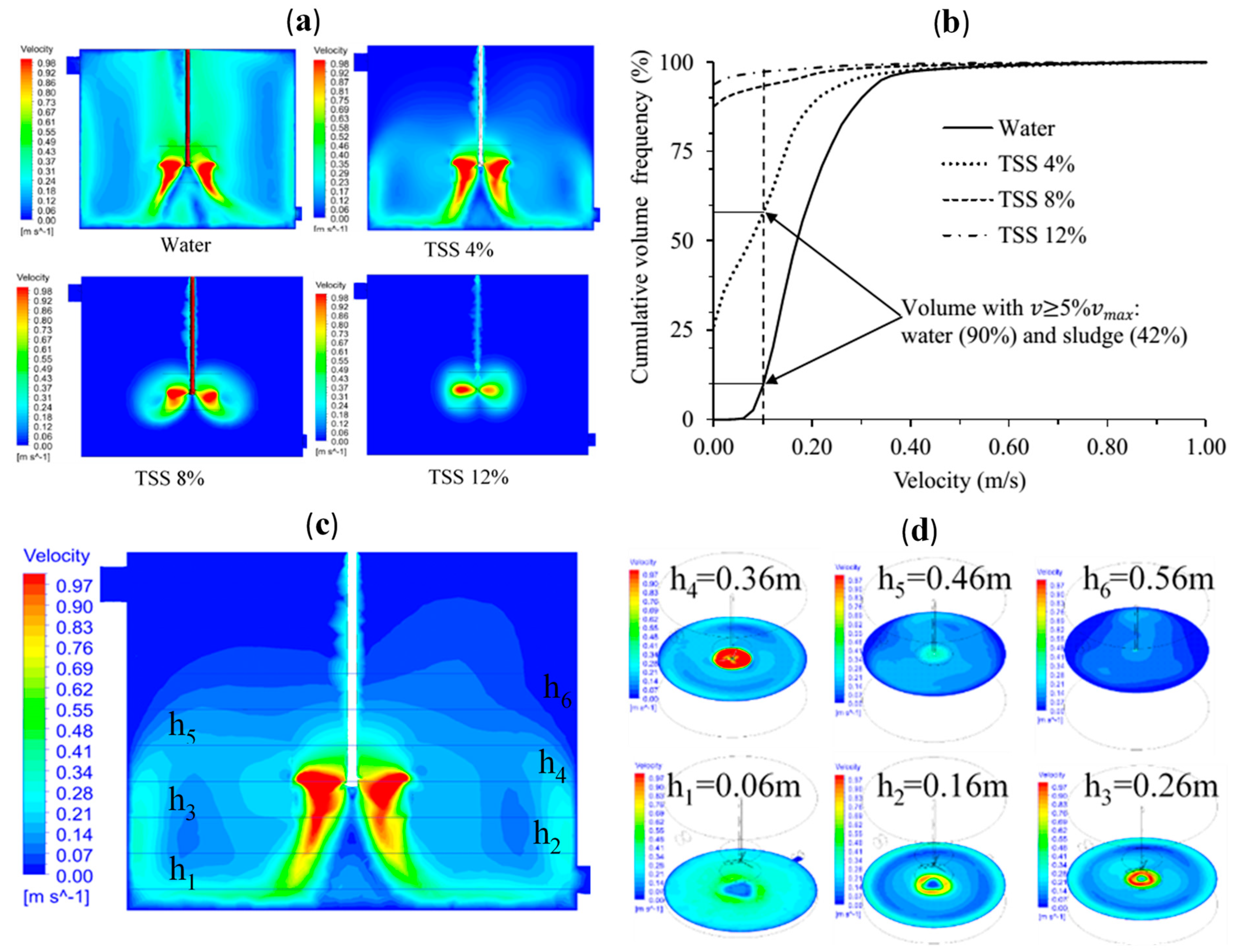
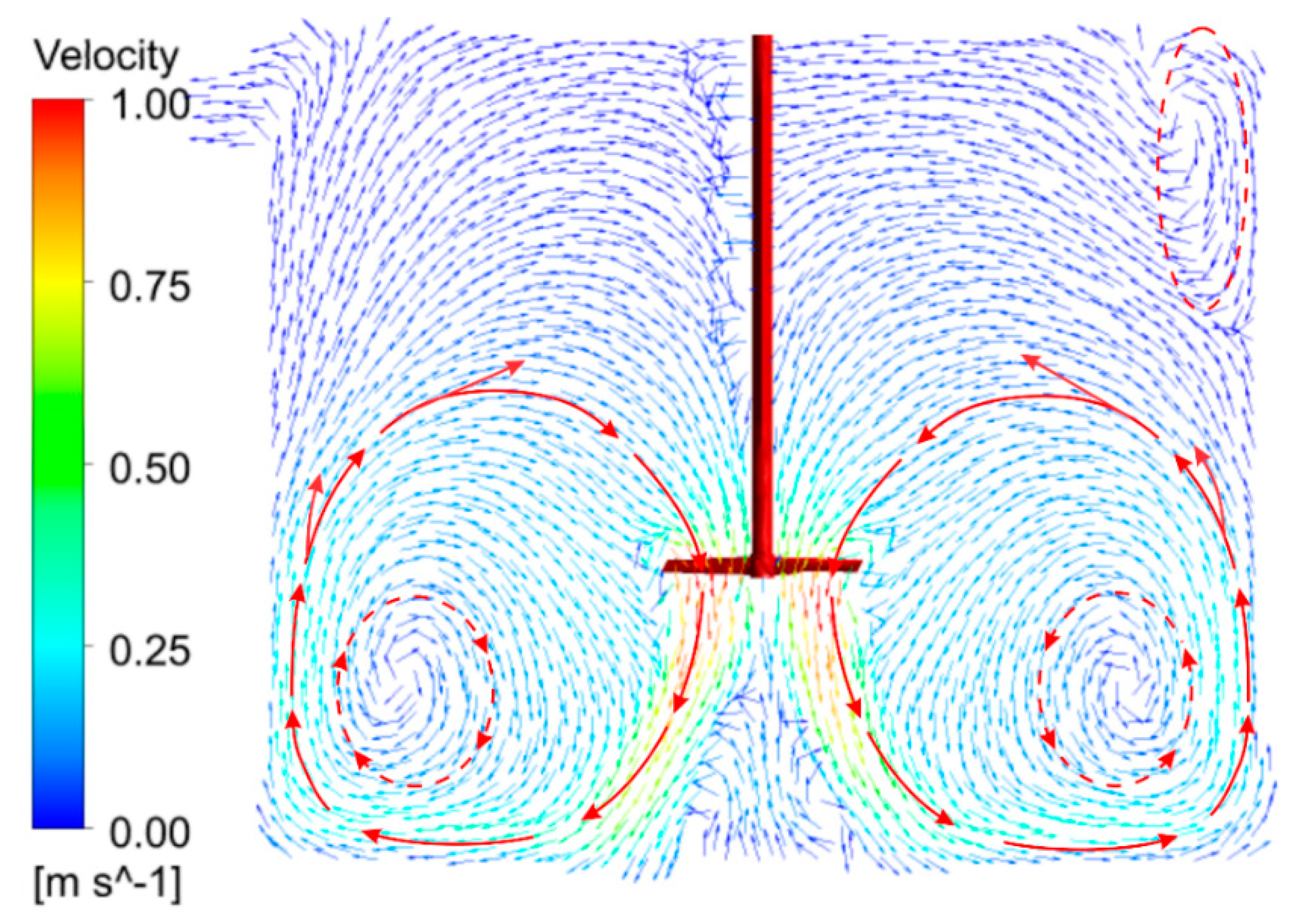
3.1. CFD Model Simulation
Compartmentalization of AD
- Zone one: high-velocity zone (C3). This zone is near the impeller with a velocity magnitude 0.6*maximum velocity (). The local residence time in this zone is very short due to the high flux exchange. is the sludge velocity near the mixer impeller tip.
- Zone two: medium velocity zone. The velocity in this zone ranges between . This zone is the outermost of the digester volume with a large diameter with low radial velocity; therefore, the recirculation time is longer. Instead of assuming the zone is a single compartment, it is decided to implement two compartments in series (C1 and C5) to make the residence time more realistic.
- Zone three: low-velocity zone. A zone with a velocity magnitude of . This zone is divided into two compartments in series (C2 and C4), such as zone two.
- Zone four: Stagnant zone. A very low velocity , and it includes C6, C7, and C8.
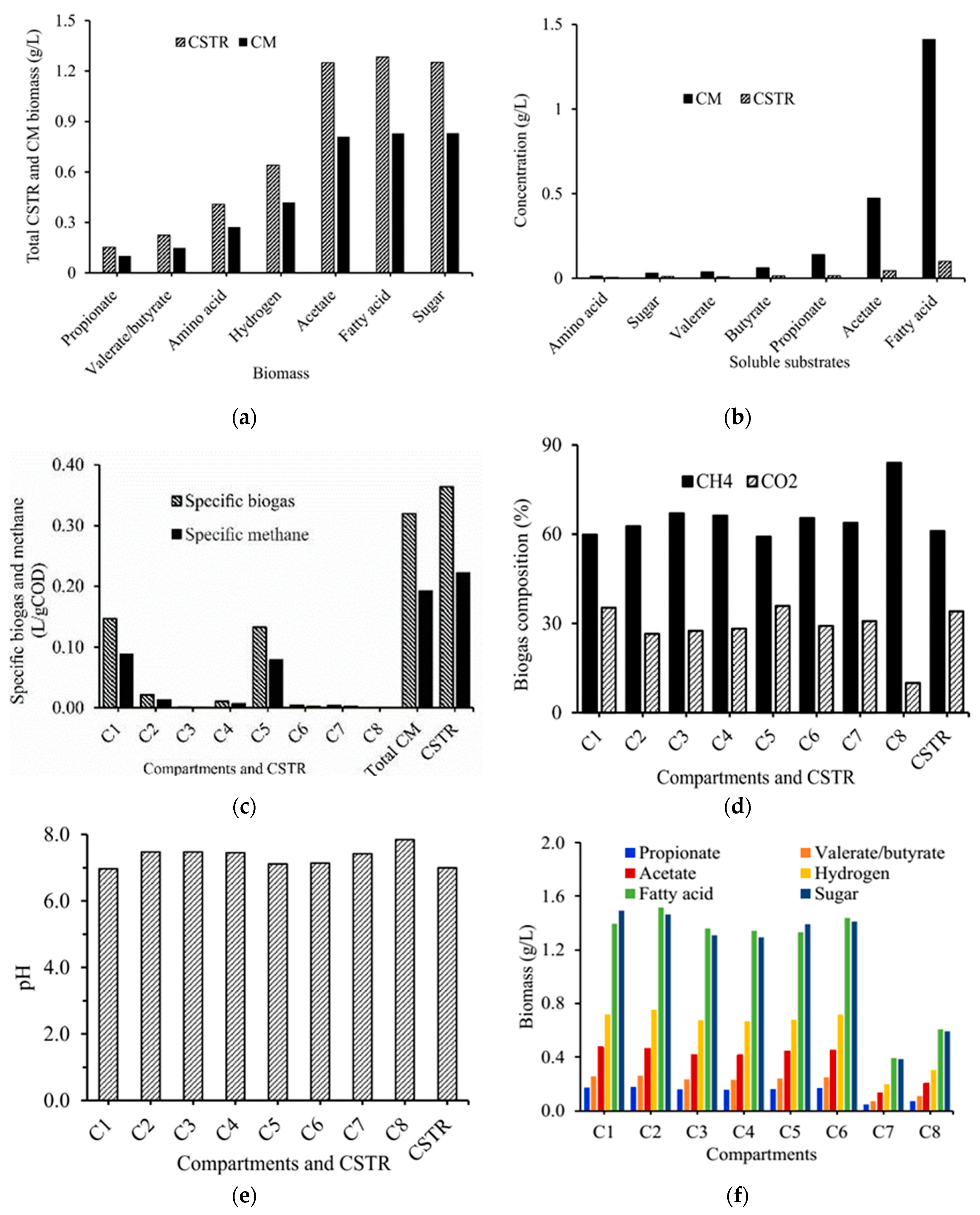
3.2. Non-Ideal Mixing Kinetics Modelling
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Batstone, D.; Keller, J.; Angelidaki, I.; Kalyuzhnyi, S.; Pavlostathis, S.; Rozzi, A.; Sanders, W.; Siegrist, H.; Vavilin, V. The IWA Anaerobic Digestion Model No 1 (ADM1). Water Sci. Technol. 2002, 45, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Polizzi, C.; Alatriste-Mondragón, F.; Munz, G. Modeling the Disintegration Process in Anaerobic Digestion of Tannery Sludge and Fleshing. Front. Environ. Sci. 2017, 5, 1–10. [Google Scholar] [CrossRef]
- Kil, H.; Xi, Y.; Li, D. A new waste characterization method for the anaerobic digestion based on ADM1. Chem. Eng. Commun. 2017, 204, 1428–1444. [Google Scholar] [CrossRef]
- Poggio, D.; Walker, M.; Nimmo, W.; Ma, L.; Pourkashanian, M. Modelling the anaerobic digestion of solid organic waste—Substrate characterisation method for ADM1 using a combined biochemical and kinetic parameter estimation approach. Waste Manag. 2016, 53, 40–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donoso-Bravo, A.; Sadino-Riquelme, C.; Gomez, D.; Segura, C.; Valdebenito, E.; Hansen, F. Modelling of an anaerobic plug-flow reactor. Process analysis and evaluation approaches with non-ideal mixing considerations. Bioresour. Technol. 2018, 260, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Conti, F.; Wiedemann, L.; Sonnleitner, M.; Saidi, A.; Goldbrunner, M. Monitoring the mixing of an artificial model substrate in a scale-down laboratory digester. Renew. Energy 2019, 132, 351–362. [Google Scholar] [CrossRef]
- Wiedemann, L.; Conti, F.; Saidi, A.; Sonnleitner, M.; Goldbrunner, M. Modeling Mixing in Anaerobic Digesters with Computational Fluid Dynamics Validated by Experiments. Chem. Eng. Technol. 2018, 41, 2101–2110. [Google Scholar] [CrossRef]
- Cortada-Garcia, M.; Dore, V.; Mazzei, L.; Angeli, P. Experimental and CFD studies of power consumption in the agitation of highly viscous shear thinning fluids. Chem. Eng. Res. Des. 2017, 119, 171–182. [Google Scholar] [CrossRef]
- Bridgeman, J. Computational fluid dynamics modelling of sewage sludge mixing in an anaerobic digester. Adv. Eng. Softw. 2012, 44, 54–62. [Google Scholar] [CrossRef]
- Yu, L.; Ma, J.; Chen, S. Numerical simulation of mechanical mixing in high solid anaerobic digester. Bioresour. Technol. 2011, 102, 1012–1018. [Google Scholar] [CrossRef]
- Wu, B. Computational Fluid Dynamics Investigation of Turbulence Models for Non-Newtonian Fluid Flow in Anaerobic Digesters. Environ. Sci. Technol. 2010, 44, 8989–8995. [Google Scholar] [CrossRef] [PubMed]
- Wu, B. Integration of mixing, heat transfer, and biochemical reaction kinetics in anaerobic methane fermentation. Biotechnol. Bioeng. 2012, 109, 2864–2874. [Google Scholar] [CrossRef] [PubMed]
- Rezavand, M.; Winkler, D.; Sappl, J.; Seiler, L.; Meister, M.; Rauch, W. A fully Lagrangian computational model for the integration of mixing and biochemical reactions in anaerobic digestion. Comput. Fluids 2019, 181, 224–235. [Google Scholar] [CrossRef]
- Gaden, D.L.F.; Bibeau, E.L. A general three-dimensional extension to ADM1: The importance of an integrated fluid flow model. In Proceedings of the IWA/WEF Wastewater Treatment Seminar, San Francisco, CA, USA, 3–8 November 2013; Volume 4, pp. 318–321. [Google Scholar]
- Delafosse, A.; Collignon, M.-L.; Calvo, S.; Delvigne, F.; Crine, M.; Thonart, P.; Toye, D. CFD-based compartment model for description of mixing in bioreactors. Chem. Eng. Sci. 2014, 106, 76–85. [Google Scholar] [CrossRef]
- Delafosse, A.; Delvigne, F.; Collignon, M.L.; Crine, M.; Thonart, P.; Toye, D. Development of a compartment model based on CFD simulations for description of mixing in bioreactors. Biotechnol. Agron. Soc. Environ. 2010, 14, 517–522. [Google Scholar]
- Gresch, M.; Brügger, R.; Meyer, A.; Gujer, W. Compartmental models for continuous flow reactors derived from CFD simulations. Environ. Sci. Technol. 2009, 43, 2381–2387. [Google Scholar] [CrossRef]
- Wells, G.J.; Ray, W.H. Methodology for modeling detailed imperfect mixing effects in complex reactors. AIChE J. 2005, 51, 1508–1520. [Google Scholar] [CrossRef]
- Delafosse, A.; Calvo, S.; Collignon, M.-L.; Delvigne, F.; Crine, M.; Toye, D. Euler–Lagrange approach to model heterogeneities in stirred tank bioreactors—Comparison to experimental flow characterization and particle tracking. Chem. Eng. Sci. 2015, 134, 457–466. [Google Scholar] [CrossRef]
- Le Moullec, Y.; Gentric, C.; Potier, O.; Leclerc, J. Comparison of systemic, compartmental and CFD modelling approaches: Application to the simulation of a biological reactor of wastewater treatment. Chem. Eng. Sci. 2010, 65, 343–350. [Google Scholar] [CrossRef]
- Spann, R.; Gernaey, K.V.; Sin, G. A compartment model for risk-based monitoring of lactic acid bacteria cultivations. Biochem. Eng. J. 2019, 151. [Google Scholar] [CrossRef]
- Jourdan, N.; Neveux, T.; Potier, O.; Kanniche, M.; Wicks, J.; Nopens, I.; Rehman, U.; Le Moullec, Y. Compartmental Modelling in chemical engineering: A critical review. Chem. Eng. Sci. 2019, 210, 115196. [Google Scholar] [CrossRef]
- Öner, M.; Bach, C.; Tajsoleiman, T.; Molla, G.S.; Freitag, M.F.; Stocks, S.M.; Abildskov, J.; Krühne, U.; Sin, G. Scale-up Modeling of a Pharmaceutical Crystallization Process via Compartmentalization Approach. Comput. Aided Chem. Eng. 2018, 44, 181–186. [Google Scholar]
- Šafarič, L.; Yekta, S.S.; Ejlertsson, J.; Safari, M.; Najafabadi, H.N.; Karlsson, A.; Ometto, F.; Svensson, B.H.; Björn, A.; Yekta, S.; et al. A Comparative Study of Biogas Reactor Fluid Rheology—Implications for Mixing Profile and Power Demand. Processes 2019, 7, 700. [Google Scholar] [CrossRef] [Green Version]
- Wu, B. CFD investigation of turbulence models for mechanical agitation of non-Newtonian fluids in anaerobic digesters. Water Res. 2011, 45, 2082–2094. [Google Scholar] [CrossRef]
- ANSYS. ANSYS Fluent Theory Guide, 15317; ANSYS: Fort Canonville, PA, USA, 2013. [Google Scholar]
- Craig, K.; Nieuwoudt, M.; Niemand, L. CFD simulation of anaerobic digester with variable sewage sludge rheology. Water Res. 2013, 47, 4485–4497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eshtiaghi, N.; Markis, F.; Yap, S.D.; Baudez, J.-C.; Slatter, P. Rheological characterisation of municipal sludge: A review. Water Res. 2013, 47, 5493–5510. [Google Scholar] [CrossRef] [PubMed]
- Slatter, P.T. The rheological characterisation of sludges. Water Sci. Technol. 1997, 36, 9–18. [Google Scholar] [CrossRef]
- Alvarado, A.; Vedantam, S.; Goethals, P.; Nopens, I. A compartmental model to describe hydraulics in a full-scale waste stabilization pond. Water Res. 2012, 46, 521–530. [Google Scholar] [CrossRef]
- Tajsoleiman, T.; Spann, R.; Bach, C.; Gernaey, K.V.; Gernaey, K.V.; Krühne, U. A CFD based automatic method for compartment model development. Comput. Chem. Eng. 2019, 123, 236–245. [Google Scholar] [CrossRef]
- Rosen, C.; Jeppsson, U. Aspects on ADM1 Implementation within the BSM2 Framework; Tech. Rep.; Lund University: Lund, Sweden, 2006; pp. 1–37. [Google Scholar]
- Nopens, I.; Batstone, D.J.; Copp, J.B.; Jeppsson, U.; Volcke, E.I.; Alex, J.; Vanrolleghem, P. An ASM/ADM model interface for dynamic plant-wide simulation. Water Res. 2009, 43, 1913–1923. [Google Scholar] [CrossRef]
| TSS (%) | K | n | |
|---|---|---|---|
| 4% | 0.02 | 2.23 | 0.57 |
| 8% | 0.16 | 19.89 | 0.51 |
| 12% | 88.44 | 75.92 | 0.34 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tobo, Y.M.; Bartacek, J.; Nopens, I. Linking CFD and Kinetic Models in Anaerobic Digestion Using a Compartmental Model Approach. Processes 2020, 8, 703. https://doi.org/10.3390/pr8060703
Tobo YM, Bartacek J, Nopens I. Linking CFD and Kinetic Models in Anaerobic Digestion Using a Compartmental Model Approach. Processes. 2020; 8(6):703. https://doi.org/10.3390/pr8060703
Chicago/Turabian StyleTobo, Yohannis Mitiku, Jan Bartacek, and Ingmar Nopens. 2020. "Linking CFD and Kinetic Models in Anaerobic Digestion Using a Compartmental Model Approach" Processes 8, no. 6: 703. https://doi.org/10.3390/pr8060703
APA StyleTobo, Y. M., Bartacek, J., & Nopens, I. (2020). Linking CFD and Kinetic Models in Anaerobic Digestion Using a Compartmental Model Approach. Processes, 8(6), 703. https://doi.org/10.3390/pr8060703






