Catalytic and Non-Catalytic Treatment of Industrial Wastewater under the Exposure of Non-Thermal Plasma Jet
Abstract
1. Introduction
2. Materials and Methods
2.1. Water Sampling
2.2. Plasma Treatment Setup
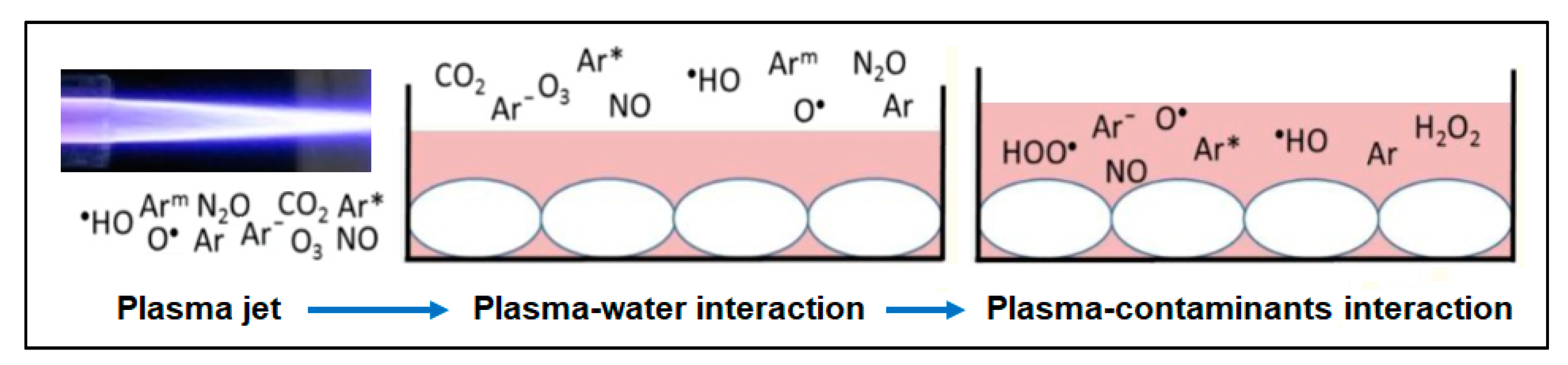
2.3. Plasma Chemistry
2.4. Plasma Inactivation of Bacteria
3. Results and Discussion
3.1. Water Quality Parameters
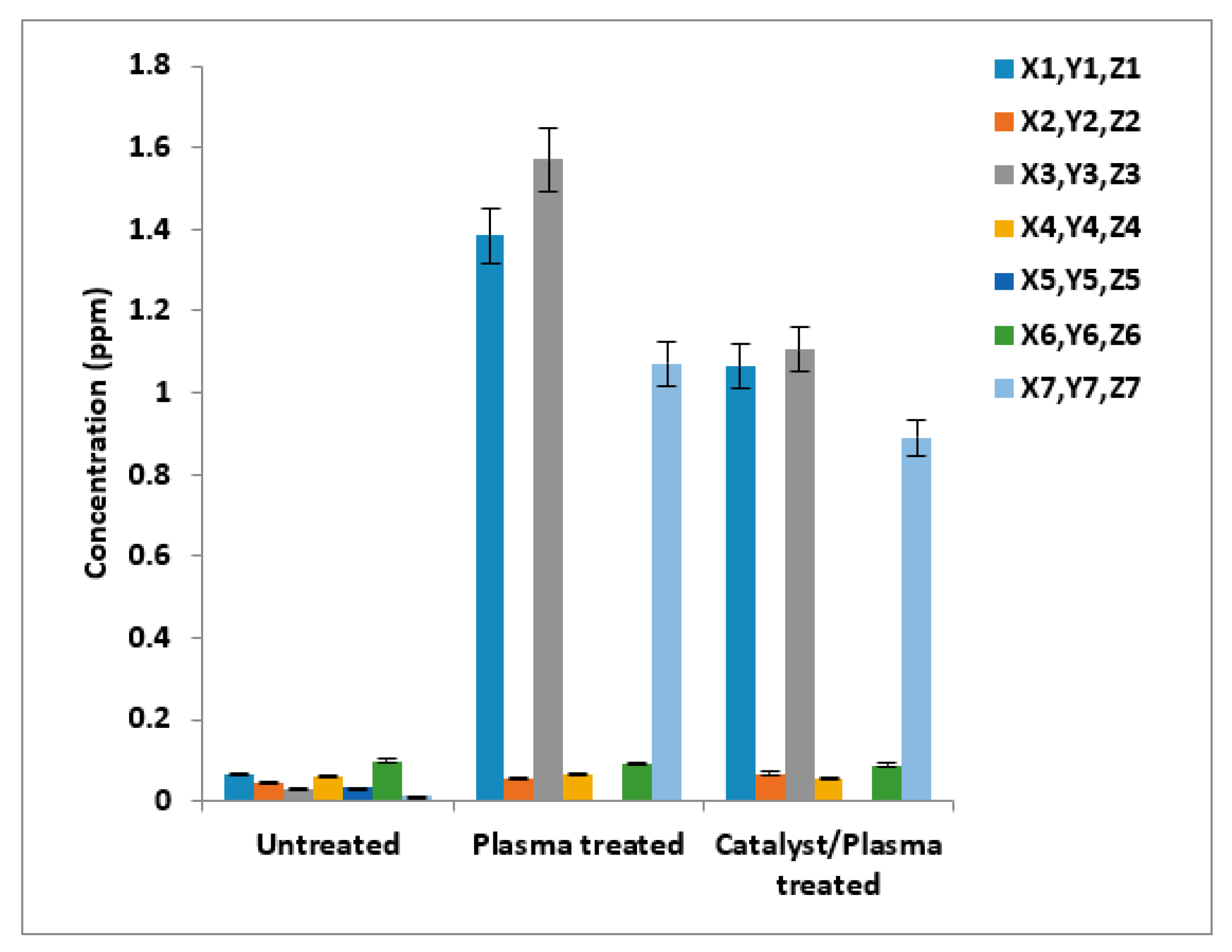
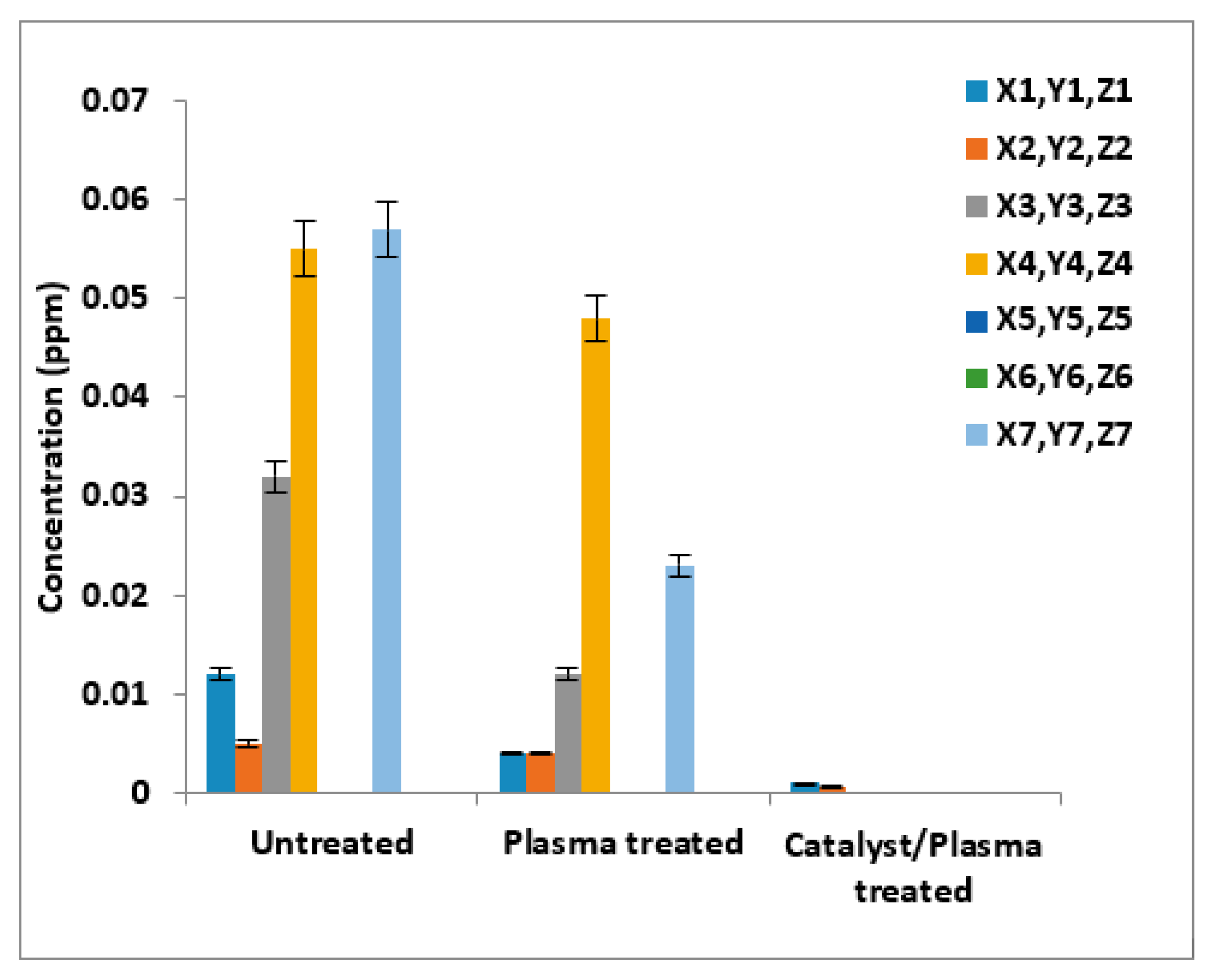
3.2. Identification of Heavy Metals
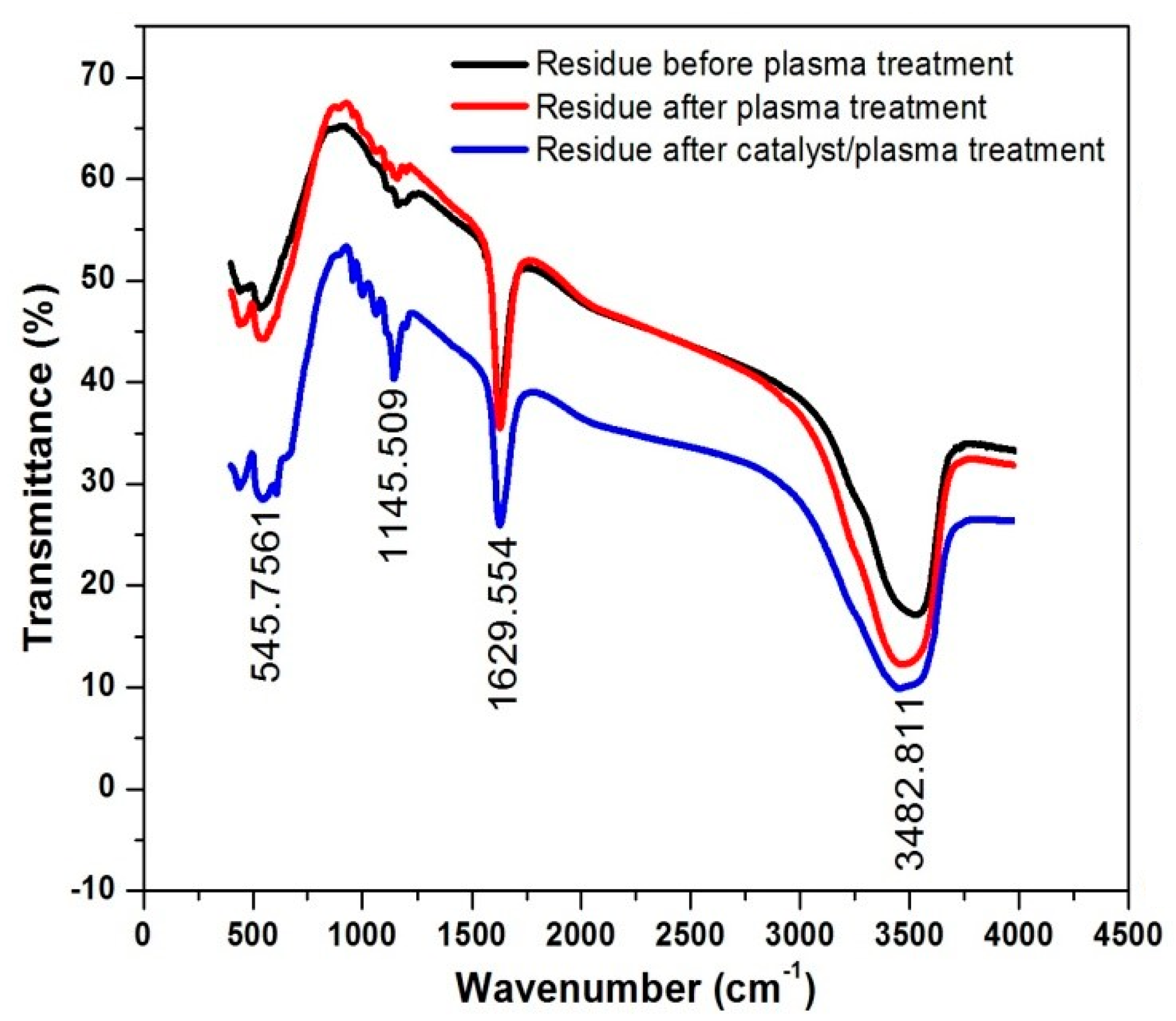
3.3. FT-IR and XRD Analysis of Residue
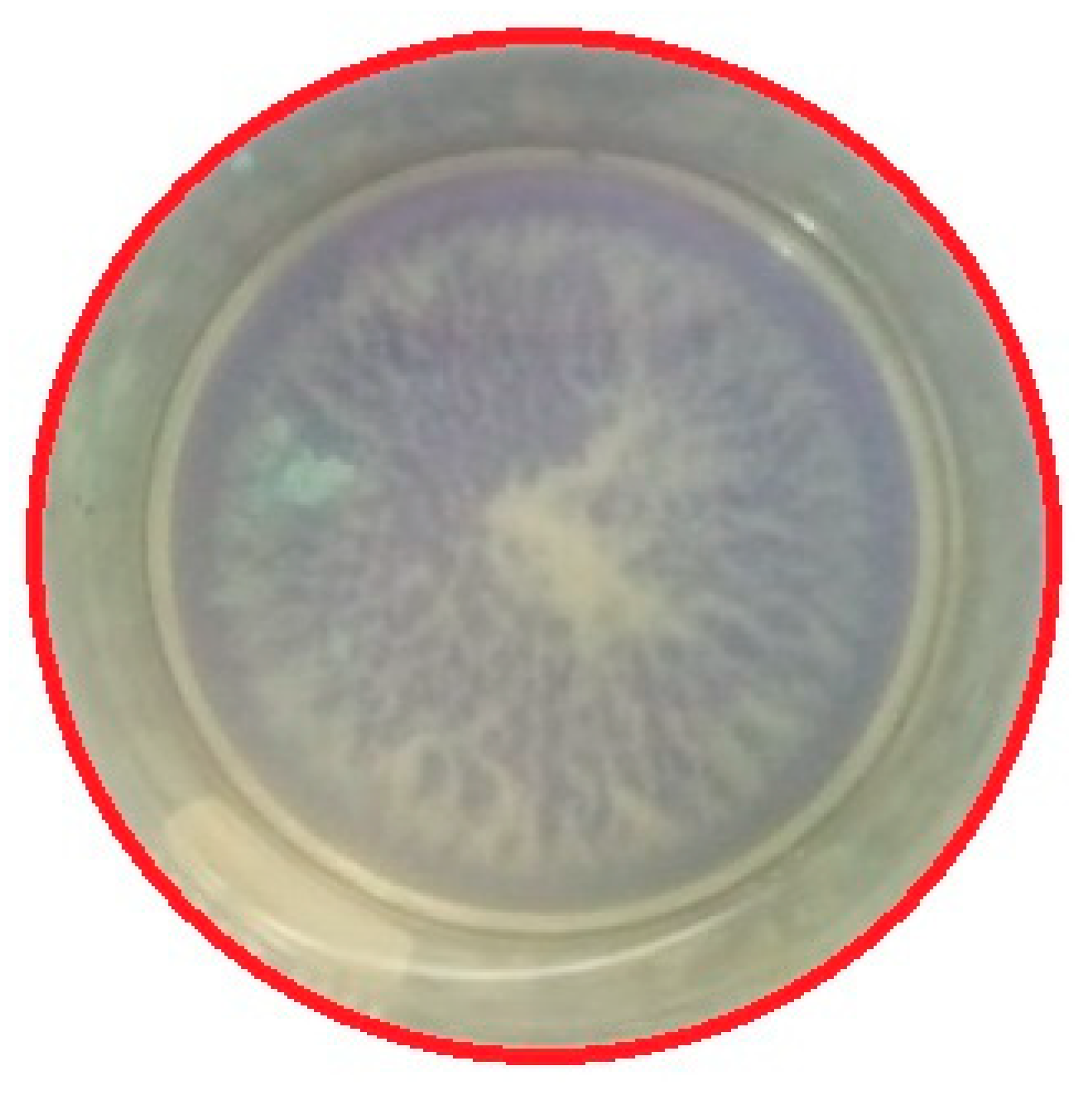
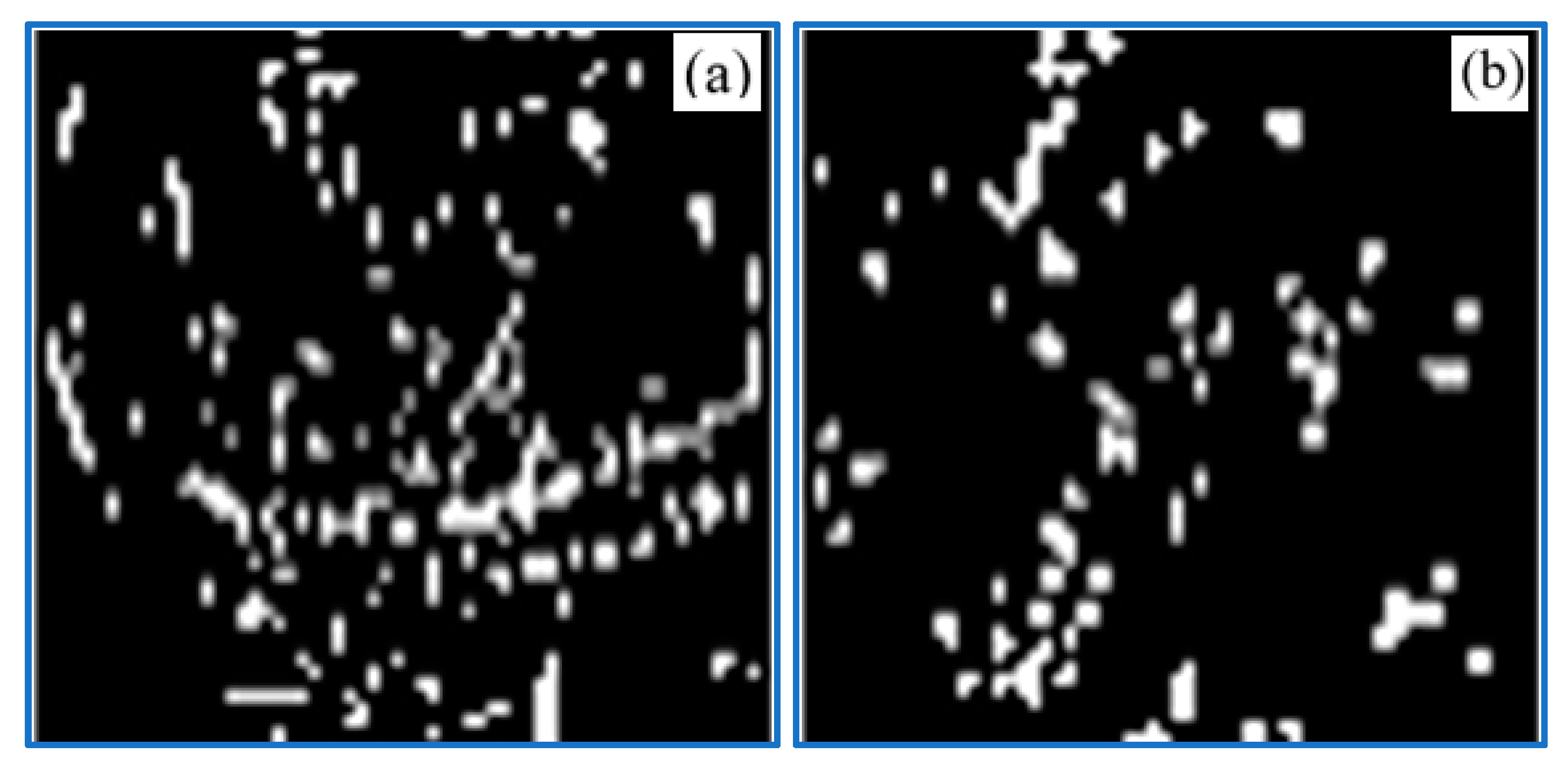
3.4. Effect of Plasma Treatment on Bacteria Inactivation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Droste, R.L.; Gehr, R.L. Theory and Practice of Water and Wastewater Treatment, 2nd ed.; John Wiley and Sons: Hoboken, NJ, USA, 2018. [Google Scholar]
- Foster, J.E. Plasma-based water purification: Challenges and prospects for the future. Phys. Plasmas 2017, 24, 055501. [Google Scholar] [CrossRef]
- Shannon, M.A.; Bohn, P.W.; Elimelech, M.; Georgiadis, J.G.; Marinas, B.J.; Mayes, A.M. Science and technology for water purification in the coming decades. Nature 2010, 452, 301–310. [Google Scholar] [CrossRef]
- John, D.Z. Handbook of Drinking Water Quality: Standards and Controls; Van Nostrand Reinhold: New York, NY, USA, 1990. [Google Scholar]
- Chandana, L.; Reddy, P.M.K.; Subrahmanyam, C. Atmospheric pressure non-thermal plasma jet for the degradation of methylene blue in aqueous medium. Chem. Eng. J. 2015, 282, 116–122. [Google Scholar] [CrossRef]
- Magureanu, M.; Piroi, D.; Mandache, N.B.; David, V.; Medvedovici, A.; Parvulescu, V.I. Degradation of pharmaceutical compound pentoxifylline in water by non-thermal plasma treatment. Water Res. 2010, 44, 3445–3453. [Google Scholar] [CrossRef] [PubMed]
- Dojčinović, B.; Roglic, G.; Obradović, B.M.; Kuraica, M.M.; Kostić, M.M.; Nešić, J.; Manojlović, D. Decolorization of reactive textile dyes using water falling film dielectric barrier discharge. J. Hazard. Mater. 2011, 192, 763–771. [Google Scholar] [CrossRef]
- Garcia, M.C.; Mora, M.; Esquivel, D.; Foster, J.E.; Rodero, A.; Jiménez-Sanchidrián, C.; Romero-Salguero, F.J. Microwave atmospheric pressure plasma jets for wastewater treatment: Degradation of methylene blue as a model dye. Chemosphere 2017, 180, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Raza, H.A.; Shafiq, M.; Naeem, M.; Naz, M.Y. Enhancement of mechanical and structural traits of aluminum using low energy plasma focus device. Chem. Sel. 2019, 4, 5348–5354. [Google Scholar] [CrossRef]
- Tendero, C.; Tixier, C.; Tristant, P.; Desmaison, J.; Leprince, P. Atmospheric pressure plasmas: A review. Spectrochim. Acta Part B At. Spectrosc. 2006, 61, 2–30. [Google Scholar] [CrossRef]
- Schmidt-Bleker, A.; Winter, J.; Bösel, A.; Reuter, S.; Weltmann, K.-D. On the plasma chemistry of a cold atmospheric argon plasma jet with shielding gas device. Plasma Sources Sci. Technol. 2015, 25, 15005. [Google Scholar] [CrossRef]
- Naz, M.Y.; Shukrullah, S.; Ghaffar, A.; Rehman, N.U.; Sagir, M. A low frequency dielectric barrier discharge system design for textile treatment. Synth. React. Inorg. Met. Chem. 2015, 46, 104–109. [Google Scholar] [CrossRef]
- Benetoli, L.O.D.B.; Cadorin, B.M.; Baldissarelli, V.Z.; Geremias, R.; De Souza, I.G.; Debacher, N. Pyrite-enhanced methylene blue degradation in non-thermal plasma water treatment reactor. J. Hazard. Mater. 2012, 237, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Zukalova, M.; Zukal, A.; Kavan, L.; Nazeeruddin, M.K.; Liska, P.; Grätzel, M. Organized Mesoporous TiO2Films Exhibiting Greatly Enhanced Performance in Dye-Sensitized Solar Cells. Nano Lett. 2005, 5, 1789–1792. [Google Scholar] [CrossRef]
- Sugiarto, A.T.; Ito, S.; Ohshima, T.; Sato, M.; Skalný, J.D. Oxidative decoloration of dyes by pulsed discharge plasma in water. J. Electrost. 2003, 58, 135–145. [Google Scholar] [CrossRef]
- Tichonovas, M.; Krugly, E.; Račys, V.; Hippler, R.; Kauneliene, V.; Stasiulaitiene, I.; Martuzevičius, D. Degradation of various textile dyes as wastewater pollutants under dielectric barrier discharge plasma treatment. Chem. Eng. J. 2013, 229, 9–19. [Google Scholar] [CrossRef]
- Malik, M.A. Synergistic effect of plasmacatalyst and ozone in a pulsed corona discharge reactor on the decomposition of organic pollutants in water. Plasma Sources Sci. Technol. 2003, 12, S26–S32. [Google Scholar] [CrossRef]
- Linsebigler, A.L.; Lu, G.; Yates, J.T. Photocatalysis on TiO2 surfaces: Principles, mechanisms, and selected results. Chem. Rev. 1995, 95, 735–758. [Google Scholar] [CrossRef]
- Ghezzar, M.; Abdelmalek, F.; Belhadj, M.; Benderdouche, N.; Addou, A. Enhancement of the bleaching and degradation of textile wastewaters by Gliding arc discharge plasma in the presence of TiO2 catalyst. J. Hazard. Mater. 2009, 164, 1266–1274. [Google Scholar] [CrossRef]
- Zhong, D.; Liu, Y.-H.; Cheung, N.-T.; Kan, C.; Chua, H. A parameter study of the effect of a plasma-induced ozone colour-fading process on sulphur-dyed cotton fabric. Processes 2018, 6, 81. [Google Scholar] [CrossRef]
- Hu, C.; Yu, J.C.-M.; Hao, Z.; Wong, P.K. Effects of acidity and inorganic ions on the photocatalytic degradation of different azo dyes. Appl. Catal. B Environ. 2003, 46, 35–47. [Google Scholar] [CrossRef]
- Moussa, D.; Abdelmalek, F.; Benstaali, B.; Addou, A.; Hnatiuc, E.; Brisset, J. Acidity control of the gliding arc treatments of aqueous solutions: Application to pollutant abatement and biodecontamination. Eur. Phys. J. Appl. Phys. 2004, 29, 189–199. [Google Scholar] [CrossRef]
- Bisschops, I.; Spanjers, H. Literature review on textile wastewater characterization. Environ. Technol. 2003, 24, 1399–1411. [Google Scholar] [CrossRef]
- Yang, Y.; Gutsol, A.; Fridman, A.; Cho, Y.I. Removal of CaCO3 scales on a filter membrane using plasma discharge in water. Int. J. Heat Mass Transf. 2009, 52, 4901–4906. [Google Scholar] [CrossRef]
- Ke, Z.; Huang, Q.; Zhang, H.; Yu, Z. Reduction and removal of aqueous Cr(VI) by glow discharge plasma at the gas–solution interface. Environ. Sci. Technol. 2011, 45, 7841–7847. [Google Scholar] [CrossRef] [PubMed]
- Icopini, G.A.; Long, D.T. Speciation of aqueous chromium by use of solid-phase extractions in the field. Environ. Sci. Technol. 2002, 36, 2994–2999. [Google Scholar] [CrossRef]
- Cserfalvi, T.; Mezei, P. Direct solution analysis by glow discharge: Electrolyte-cathode discharge spectrometry. J. Anal. At. Spectrom. 1994, 9, 345. [Google Scholar] [CrossRef]
- Parshetti, G.; Saratale, G.; Telke, A.; Govindwar, S.P. Biodegradation of hazardous triphenylmethane dye methyl violet by Rhizobium radiobacter (MTCC 8161). J. Basic Microbiol. 2009, 49, S36–S42. [Google Scholar] [CrossRef] [PubMed]
- Şolpan, D.; Duran, S.; Saraydin, D.; Guven, O. Adsorption of methyl violet in aqueous solutions by poly(acrylamide-co-acrylic acid) hydrogels. Radiat. Phys. Chem. 2003, 66, 117–127. [Google Scholar] [CrossRef]
- Sharma, A.K.; Desnavi, S.; Dixit, C.; Varshney, U.; Sharma, A. Extraction of nickel nanoparticles from electroplating waste and their application in production of bio-diesel from biowaste. Int. J. Chem. Eng. Appl. 2015, 6, 156. [Google Scholar] [CrossRef]
- Riaz, S.; Fatima, S.; Naseem, S. Bacterial removal from water by using Al2O3 and FeO nanoparticles. In Proceedings of the World Congress on Advances in Civil, Environmental and Materials Research (ACEM’16), Jeju Island, Korea, 28 August–1 September 2016; Available online: http://www.i-asem.org/publication_conf/acem16/6.ICAM16/T3H.6.MR373_1464F1.pdf (accessed on 24 March 2020).
- Vo, Q.; Phung, D.; Nguyen, Q.V.; Thi, H.H.; Nhat, H.N.T.; Phong, N.T.P.; Bach, L.-G.; Van Tan, L. Controlled synthesis of triangular silver nanoplates by Gelatin-Chitosan mixture and the influence of their shape on antibacterial activity. Processes 2019, 7, 873. [Google Scholar] [CrossRef]







| Water Sample | Source |
|---|---|
| X1, X2 and X3 | Tap water of different areas of Faisalabad |
| X4 | Dye sample of textile industry in Faisalabad |
| X5 | Industrial wastewater of Faisalabad |
| X6 | Sample of Chenab river in Faisalabad |
| X7 | Wastewater treatment plant |
| Oxidant | Oxidation Potential (V) |
|---|---|
| Hydroxyl radical (OH) | 2.80 |
| Ozone (O3) | 2.07 |
| Hydrogen peroxide (H2O2) | 1.77 |
| Permanganate ion | 1.67 |
| Chlorine oxide | 1.50 |
| Chlorine | 1.36 |
| O• | 2.42 |
| Sample | pH | Conductivity | TDS | Hardness | Sulfates | Phosphates | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Value | µS/cm | mg/L | mg/L | mg/L | mg/L | |||||||
| X1 | 8.4 | 0.168 | 2020 | 40.4 | 1030 | 20.6 | 40 | 0.8 | 59 | 1.18 | 3.5 | 0.07 |
| Y1 | 8.7 | 0.174 | 2040 | 40.8 | 1040 | 20.8 | 56 | 1.12 | 48 | 0.96 | 3.3 | 0.066 |
| Z1 | 8.6 | 0.172 | 2000 | 40 | 1020 | 20.4 | 56 | 1.12 | 40 | 0.8 | 2.6 | 0.052 |
| X2 | 7.9 | 0.158 | 830 | 16.6 | 420 | 8.4 | 384 | 7.68 | 45 | 0.9 | 3.3 | 0.066 |
| Y2 | 8.2 | 0.164 | 820 | 16.4 | 420 | 8.4 | 408 | 8.16 | 51 | 1.02 | 2.6 | 0.052 |
| Z2 | 8.4 | 0.168 | 840 | 16.8 | 430 | 8.6 | 400 | 8 | 42 | 0.84 | 2.9 | 0.058 |
| X3 | 7.9 | 0.158 | 4660 | 93.2 | 2370 | 27.4 | 456 | 9.12 | 58 | 1.16 | 2.6 | 0.052 |
| Y3 | 8.3 | 0.166 | 4650 | 93 | 2370 | 27.4 | 504 | 8.08 | 60 | 1.2 | 2.8 | 0.056 |
| Z3 | 8.3 | 0.166 | 4670 | 93.4 | 2390 | 27.8 | 480 | 9.6 | 58 | 1.16 | 3.4 | 0.068 |
| X4 | 12.2 | 0.244 | 8040 | 98.8 | 4100 | 32 | 108 | 2.16 | 44 | 0.88 | 2.8 | 0.056 |
| Y4 | 12 | 0.24 | 7770 | 95.4 | 3960 | 39.2 | 97 | 1.94 | 54 | 1.08 | 3.3 | 0.066 |
| Z4 | 11.8 | 0.236 | 7470 | 84.4 | 3810 | 36.2 | 68 | 1.36 | 54 | 1.08 | 3.2 | 0.064 |
| X5 | 7.8 | 0.156 | 1220 | 24.4 | 620 | 12.4 | 416 | 8.32 | 42 | 0.84 | 3.4 | 0.068 |
| Y5 | 8.3 | 0.166 | 1220 | 24.4 | 620 | 12.4 | 416 | 8.32 | 58 | 1.16 | 3.6 | 0.072 |
| Z5 | 8.5 | 0.17 | 1230 | 24.6 | 630 | 12.6 | 440 | 8.8 | 46 | 0.92 | 3.3 | 0.066 |
| X6 | 7.9 | 0.158 | 440 | 8.8 | 230 | 4.6 | 184 | 3.68 | 48 | 0.96 | 3.5 | 0.07 |
| Y6 | 8.2 | 0.164 | 470 | 9.4 | 240 | 4.8 | 192 | 3.84 | 52 | 1.04 | 3.2 | 0.064 |
| Z6 | 8.3 | 0.166 | 460 | 9.2 | 240 | 4.8 | 200 | 4 | 44 | 0.88 | 3.5 | 0.07 |
| X7 | 7.8 | 0.156 | 5350 | 67 | 2740 | 34.8 | 872 | 12.44 | 46 | 0.92 | 2.9 | 0.058 |
| Y7 | 8.2 | 0.164 | 5420 | 88.4 | 2770 | 55.4 | 792 | 15.84 | 48 | 0.96 | 3.5 | 0.07 |
| Z7 | 8.1 | 0.162 | 5430 | 88.6 | 2770 | 55.4 | 944 | 0.8 | 40 | 0.8 | 3.6 | 0.072 |
| Standard Water Quality Parameters | Range |
|---|---|
| pH | 6.5–8.5 |
| Conductivity | 200–800 µS/cm |
| TDS | 1000 mg/L |
| Hardness | <60 mg/L = soft water 60–120 mg/L = moderately hard 120–180 mg/L = hard >180 mg/L = very hard |
| Sulfates | 250 mg/L |
| Phosphates | 0.05–0.1 mg/L |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shukrullah, S.; Bashir, W.; Altaf, N.U.H.; Khan, Y.; Al-Arainy, A.A.; Sheikh, T.A. Catalytic and Non-Catalytic Treatment of Industrial Wastewater under the Exposure of Non-Thermal Plasma Jet. Processes 2020, 8, 667. https://doi.org/10.3390/pr8060667
Shukrullah S, Bashir W, Altaf NUH, Khan Y, Al-Arainy AA, Sheikh TA. Catalytic and Non-Catalytic Treatment of Industrial Wastewater under the Exposure of Non-Thermal Plasma Jet. Processes. 2020; 8(6):667. https://doi.org/10.3390/pr8060667
Chicago/Turabian StyleShukrullah, Shazia, Warda Bashir, Noor Ul Huda Altaf, Yasin Khan, Abdulrehman Ali Al-Arainy, and Toqeer Ahmad Sheikh. 2020. "Catalytic and Non-Catalytic Treatment of Industrial Wastewater under the Exposure of Non-Thermal Plasma Jet" Processes 8, no. 6: 667. https://doi.org/10.3390/pr8060667
APA StyleShukrullah, S., Bashir, W., Altaf, N. U. H., Khan, Y., Al-Arainy, A. A., & Sheikh, T. A. (2020). Catalytic and Non-Catalytic Treatment of Industrial Wastewater under the Exposure of Non-Thermal Plasma Jet. Processes, 8(6), 667. https://doi.org/10.3390/pr8060667




