Solar Photo-Assisted Degradation of Bipyridinium Herbicides at Circumneutral pH: A Life Cycle Assessment Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Analytical Techniques
2.3. Experimental Setup
2.4. Experimental Design
2.5. Kinetic Experiments
2.6. Phytotoxicity Assays
2.7. Environmental Assessment
2.8. Cost Analysis
3. Results and Discussion
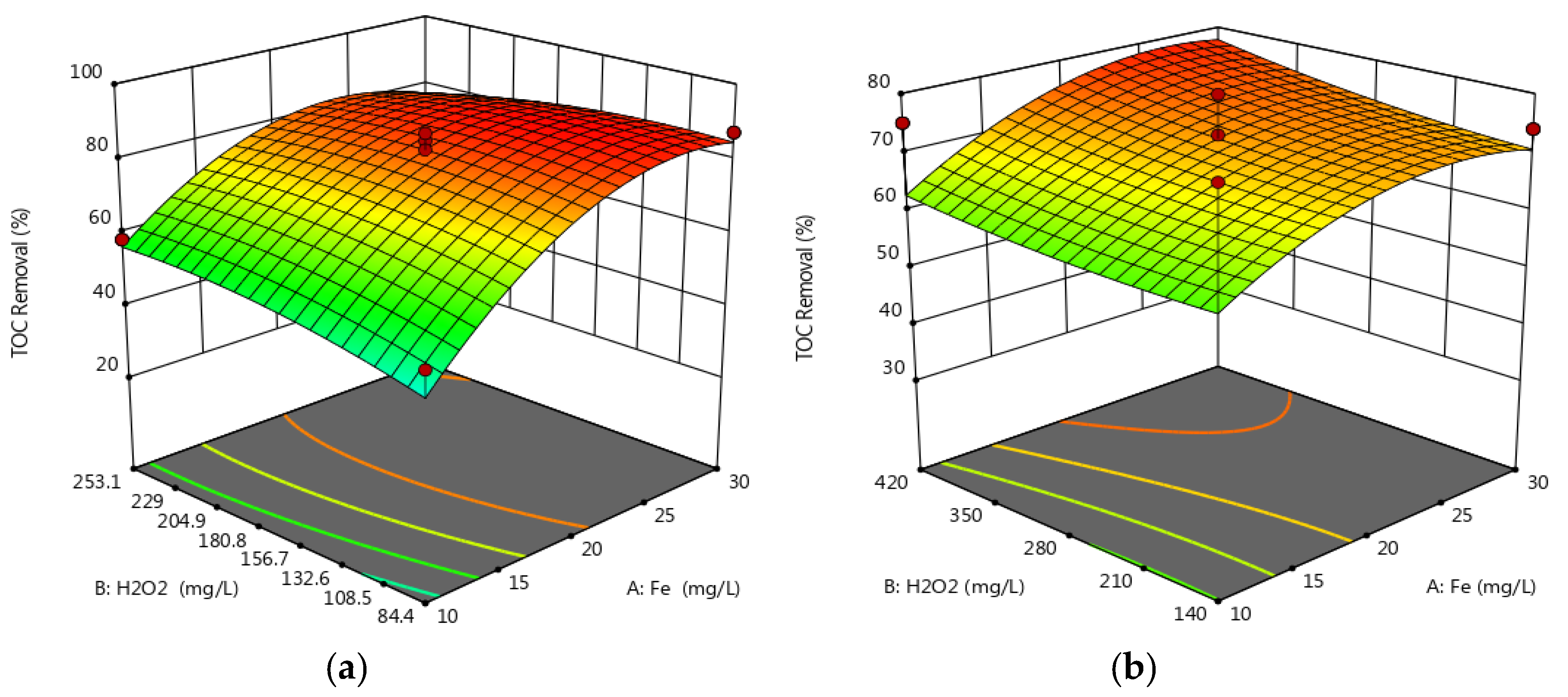
3.1. Multivariate Analysis of the Photo-Fenton Process
3.2. Kinetic Model for Paraquat and Diquat Removal
3.3. Phytotoxicity Assay
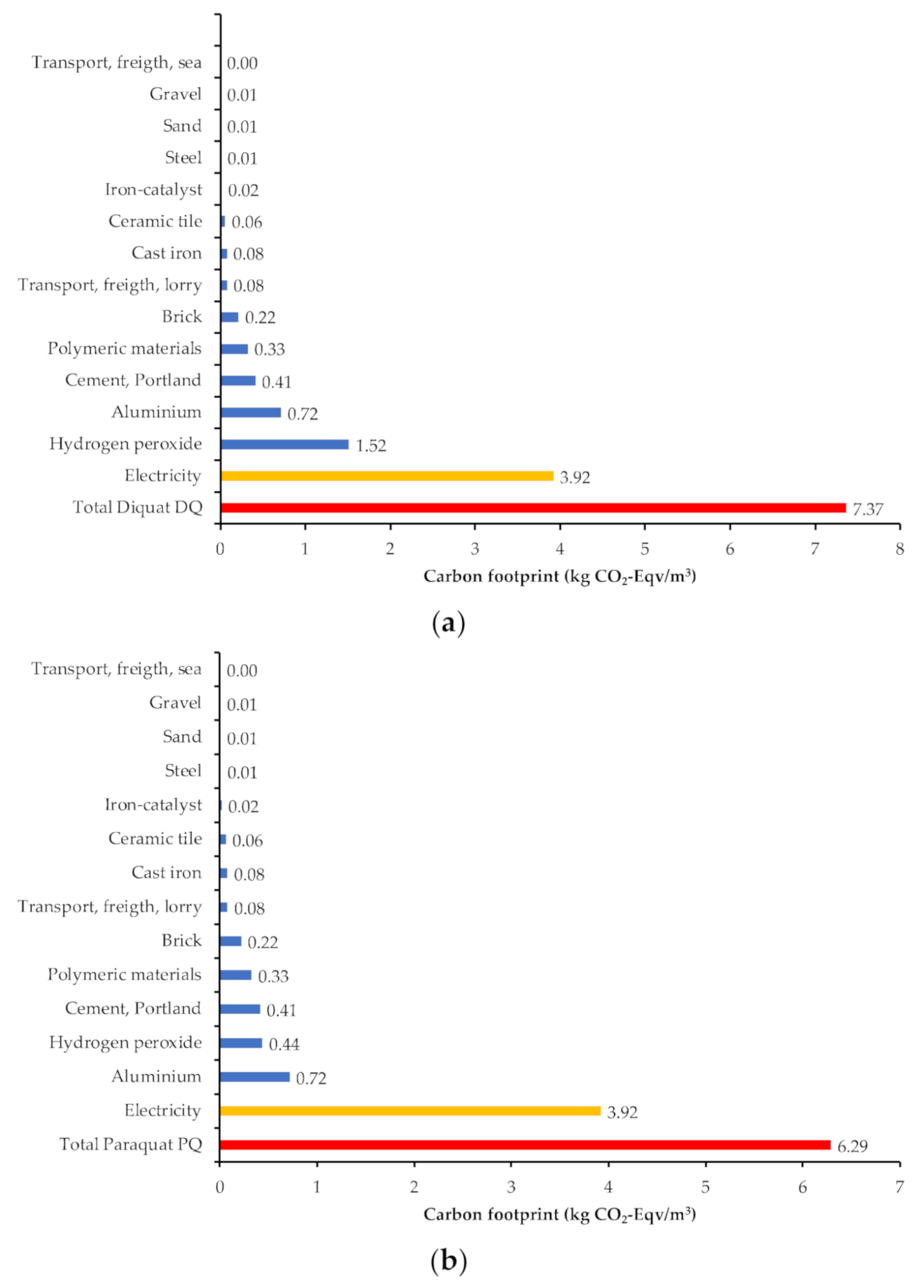
3.4. Environmental Analysis of the Treatment Process
3.5. Costs Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gao, L.; Liu, J.; Yuan, H.; Deng, X. Solid phase microextraction combined with GC-MS for determination of diquat and paraquat residues in water. Chromatographia 2015, 125–130. [Google Scholar] [CrossRef]
- Nakajima, M.; Nagao, M.; Iwasa, M.; Monma-Ohtaki, J.; Maeno, Y.; Koyama, H.; Seko-Nakamura, Y.; Isobe, I.; Takatori, T. Purification and characterization of diquat (1,1%-ethylene-2,2%-dipyridylium)-metabolizing enzyme from paraquat-resistant rat liver cytosol. Toxicology 2000, 154, 55–66. [Google Scholar] [CrossRef]
- Jaiswal, D.K.; Verma, J.P.; Yadav, J. Microbe induced degradation of pesticides in agricultural soils. Microbe Induc. Degrad. Pestic. 2016, 167–189. [Google Scholar] [CrossRef]
- Jafarinejad, S. Recent advances in determination of herbicide paraquat in environmental waters and its removal from aqueous solutions: A review. Int. Res. J. Appl. Basic Sci. 2015, 9, 1758–1774. [Google Scholar]
- Zahedi, F.; Behpour, M.; Ghoreishi, S.M.; Khalilian, H. Photocatalytic degradation of paraquat herbicide in the presence TiO2 nanostructure thin films under visible and sun light irradiation using continuous flow photoreactor. Sol. Energy 2015, 120, 287–295. [Google Scholar] [CrossRef]
- Camacho-Morales, R.L.; Guillén-Navarro, K.; Sánchez, J.E. Degradation of the herbicide paraquat by macromycetes isolated from southeastern Mexico. 3 Biotech. 2017, 7, 324. [Google Scholar] [CrossRef]
- Amondham, W.; Parkpian, P.; Polprasert, C.; Delaune, R.D.; Jugsujinda, A. Paraquat adsorption, degradation, and remobilization in tropical soils of Thailand. J. Environ. Sci. Health Part B Pestic. Food Contam. Agric. Wastes 2006, 41, 485–507. [Google Scholar] [CrossRef]
- Huang, Y.; Zhan, H.; Bhatt, P.; Chen, S. Paraquat degradation from contaminated environments: Current achievements and perspectives. Front. Microbiol. 2019, 10, 1754. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yen, J.; Hsieh, Y.; Chen, Y. Dissipation of 2, 4-d glyphosate and paraquat in river water. Water Air Soil Poll. 1994, 72, 1–7. [Google Scholar] [CrossRef]
- Cartaxo, M.; Borges, C.; Pereira, M.; Mendonça, M. Electrochemical oxidation of paraquat in neutral medium. Electrochim. Acta 2015, 176, 1010–1018. [Google Scholar] [CrossRef]
- Santos, M.; Schaule, G.; Alves, A.; Madeira, L. Adsorption of paraquat herbicide on deposits from drinking water networks. Chem. Eng. J. 2013, 229, 324–333. [Google Scholar] [CrossRef]
- Trovó, A.; Gomes-Junior, O.; Machado, A.; Borges-Neto, W.; Silva, J. Degradation of the herbicide paraquat by photo-Fenton process: Optimization by experimental design and toxicity assessment. J. Braz. Chem. Soc. 2013, 24, 76–84. [Google Scholar] [CrossRef]
- Klamerth, N.; Malato, S.; Maldonado, M.; Agüera, A.; Fernández-Alba, A. Application of photo-Fenton as a tertiary treatment of emerging contaminants in municipal wastewater. Environ. Sci. Technol. 2010, 44, 1792–1798. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.; Alves, A.; Madeira, L. Paraquat removal from water by oxidation with Fenton’s reagent. Chem. Eng. J. 2011, 175, 279–290. [Google Scholar] [CrossRef]
- Nakamura, T.; Kawasaki, N.; Ogawa, H.; Tanada, S.; Kogirima, M.; Imaki, M. Adsorption removal of paraquat and diquat onto activated carbon at different adsorption temperature. Toxicol. Environ. Chem. 1999, 70, 275–280. [Google Scholar] [CrossRef]
- Tsai, W.; Chen, H. Adsorption kinetics of herbicide paraquat in aqueous solution onto a low-cost adsorbent, swine-manure-derived biochar. Int. J. Environ. Sci. Technol. 2013, 10, 1349–1356. [Google Scholar] [CrossRef]
- Rytwo, G.; Nir, S.; Margulies, L. Adsorption and interactions of diquat and paraquat with montmorillonite. Soil Sci. Soc. Am. J. 1996, 60, 601–610. [Google Scholar] [CrossRef]
- Tsai, W.; Hsien, K.; Chang, Y.L.C. Removal of herbicide paraquat from an aqueous solution by adsorption onto spent and treated diatomaceous earth. Bioresour. Technol. 2005, 96, 657–663. [Google Scholar] [CrossRef]
- Tsai, W.; Lai, C.; Hsien, K. Adsorption kinetics of herbicide paraquat from aqueous solution onto activated bleaching earth. Chemosphere 2004, 55, 829–837. [Google Scholar] [CrossRef]
- Hsu, S.; Pan, T. Adsorption of paraquat using methacrylic acid-modified rice husk. Bioresour. Technol. 2007, 98, 3617–3621. [Google Scholar] [CrossRef]
- Ibrahim, K.; Jbara, H. Removal of paraquat from synthetic wastewater using phillipsite–faujasite tuff from Jordan. J. Hazard. Mater. 2009, 163, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Dhaouadi, A.; Adhoum, N. Degradation of paraquat herbicide by electrochemical advanced oxidation methods. J. Electroanal. Ehem. 2009, 637, 33–42. [Google Scholar] [CrossRef]
- Pouran, S.R.; Abdul Aziz, A.R.; Wan Daud, W.A. Review on the advances in photo-Fenton oxidation system for recalcitrant wastewaters. J. Ind. Eng. Chem. 2015, 21, 53–69. [Google Scholar] [CrossRef]
- Zapata, A.; Oller, I.; Sirtori, C.; Rodríguez, A.; Sánchez-Pérez, J.A.; López, A.; Mezcua, M.; Malato, S. Decontamination of industrial wastewater containing pesticides by combining large-scale homogeneous solar photocatalysis and biological treatment. Chem. Eng. J. 2010, 160, 447–456. [Google Scholar] [CrossRef]
- Pignatello, J.J.; Oliveros, E.; MacKay, A. Advanced oxidation processes for organic contaminant destruction based on the Fenton reaction and related chemistry. Crit. Rev. Env. Sci. Technol. 2006, 36, 1–84. [Google Scholar] [CrossRef]
- Dhaouadi, A.; Adhoum, N. Heterogeneous catalytic wet peroxide oxidation of paraquat in the presence of modified activated carbon. Appl. Catal. B Environ. 2010, 97, 227–235. [Google Scholar] [CrossRef]
- Florencio, M.H.; Pires, E.; Castro, A.L.; Nunes, M.R.; Borges, C.; Costa, F.M. Photodegradation of Diquat and Paraquat in aqueous solutions by titanium dioxide: Evolution of degradation reactions and characterization of intermediates. Chemosphere 2004, 55, 345–355. [Google Scholar] [CrossRef]
- Sorolla, M.G.; Dalida, M.L.; Khemthong, P.; Grisdanurak, N. Photocatalytic degradation of paraquat using nano-sized Cu-TiO2/SBA-15 under UV and visible light. Int. J. Environ. Sci. 2012, 24, 1125–1132. [Google Scholar] [CrossRef]
- Brillas, E.; Sirés, I.; Oturan, M.A. Electro-Fenton process and related electrochemical technologies based on Fenton’s reaction chemistry. Chem. Rev. 2009, 109, 6570–6631. [Google Scholar] [CrossRef]
- Foteinis, S.; Durán, A.; Chatzisymeon, E. Environmental sustainability of the solar photo-Fenton process for wastewater treatment and pharmaceuticals mineralization at semi-industrial scale. Sci. Total Environ. 2018, 612, 605–612. [Google Scholar] [CrossRef]
- Vasquez-Medrano, R.; Prato-Garcia, D.; Vedrenne, M. Chapter 4. Ferrioxalate-mediated processes, advanced oxidation processes for waste. In Water Treatment Emerging Green Chemical Technology; Academic Press: New York, NY, USA, 2018; pp. 89–113. [Google Scholar] [CrossRef]
- Jeong, J.; Yoon, J. pH effect on OH radical production in photo/ferrioxalate system. Water. Res. 2005, 39, 2893–2900. [Google Scholar] [CrossRef] [PubMed]
- Selvam, K.; Muruganandham, M.; Swaminathan, M. Enhanced heterogeneous ferrioxalate photo-Fenton degradation of reactive orange 4 by solar light. Sol. Energy. Mater. Sol. Cells 2005, 89, 61–74. [Google Scholar] [CrossRef]
- Nogueira, R.F.P.; Oliveira, M.C.; Paterlini, W.C. Simple and fast spectrophotometric determination of H2O2 in photo-Fenton reactions using metavanadate. Talanta 2005, 66, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, R.F.P.; Guimaräes, J.R. Photodegradation of dichloroacetic acid and 2, 4-dichlorophenol by ferrioxalate/H2O2 system. Water. Res. 2000, 34, 895–901. [Google Scholar] [CrossRef]
- EPA, Ecological Effects Test Guidelines. OCSPP 850.4100: Seedling Emergence and Seedling Growth; Environmental Protection Agency: Washington, DC, USA, 2012. [Google Scholar]
- Ioannou, L.A.; Fatta-Kassinos, D. Solar photo-Fenton oxidation against the bioresistant fractions of winery wastewater. J. Environ. Chem. Eng. 2013, 1, 703–712. [Google Scholar] [CrossRef]
- Chatzisymeon, E.; Foteinis, S.; Mantzavinos, D.; Tsoutsos, T. Life cycle assessment of advanced oxidation processes for olive mill wastewater treatment. J. Clean. Prod. 2013, 54, 229–234. [Google Scholar] [CrossRef]
- Hauschild, M.Z.; Rosenbaum, R.K.; Olsen, S.I. Life Cycle Assessment. Theory and Practice; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Blanco, J.; Malato, S. Solar Detoxification; UNESCO: Paris, France, 2003. [Google Scholar]
- Liu, W.; Andrews, S.A.; Stefan, M.I.; Bolton, J.R. Optimal methods for quenching H2O2 residuals prior to UFC testing. Water Res. 2003, 37, 3697–3703. [Google Scholar] [CrossRef]
- Gerber, M.D.; Thomaz, L.J.; Correa, L.; Pereira-Neto, J.E.; Correa, É.K. Phytotoxicity of effluents from swine slaughterhouses using lettuce and cucumber seeds as bioindicators. Sci. Total Environ. 2017, 592, 86–90. [Google Scholar] [CrossRef]
- Mendes, P.M.; Becker, R.; Corrêa, L.B.; Bianchi, I.; Dai-Prá, M.A.; Lucia, T.; Corrêa, E.K. Phytotoxicity as an indicator of stability of broiler production residues. J. Environ. Manag. 2016, 167, 156–159. [Google Scholar] [CrossRef]
- Zucconi, F.; Pera, A.; Forte, M.; Bertoldi, M. Evaluating toxicity in immature compost. Biocycle 1981, 22, 54–57. [Google Scholar]
- Klöpffer, W.; Grahl, B. Life Cycle Assessment (LCA): A Guide to Best Practice; Wiley-VCH: Weinheim, Germany, 2014. [Google Scholar]
- Goedkoop, M.J.; Heijungs, R.; Huijbregts, M.; De Schryver, A.; Struijs, J.; Van Zelm, R. ReCiPe 2008, a Life Cycle Impact Assessment Method which Comprises Harmonised Category Indicators at the Midpoint and the Endpoint Level, 1st ed.; Report I: Characterization; Ministerie van Volkshuisvesting, Ruimtelijke Ordening en Milieubeheer (VROM): The Hague, The Netherlands, 2009.
- Wernet, G.; Bauer, C.; Steubing, B.; Reinhard, J.; Moreno-Ruiz, E.; Weidema, B. The ecoinvent database version 3 (part I): Overview and methodology. Int. J. Life Cycle. Assess. 2016, 21, 1218–1230. [Google Scholar] [CrossRef]
- Ioannou-Ttofa, L.; Foteinis, S.; Chatzisymeon, E.; Michael-Kordatou, I.; Fatta-Kassinos, D. Life cycle assessment of solar-driven oxidation as a polishing step of secondary-treated urban effluents. J. Chem. Technol. Biotechnol. 2017, 92, 1315–1327. [Google Scholar] [CrossRef]
- Paterlini, W.C.; Nogueira, R.F.P. Multivariate analysis of photo-Fenton degradation of the herbicides tebuthiuron, diuron and 2, 4-D. Chemosphere 2005, 58, 1107–1116. [Google Scholar] [CrossRef] [PubMed]
- Brink, A.; Sheridan, C.M.; Harding, K.G. The Fenton oxidation of biologically treated paper and pulp mill effluents: A performance and kinetic study. Process. Saf. Environ. 2017, 107, 206–215. [Google Scholar] [CrossRef]
- Behnajady, M.A.; Modirshahla, N.; Ghanbary, F. A kinetic model for the decolorization of C.I. Acid Yellow 23 by Fenton process. J. Hazard. Mater. 2007, 148, 98–102. [Google Scholar] [CrossRef]
- Ravindran, B.; Sheena-Kumari, S.K.; Stenstrom, T.A.; Bux, F. Evaluation of phytotoxicity effect on selected crops using treated and untreated wastewater from different configurative domestic wastewater plants. Environ. Technol. 2016, 37, 1782–1789. [Google Scholar] [CrossRef] [PubMed]
- Welter, J.B.; Soares, E.V.; Rotta, E.H.; Seibert, D. Bioassays and Zahn-Wellens test assessment on landfill leachate treated by photo-Fenton process. J. Environ. Chem. Eng. 2018, 6, 1390–1395. [Google Scholar] [CrossRef]
- Hanafi, F.; Belaoufi, A.; Mountadar, M.; Assobhei, O. Augmentation of biodegradability of olive mill wastewater by electrochemical pre-treatment: Effect on phytotoxicity and operating cost. J. Hazard. Mater. 2011, 190, 94–99. [Google Scholar] [CrossRef]
- Expósito, A.J.; Monteagudo, J.M.; Durán, A.; Martín, I.S.; González, L. Study of the intensification of solar photo-Fenton degradation of carbamazepine with ferrioxalate complexes and ultrasound. J. Hazard. Mater. 2018, 342, 597–605. [Google Scholar] [CrossRef]
- Garcia-Herrero, I.; Margallo, M.; Onandía, R.; Aldaco, R.; Irabien, A. Life cycle assessment model for the chlor-alkali process: A comprehensive review of resources and available technologies. Sustain. Prod. Consum. 2017, 12, 44–58. [Google Scholar] [CrossRef]
- Hong, J.; Chen, W.; Wang, Y.; Xu, C.; Xu, X. Life cycle assessment of caustic soda production: A case study in China. J. Clean. Prod. 2014, 66, 113–120. [Google Scholar] [CrossRef]
- Muñoz, I.; Rieradevall, J.; Torrades, F.; Peral, J.; Domènech, X. Environmental assessment of different solar driven advanced oxidation processes. Sol. Energy 2005, 79, 369–375. [Google Scholar] [CrossRef]
- Serra, A.; Domenech, X.; Brillas, E.; Peral, J. Life cycle assessment of solar photo-Fenton and solar photoelectro-Fenton processes used for the degradation of aqueous a-methylphenylglycine. J. Environ. Monitor. 2011, 13, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Samanta, C. Direct synthesis of hydrogen peroxide from hydrogen and oxygen: An overview of recent developments in the process. Appl. Catal. A Gen. 2008, 350, 133–149. [Google Scholar] [CrossRef]
- García-Montaño, J.; Ruiz, N.; Muñoz, I.; Domènech, X.; García-Hortal, J.A.; Torrades, F.; Peral, J. Environmental assessment of different photo-Fenton approaches for commercial reactive dye removal. J. Hazard. Mater. 2006, 138, 218–225. [Google Scholar] [CrossRef]
- Liu, G.; Müller, D.B. Addressing sustainability in the aluminum industry: A critical review of life cycle assessments. J. Clean. Prod. 2012, 35, 108–117. [Google Scholar] [CrossRef]
- Salas, D.A.; Ramirez, D.A.; Rodriguez, C.R.; Petroche, D.M.; Boero, A.J.; Duque, J. Environmental impacts, life cycle assessment and potential improvement measures for cement production: A literature review. J. Clean. Prod. 2016, 113, 114–122. [Google Scholar] [CrossRef]
- Stafford, F.N.; Raupp-Pereira, F.; Labrincha, J.A.; Hotza, D. Life cycle assessment of the production of cement: A Brazilian case study. J. Clean. Prod. 2016, 137, 1293–1299. [Google Scholar] [CrossRef]
- Ukwatta, A.; Mohajerani, A.; Setunge, S.; Eshtiaghi, N. A study of gas emissions during the firing process from bricks incorporating biosolids. Waste Manage. 2018, 74, 413–426. [Google Scholar] [CrossRef]
- GmbH, P. Final Report-Life Cycle Assessment (LCA) of PVC and of Principal Competing Materials; European Commission: Brussels, Belgium, 2004. [Google Scholar]
- Arvesen, A.; Hauan, I.B.; Bolsøy, B.M.; Hertwich, E.G. Life cycle assessment of transport of electricity via different voltage levels: A case study for Nord-Trøndelag County in Norway. Appl. Energy 2015, 157, 144–151. [Google Scholar] [CrossRef]
- Rodrigues, C.S.D.; Madeira, L.M.; Boaventura, R.A.R. Optimization and economic analysis of textile wastewater treatment by photo-Fenton process under artificial and simulated solar radiation. Ind. Eng. Chem. Res. 2013, 52, 13313–13324. [Google Scholar] [CrossRef]
- Trapido, M.; Tenno, T.; Goi, A.; Dulova, N.; Kattel, E.; Klauson, D.; Klein, K.; Tenno, T.; Viisimaa, M. Bio-recalcitrant pollutants removal from wastewater with combination of the Fenton treatment and biological oxidation. J. Water. Process Eng. 2017, 16, 277–282. [Google Scholar] [CrossRef]
- Wang, N.; Zheng, T.; Zhang, G.; Wang, P. A review on Fenton-like processes for organic wastewater treatment. J. Environ. Chem. Eng. 2016, 4, 762–787. [Google Scholar] [CrossRef]
- Silva, T.F.C.V.; Fonseca, A.; Saraiva, I.; Boaventura, R.A.R.; Vilar, V.J.P. Scale-up and cost analysis of a photo-Fenton system for sanitary landfill leachate treatment. Chem. Eng. J. 2016, 283, 76–88. [Google Scholar] [CrossRef]
- Gar, M.; Taw, A.; Ookawara, S. Degradation of four pharmaceuticals by solar photo-Fenton process: Kinetics and costs estimation. J. Environ. Chem. Eng. 2015, 3, 46–51. [Google Scholar] [CrossRef]
- Santos-Juanes, L.; Ballesteros, M.M.; Ortega, E.; Cabrera, A.; Román, I.M.; Casas, J.L.; Sánchez, J.A. Economic evaluation of the photo- Fenton process. Mineralization level and reaction time: The keys for increasing plant efficiency. J. Hazard. Mater. 2011, 186, 1924–1929. [Google Scholar] [CrossRef]
- Soares, P.; Souza, R.; Soler, J.; Silva, T.F.C.V.; Guelli, S.M.A.; Souza, U.; Boaventura, R.A.R.; Vilar, V.J.P. Remediation of a synthetic textile wastewater from polyester-cotton dyeing combining biological and photochemical oxidation processes. Sep. Purif. Technol. 2017, 172, 450–462. [Google Scholar] [CrossRef]
- Spasiano, D.; Marotta, R.; Malato, S.; Fernandez, P.; Di Somma, I. Solar photocatalysis: Materials, reactors, some commercial, and pre-industrialized applications. A comprehensive approach. Appl. Catal. B Environ. 2015, 170–171, 90–123. [Google Scholar] [CrossRef]


| Models | Equation | Parameter | r2 |
|---|---|---|---|
| Diquat (DQ) | |||
| First-order | k = 0.013 L kJ−1 | 0.71 | |
| Second-order | k = 0.175e−3 L2 mg−1 kJ−1 | 0.90 | |
| BMG | m = 7.96 kJ L−1, b = 1.21 | 0.98 | |
| Paraquat (PQ) | |||
| First-order | k = 0.003 L kJ−1 | 0.57 | |
| Second-order | k = 0.47e−4L2 mg−1 kJ−1 | 0.83 | |
| BMG | m = 26.18 kJ L−1, b = 1.44 | 0.99 | |
| Treatment | Germination index (%) (RSD) |
|---|---|
| Diquat (DQ) | |
| Control (distilled water) | 100 (0) |
| Untreated DQ solution | 4.7 (0.68) |
| Treated DQ solution | 55.8 (3.39) |
| Paraquat (PQ) | |
| Control (distilled water) | 100 (0) |
| Untreated PQ solution | 16.5 (2.79) |
| Treated PQ solution | 59.7 (0.87) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teutli-Sequeira, A.; Vasquez-Medrano, R.; Prato-Garcia, D.; Ibanez, J.G. Solar Photo-Assisted Degradation of Bipyridinium Herbicides at Circumneutral pH: A Life Cycle Assessment Approach. Processes 2020, 8, 1117. https://doi.org/10.3390/pr8091117
Teutli-Sequeira A, Vasquez-Medrano R, Prato-Garcia D, Ibanez JG. Solar Photo-Assisted Degradation of Bipyridinium Herbicides at Circumneutral pH: A Life Cycle Assessment Approach. Processes. 2020; 8(9):1117. https://doi.org/10.3390/pr8091117
Chicago/Turabian StyleTeutli-Sequeira, Alejandra, Ruben Vasquez-Medrano, Dorian Prato-Garcia, and Jorge G. Ibanez. 2020. "Solar Photo-Assisted Degradation of Bipyridinium Herbicides at Circumneutral pH: A Life Cycle Assessment Approach" Processes 8, no. 9: 1117. https://doi.org/10.3390/pr8091117
APA StyleTeutli-Sequeira, A., Vasquez-Medrano, R., Prato-Garcia, D., & Ibanez, J. G. (2020). Solar Photo-Assisted Degradation of Bipyridinium Herbicides at Circumneutral pH: A Life Cycle Assessment Approach. Processes, 8(9), 1117. https://doi.org/10.3390/pr8091117






