Molecular Interpretation of Pharmaceuticals’ Adsorption on Carbon Nanomaterials: Theory Meets Experiments
Abstract
1. Introduction
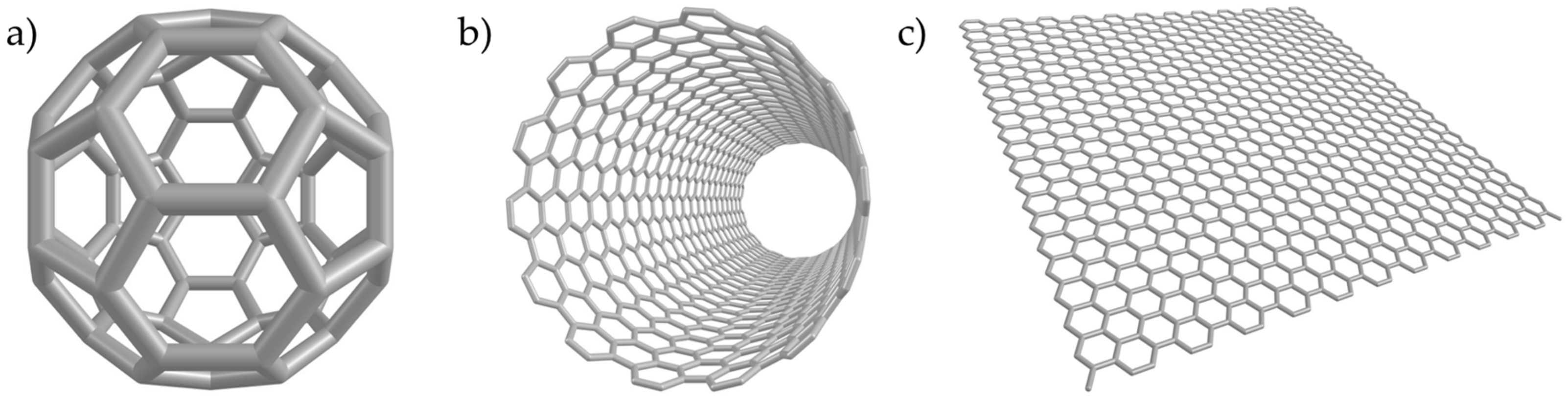
2. CNMs Models
3. Antimicrobials
3.1. Fluoroquinolones
3.2. Other Antimicrobials
4. Cytostatic Compounds
4.1. Platinum Anticancer Compounds
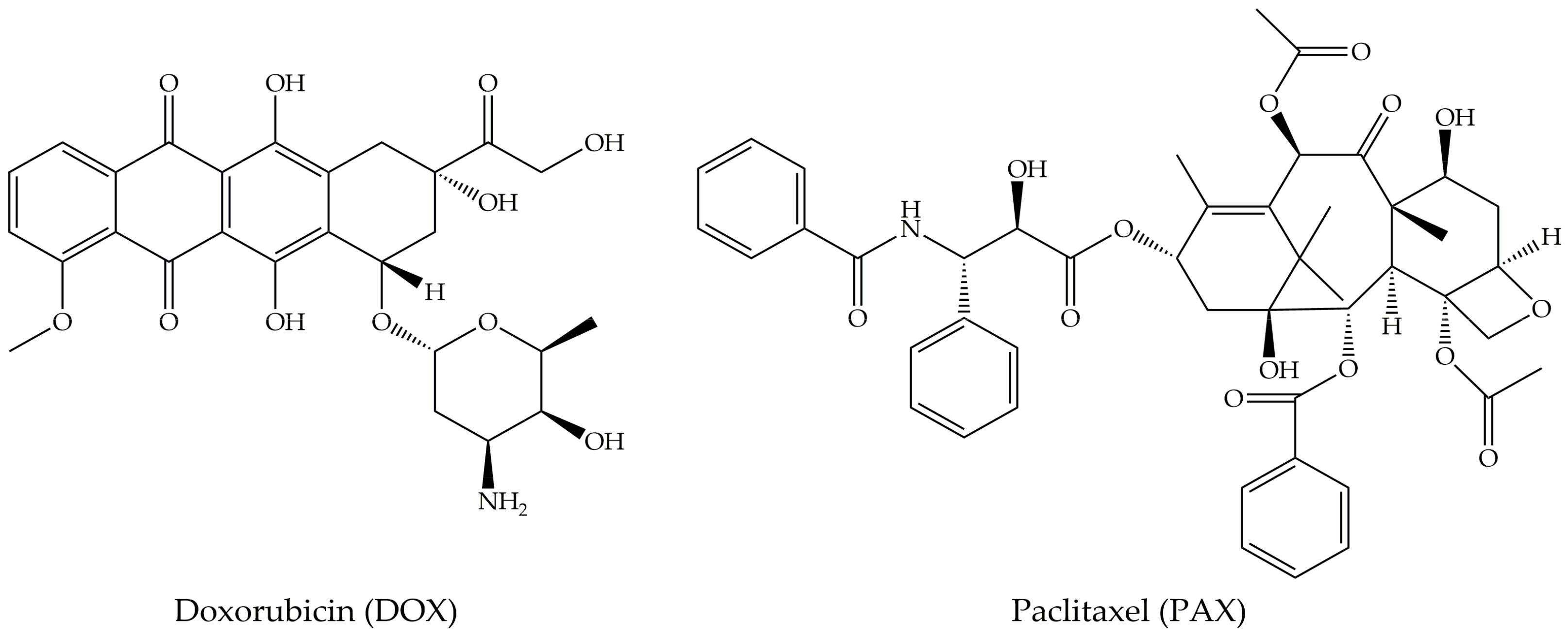
4.2. Doxorubicin
4.3. Paclitaxel
4.4. Other Cytostatics
5. Conclusions and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kroto, H.W.; Heath, J.R.; O’Brien, S.C.; Curl, R.F.; Smalley, R.E. C60: Buckminsterfullerene. Nature 1985, 318, 162–163. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Bacon, R. Growth, structure, and properties of graphite whiskers. J. Appl. Phys. 1960, 31, 283–290. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef]
- Mohan, V.B.; Lau, K.T.; Hui, D.; Bhattacharyya, D. Graphene-based materials and their composites: A review on production, applications and product limitations. Compos. Part B Eng. 2018, 142, 200–220. [Google Scholar] [CrossRef]
- Lawal, A.T. Graphene-based nano composites and their applications. A review. Biosens. Bioelectron. 2019, 141, 111384. [Google Scholar] [CrossRef] [PubMed]
- Apul, O.G.; Karanfil, T. Adsorption of synthetic organic contaminants by carbon nanotubes: A critical review. Water Res. 2014, 68, 34–55. [Google Scholar] [CrossRef]
- Loh, K.P.; Bao, Q.; Ang, P.K.; Yang, J. The chemistry of graphene. J. Mater. Chem. 2010, 20, 2277–2289. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Z.; Zhang, Y. The application of carbon nanotubes in target drug delivery systems for cancer therapies. Nanoscale Res. Lett. 2011, 6, 555. [Google Scholar] [CrossRef]
- Ebbesen, T.W.; Lezec, H.J.; Hiura, H.; Bennett, J.W.; Ghaemi, H.F.; Thio, T. Electrical conductivity of individual carbon nanotubes. Nature 1996, 382, 54–56. [Google Scholar] [CrossRef]
- White, C.T.; Todorov, T.N. Carbon nanotubes as long ballistic conductors. Nature 1998, 393, 240–241. [Google Scholar] [CrossRef]
- Wei, B.Q.; Vajtai, R.; Ajayan, P.M. Reliability and current carrying capacity of carbon nanotubes. Appl. Phys. Lett. 2001, 79, 1172–1174. [Google Scholar] [CrossRef]
- Hills, G.; Lau, C.; Wright, A.; Fuller, S.; Bishop, M.D.; Srimani, T.; Kanhaiya, P.; Ho, R.; Amer, A.; Stein, Y.; et al. Modern microprocessor built from complementary carbon nanotube transistors. Nature 2019, 572, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Treacy, M.M.J.; Ebbesen, T.W.; Gibson, J.M. Exceptionally high Young’s modulus observed for individual carbon nanotubes. Nature 1996, 381, 678–680. [Google Scholar] [CrossRef]
- Lu, J.P. Elastic properties of carbon nanotubes and nanoropes. Phys. Rev. Lett. 1997, 79, 1297–1300. [Google Scholar] [CrossRef]
- Wong, E.W.; Sheehan, P.E.; Lieber, C.M. Nanobeam mechanics: Elasticity, strength, and toughness of nanorods and nanotubes. Science 1997, 277, 1971–1975. [Google Scholar] [CrossRef]
- Schwamb, T.; Burg, B.R.; Schirmer, N.C.; Poulikakos, D. An electrical method for the measurement of the thermal and electrical conductivity of reduced graphene oxide nanostructures. Nanotechnology 2009, 20, 405704. [Google Scholar] [CrossRef]
- De Volder, M.F.L.; Tawfick, S.H.; Baughman, R.H.; Hart, A.J. Carbon Nanotubes: Present and Future Commercial Applications. Science 2013, 339, 535–539. [Google Scholar] [CrossRef]
- Krätschmer, W.; Lamb, L.D.; Fostiropoulos, K.; Huffman, D.R. Solid C60: A new form of carbon. Nature 1990, 347, 354–358. [Google Scholar] [CrossRef]
- Thakral, S.; Mehta, R.M. Fullerenes: An introduction and overview of their biological properties. Indian J. Pharm. Sci. 2006, 68, 13–19. [Google Scholar] [CrossRef]
- Geckeler, K.E.; Samal, S. Syntheses and properties of macromolecular fullerenes, a review. Polym. Int. 1999, 48, 743–757. [Google Scholar] [CrossRef]
- Goodarzi, S.; Da Ros, T.; Conde, J.; Sefat, F.; Mozafari, M. Fullerene: Biomedical engineers get to revisit an old friend. Mater. Today 2017, 20, 460–480. [Google Scholar] [CrossRef]
- Acquah, S.F.A.; Penkov, A.V.; Markelov, D.A.; Semisalova, A.S.; Leonhardt, B.E.; Magi, J.M. Review-The beautiful molecule: 30 years of C60 and its derivatives. ECS J. Solid State Sci. Technol. 2017, 6, M3155–M3162. [Google Scholar] [CrossRef]
- Castro, E.; Garcia, A.H.; Zavala, G.; Echegoyen, L. Fullerenes in biology and medicine. J. Mater. Chem. B 2017, 5, 6523–6535. [Google Scholar] [CrossRef] [PubMed]
- Dresselhaus, M.S.; Dresselhaus, G.; Charlier, J.C.; Hernández, E. Electronic, thermal and mechanical properties of carbon nanotubes. Philos. Trans. Math. Phys. Eng. Sci. 2004, 362, 2065–2098. [Google Scholar] [CrossRef]
- Hou, P.X.; Bai, S.; Yang, Q.H.; Liu, C.; Cheng, H.M. Multi-step purification of carbon nanotubes. Carbon 2002, 40, 81–85. [Google Scholar] [CrossRef]
- Hirlekar, R.; Yamagar, M.; Garse, H.; Vij, M.; Kadam, V. Carbon Nanotubes and Its Applications: a Review. Asian J. Pharm. Clin. Res. 2009, 2, 17–27. [Google Scholar]
- Chico, L.; Crespi, V.; Benedict, L.; Louie, S.; Cohen, M. Pure Carbon Nanoscale Devices: Nanotube Heterojunctions. Phys. Rev. Lett. 1996, 76, 971–974. [Google Scholar] [CrossRef]
- Tessonnier, J.P.; Su, D.S. Recent progress on the growth mechanism of carbon nanotubes: A review. ChemSusChem 2011, 4, 824–847. [Google Scholar] [CrossRef]
- Thostenson, E.T.; Ren, Z.; Chou, T.-W. Advances in the science and technology of carbon nanotubes and their composites: A review. Compos. Sci. Technol. 2001, 61, 1899–1912. [Google Scholar] [CrossRef]
- Abbasi, E.; Aval, S.F.; Akbarzadeh, A.; Milani, M.; Nasrabadi, H.T.; Woo joo, S.; Hanifehpour, Y.; Nejati-Koshki, K.; Pashaei-Asl, R. Dendrimers: Synthesis, applications, and properties. Nanoscale Res. Lett. 2014, 9, 247. [Google Scholar] [CrossRef]
- Gao, C.; Guo, Z.; Liu, J.H.; Huang, X.J. The new age of carbon nanotubes: An updated review of functionalized carbon nanotubes in electrochemical sensors. Nanoscale 2012, 4, 1948–1963. [Google Scholar] [CrossRef]
- Negri, V.; Pacheco-Torres, J.; Calle, D.; López-Larrubia, P. Carbon Nanotubes in Biomedicine. Top. Curr. Chem. 2020, 378, 15. [Google Scholar] [CrossRef] [PubMed]
- Kuila, T.; Bose, S.; Mishra, A.K.; Khanra, P.; Kim, N.H.; Lee, J.H. Chemical functionalization of graphene and its applications. Prog. Mater. Sci. 2012, 57, 1061–1105. [Google Scholar] [CrossRef]
- Craciun, M.F.; Khrapach, I.; Barnes, M.D.; Russo, S. Properties and applications of chemically functionalized graphene. J. Phys. Condens. Matter 2013, 25, 423201. [Google Scholar] [CrossRef] [PubMed]
- Sprinkle, M.; Ruan, M.; Hu, Y.; Hankinson, J.; Rubio-Roy, M.; Zhang, B.; Wu, X.; Berger, C.; de Heer, W.A. Scalable templated growth of graphene nanoribbons on SiC. Nat. Nanotechnol. 2010, 5, 727–731. [Google Scholar] [CrossRef] [PubMed]
- Avouris, P.; Dimitrakopoulos, C. Graphene: Synthesis and applications. Mater. Today 2012, 15, 86–97. [Google Scholar] [CrossRef]
- Park, S.; An, J.; Jung, I.; Piner, R.D.; An, S.J.; Li, X.; Velamakanni, A.; Ruoff, R.S. Colloidal Suspensions of Highly Reduced Graphene Oxide in a Wide Variety of Organic Solvents. Nano Lett. 2009, 9, 1593–1597. [Google Scholar] [CrossRef]
- Kim, J.; Cote, L.J.; Kim, F.; Yuan, W.; Shull, K.R.; Huang, J. Graphene Oxide Sheets at Interfaces. J. Am. Chem. Soc. 2010, 132, 8180–8186. [Google Scholar] [CrossRef]
- Kim, F.; Cote, L.J.; Huang, J. Graphene Oxide: Surface Activity and Two-Dimensional Assembly. Adv. Mater. 2010, 22, 1954–1958. [Google Scholar] [CrossRef]
- Guo, F.; Kim, F.; Han, T.H.; Shenoy, V.B.; Huang, J.; Hurt, R.H. Hydration-Responsive Folding and Unfolding in Graphene Oxide Liquid Crystal Phases. ACS Nano 2011, 23, 8019–8025. [Google Scholar] [CrossRef] [PubMed]
- Bagri, A.; Mattevi, C.; Acik, M.; Chabal, Y.J.; Chhowalla, M.; Shenoy, V.B. Structural evolution during the reduction of chemically derived graphene oxide. Nat. Chem. 2010, 2, 581–587. [Google Scholar] [CrossRef]
- Smalley, R.E.; Yakobson, B.I. The future of the fullerenes. Solid State Commun. 1998, 107, 597–606. [Google Scholar] [CrossRef]
- Ruhl, G.; Wittmann, S.; Koenig, M.; Neumaier, D. The integration of graphene into microelectronic devices. Beilstein J. Nanotechnol. 2017, 8, 1056–1064. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Y.; Xu, J.; Li, S.; Zhang, F.; Ye, Q.; Zhai, X.; Zhao, X. Molecular dynamics study of binding strength and drug diffusion of graphene-based drug delivery systems. J. Funct. Mater. 2015, 46, 16052–16058. [Google Scholar]
- Li, X.; Zhi, L. Graphene hybridization for energy storage applications. Chem. Soc. Rev. 2018, 47, 3189–3216. [Google Scholar] [CrossRef] [PubMed]
- Kharissova, O.V.; Kharisov, B.I.; Oliva González, C.M. Carbon-Carbon Allotropic Hybrids and Composites: Synthesis, Properties, and Applications. Ind. Eng. Chem. Res. 2019, 58, 3921–3948. [Google Scholar] [CrossRef]
- Raphey, V.R.; Henna, T.K.; Nivitha, K.P.; Mufeedha, P.; Sabu, C.; Pramod, K. Advanced biomedical applications of carbon nanotube. Mater. Sci. Eng. C 2019, 100, 616–630. [Google Scholar] [CrossRef] [PubMed]
- Dasari Shareena, T.P.; McShan, D.; Dasmahapatra, A.K.; Tchounwou, P.B. A Review on Graphene-Based Nanomaterials in Biomedical Applications and Risks in Environment and Health. Nano-Micro Lett. 2018, 10, 53. [Google Scholar] [CrossRef]
- Ahadian, S.; Davenport Huyer, L.; Estili, M.; Yee, B.; Smith, N.; Xu, Z.; Sun, Y.; Radisic, M. Moldable elastomeric polyester-carbon nanotube scaffolds for cardiac tissue engineering. Acta Biomater. 2017, 52, 81–91. [Google Scholar] [CrossRef]
- Gajendiran, M.; Choi, J.; Kim, S.J.; Kim, K.; Shin, H.; Koo, H.J.; Kim, K. Conductive biomaterials for tissue engineering applications. J. Ind. Eng. Chem. 2017, 51, 12–26. [Google Scholar] [CrossRef]
- Nongbe, M.C.; Ekou, T.; Ekou, L.; Yao, K.B.; Le Grognec, E.; Felpin, F.X. Biodiesel production from palm oil using sulfonated graphene catalyst. Renew. Energy 2017, 106, 135–141. [Google Scholar] [CrossRef]
- López-Andarias, J.; Frontera, A.; Matile, S. Anion−p Catalysis on Fullerenes. J. Am. Chem. Soc. 2017, 139, 13296–13299. [Google Scholar] [CrossRef] [PubMed]
- Esteves, L.M.; Oliveira, H.A.; Passos, F.B. Carbon nanotubes as catalyst support in chemical vapor deposition reaction: A review. J. Ind. Eng. Chem. 2018, 65, 1–12. [Google Scholar] [CrossRef]
- Li, Y.; Sun, B. Nano-delivery materials: Review of development and application in drug/gene transport. Key Eng. Mater. 2019, 803, 158–166. [Google Scholar] [CrossRef]
- Demetzos, C.; Pippa, N. Advanced drug delivery nanosystems (aDDnSs): A mini-review. Drug Deliv. 2014, 21, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yap, S.Q.; Yoong, S.L.; Nayak, T.R.; Chandra, G.W.; Ang, W.H.; Panczyk, T.; Ramaprabhu, S.; Vashist, S.K.; Sheu, F.S.; et al. Carbon nanotube bottles for incorporation, release and enhanced cytotoxic effect of cisplatin. Carbon 2012, 50, 1625–1634. [Google Scholar] [CrossRef]
- Natarajan, J.V.; Nugraha, C.; Ng, X.W.; Venkatraman, S. Sustained-release from nanocarriers: A review. J. Control. Release 2014, 193, 122–138. [Google Scholar] [CrossRef]
- Arora, D.; Jaglan, S. Nanocarriers based delivery of nutraceuticals for cancer prevention and treatment: A review of recent research developments. Trends Food Sci. Technol. 2016, 54, 114–126. [Google Scholar] [CrossRef]
- Mehra, N.K.; Palakurthi, S. Interactions between carbon nanotubes and bioactives: A drug delivery perspective. Drug Discov. Today 2016, 21, 585–597. [Google Scholar] [CrossRef]
- Yu, X.; Cheng, H.; Zhang, M.; Zhao, Y.; Qu, L.; Shi, G. Graphene-based smart materials. Nat. Rev. Mater. 2017, 2, 17046. [Google Scholar] [CrossRef]
- Yang, K.; Feng, L.; Liu, Z. Stimuli responsive drug delivery systems based on nano-graphene for cancer therapy. Adv. Drug Deliv. Rev. 2016, 105, 228–241. [Google Scholar] [CrossRef] [PubMed]
- Kazemzadeh, H.; Mozafari, M. Fullerene-based delivery systems. Drug Discov. Today 2019, 24, 898–905. [Google Scholar] [CrossRef] [PubMed]
- Datsyuk, V.; Kalyva, M.; Papagelis, K.; Parthenios, J.; Tasis, D.; Siokou, A.; Kallitsis, I.; Galiotis, C. Chemical oxidation of multiwalled carbon nanotubes. Carbon 2008, 46, 833–840. [Google Scholar] [CrossRef]
- Peng, W.; Li, H.; Liu, Y.; Song, S. A review on heavy metal ions adsorption from water by graphene oxide and its composites. J. Mol. Liq. 2017, 230, 496–504. [Google Scholar] [CrossRef]
- Wang, B.; Wang, J.; Zhu, J. Fluorination of graphene: A Spectroscopic and microscopic study. ACS Nano 2014, 8, 1862–1870. [Google Scholar] [CrossRef]
- Kabdulov, M.; Jansen, M.; Amsharov, K.Y. Bottom-up C60 fullerene construction from a fluorinated C 60H21F9 precursor by laser-induced tandem cyclization. Chem. A Eur. J. 2013, 19, 17262–17266. [Google Scholar] [CrossRef]
- Adamska, M.; Narkiewicz, U. Fluorination of Carbon Nanotubes — A Review. J. Fluor. Chem. 2017, 200, 179–189. [Google Scholar] [CrossRef]
- Wang, S.; Li, S.; Hou, C.; Ma, G.; Wang, H.; Wu, J.; Hao, X.; Zhang, H. Functionalization of multiwalled carbon nanotubes by amidation and Michael addition reactions and the effect of the functional chains on the properties of waterborne polyurethane composites. J. Appl. Polym. Sci. 2018, 135, 46757. [Google Scholar] [CrossRef]
- Bjelaković, M.S.; Kop, T.J.; Vlajić, M.; DorCevie, J.; Milić, D.R. Design, synthesis, and characterization of fullerene-peptide-steroid covalent hybrids. Tetrahedron 2014, 70, 8564–8570. [Google Scholar] [CrossRef]
- Zheng, L.; Zhen, W. Preparation and characterization of amidated graphene oxide and its effect on the performance of poly(lactic acid). Iran. Polym. J. 2018, 27, 239–252. [Google Scholar] [CrossRef]
- Hsu, M.H.; Chuang, H.; Cheng, F.Y.; Huang, Y.P.; Han, C.C.; Pao, K.C.; Chou, S.C.; Shieh, F.K.; Tsai, F.Y.; Lin, C.C.; et al. Simple and highly efficient direct thiolation of the surface of carbon nanotubes. RSC Adv. 2014, 4, 14777–14780. [Google Scholar] [CrossRef]
- Frare, M.C.; Pilot, R.; De Filippo, C.C.; Weber, V.; Signorini, R.; Maggini, M.; Bozio, R. Fullerene functionalized gold nanoparticles for optical limiting of continuous wave lasers. Appl. Phys. B Lasers Opt. 2019, 125, 47. [Google Scholar] [CrossRef]
- Pereira De Sousa, I.; Buttenhauser, K.; Suchaoin, W.; Partenhauser, A.; Perrone, M.; Matuszczak, B.; Bernkop-Schnürch, A. Thiolated graphene oxide as promising mucoadhesive carrier for hydrophobic drugs. Int. J. Pharm. 2016, 509, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Wang, Y. Covalently crosslinked graphene oxide membranes by esterification reactions for ions separation. J. Mater. Chem. A 2015, 3, 4405–4412. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, L.; Li, X.; Li, B.; Wang, S. Preparation and Characterization of Thermal Conductive Composite Membranes of Aligned Esterified Carbon Nanotubes/Poly(vinylidene fluoride). Polym. Plast. Technol. Eng. 2015, 54, 515–522. [Google Scholar] [CrossRef]
- Wang, H.; Gu, W.; Xiao, N.; Ye, L.; Xu, Q. Chlorotoxin-conjugated graphene oxide for targeted delivery of an anticancer drug. Int. J. Nanomedicine 2014, 9, 1433–1442. [Google Scholar]
- Rana, V.K.; Choi, M.C.; Kong, J.Y.; Kim, G.Y.; Kim, M.J.; Kim, S.H.; Mishra, S.; Singh, R.P.; Ha, C.S. Synthesis and drug-delivery behavior of chitosan-functionalized graphene oxide hybrid nanosheets. Macromol. Mater. Eng. 2011, 296, 131–140. [Google Scholar] [CrossRef]
- Kavitha, T.; Haider Abdi, S.I.; Park, S.Y. pH-sensitive nanocargo based on smart polymer functionalized graphene oxide for site-specific drug delivery. PPhys. Chem. Chem. Phys. 2013, 15, 5176–5185. [Google Scholar] [CrossRef]
- Xu, H.; Fan, M.; Elhissi, A.M.A.; Zhang, Z.; Wan, K.W.; Ahmed, W.; Phoenix, D.A.; Sun, X. PEGylated graphene oxide for tumor-targeted delivery of paclitaxel. Nanomedicine 2015, 10, 1247–1262. [Google Scholar] [CrossRef]
- Kam, N.W.S.; Dai, H. Single walled carbon nanotubes for transport and delivery of biological cargos. Phys. Status Solidi Basic Res. 2006, 243, 3561–3566. [Google Scholar]
- Cho, Y.; Choi, Y. Graphene oxide-photosensitizer conjugate as a redox-responsive theranostic agent. Chem. Commun. 2012, 48, 9912–9914. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Zhao, T.; Wu, W.; Wu, F.; Li, L.; Qian, J.; Xu, R.; Wu, H.; Albishri, H.M.; Al-Bogami, A.S.; et al. Free-standing hierarchically sandwich-type tungsten disulfide nanotubes/graphene anode for lithium-ion batteries. Nano Lett. 2014, 14, 5899–5904. [Google Scholar] [CrossRef]
- Wu, C.S.; Liao, H.T. Study on the preparation and characterization of biodegradable polylactide/multi-walled carbon nanotubes nanocomposites. Polymer (Guildf) 2007, 48, 4449–4458. [Google Scholar] [CrossRef]
- Meng, N.; Su, Y.T.; Zhou, N.; Zhang, M.; Shao, M.; Fan, Y.; Zhu, H.; Yuan, P.; Chi, C.; Xiao, Y. Carboxylated graphene oxide functionalized with β-cyclodextrin—Engineering of a novel nanohybrid drug carrier. Int. J. Biol. Macromol. 2016, 93, 117–122. [Google Scholar] [CrossRef]
- Kumar, M.; Chung, J.S.; Kong, B.S.; Kim, E.J.; Hur, S.H. Synthesis of graphene-polyurethane nanocomposite using highly functionalized graphene oxide as pseudo-crosslinker. Mater. Lett. 2013, 106, 319–321. [Google Scholar] [CrossRef]
- Gan, S.; Zakaria, S.; Chia, C.H.; Chen, R.S.; Jeyalaldeen, N. Physico-mechanical properties of a microwave-irradiated kenaf carbamate/graphene oxide membrane. Cellulose 2015, 22, 3851–3863. [Google Scholar] [CrossRef]
- Tao, C.A.; Wang, J.; Qin, S.; Lv, Y.; Long, Y.; Zhu, H.; Jiang, Z. Fabrication of pH-sensitive graphene oxide-drug supramolecular hydrogels as controlled release systems. J. Mater. Chem. 2012, 22, 24856–24861. [Google Scholar] [CrossRef]
- Shi, J.; Liu, Y.; Wang, L.; Gao, J.; Zhang, J.; Yu, X.; Ma, R.; Liu, R.; Zhang, Z. A tumoral acidic pH-responsive drug delivery system based on a novel photosensitizer (fullerene) for in vitro and in vivo chemo-photodynamic therapy. Acta Biomater. 2014, 10, 1280–1291. [Google Scholar] [CrossRef]
- Qiu, J.; Zhang, R.; Li, J.; Sang, Y.; Tang, W.; Gil, P.R.; Liu, H. Fluorescent graphene quantum dots as traceable, pH-sensitive drug delivery systems. Int. J. Nanomedicine 2015, 10, 6709–6724. [Google Scholar]
- Qin, Y.; Chen, J.; Bi, Y.; Xu, X.; Zhou, H.; Gao, J.; Hu, Y.; Zhao, Y.; Chai, Z. Near-infrared light remote-controlled intracellular anti-cancer drug delivery using thermo/pH sensitive nanovehicle. Acta Biomater. 2015, 17, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Mo, Y.; Wang, H.; Liu, J.; Lan, Y.; Guo, R.; Zhang, Y.; Xue, W.; Zhang, Y. Controlled release and targeted delivery to cancer cells of doxorubicin from polysaccharide-functionalised single-walled carbon nanotubes. J. Mater. Chem. B 2015, 3, 1846–1855. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Cui, L.; Kong, N.; Barrow, C.J.; Yang, W. RAFT controlled synthesis of graphene/polymer hydrogel with enhanced mechanical property for pH-controlled drug release. Eur. Polym. J. 2014, 50, 9–17. [Google Scholar] [CrossRef]
- Liang, S.; Zhao, Y.; Adronov, A. Selective and reversible noncovalent functionalization of single-walled carbon nanotubes by a pH-responsive vinylogous tetrathiafulvalene-fluorene copolymer. J. Am. Chem. Soc. 2014, 136, 970–977. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, D.J.; Kwag, D.S.; Lee, U.Y.; Youn, Y.S.; Lee, E.S. Acid pH-activated glycol chitosan/fullerene nanogels for efficient tumor therapy. Carbohydr. Polym. 2014, 101, 692–698. [Google Scholar] [CrossRef]
- Depan, D.; Shah, J.; Misra, R.D.K. Controlled release of drug from folate-decorated and graphene mediated drug delivery system: Synthesis, loading efficiency, and drug release response. Mater. Sci. Eng. C 2011, 31, 1305–1312. [Google Scholar] [CrossRef]
- Ardeshirzadeh, B.; Anaraki, N.A.; Irani, M.; Rad, L.R.; Shamshiri, S. Controlled release of doxorubicin from electrospun PEO/chitosan/graphene oxide nanocomposite nanofibrous scaffolds. Mater. Sci. Eng. C 2015, 48, 384–390. [Google Scholar] [CrossRef]
- Yoon, H.J.; Shanker, A.; Wang, Y.; Kozminsky, M.; Jin, Q.; Palanisamy, N.; Burness, M.L.; Azizi, E.; Simeone, D.M.; Wicha, M.S.; et al. Tunable Thermal-Sensitive Polymer–Graphene Oxide Composite for Efficient Capture and Release of Viable Circulating Tumor Cells. Adv. Mater. 2016, 28, 4891–4897. [Google Scholar] [CrossRef]
- Teodorescu, F.; Oz, Y.; Quéniat, G.; Abderrahmani, A.; Foulon, C.; Lecoeur, M.; Sanyal, R.; Sanyal, A.; Boukherroub, R.; Szunerits, S. Photothermally triggered on-demand insulin release from reduced graphene oxide modified hydrogels. J. Control. Release 2017, 246, 164–173. [Google Scholar] [CrossRef]
- Pistone, A.; Iannazzo, D.; Ansari, S.; Milone, C.; Salamò, M.; Galvagno, S.; Cirmi, S.; Navarra, M. Tunable doxorubicin release from polymer-gated multiwalled carbon nanotubes. Int. J. Pharm. 2016, 515, 30–36. [Google Scholar] [CrossRef]
- Hashemi, M.; Yadegari, A.; Yazdanpanah, G.; Omidi, M.; Jabbehdari, S.; Haghiralsadat, F.; Yazdian, F.; Tayebi, L. Normalization of doxorubicin release from graphene oxide: New approach for optimization of effective parameters on drug loading. Biotechnol. Appl. Biochem. 2017, 64, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Li, Y.; Han, S.; Ma, J. Adsorptive removal of antibiotics from aqueous solution using carbon materials. Chemosphere 2016, 153, 365–385. [Google Scholar] [CrossRef] [PubMed]
- Gehrke, I.; Geiser, A.; Somborn-schulz, A. Innovations in nanotechnology for water treatment. Nanotechnol. Sci. Appl. 2015, 8, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Rivera-utrilla, J.; Sánchez-polo, M.; Ferro-garcía, M.Á.; Prados-joya, G.; Ocampo-Perez, R. Pharmaceuticals as emerging contaminants and their removal from water. A review. Chemosphere 2013, 9, 1268–1287. [Google Scholar] [CrossRef] [PubMed]
- Gothwal, R.; Shashidhar, T. Antibiotic Pollution in the Environment: A Review. Clean - Soil, wat 2015, 43, 479–489. [Google Scholar] [CrossRef]
- Tiwari, B.; Sellamuthu, B.; Ouarda, Y.; Drogui, P.; Tyagi, R.D.; Buelna, G. Review on fate and mechanism of removal of pharmaceutical pollutants from wastewater using biological approach. Bioresour. Technol. 2017, 224, 1–12. [Google Scholar] [CrossRef]
- Quesada, H.B.; Baptista, A.T.A.; Cusioli, L.F.; Seibert, D.; de Oliveira Bezerra, C.; Bergamasco, R. Surface water pollution by pharmaceuticals and an alternative of removal by low-cost adsorbents: A review. Chemosphere 2019, 222, 766–780. [Google Scholar] [CrossRef]
- Santhosh, C.; Velmurugan, V.; Jacob, G.; Jeong, S.K.; Grace, A.N.; Bhatnagar, A. Role of nanomaterials in water treatment applications: A review. Chem. Eng. J. 2016, 306, 1116–1137. [Google Scholar] [CrossRef]
- Patel, M.; Kumar, R.; Kishor, K.; Mlsna, T.; Pittman, C.U.; Mohan, D. Pharmaceuticals of Emerging Concern in Aquatic Systems: Chemistry, Occurrence, Effects, and Removal Methods. Chem. Rev. 2019, 119, 3510–3673. [Google Scholar] [CrossRef]
- Kanakaraju, D.; Glass, B.D.; Oelgemöller, M. Advanced oxidation process-mediated removal of pharmaceuticals from water: A review. J. Environ. Manage. 2018, 219, 189–207. [Google Scholar] [CrossRef]
- Miklos, D.B.; Remy, C.; Jekel, M.; Linden, K.G.; Drewes, J.E.; Hübner, U. Evaluation of advanced oxidation processes for water and wastewater treatment – A critical review. Water Res. 2018, 139, 118–131. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.; Khan, M.Z.A.; Shahane, S.; Rai, D.; Chauhan, D.; Kant, C.; Chaudhary, V.K. Emerging Pollutants in Aquatic Environment: Source, Effect, and Challenges in Biomonitoring and Bioremediation- A Review. Pollution 2020, 6, 99–113. [Google Scholar]
- Vieno, N.; Tuhkanen, T.; Kronberg, L. Removal of pharmaceuticals in drinking water treatment: Effect of chemical coagulation. Environ. Technol. 2006, 27, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.T.; Hai, F.I.; Al-aboud, T.M. Chemical coagulation-based processes for trace organic contaminant removal: Current state and future potential. J. Environ. Manage. 2012, 111, 195–207. [Google Scholar] [CrossRef]
- Yu-Chen Lin, A.; Hsueh, J.H.-F.; Hong, P.K.A. Removal of antineoplastic drugs cyclophosphamide, ifosfamide, and 5-fluorouracil and a vasodilator drug pentoxifylline from wastewaters by ozonation. Environ. Sci. Pollut. Res. 2015, 22, 508–515. [Google Scholar]
- Wei, C.; Zhang, F.; Hu, Y.; Feng, C.; Wu, H. Ozonation in water treatment: The generation, basic properties of ozone and its practical application. Rev. Chem. Eng. 2017, 33, 49–89. [Google Scholar] [CrossRef]
- Ikehata, K.; Jodeiri Naghashkar, N.; Gamal El-Din, M. Degradation of aqueous pharmaceuticals by ozonation and advanced oxidation processes: A review. Ozone Sci. Eng. 2006, 28, 353–414. [Google Scholar] [CrossRef]
- Rodriguez-Mozaz, S.; Ricart, M.; Köck-Schulmeyer, M.; Guasch, H.; Bonnineau, C.; Proia, L.; de Alda, M.L.; Sabater, S.; Barceló, D. Pharmaceuticals and pesticides in reclaimed water: Efficiency assessment of a microfiltration-reverse osmosis (MF-RO) pilot plant. J. Hazard. Mater. 2015, 282, 165–173. [Google Scholar] [CrossRef]
- Joo, S.H.; Tansel, B. Novel technologies for reverse osmosis concentrate treatment: A review. J. Environ. Manage. 2015, 150, 322–335. [Google Scholar] [CrossRef]
- Wang, X.; Yin, R.; Zeng, L.; Zhu, M. A review of graphene-based nanomaterials for removal of antibiotics from aqueous environments. Environ. Pollut. 2019, 253, 100–110. [Google Scholar] [CrossRef]
- Khan, A.; Wang, J.; Li, J.; Wang, X.; Chen, Z.; Alsaedi, A.; Hayat, T.; Chen, Y.; Wang, X. The role of graphene oxide and graphene oxide-based nanomaterials in the removal of pharmaceuticals from aqueous media: A review. Environ. Sci. Pollut. Res. 2017, 24, 7938–7958. [Google Scholar] [CrossRef] [PubMed]
- Carmalin Sophia, A.; Lima, E.C.; Allaudeen, N.; Rajan, S. Application of graphene based materials for adsorption of pharmaceutical traces from water and wastewater- a review. Desalin. Water Treat. 2016, 57, 27573–27586. [Google Scholar] [CrossRef]
- Son, K.H.; Hong, J.H.; Lee, J.W. Carbon nanotubes as cancer therapeutic carriers and mediators. Int. J. Nanomed. 2016, 11, 5163–5185. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, V.; Yadav, P.; Bhattacharya, S.S.; Mishra, A.K.; Verma, N.; Verma, A.; Pandit, J.K. Carbon Nanotubes: An Emerging Drug Carrier for Targeting Cancer Cells. J. Drug Deliv. 2014, 2014, 670815. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Pant, A.; Chin, C.F.; Ang, W.H.; Ménard-Moyon, C.; Nayak, T.R.; Gibson, D.; Ramaprabhu, S.; Panczyk, T.; Bianco, A.; et al. In vivo biodistribution of platinum-based drugs encapsulated into multi-walled carbon nanotubes. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 1465–1475. [Google Scholar] [CrossRef] [PubMed]
- Shim, G.; Kim, M.G.; Park, J.Y.; Oh, Y.K. Graphene-based nanosheets for delivery of chemotherapeutics and biological drugs. Adv. Drug Deliv. Rev. 2016, 105, 205–227. [Google Scholar] [CrossRef]
- Song, W.; Yang, T.; Wang, X.; Sun, Y.; Ai, Y.; Sheng, G.; Hayat, T.; Wang, X. Experimental and theoretical evidence for competitive interactions of tetracycline and sulfamethazine with reduced graphene oxides. Environ. Sci. Nano 2016, 3, 1318–1326. [Google Scholar] [CrossRef]
- Wang, F.; Ma, S.; Si, Y.; Dong, L.; Wang, X.; Yao, J.; Chen, H.; Yi, Z.; Yao, W.; Xing, B. Interaction mechanisms of antibiotic sulfamethoxazole with various graphene-based materials and multiwall carbon nanotubes and the effect of humic acid in water. Carbon 2017, 114, 671–678. [Google Scholar] [CrossRef]
- Zhao, L.; Dong, P.; Xie, J.; Li, J.; Wu, L.; Yang, S.-T.; Luo, J. Porous graphene oxide-chitosan aerogel for tetracycline removal. Mater. Res. Express 2013, 1, 15601. [Google Scholar] [CrossRef]
- Tang, Y.; Guo, H.; Xiao, L.; Yu, S.; Gao, N.; Wang, Y. Synthesis of reduced graphene oxide/magnetite composites and investigation of their adsorption performance of fluoroquinolone antibiotics. Colloids Surfaces A Physicochem. Eng. Asp. 2013, 424, 74–80. [Google Scholar] [CrossRef]
- Chen, H.; Gao, B.; Li, H. Removal of sulfamethoxazole and ciprofloxacin from aqueous solutions by graphene oxide. J. Hazard. Mater. 2015, 282, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Rostamian, R.; Behnejad, H. A unified platform for experimental and quantum mechanical study of antibiotic removal from water. J. Water Process Eng. 2017, 17, 207–215. [Google Scholar] [CrossRef]
- Li, M.F.; Liu, Y.G.; Zeng, G.M.; Liu, N.; Liu, S.B. Graphene and graphene-based nanocomposites used for antibiotics removal in water treatment: A review. Chemosphere 2019, 226, 360–380. [Google Scholar] [CrossRef]
- Ho, Y.S. Review of second-order models for adsorption systems. J. Hazard. Mater. 2006, 136, 681–689. [Google Scholar] [CrossRef]
- Wang, F.; Yang, B.; Wang, H.; Song, Q.; Tan, F.; Cao, Y. Removal of ciprofloxacin from aqueous solution by a magnetic chitosan grafted graphene oxide composite. J. Mol. Liq. 2016, 222, 188–194. [Google Scholar] [CrossRef]
- Rostamian, R.; Amiri, N.; Behnejad, H. How does graphene nanosheet affect the pharmaceutical adsorption? A comprehensive insight from molecular dynamics simulation, quantum mechanics and experimental study. J. Mol. Liq. 2018, 269, 29–37. [Google Scholar] [CrossRef]
- Farahani, B.V.; Behbahani, G.R.; Javadi, N. Functionalized multi walled carbon nanotubes as a carrier for doxorubicin: Drug adsorption study and statistical optimization of drug loading by factorial design methodology. J. Braz. Chem. Soc. 2016, 27, 694–705. [Google Scholar] [CrossRef]
- Sabna, V.; Thampi, S.G.; Chandrakaran, S. Adsorption of crystal violet onto functionalised multi-walled carbon nanotubes: Equilibrium and kinetic studies. Ecotoxicol. Environ. Saf. 2016, 134, 390–397. [Google Scholar] [CrossRef]
- Jorgensen, W.L. The Many Roles of Computation in Drug Discovery. Science 2004, 303, 1813–1818. [Google Scholar] [CrossRef]
- Sliwoski, G.; Kothiwale, S.; Meiler, J.; Lowe, E.W.J. Computational methods in drug discovery. Pharmacol. Rev. 2014, 66, 334–395. [Google Scholar] [CrossRef]
- Ruiz, V.G.; Liu, W.; Tkatchenko, A. Density-functional theory with screened van der Waals interactions applied to atomic and molecular adsorbates on close-packed and non-close-packed surfaces. Phys. Rev. B 2016, 93, 35118. [Google Scholar] [CrossRef]
- Tang, Q.; Zhou, Z.; Chen, Z. Innovation and discovery of graphene-like materials via density-functional theory computations. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2015, 5, 360–379. [Google Scholar] [CrossRef]
- Botu, V.; Ramprasad, R. Adaptive machine learning framework to accelerate ab initio molecular dynamics. Int. J. Quantum Chem. 2015, 115, 1074–1083. [Google Scholar] [CrossRef]
- Cramer, C.J. Essentials of Computational Chemistry: Theories and Models; Wiley: Chichester, UK, 2004; ISBN 0470091827. [Google Scholar]
- Frenkel, D.; Smit, B. Understanding Molecular Simulation: From Algorithms to Applications, 2nd ed.; Academic Press: Harcourt, FL, USA, 2002; ISBN 0122673514. [Google Scholar]
- Huang, J.; MacKerell Jr, A.D. CHARMM36 all-atom additive protein force field: Validation based on comparison to NMR data. J. Comput. Chem. 2013, 34, 2135–2145. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Tran, A.; Allsopp, M.; Lim, J.B.; Hénin, J.; Klauda, J.B. CHARMM36 United Atom Chain Model for Lipids and Surfactants. J. Phys. Chem. B 2014, 118, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Dickson, C.J.; Madej, B.D.; Skjevik, Å.A.; Betz, R.M.; Teigen, K.; Gould, I.R.; Walker, R.C. Lipid14: The Amber Lipid Force Field. J. Chem. Theory Comput. 2014, 10, 865–879. [Google Scholar] [CrossRef] [PubMed]
- Cornell, W.D.; Cieplak, P.; Bayly, C.I.; Gould, I.R.; Merz, K.M.; Ferguson, D.M.; Spellmeyer, D.C.; Fox, T.; Caldwell, J.W.; Kollman, P.A. A Second Generation Force Field for the Simulation of Proteins, Nucleic Acids, and Organic Molecules. J. Am. Chem. Soc. 1995, 117, 5179–5197. [Google Scholar] [CrossRef]
- Pechlaner, M.; Reif, M.M.; Oostenbrink, C. Reparametrisation of united-atom amine solvation in the GROMOS force field. Mol. Phys. 2017, 115, 1144–1154. [Google Scholar] [CrossRef]
- Müller-Dethlefs, K.; Hobza, P. Noncovalent Interactions: A Challenge for Experiment and Theory. Chem. Rev. 2000, 100, 143–167. [Google Scholar] [CrossRef]
- Pettersson, I.; Liljefors, T. Benzene-benzene (phenyl-phenyl) interactions in MM2/MMP2 molecular mechanics calculations. J. Comput. Chem. 1987, 8, 1139–1145. [Google Scholar] [CrossRef]
- Hobza, P.; Selzle, H.L.; Schlag, E.W. Floppy structure of the benzene dimer: Ab initio calculation on the structure and dipole moment. J. Chem. Phys. 1990, 93, 5893–5897. [Google Scholar] [CrossRef]
- Hentschke, R.; Winkler, R.G. Molecular dynamics simulation study of the adsorption of chain alkanes from solution onto graphite. J. Chem. Phys. 1993, 99, 5528–5534. [Google Scholar] [CrossRef]
- Hobza, P.; Selzle, H.L.; Schlag, E.W. Potential Energy Surface of the Benzene Dimer: Ab Initio Theoretical Study. J. Am. Chem. Soc. 1994, 116, 3500–3506. [Google Scholar] [CrossRef]
- Tsuzuki, S.; Uchimaru, T.; Mikami, M.; Tanabe, K. Basis set effects on the calculated bonding energies of neutral benzene dimers: Importance of diffuse polarization functions. Chem. Phys. Lett. 1996, 252, 206–210. [Google Scholar] [CrossRef]
- Tsuzuki, S.; Honda, K.; Uchimaru, T.; Mikami, M. Ab initio calculations of structures and interaction energies of toluene dimers including CCSD(T) level electron correlation correction. J. Chem. Phys. 2005, 122, 144323. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. Multicoefficient extrapolated density functional theory studies of π⋯π interactions: The benzene dimer. J. Phys. Chem. A 2005, 109, 4209–4212. [Google Scholar] [CrossRef]
- Lee, E.C.; Kim, D.; Jurečka, P.; Tarakeshwar, P.; Hobza, P.; Kim, K.S. Understanding of assembly phenomena by aromatic-aromatic interactions: Benzene Dimer and the substituted systems. J. Phys. Chem. A 2007, 111, 3446–3457. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the Damping Function in Dispersion Corrected Density Functional Theory. J. Comput. Chem. 2012, 32, 174–182. [Google Scholar] [CrossRef]
- Schnell, M.; Erlekam, U.; Bunker, P.R.; Vonhelden, G.; Grabow, J.U.; Meijer, G.; Vanderavoird, A. Structure of the benzene dimer - Governed by dynamics. Angew. Chem. Int. Ed. 2013, 52, 5180–5183. [Google Scholar] [CrossRef]
- Miliordos, E.; Aprà, E.; Xantheas, S.S. Benchmark theoretical study of the π-π binding energy in the benzene dimer. J. Phys. Chem. A 2014, 118, 7568–7578. [Google Scholar] [CrossRef]
- Zhan, Y.Z.; Zhao, X.; Wang, W. Theoretical study of the interaction energy of benzodifuranone dye molecule rings. Color. Technol. 2017, 133, 50–56. [Google Scholar] [CrossRef]
- Sherrill, C.D.; Takatani, T.; Hohenstein, E.G. An assessment of theoretical methods for nonbonded interactions: Comparison to complete basis set limit coupled-cluster potential energy curves for the benzene dimer, the methane dimer, benzene-methane, and benzene-H2S. J. Phys. Chem. A 2009, 113, 10146–10159. [Google Scholar] [CrossRef] [PubMed]
- Ruuska, H.; Pakkanen, T.A. Ab initio study of interlayer interaction of graphite: Benzene-coronene and coronene dimer two-layer models. J. Phys. Chem. B 2001, 105, 9541–9547. [Google Scholar] [CrossRef]
- Chakarova-Käck, S.D.; Schröder, E.; Lundqvist, B.I.; Langreth, D.C. Application of van der Waals density functional to an extended system: Adsorption of benzene and naphthalene on graphite. Phys. Rev. Lett. 2006, 96, 146107. [Google Scholar] [CrossRef]
- Zeinalipour-Yazdi, C.D.; Pullman, D.P. Correlation of polarizabilities with Van der Waals interactions in π-systems. J. Phys. Chem. B 2006, 110, 24260–24265. [Google Scholar] [CrossRef]
- Hsun Su, Y.; Kai Wu, Y.; Tu, S.L.; Chang, S.J. Electrostatic studies of π-π Interaction for benzene stacking on a graphene layer. Appl. Phys. Lett. 2011, 99, 163102. [Google Scholar] [CrossRef]
- De Moraes, E.E.; Tonel, M.Z.; Fagan, S.B.; Barbosa, M.C. Density functional theory study of π-aromatic interaction of benzene, phenol, catechol, dopamine isolated dimers and adsorbed on graphene surface. J. Mol. Model. 2019, 25, 302. [Google Scholar] [CrossRef]
- Ershova, O.V.; Lillestolen, T.C.; Bichoutskaia, E. Study of polycyclic aromatic hydrocarbons adsorbed on graphene using density functional theory with empirical dispersion correction. Phys. Chem. Chem. Phys. 2010, 12, 6309–6329. [Google Scholar] [CrossRef]
- Falconer, J.L.; Madix, R.J. Desorption rate isotherms in flash desorption analysis. J. Catal. 1977, 48, 262–268. [Google Scholar] [CrossRef]
- Chai, J.-D.; Head-Gordon, M. Systematic optimization of long-range corrected hybrid density functionals. J. Chem. Phys. 2008, 128, 84106. [Google Scholar] [CrossRef]
- Chai, J.-D.; Head-Gordon, M. Long-range corrected hybrid density functionals with damped atom–atom dispersion corrections. Phys. Chem. Chem. Phys. 2008, 10, 6615–6620. [Google Scholar] [CrossRef] [PubMed]
- Boateng, L.K.; Heo, J.; Flora, J.R.V.; Park, Y.G.; Yoon, Y. Molecular level simulation of the adsorption of bisphenol A and 17a-ethinyl estradiol onto carbon nanomaterials. Sep. Purif. Technol. 2013, 116, 471–478. [Google Scholar] [CrossRef]
- Ding, N.; Chen, X.; Wu, C.M.L. Interactions between polybrominated diphenyl ethers and graphene surface: A DFT and MD investigation. Environ. Sci. Nano 2014, 1, 55–63. [Google Scholar] [CrossRef]
- Conti, S.; Cecchini, M. Accurate and efficient calculation of the desorption energy of small molecules from graphene. J. Phys. Chem. C 2015, 119, 1867–1879. [Google Scholar] [CrossRef]
- Comer, J.; Chen, R.; Poblete, H.; Vergara-jaque, A.; Riviere, J.E. Predicting Adsorption Affinities of Small Molecules on Carbon Nanotubes Using Molecular Dynamics Simulation. ACS Nano 2015, 9, 11761–11774. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Wang, X.; Ai, Y.; Tan, X.; Hayat, T.; Hu, W.; Wang, X. Experimental and theoretical studies on competitive adsorption of aromatic compounds on reduced graphene oxides. J. Mater. Chem. A 2016, 4, 5654–5662. [Google Scholar] [CrossRef]
- Tang, H.; Zhao, Y.; Yang, X.; Liu, D.; Shan, S.; Cui, F.; Xing, B. Understanding the pH-dependent adsorption of ionizable compounds on graphene oxide using molecular dynamics simulations. Environ. Sci. Nano 2017, 4, 1935–1943. [Google Scholar] [CrossRef]
- Calbo, J.; López-Moreno, A.; de Juan, A.; Comer, J.; Ortí, E.; Pérez, E.M. Understanding Noncovalent Interactions of Small Molecules with Carbon Nanotubes. Chem. Eur. J. 2017, 23, 12909–12916. [Google Scholar] [CrossRef]
- Ulbricht, H.; Zacharia, R.; Cindir, N.; Hertel, T. Thermal desorption of gases and solvents from graphite and carbon nanotube surfaces. Carbon 2006, 44, 2931–2942. [Google Scholar] [CrossRef]
- Tait, S.L.; Dohnálek, Z.; Campbell, C.T.; Kay, B.D. n-alkanes on Pt(111) and on C (0001) Pt (111): Chain length dependence of kinetic desorption parameters. J. Chem. Phys. 2006, 125, 234308. [Google Scholar] [CrossRef]
- Lazar, P.; Karlický, F.; Jurecka, P.; Kocman, M.; Otyepková, E.; Šafářová, K.; Otyepka, M. Adsorption of small organic molecules on graphene. J. Am. Chem. Soc. 2013, 135, 6372–6377. [Google Scholar] [CrossRef] [PubMed]
- Scuseria, G.E. The open-shell restricted Hartree—Fock singles and doubles coupled-cluster method including triple excitations CCSD (T): application to C+3. Chem. Phys. Lett. 1991, 176, 27–35. [Google Scholar] [CrossRef]
- Riley, K.E.; Pitoňák, M.; Jurečka, P.; Hobza, P. Stabilization and Structure Calculations for Noncovalent Interactions in Extended Molecular Systems Based on Wave Function and Density Functional Theories. Chem. Rev. 2010, 110, 5023–5063. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Truhlar, D.G. Density Functionals with Broad Applicability in Chemistry. Acc. Chem. Res. 2008, 41, 157–167. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other function. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef]
- Klimeš, J.; Bowler, D.R.; Michaelides, A. Chemical accuracy for the van der Waals density functional. J. Phys. Condens. Matter 2009, 22, 22201. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef]
- Perdew, J.; Chevary, J.; Vosko, S.; Jackson, K.; Pederson, M.; Singh, D.; Fiolhais, C. Atoms, molecules, solids, and surfaces: Applications of the generalized gradient approximation for exchange and correlation. Phys. Rev. B 1992, 46, 6671–6687. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Perdew, J.P. Generalized gradient approximation for the exchange-correlation hole of a many-electron system. Phys. Rev. B 2014, 54, 16533–16539. [Google Scholar] [CrossRef]
- Credendino, R.; Minenkov, Y.; Liguori, D.; Piemontesi, F.; Melchior, A.; Morini, G.; Tolazzi, M.; Cavallo, L. Accurate experimental and theoretical enthalpies of association of TiCl4 with typical Lewis bases used in heterogeneous Ziegler-Natta catalysis. Phys. Chem. Chem. Phys. 2017, 19, 26996–27006. [Google Scholar] [CrossRef] [PubMed]
- Bruch, L.W. Theory of physisorption interactions. Surf. Sci. 1983, 125, 194–217. [Google Scholar] [CrossRef]
- Whitten, J.L.; Yang, H. Theory of chemisorption on metal surfaces. Surf. Sci. Rep. 1966, 218, 55–124. [Google Scholar]
- Srinivasan, J.; Cheatham, T.E.; Cieplak, P.; Kollman, P.A.; Case, D.A. Continuum Solvent Studies of the Stability of DNA, RNA, and Phosphoramidate–DNA Helices. J. Am. Chem. Soc. 1998, 120, 9401–9409. [Google Scholar] [CrossRef]
- Carvalho, I.T.; Santos, L. Antibiotics in the aquatic environments: A review of the European scenario. Environ. Int. 2016, 94, 736–757. [Google Scholar] [CrossRef]
- Rusu, A.; Hancu, G.; Uivaroşi, V. Fluoroquinolone pollution of food, water and soil, and bacterial resistance. Env. Chem Lett 2015, 13, 21–36. [Google Scholar] [CrossRef]
- Kümmerer, K. Chemosphere Antibiotics in the aquatic environment – A review – Part I. Chemosphere 2009, 75, 417–434. [Google Scholar] [CrossRef]
- Felis, E.; Kalka, J.; Sochacki, A.; Kowalska, K.; Bajkacz, S.; Harnisz, M.; Korzeniewska, E. Antimicrobial pharmaceuticals in the aquatic environment - occurrence and environmental implications. Eur. J. Pharmacol. 2020, 866, 172813. [Google Scholar] [CrossRef]
- Gil-Gil, T.; Laborda, P.; Sanz-García, F.; Hernando-Amado, S.; Blanco, P.; Martínez, J.L. Antimicrobial resistance: A multifaceted problem with multipronged solutions. Microbiologyopen 2019, 8, e945. [Google Scholar] [CrossRef]
- White, A.; Hughes, J.M. Critical Importance of a One Health Approach to Antimicrobial Resistance. Ecohealth 2019, 16, 404–409. [Google Scholar] [CrossRef]
- Miao, H.; Teng, Z.; Wang, C.; Chong, H.; Wang, G. Recent Progress in Two-Dimensional Antimicrobial Nanomaterials. Chem. Eur. J. 2019, 25, 929–944. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Leong, K.Y.; Ng, H.Y. Anaerobic treatment of pharmaceutical wastewater: A critical review. Bioresour. Technol. 2017, 245, 1238–1244. [Google Scholar] [CrossRef] [PubMed]
- Pazda, M.; Kumirska, J.; Stepnowski, P.; Mulkiewicz, E. Antibiotic resistance genes identified in wastewater treatment plant systems – A review. Sci. Total Environ. 2019, 697, 134023. [Google Scholar] [CrossRef]
- Rodríguez-Molina, D.; Mang, P.; Schmitt, H.; Chifiriuc, M.C.; Radon, K.; Wengenroth, L. Do wastewater treatment plants increase antibiotic resistant bacteria or genes in the environment? Protocol for a systematic review. Syst. Rev. 2019, 8, 304. [Google Scholar] [CrossRef]
- Li, H.; Zhang, D.; Han, X.; Xing, B. Adsorption of antibiotic ciprofloxacin on carbon nanotubes: pH dependence and thermodynamics. Chemosphere 2014, 95, 150–155. [Google Scholar] [CrossRef]
- Peng, H.; Pan, B.; Wu, M.; Liu, Y.; Zhang, D.; Xing, B. Adsorption of ofloxacin and norfloxacin on carbon nanotubes: Hydrophobicity- and structure-controlled process. J. Hazard. Mater. 2012, 233–234, 89–96. [Google Scholar] [CrossRef]
- Ncibi, M.C.; Sillanpää, M. Optimized removal of antibiotic drugs from aqueous solutions using single, double and multi-walled carbon nanotubes. J. Hazard. Mater. 2015, 298, 102–110. [Google Scholar] [CrossRef]
- Peixoto, P.S.; Tóth, I.V.; Segundo, M.A.; Lima, J.L.F.C. Fluoroquinolones and sulfonamides: features of their determination in water. A review. Int. J. Environ. Anal. Chem. 2016, 96, 185–202. [Google Scholar] [CrossRef]
- Ghadim, E.E.; Manouchehri, F.; Soleimani, G.; Hosseini, H.; Kimiagar, S.; Nafisi, S. Adsorption properties of tetracycline onto graphene oxide: Equilibrium, kinetic and thermodynamic studies. PLoS ONE 2013, 8, e79254. [Google Scholar] [CrossRef]
- Chang, P.; Jiang, W.; Li, Z.; Jean, J.; Kuo, C. Pharmaceutical Analysis Antibiotic tetracycline in the environments — A review. Res. Rev. J. Pharm. Anal. 2015, 4, 15–40. [Google Scholar]
- Wei, J.; Sun, W.; Pan, W.; Yu, X.; Sun, G.; Jiang, H. Comparing the effects of different oxygen-containing functional groups on sulfonamides adsorption by carbon nanotubes: Experiments and theoretical calculation. Chem. Eng. J. 2017, 312, 167–179. [Google Scholar] [CrossRef]
- Veclani, D.; Melchior, A. Adsorption of ciprofloxacin on carbon nanotubes: Insights from molecular dynamics simulations. J. Mol. Liq. 2020, 298, 111977. [Google Scholar] [CrossRef]
- He, L.; Liu, F.; Zhao, M.; Qi, Z.; Sun, X.; Afzal, M.Z.; Sun, X.; Li, Y.; Hao, J.; Wang, S. Electronic-property dependent interactions between tetracycline and graphene nanomaterials in aqueous solution. J. Environ. Sci. (China) 2018, 66, 286–294. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, J.; Ren, X.; Tan, X.; Hayat, T.; Alsaedi, A.; Cheng, C.; Chen, C. Impact of graphene oxide on the antibacterial activity of antibiotics against bacteria. Environ. Sci. Nano 2017, 4, 1016–1024. [Google Scholar] [CrossRef]
- Chaban, V.V.; Savchenko, T.I.; Kovalenko, S.M.; Prezhdo, O. V Heat-Driven Release of a Drug Molecule from Carbon Nanotubes: A Molecular Dynamics Study. J. Phys. Chem. B 2010, 114, 13481–13486. [Google Scholar] [CrossRef] [PubMed]
- Ai, Y.; Liu, Y.; Huo, Y.; Zhao, C.; Sun, L.; Han, B.; Cao, X.; Wang, X. Insights into the adsorption mechanism and dynamic behavior of tetracycline antibiotics on reduced graphene oxide (RGO) and graphene oxide (GO) materials. Environ. Sci. Nano 2019, 6, 3336–3348. [Google Scholar] [CrossRef]
- Redgrave, L.S.; Sutton, S.B.; Webber, M.A.; Piddock, L.J. V Fluoroquinolone resistance: mechanisms, impact on bacteria, and role in evolutionary success. Trends Microbiol. 2014, 22, 438–445. [Google Scholar] [CrossRef]
- Janencko, N.; Pokludova, L.; Blahova, J.; Svobodova, Z.; Literak, I. Implications of fluoroquinolone contamination for the aquatic environment—A review. Environ. Toxicol. Chem. 2016, 35, 2647–2656. [Google Scholar] [CrossRef]
- Wammer, K.H.; Korte, A.R.; Lundeen, R.A.; Sundberg, J.E.; Mcneill, K.; Arnold, W.A. Direct photochemistry of three fluoroquinolone antibacterials: Norfloxacin, ofloxacin, and enrofloxacin. Water Res. 2012, 47, 439–448. [Google Scholar] [CrossRef]
- Collignon, P.; Powers, J.H.; Chiller, T.M.; Aidara-Kane, A.; Aarestrup, F.M. World Health Organization Ranking of Antimicrobials According to Their Importance in Human Medicine: A Critical Step for Developing Risk Management Strategies for the Use of Antimicrobials in Food Production Animals. Clin. Infect. Dis. 2009, 49, 132–141. [Google Scholar] [CrossRef]
- Nurchi, V.M.; Crisponi, G.; Lachowicz, J.I.; Zoroddu, M.A.; Peana, M.; Medici, S.; Veclani, D.; Tolazzi, M.; Melchior, A. Fluoroquinolones: A micro-species equilibrium in the protonation of amphoteric compounds. Eur. J. Pharm. Sci. 2016, 93, 380–391. [Google Scholar] [CrossRef] [PubMed]
- Patiño, Y.; Díaz, E.; Ordóñez, S.; Gallegos-suarez, E.; Guerrero-ruiz, A. Adsorption of emerging pollutants on functionalized multiwall carbon nanotubes. Chemosphere 2015, 136, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Shen, J.; Zhuo, N.; Tian, Z.; Xu, P.; Yang, Z.; Yang, W. Interactions between Antibiotics and Graphene-Based Materials in Water: A Comparative Experimental and Theoretical Investigation. ACS Appl. Mater. Interfaces 2016, 8, 24273–24280. [Google Scholar] [CrossRef]
- Dong, S.; Sun, Y.; Wu, J.; Wu, B.; Creamer, A.E.; Gao, B. Graphene oxide as filter media to remove levofloxacin and lead from aqueous solution. Chemosphere 2016, 150, 759–764. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Goel, N.; Kumar, V.; Tikoo, K.; Singhal, S. Removal of fluoroquinolone from aqueous solution using graphene oxide: experimental and computational elucidation. Environ. Sci. Pollut. Res. 2018, 25, 2942–2957. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Tsang, D.C.W.; Chen, F.; Li, S.; Yang, X. Ciprofloxacin adsorption on graphene and granular activated carbon: Kinetics, isotherms, and effects of solution chemistry. Environ. Technol. (United Kingdom) 2015, 36, 3094–3102. [Google Scholar] [CrossRef]
- Fei, Y.; Li, Y.; Han, S.; Ma, J. Adsorptive removal of ciprofloxacin by sodium alginate/graphene oxide composite beads from aqueous solution. J. Colloid Interface Sci. 2016, 484, 196–204. [Google Scholar] [CrossRef]
- Wu, S.; Zhao, X.; Li, Y.; Zhao, C.; Du, Q.; Sun, J.; Wang, Y.; Peng, X.; Xia, Y.; Wang, Z.; et al. Adsorption of ciprofloxacin onto biocomposite fibers of graphene oxide/calcium alginate. Chem. Eng. J. 2013, 230, 389–395. [Google Scholar] [CrossRef]
- Ma, J.; Yang, M.; Yu, F.; Zheng, J. Water-enhanced Removal of Ciprofloxacin from Water by Porous Graphene Hydrogel. Sci. Rep. 2015, 5, 13578. [Google Scholar] [CrossRef]
- Yu, F.; Ma, J.; Bi, D. Enhanced adsorptive removal of selected pharmaceutical antibiotics from aqueous solution by activated graphene. Environ. Sci. Pollut. Res. 2015, 22, 4715–4724. [Google Scholar] [CrossRef]
- Carabineiro, S.A.C.; Thavorn-Amornsri, T.; Pereira, M.F.R.; Serp, P.; Figueiredo, J.L. Comparison between activated carbon, carbon xerogel and carbon nanotubes for the adsorption of the antibiotic ciprofloxacin. Catal. Today 2012, 186, 29–34. [Google Scholar] [CrossRef]
- Yu, F.; Sun, S.; Han, S.; Zheng, J.; Ma, J. Adsorption removal of ciprofloxacin by multi-walled carbon nanotubes with different oxygen contents from aqueous solutions. Chem. Eng. J. 2016, 285, 588–595. [Google Scholar] [CrossRef]
- Meher, J.G.; Kesharwani, P.; Chaurasia, M.; Singh, A.; Chourasia, M.K. Chapter 14 - Carbon Nanotubes (CNTs): A Novel Drug Delivery Tool in Brain Tumor Treatment. In Nanotechnology-Based Targeted Drug Delivery Systems for Brain Tumors; Kesharwani, P., Gupta, U., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 375–396. ISBN 978-0-12-812218-1. [Google Scholar]
- Grossman, T.H. Tetracycline antibiotics and resistance. Cold Spring Harb. Perspect. Med. 2016, 6, a025387. [Google Scholar] [CrossRef] [PubMed]
- Chukwudi, C.U. rRNA Binding Sites and the Molecular Mechanism of Action of the Tetracyclines. Antimicrob Agents Chemother. 2016, 60, 4433–4441. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Liu, M.; Li, J.; Yang, S.; Sun, Y.; Sun, Q.; Wang, W.; Lu, L.; Zhang, K.; Xu, J.; et al. A review on pollution situation and treatment methods of tetracycline in groundwater. Sep. Sci. Technol. 2020, 55, 1005–1021. [Google Scholar] [CrossRef]
- Gao, Y.; Li, Y.; Zhang, L.; Huang, H.; Hu, J.; Shah, S.M.; Su, X. Adsorption and removal of tetracycline antibiotics from aqueous solution by graphene oxide. J. Colloid Interface Sci. 2012, 368, 540–546. [Google Scholar] [CrossRef]
- Yuan, X.; Wu, Z.; Zhong, H.; Wang, H.; Chen, X.; Leng, L.; Jiang, L.; Xiao, Z.; Zeng, G. Fast removal of tetracycline from wastewater by reduced graphene oxide prepared via microwave-assisted ethylenediamine–N,N’–disuccinic acid induction method. Environ. Sci. Pollut. Res. 2016, 23, 18657–18671. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, B.; Zhang, L.; Huang, J.; Chen, F.; Yang, Z.; Yao, J.; Zhang, Z. Controlled assembly of Fe3O4 magnetic nanoparticles on graphene oxide. Nanoscale 2011, 3, 1446–1450. [Google Scholar] [CrossRef]
- Yu, F.; Ma, J.; Han, S. Adsorption of tetracycline from aqueous solutions onto multi-walled carbon nanotubes with different oxygen contents. Sci. Rep. 2015, 4, 5326. [Google Scholar] [CrossRef]
- Fukui, K.; Yonezawa, T.; Nagata, C.; Shingu, H. Molecular Orbital Theory of Orientation in Aromatic, Heteroaromatic, and Other Conjugated Molecules. J. Chem. Phys. 1954, 22, 1433–1442. [Google Scholar] [CrossRef]
- Fukui, K. Role of Frontier Orbitals in Chemical Reactions. Science 1982, 218, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Chen, W.; Zheng, S.; Xu, Z.; Zhu, D. Adsorption of Sulfonamide Antibiotics to Multiwalled Carbon Nanotubes. Langmuir 2009, 25, 11608–11613. [Google Scholar] [CrossRef] [PubMed]
- Le-Minh, N.; Khan, S.J.; Drewes, J.E.; Stuetz, R.M. Fate of antibiotics during municipal water recycling treatment processes. Water Res. 2010, 44, 4295–4323. [Google Scholar] [CrossRef]
- Arsawang, U.; Saengsawang, O.; Rungrotmongkol, T.; Sornmee, P.; Wittayanarakul, K.; Remsungnen, T.; Hannongbua, S. How do carbon nanotubes serve as carriers for gemcitabine transport in a drug delivery system? J. Mol. Graph. Model. 2011, 29, 591–596. [Google Scholar] [CrossRef]
- Lim, D.J.; Sim, M.; Oh, L.; Lim, K.; Park, H. Carbon-based drug delivery carriers for cancer therapy. Arch. Pharm. Res. 2014, 37, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.R.; Qiao, P. Drug Delivery in Cancer Therapy, Quo Vadis? Mol. Pharm. 2018, 15, 3603–3616. [Google Scholar] [CrossRef] [PubMed]
- Danhier, F.; Feron, O.; Préat, V. To exploit the tumor microenvironment: Passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J. Control. Release 2010, 148, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chang, V.W.C.; Giannis, A.; Wang, J.Y. Removal of cytostatic drugs from aquatic environment: A review. Sci. Total Environ. 2013, 445–446, 281–298. [Google Scholar] [CrossRef]
- Amable, L. Cisplatin resistance and opportunities for precision medicine. Pharmacol. Res. 2016, 106, 27–36. [Google Scholar] [CrossRef]
- Jung, Y.; Lippard, S.J. Direct cellular responses to platinum-induced DNA damage. Chem Rev 2007, 107, 1387–1407. [Google Scholar] [CrossRef]
- Browning, R.J.; Reardon, P.J.T.; Parhizkar, M.; Pedley, R.B.; Edirisinghe, M.; Knowles, J.C.; Stride, E. Drug Delivery Strategies for Platinum-Based Chemotherapy. ACS Nano 2017, 11, 8560–8578. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, T.C.; Suntharalingam, K.; Lippard, S.J. The Next Generation of Platinum Drugs: Targeted Pt(II) Agents, Nanoparticle Delivery, and Pt(IV) Prodrugs. Chem. Rev. 2016, 116, 3436–3486. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.; Xiao, H.; Li, C.; Dai, Y.; Cheng, Z.; Hou, Z.; Lin, J. Inorganic nanocarriers for platinum drug delivery. Mater. Today 2015, 18, 554–564. [Google Scholar] [CrossRef]
- Veclani, D.; Melchior, A.; Tolazzi, M.; Cerón-Carrasco, J.P. Using Theory to Reinterpret the Kinetics of Monofunctional Platinum Anticancer Drugs: Stacking Matters. J. Am. Chem. Soc. 2018, 140, 14024–14027. [Google Scholar] [CrossRef]
- Lucas, M.F.A.; Pavelka, M.; Alberto, M.E.; Russo, N. Neutral and Acidic Hydrolysis Reactions of the Third Generation Anticancer Drug Oxaliplatin. J. Phys. Chem. B 2009, 113, 831–838. [Google Scholar] [CrossRef]
- Pavelka, M.; Lucas, M.F.A.; Russo, N. On the hydrolysis mechanism of the second-generation anticancer drug carboplatin. Chem. Eur. J. 2007, 13, 10108–10116. [Google Scholar] [CrossRef]
- Melchior, A.; Martínez, J.M.; Pappalardo, R.R.; Sánchez Marcos, E. Hydration of Cisplatin Studied by an Effective Ab Initio Pair Potential Including Solute–Solvent Polarization. J. Chem. Theor. Comp. 2013, 9, 4562–4573. [Google Scholar] [CrossRef]
- Melchior, A.; Sánchez Marcos, E.; Pappalardo, R.R.; Martínez, J.M. Comparative study of the hydrolysis of a third- and a first-generation platinum anticancer complexes. Theor. Chem. Acc. 2011, 128, 627–638. [Google Scholar] [CrossRef]
- Melchior, A.; Tolazzi, M.; Martínez, J.M.; Pappalardo, R.R.; Sánchez Marcos, E. Hydration of two cisplatin aqua-derivatives studied by quantum mechanics and molecular dynamics simulations. J. Chem. Theory Comput. 2015, 11, 1735–1744. [Google Scholar] [CrossRef] [PubMed]
- Dell’Anna, M.M.; Censi, V.; Carrozzini, B.; Caliandro, R.; Denora, N.; Franco, M.; Veclani, D.; Melchior, A.; Tolazzi, M.; Mastrorilli, P. Triphenylphosphane Pt(II) complexes containing biologically active natural polyphenols: Synthesis, crystal structure, molecular modeling and cytotoxic studies. J. Inorg. Biochem. 2016, 163. [Google Scholar] [CrossRef]
- Kazemi-Beydokhti, A.; Zeinali Heris, S.; Jaafari, M.R. Investigation of different methods for cisplatin loading using single-walled carbon nanotube. Chem. Eng. Res. Des. 2016, 112, 56–63. [Google Scholar] [CrossRef]
- Ajima, K.; Murakami, T.; Mizoguchi, Y.; Tsuchida, K.; Ichihashi, T.; Iijima, S.; Yudasaka, M. Enhancement of in vivo anticancer effects of cisplatin by incorporation inside single-wall carbon nanohorns. ACS Nano 2008, 2, 2057–2064. [Google Scholar] [CrossRef] [PubMed]
- Ajima, K.; Yudasaka, M.; Maigné, A.; Miyawaki, J.; Iijima, S. Effect of functional groups at hole edges on cisplatin release from inside single-wall carbon nanohorns. J. Phys. Chem. B 2006, 110, 5773–5778. [Google Scholar] [CrossRef] [PubMed]
- Hampel, S.; Kunze, D.; Haase, D.; Krämer, K.; Rauschenbach, M.; Ritschel, M.; Leonhardt, A.; Thomas, J.; Oswald, S.; Hoffmann, V.; et al. Carbon nanotubes filled with a chemotherapeutic agent: A nanocarrier mediates inhibition of tumor cell growth. Nanomedicine 2008, 3, 175–182. [Google Scholar] [CrossRef]
- Smailii, P.; Pakroo, R.; Mohammadkhani, R.; Jafarian, V.; Kabiri Esfahani, F.; Hassani, L. Decorations of graphene oxide with cisplatin toward investigation of fluorescence quencher on regulatory sequence of BRCA1 and BRCA2. J. Iran. Chem. Soc. 2020, 17, 127–134. [Google Scholar] [CrossRef]
- Sumathra, M.; Sadasivuni, K.K.; Suresh Kumar, S.; Rajan, M. Cisplatin-Loaded Graphene Oxide/Chitosan/Hydroxyapatite Composite as a Promising Tool for Osteosarcoma-Affected Bone Regeneration. ACS Omega 2018, 3, 14620–14633. [Google Scholar] [CrossRef]
- Tian, L.; Pei, X.; Zeng, Y.; He, R.; Li, Z.; Wang, J.; Wan, Q.; Li, X. Functionalized nanoscale graphene oxide for high efficient drug delivery of cisplatin. J. Nanoparticle Res. 2014, 16, 2709. [Google Scholar] [CrossRef]
- Duma, A.; Prodana, M.; Demetrescu, I. Cisplatin functionalization of multiwall carbon nanotubes. UPB Sci. Bull. Ser. B Chem. Mater. Sci. 2014, 76, 49–58. [Google Scholar]
- Almeida, E.R.; De Souza, L.A.; De Almeida, W.B.; Dos Santos, H.F. Molecular dynamics of carbon nanohorns and their complexes with cisplatin in aqueous solution. J. Mol. Graph. Model. 2019, 89, 167–177. [Google Scholar] [CrossRef]
- Prylutskyy, Y.I.; Cherepanov, V.V.; Evstigneev, M.P.; Kyzyma, O.A.; Petrenko, V.I.; Styopkin, V.I.; Bulavin, L.A.; Davidenko, N.A.; Wyrzykowski, D.; Woziwodzka, A.; et al. Structural self-organization of C60 and cisplatin in physiological solution. Phys. Chem. Chem. Phys. 2015, 17, 26084–26092. [Google Scholar] [CrossRef]
- Tripisciano, C.; Kraemer, K.; Taylor, A.; Borowiak-Palen, E. Single-wall carbon nanotubes based anticancer drug delivery system. Chem. Phys. Lett. 2009, 478, 200–205. [Google Scholar] [CrossRef]
- De Souza, L.A.; Nogueira, C.A.S.; Lopes, J.F.; Dos Santos, H.F.; De Almeida, W.B. DFT study of cisplatin@carbon nanohorns complexes. J. Inorg. Biochem. 2013, 129, 71–83. [Google Scholar] [CrossRef] [PubMed]
- De Souza, L.A.; Nogueira, C.A.S.; Lopes, J.F.; Dos Santos, H.F.; De Almeida, W.B. Theoretical study of the formation of inclusion complex between cisplatin and single-wall carbon nanotube. J. Phys. Chem. C 2015, 119, 8394–8401. [Google Scholar] [CrossRef]
- De Souza, L.A.; Nogueira, C.A.S.; Ortega, P.F.R.; Lopes, J.F.; Calado, H.D.R.; Lavall, R.L.; Silva, G.G.; Dos Santos, H.F.; De Almeida, W.B. Inclusion complex between cisplatin and single-walled carbon nanotube: An integrated experimental and theoretical approach. Inorganica Chim. Acta 2016, 447, 38–44. [Google Scholar] [CrossRef]
- De Souza, L.A.; Dos Santos, H.F.; Costa, L.T.; De Almeida, W.B. Inclusion complexes between cisplatin and oxidized carbon nanostructures: A theoretical approach. J. Inorg. Biochem. 2018, 178, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Del Cuevas-Flores, M.R.; Garcia-Revilla, M.A.; Bartolomei, M. Noncovalent interactions between cisplatin and graphene prototypes. J. Comput. Chem. 2018, 39, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Panczyk, T.; Jagusiak, A.; Pastorin, G.; Ang, W.H.; Narkiewicz-Michalek, J. Molecular dynamics study of cisplatin release from carbon nanotubes capped by magnetic nanoparticles. J. Phys. Chem. C 2013, 117, 17327–17336. [Google Scholar] [CrossRef]
- Panczyk, T.; Da Ros, T.; Pastorin, G.; Jagusiak, A.; Narkiewicz-Michalek, J. Role of intermolecular interactions in assemblies of nanocontainers composed of carbon nanotubes and magnetic nanoparticles: A molecular dynamics study. J. Phys. Chem. C 2014, 118, 1353–1363. [Google Scholar] [CrossRef]
- Panczyk, T.; Wolski, P.; Konczak, L.; Narkiewicz-Michalek, J. Sidewall functionalization of carbon nanotubes as a method of controlling structural transformations of the magnetically triggered nanocontainer: A molecular dynamics study. J. Phys. Chem. C 2015, 119, 8373–8381. [Google Scholar] [CrossRef]
- Mejri, A.; Vardanega, D.; Tangour, B.; Gharbi, T.; Picaud, F. Encapsulation into carbon nanotubes and release of anticancer cisplatin drug molecule. J. Phys. Chem. B 2015, 119, 604–611. [Google Scholar] [CrossRef]
- Mehrjouei, E.; Akbarzadeh, H.; Shamkhali, A.N.; Abbaspour, M.; Salemi, S.; Abdi, P. Delivery of Cisplatin Anti-Cancer Drug from Carbon, Boron Nitride, and Silicon Carbide Nanotubes Forced by Ag-Nanowire: A Comprehensive Molecular Dynamics Study. Mol. Pharm. 2017, 14, 2273–2284. [Google Scholar] [CrossRef]
- Hosni, Z.; Bessrour, R.; Tangour, B. 195Pt chemical shift ability to control the antitumor drug cisplatin encapsulated into carbon nanotubes: A theoretical study. J. Comput. Theor. Nanosci. 2014, 11, 318–323. [Google Scholar] [CrossRef]
- Stephens, P.J.; Devlin, F.J.; Chabalowski, C.F.; Frisch, M.J. Ab Initio Calculation of Vibrational Absorption and Circular Dichroism Spectra Using Density Functional Force Fields. J. Phys. Chem. 1994, 98, 11623–11627. [Google Scholar] [CrossRef]
- Cagel, M.; Grotz, E.; Bernabeu, E.; Moretton, M.A.; Chiappetta, D.A. Doxorubicin: nanotechnological overviews from bench to bedside. Drug Discov. Today 2017, 22, 270–281. [Google Scholar] [CrossRef] [PubMed]
- Bouma, J.; Beijnen, J.H.; Bult, A.; Underberg, W.J.M. Anthracycline antitumour agents. Pharm. Weekbl. 1986, 8, 109–133. [Google Scholar] [CrossRef]
- Yang, F.; Teves, S.S.; Kemp, C.J.; Henikoff, S. Doxorubicin, DNA torsion, and chromatin dynamics. Biochim. Biophys. Acta Rev. Cancer 2014, 1845, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Shafei, A.; El-Bakly, W.; Sobhy, A.; Wagdy, O.; Reda, A.; Aboelenin, O.; Marzouk, A.; El Habak, K.; Mostafa, R.; Ali, M.A.; et al. A review on the efficacy and toxicity of different doxorubicin nanoparticles for targeted therapy in metastatic breast cancer. Biomed. Pharmacother. 2017, 95, 1209–1218. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Zhang, J.J.; Cheng, F.F.; Zheng, T.T.; Wang, C.; Zhu, J.J. Green and facile synthesis of highly biocompatible graphene nanosheets and its application for cellular imaging and drug delivery. J. Mater. Chem. 2011, 21, 12034–12040. [Google Scholar] [CrossRef]
- Sadaf, S.; Walder, L. Doxorubicin Adsorbed on Carbon Nanotubes: Helical Structure and New Release Trigger. Adv. Mater. Interfaces 2017, 4, 1700649. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, S.T.; Wang, Y.; Liu, Y.; Wang, H. Adsorption and desorption of doxorubicin on oxidized carbon nanotubes. Colloids Surf. B Biointerfaces 2012, 97, 62–69. [Google Scholar] [CrossRef]
- Wang, F.; Liu, B.; Ip, A.C.F.; Liu, J. Orthogonal adsorption onto nano-graphene oxide using different intermolecular forces for multiplexed delivery. Adv. Mater. 2013, 25, 4087–4092. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Zhao, X.; Li, Y.; Du, Q.; Sun, J.; Wang, Y.; Wang, X.; Xia, Y.; Wang, Z.; Xia, L. Adsorption properties of doxorubicin hydrochloride onto graphene oxide: Equilibrium, kinetic and thermodynamic studies. Materials 2013, 6, 2026–2042. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Zhao, X.; Cui, Z.; Zhao, C.; Wang, Y.; Du, L.; Li, Y. Cytotoxicity of graphene oxide and graphene oxide loaded with doxorubicin on human multiple myeloma cells. Int. J. Nanomed. 2014, 9, 1413–1421. [Google Scholar]
- Zhang, W.; Guo, Z.; Huang, D.; Liu, Z.; Guo, X.; Zhong, H. Synergistic effect of chemo-photothermal therapy using PEGylated graphene oxide. Biomaterials 2011, 32, 8555–8561. [Google Scholar] [CrossRef]
- Izadyar, A.; Farhadian, N.; Chenarani, N. Molecular dynamics simulation of doxorubicin adsorption on a bundle of functionalized CNT. J. Biomol. Struct. Dyn. 2016, 34, 1797–1805. [Google Scholar] [CrossRef]
- Mahdavi, M.; Rahmani, F.; Nouranian, S. Molecular simulation of pH-dependent diffusion, loading, and release of doxorubicin in graphene and graphene oxide drug delivery systems. J. Mater. Chem. B 2016, 4, 7441–7451. [Google Scholar] [CrossRef]
- Mirhosseini, M.M.; Rahmati, M.; Zargarian, S.S.; Khordad, R. Molecular dynamics simulation of functionalized graphene surface for high efficient loading of doxorubicin. J. Mol. Struct. 2017, 1141, 441–450. [Google Scholar] [CrossRef]
- Contreras, M.L.; Torres, C.; Villarroel, I.; Rozas, R. Molecular dynamics assessment of doxorubicin–carbon nanotubes molecular interactions for the design of drug delivery systems. Struct. Chem. 2019, 30, 369–384. [Google Scholar] [CrossRef]
- Kordzadeh, A.; Amjad-Iranagh, S.; Zarif, M.; Modarress, H. Adsorption and encapsulation of the drug doxorubicin on covalent functionalized carbon nanotubes: A scrutinized study by using molecular dynamics simulation and quantum mechanics calculation. J. Mol. Graph. Model. 2019, 88, 11–22. [Google Scholar] [CrossRef]
- Tonel, M.Z.; Martins, M.O.; Zanella, I.; Pontes, R.B.; Fagan, S.B. A first-principles study of the interaction of doxorubicin with graphene. Comput. Theor. Chem. 2017, 1115, 270–275. [Google Scholar] [CrossRef]
- Vovusha, H.; Banerjee, D.; Yadav, M.K.; Perrozzi, F.; Ottaviano, L.; Sanyal, S.; Sanyal, B. Binding Characteristics of Anticancer Drug Doxorubicin with Two-Dimensional Graphene and Graphene Oxide: Insights from Density Functional Theory Calculations and Fluorescence Spectroscopy. J. Phys. Chem. C 2018, 122, 21031–21038. [Google Scholar] [CrossRef]
- Rowinsky, E.K.; Cazenave, L.A.; Donehower, R.C. Taxol: A novel investigational antimicrotubule agent. J. Natl. Cancer Inst. 1990, 82, 1247–1259. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, B.; Yan, B. Enabling anticancer therapeutics by nanoparticle carriers: The delivery of paclitaxel. Int. J. Mol. Sci. 2011, 12, 4395–4413. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Robinson, J.T.; Tabakman, S.M.; Yang, K.; Dai, H. Carbon materials for drug delivery & cancer therapy. Mater. Today 2011, 14, 316–323. [Google Scholar]
- Berlin, J.M.; Pham, T.T.; Sano, D.; Mohamedali, K.A.; Marcano, D.C.; Myers, J.N.; Tour, J.M. Noncovalent functionalization of carbon nanovectors with an antibody enables targeted drug delivery. ACS Nano 2011, 5, 6643–6650. [Google Scholar] [CrossRef] [PubMed]
- Rezaian, M.; Maleki, R.; Dahri Dahroud, M.; Alamdari, A.; Alimohammadi, M. pH-Sensitive Co-Adsorption/Release of Doxorubicin and Paclitaxel by Carbon Nanotube, Fullerene, and Graphene Oxide in Combination with N-isopropylacrylamide: A Molecular Dynamics Study. Biomolecules 2018, 8, 127. [Google Scholar] [CrossRef] [PubMed]
- Hasanzade, Z.; Raissi, H. Investigation of graphene-based nanomaterial as nanocarrier for adsorption of paclitaxel anticancer drug: A molecular dynamics simulation study. J. Mol. Model. 2017, 23, 36. [Google Scholar] [CrossRef] [PubMed]
- Hashemzadeh, H.; Raissi, H. The functionalization of carbon nanotubes to enhance the efficacy of the anticancer drug paclitaxel: a molecular dynamics simulation study. J. Mol. Model. 2017, 23, 222. [Google Scholar] [CrossRef]
- Labrie, F. Mechanism of action and pure antiandrogenic properties of flutamide. Cancer 1993, 72, 3816–3827. [Google Scholar] [CrossRef]
- Sufrin, G.; Coffey, D.S. Flutamide. Mechanism of action of a new nonsteroidal antiandrogen. Invest. Urol. 1976, 13, 429–434. [Google Scholar]
- Kamel, M.; Raissi, H.; Morsali, A. Theoretical study of solvent and co-solvent effects on the interaction of Flutamide anticancer drug with Carbon nanotube as a drug delivery system. J. Mol. Liq. 2017, 248, 490–500. [Google Scholar] [CrossRef]
- Kamel, M.; Raissi, H.; Morsali, A.; Shahabi, M. Assessment of the adsorption mechanism of Flutamide anticancer drug on the functionalized single-walled carbon nanotube surface as a drug delivery vehicle: An alternative theoretical approach based on DFT and MD. Appl. Surf. Sci. 2018, 434, 492–503. [Google Scholar] [CrossRef]
- Tomasi, J.; Cammi, R.; Mennucci, B.; Cappelli, C.; Corni, S. Molecular properties in solution described with a continuum solvation model. Phys. Chem. Chem. Phys. 2002, 4, 5697–5712. [Google Scholar] [CrossRef]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum mechanical continuum solvation models. Chem. Rev. 2005, 105, 2999–3093. [Google Scholar] [CrossRef]
- Endrizzi, F.; Di Bernardo, P.; Zanonato, P.L.; Tisato, F.; Porchia, M.; Isse, A.A.; Melchior, A.; Tolazzi, M. Cu(i) and Ag(i) complex formation with the hydrophilic phosphine 1,3,5-triaza-7-phosphadamantane in different ionic media. How to estimate the effect of a complexing medium. Dalton Trans. 2017, 46, 1455–1466. [Google Scholar] [CrossRef]
- Dau, P.V.; Zhang, Z.; Gao, Y.; Parker, B.F.; Dau, P.D.; Gibson, J.K.; Arnold, J.; Tolazzi, M.; Melchior, A.; Rao, L. Thermodynamic, Structural, and Computational Investigation on the Complexation between UO22+ and Amine-Functionalized Diacetamide Ligands in Aqueous Solution. Inorg. Chem. 2018, 57, 2122–2131. [Google Scholar] [CrossRef]
- Gao, Y.; Dau, P.V.; Parker, B.F.; Arnold, J.; Melchior, A.; Zhang, Z.; Rao, L. Complexation of NpO2+ with Amine-Functionalized Diacetamide Ligands in Aqueous Solution: Thermodynamic, Structural, and Computational Studies. Inorg. Chem. 2018, 57, 6965–6972. [Google Scholar] [CrossRef]
- Leonzio, M.; Melchior, A.; Faura, G.; Tolazzi, M.; Zinna, F.; Di Bari, L.; Piccinelli, F. Strongly Circularly Polarized Emission from Water-Soluble Eu(III)- and Tb(III)-Based Complexes: A Structural and Spectroscopic Study. Inorg. Chem. 2017, 56, 4413–4422. [Google Scholar] [CrossRef]
- Ghadamgahi, M.; Ajloo, D. Molecular dynamic insight into the ethanol effect on Tretinoin drug delivery through carbon nanotubes. J. Nanostruct. Chem. 2014, 4, 91. [Google Scholar] [CrossRef]
- Saban, N.; Bujak, M. Hydroxyurea and hydroxamic acid derivatives as antitumor drugs. Cancer Chemother. Pharmacol. 2009, 64, 213–221. [Google Scholar] [CrossRef]
- Hesabi, M.; Behjatmanesh-Ardakani, R. Investigation of carboxylation of carbon nanotube in the adsorption of anti-cancer drug: A theoretical approach. Appl. Surf. Sci. 2018, 427, 112–125. [Google Scholar] [CrossRef]
- Sheikhi, M.; Shahab, S.; Alnajjar, R.; Ahmadianarog, M. Adsorption Properties of the New Anti-Cancer Drug Alectinib on CNT(6,6-6) Nanotube: Geometry Optimization, Molecular Structure, Spectroscopic (NMR, UV/Vis, Excited State), FMO, MEP and HOMO–LUMO Investigations. J. Clust. Sci. 2019, 30, 83–96. [Google Scholar] [CrossRef]
- Dehneshin, N.; Raissi, H.; Hasanzade, Z.; Farzad, F. Using molecular dynamics simulation to explore the binding of the three potent anticancer drugs sorafenib, streptozotocin, and sunitinib to functionalized carbon nanotubes. J. Mol. Model. 2019, 25, 159. [Google Scholar] [CrossRef] [PubMed]
- Iyer, R.; Fetterly, G.; Lugade, A.; Thanavala, Y. Sorafenib: A clinical and pharmacologic review. Expert Opin. Pharmacother. 2010, 11, 1943–1955. [Google Scholar] [CrossRef]
- Wilhelm, S.; Carter, C.; Lynch, M.; Lowinger, T.; Dumas, J.; Smith, R.A.; Schwartz, B.; Simantov, R.; Kelley, S. Discovery and development of sorafenib: A multikinase inhibitor for treating cancer. Nat. Rev. Drug Discov. 2006, 5, 835–844. [Google Scholar] [CrossRef]
- Bagcchi, S. Sunitinib still first-line therapy for metastatic renal cancer. Lancet. Oncol. 2014, 15, e420. [Google Scholar] [CrossRef]
- Nocera, M.; Baudin, E.; Pellegriti, G.; Cailleux, A.F.; Mechelany-Corone, C.; Schlumberger, M. Treatment of advanced medullary thyroid cancer with an alternating combination of doxorubicin-streptozocin and 5 FU-dacarbazine. Br. J. Cancer 2000, 83, 715–718. [Google Scholar] [CrossRef][Green Version]
- Szkudelski, T. The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol. Res. 2001, 50, 537–546. [Google Scholar]

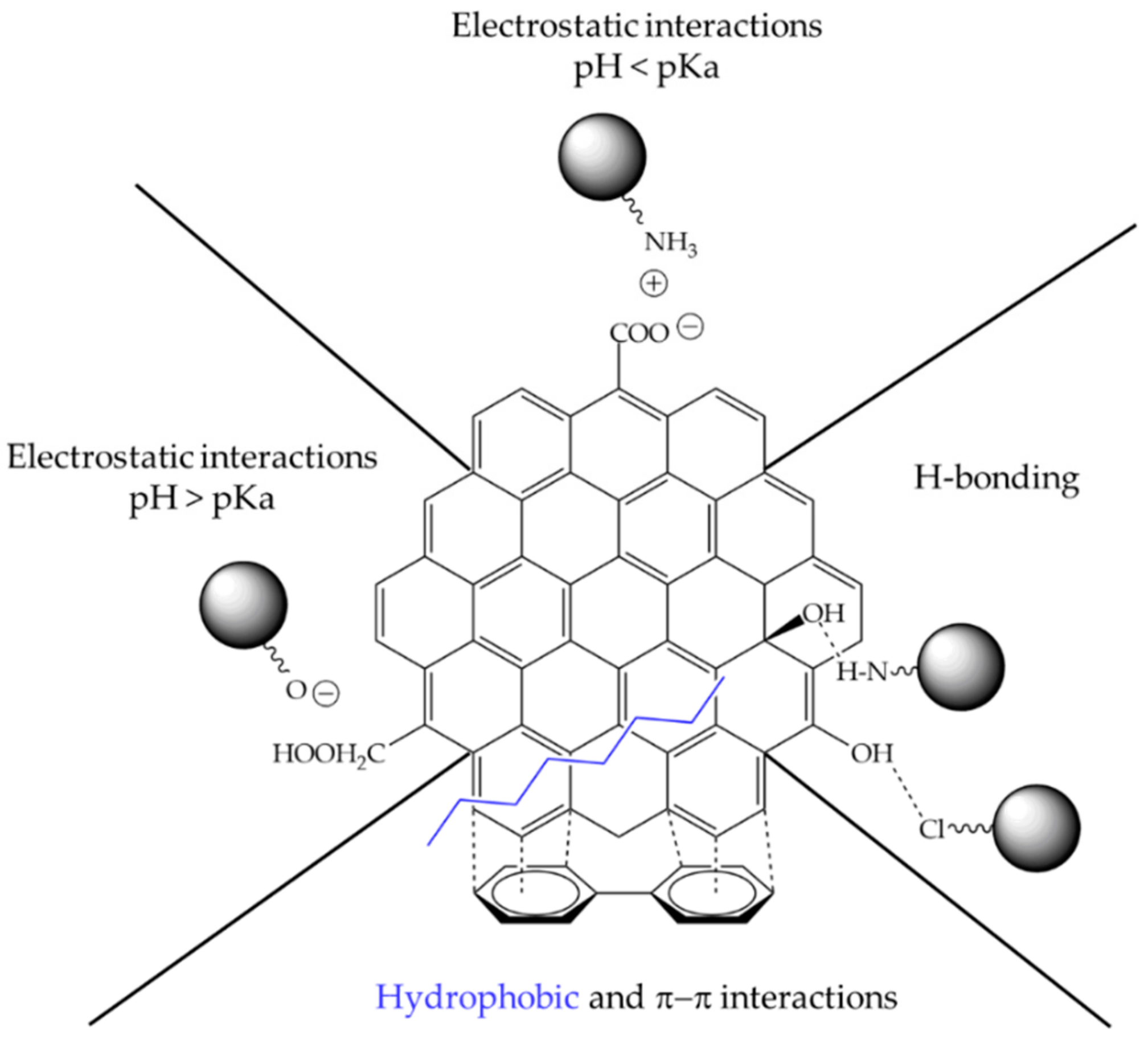

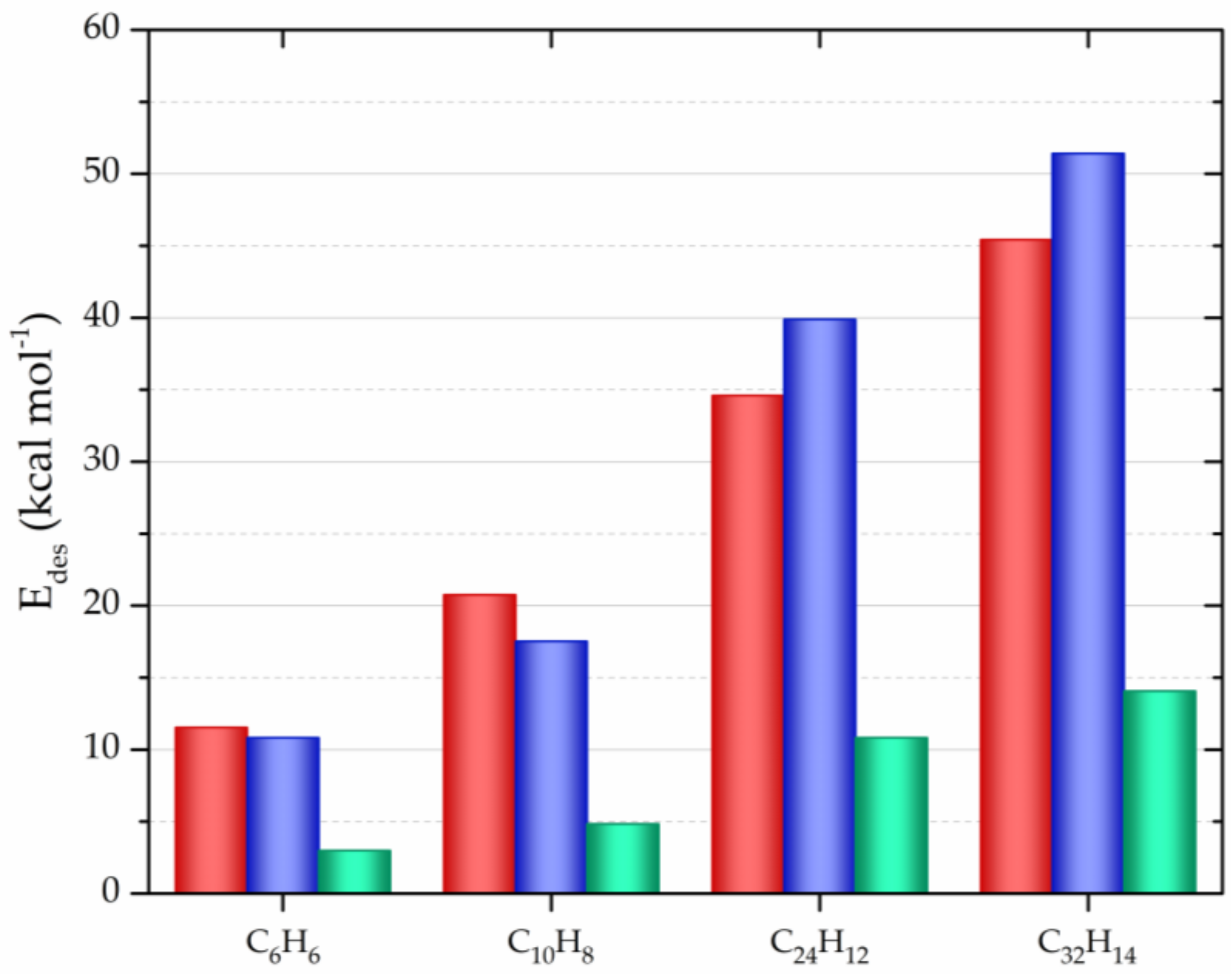
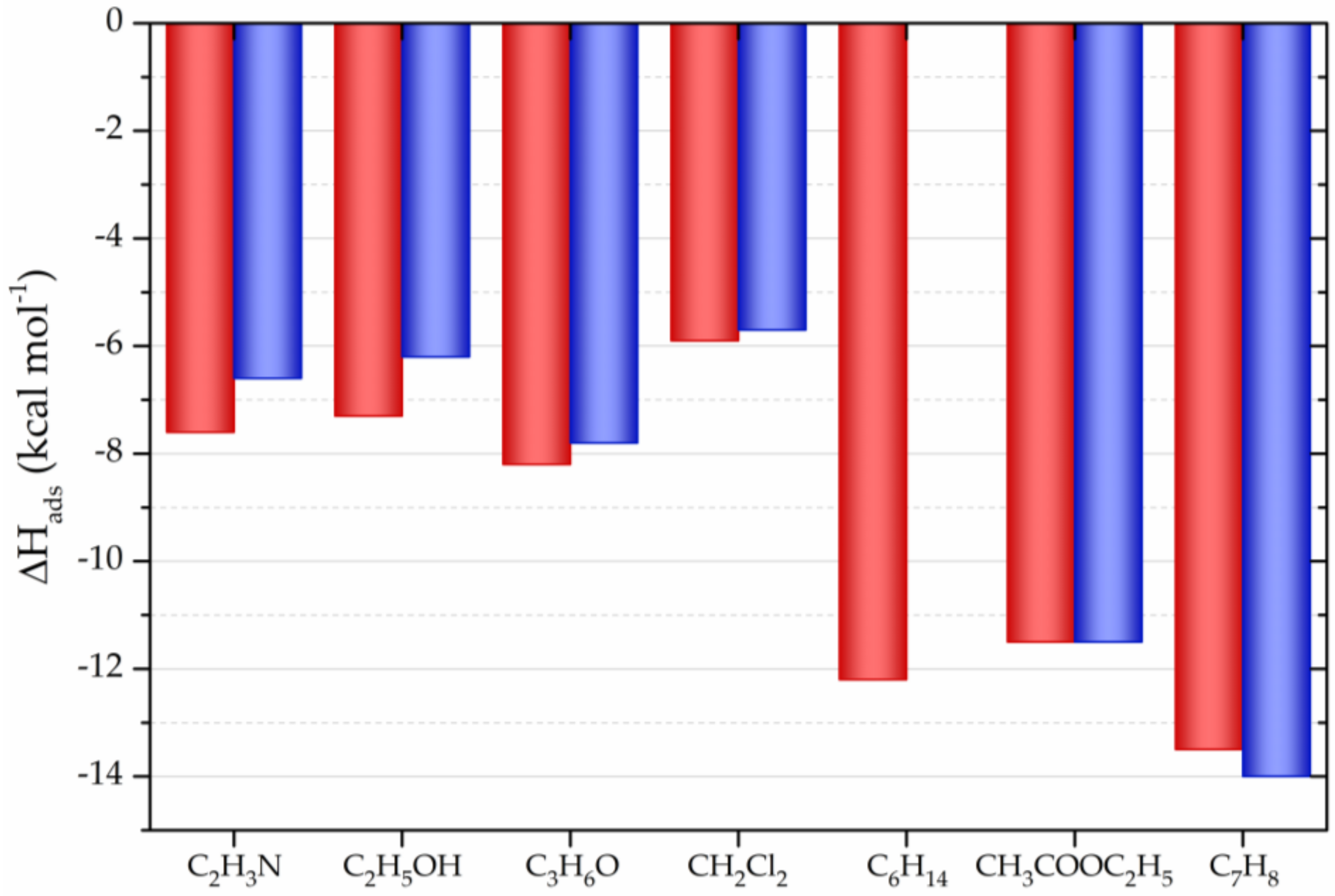
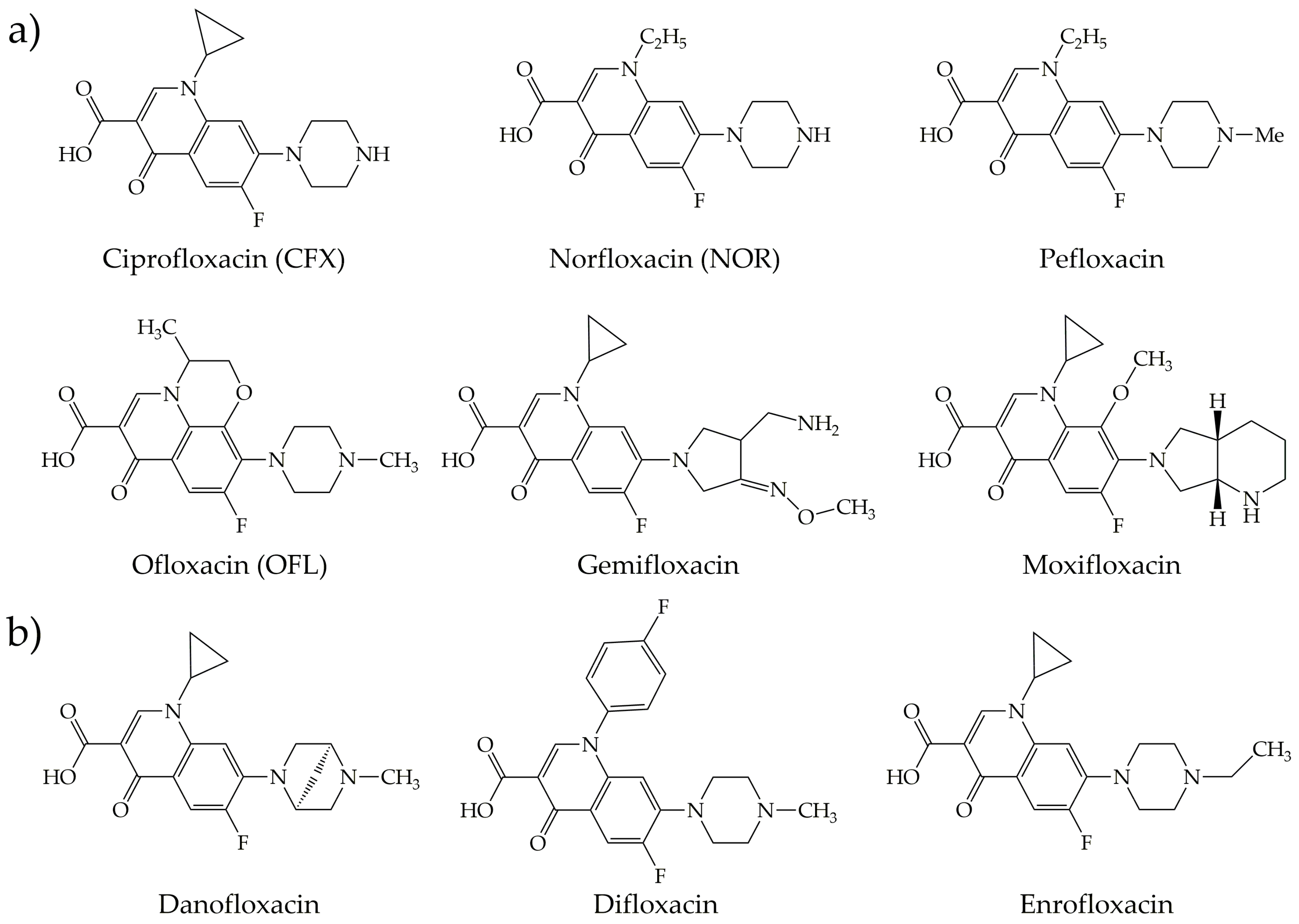
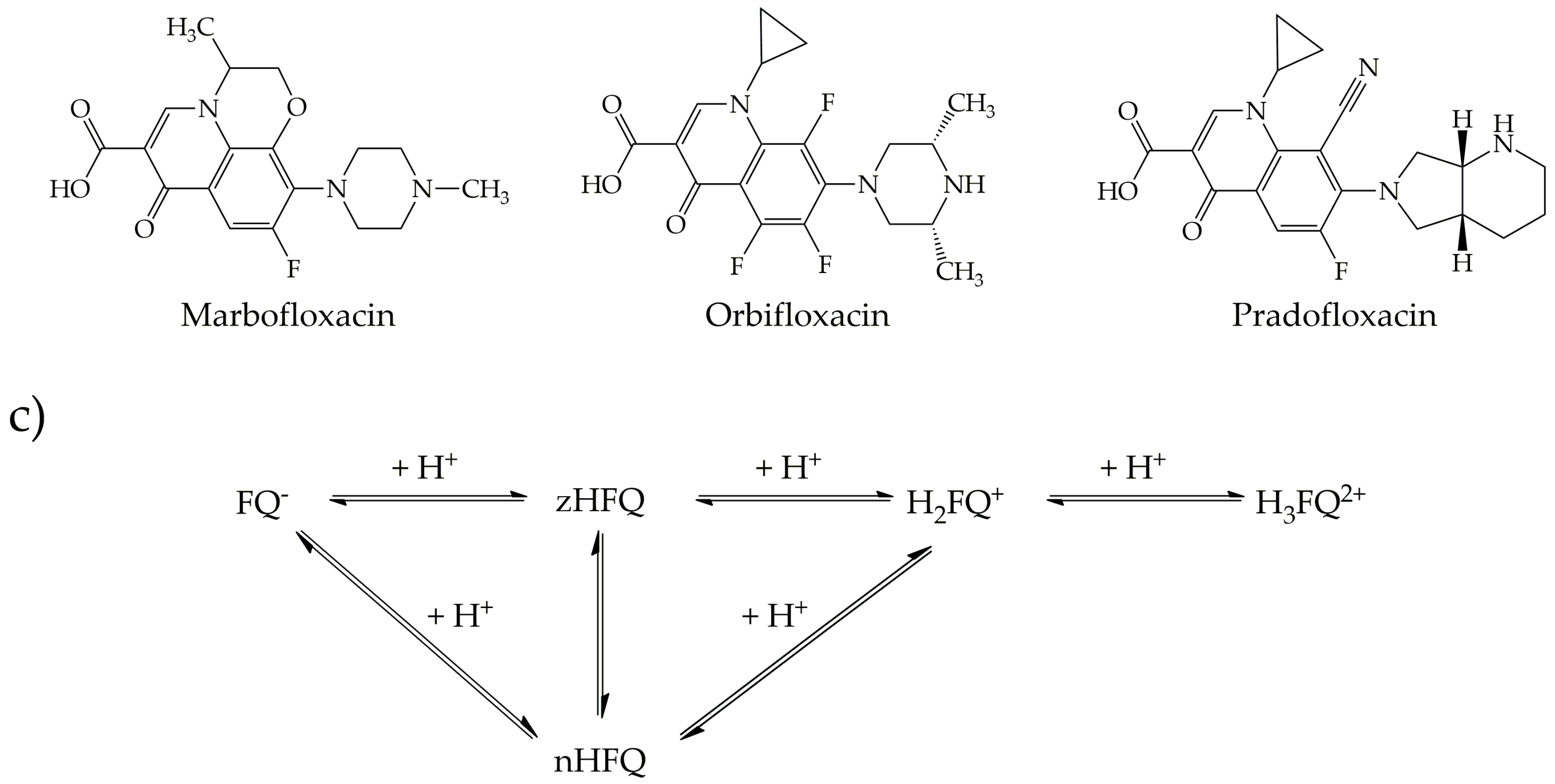
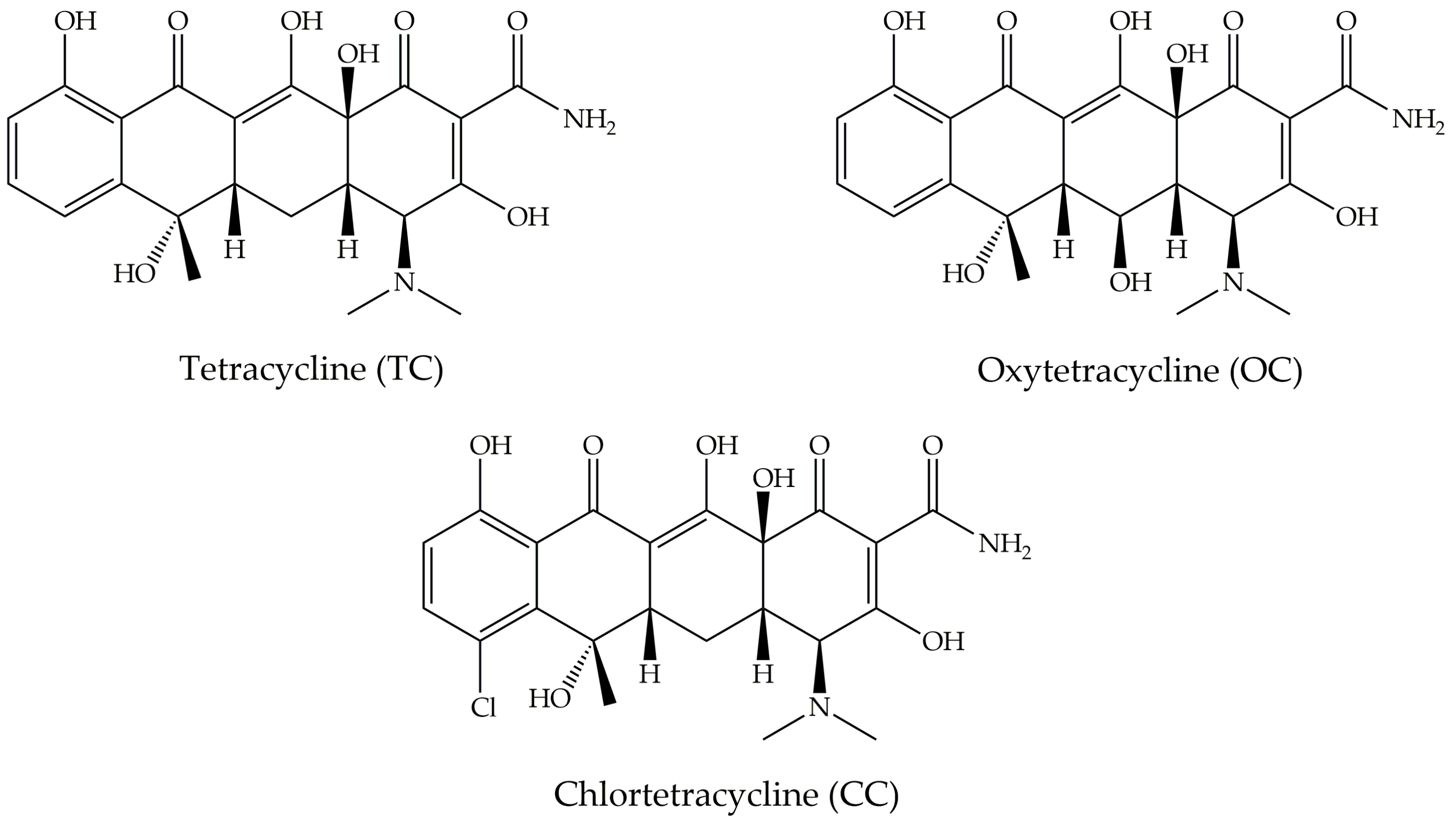
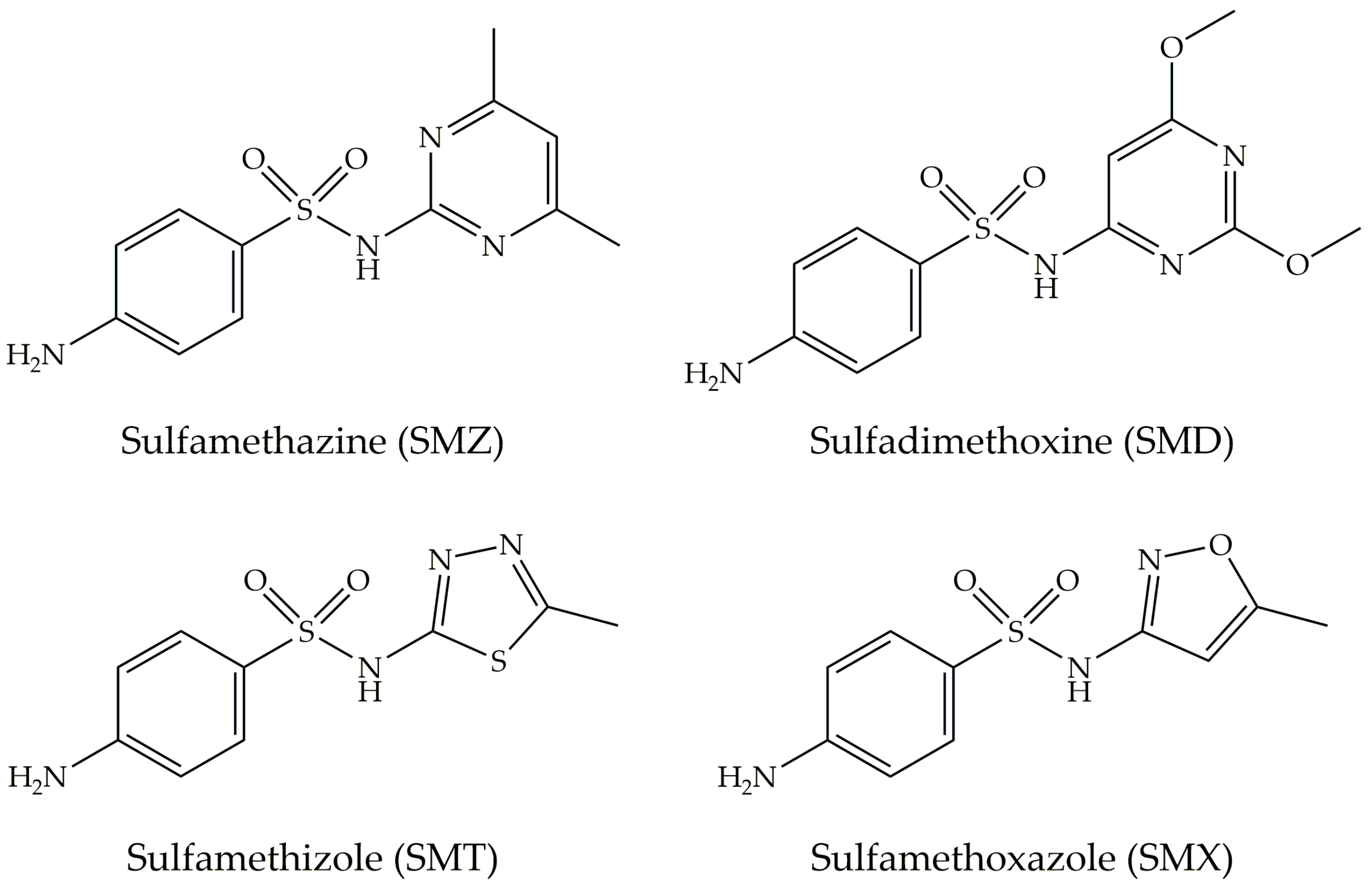
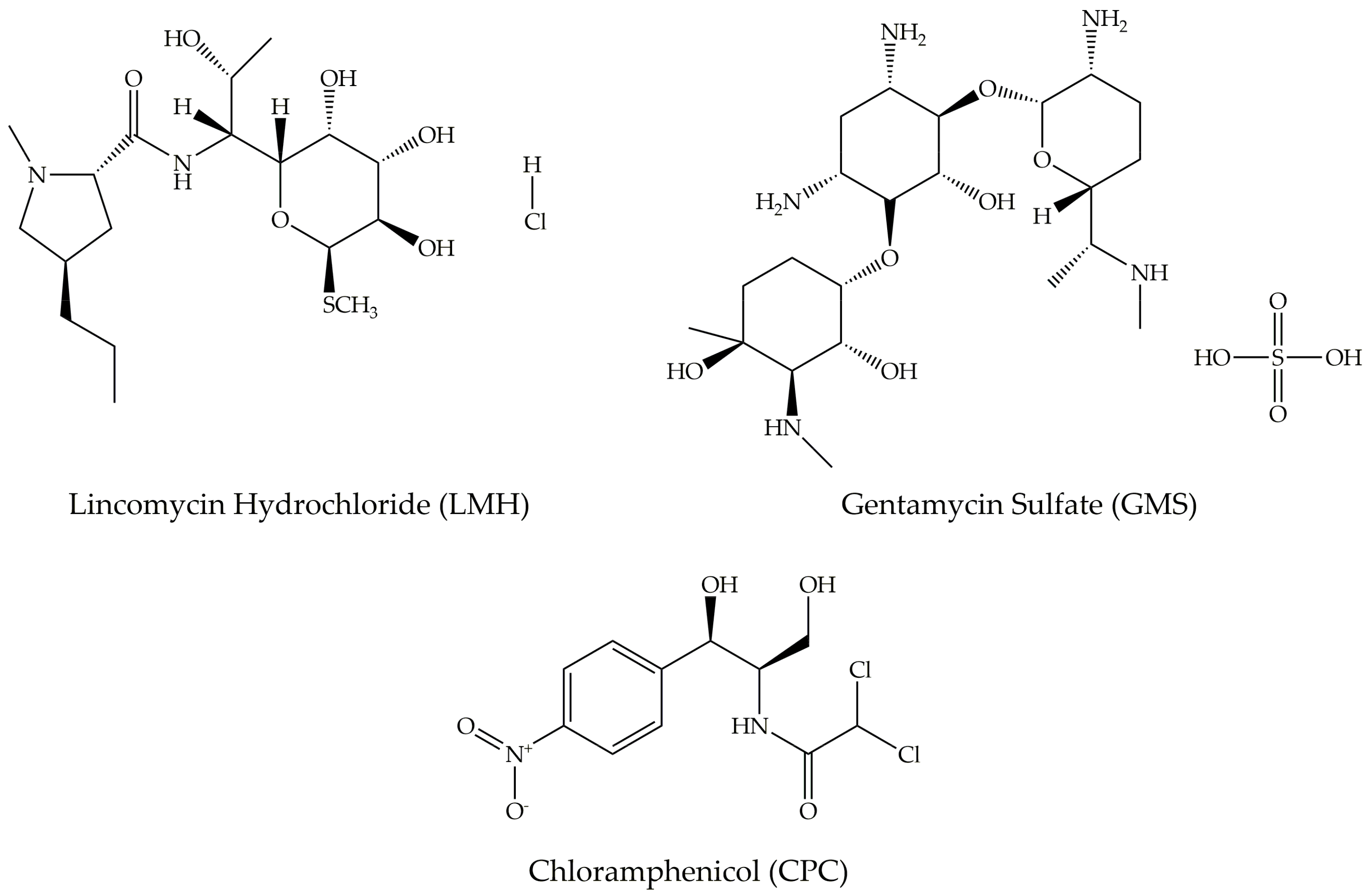
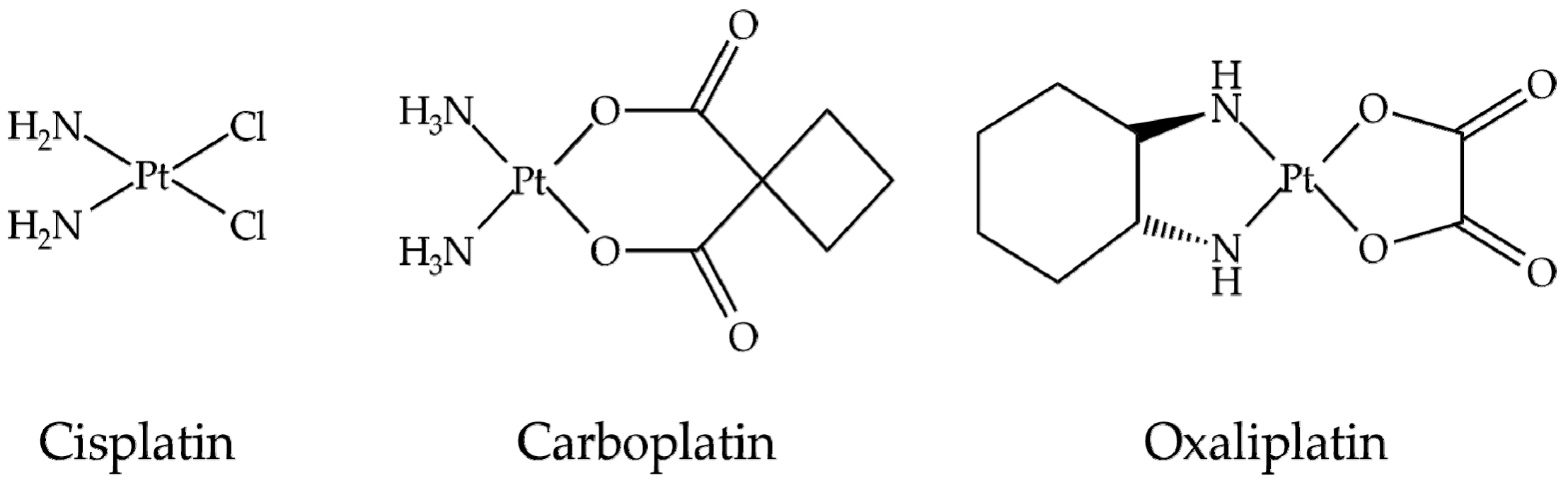


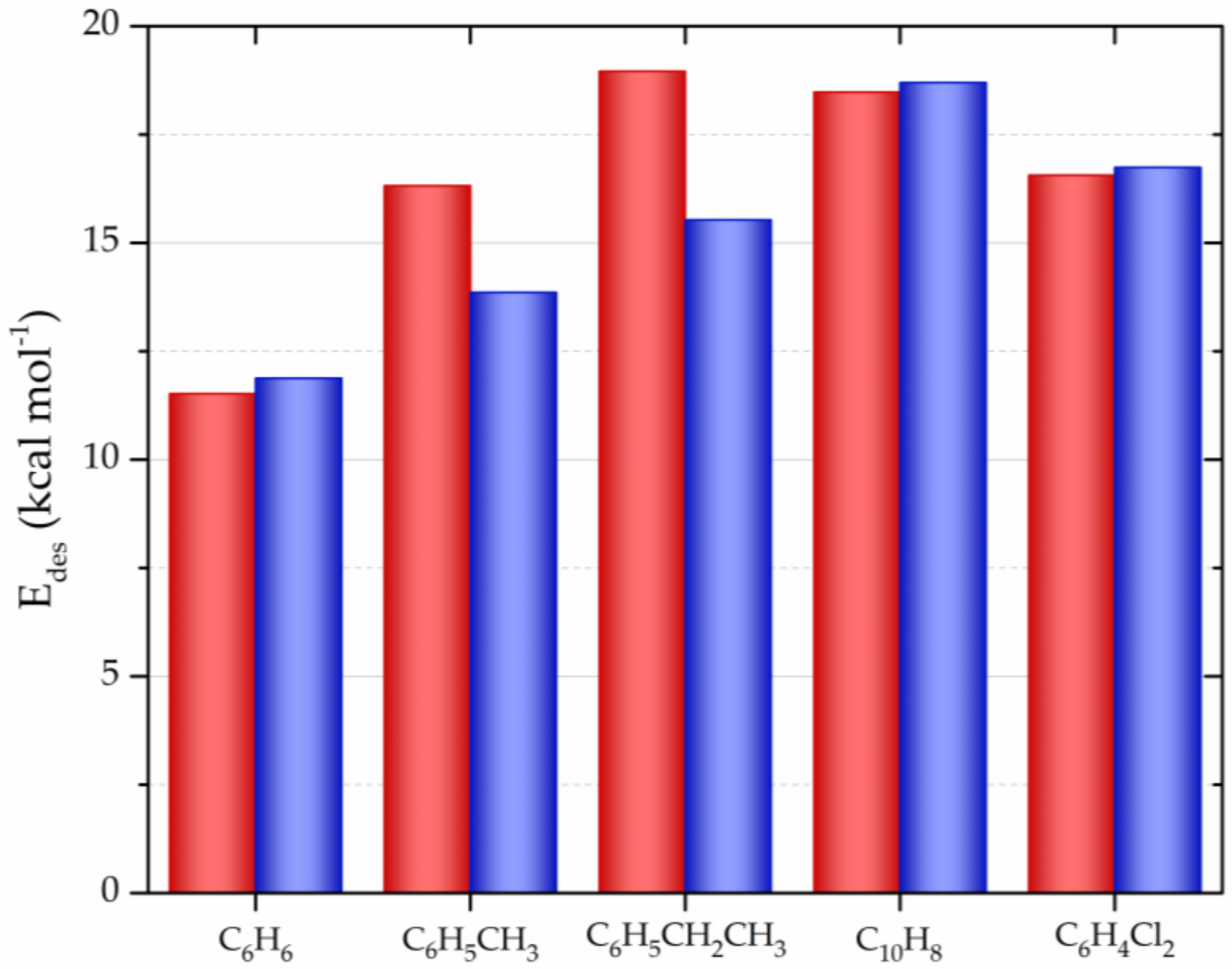
| CNM | Compound | Method | pH | T | LC | ΔX | Type of Interaction | Reference |
|---|---|---|---|---|---|---|---|---|
| MW-CNT | NA | HPLC | 7.0 | 303 | 169.08 | ΔGads = −3.1 | π–π int. H-bond | [224] |
| ΔHads = −31.6 | ||||||||
| MW-CNT-COOH | 188.68 | ΔGads = −3.0 | ||||||
| ΔHads = −32.1 | ||||||||
| MWCNT-NH2 | 135.14 | ΔGads = −3.1 | ||||||
| ΔHads = −32.3 | ||||||||
| SW-CNT | OFL | UV–vis | 7.0 | 298 | 288.40 | - | π–π int. H-bond | [208] |
| MW-CNT | 74.13 | |||||||
| SW-CNT | NOR | HPLC | 7.0 | 298 | 151.35 | - | ||
| MW-CNT | 53.70 | |||||||
| GO-MP | NOR | UV-vis | - | 298 | 127.80 | - | π–π int. H-bond | [225] |
| rGO-MP | 97.80 | - | ||||||
| GO | LEV | HPLC | - | 298 | 256.6 | - | Electro. π–π int. | [226] |
| GO | NOR | UV-vis | 24.93 | - | π–π int. H-bond | [227] | ||
| OFL | 2–12 | - | 40.65 | |||||
| CFX | 18.65 | |||||||
| SW-CNT | CFX | UV–vis | 7.0 | 298 | 907.19 | ΔGads = −9.1 | π–π int. | [207] |
| MW-CNT-COOH | 60.15 | ΔGads = −9.3 | ||||||
| MW-CNT | 382.10 | ΔGads = −9.0 | ||||||
| MW-CNT-OH | 62.59 | ΔGads = −7.8 | ||||||
| SW-CNT | CFX | UV–vis | 3–11 | 298 | 724.00 | ΔGads = −6.2 | π–π int. | [209] |
| DW-CNT | 605.00 | ΔGads = −5.9 | ||||||
| MW-CNT | 475.00 | ΔGads = −5.4 | ||||||
| G | CFX | FT-IR | 7.0 | 298 | 322.6 | ΔGads = −2.7 | π–π int. | [228] |
| GO | CFX | Florescence | 5.0 | 298 | 379.00 | ΔGads = −6.3 | Electro. π–π int. chemi ads. | [131] |
| GO-SA | CFX | UV-vis | 2–12 | 298 | 86.12 | - | π–π int. | [229] |
| GO-CA | CFX | UV-vis | 3–9 | - | 66.2 | - | IPD | [230] |
| G-H | CFX | UV-vis | - | 298 | 235.64 | - | H-bond π–π int. IPD | [231] |
| G | CFX | UV-vis | 2–10 | 298 | 145.90 | - | π–π int. | [232] |
| G-KOH | 194.60 | H-bond π–π int. EMT | ||||||
| G-NS | CFX | UV-vis | - | 298 | 147.90 | ΔGads = −1.8 | vdW Electro. | [132] |
| ΔHads = −4.2 | ||||||||
| MW-CNT | CFX | UV-vis | 5.0 | 298 | 135.00 | - | π–π int. | [233] |
| MW-CNT 2% O | CFX | UV-vis | 2–10 | 298 | 150.60 | ΔGads = −8.4 | π–π int. physic ads. | [234] |
| MW-CNT 3.2% O | 178.90 | ΔGads = −7.7 | ||||||
| MW-CNT 4.7% O | 206.00 | ΔGads = −7.6 | ||||||
| MW-CNT 5.9% O | 181.20 | ΔGads = −8.4 | ||||||
| SW-CNT out | zCFX | MD | 7.0 | 298 | - | ΔGads = −9.5 | π–π int. | [214] |
| nCFZ | ΔGads = −3.6 | |||||||
| SW-CNT in | zCFX | ΔGads = −12.2 | ||||||
| nCFZ | ΔGads = −20.9 |
| CNM | Compound | Method | pH | T | LC | ΔX | Type of Interaction | Reference |
|---|---|---|---|---|---|---|---|---|
| SW-CNT | OC | UV-vis | 3–11 | 298 | 554.00 | ΔGads = −6.4 | Electro. π–π int. | [209] |
| DW-CNT | 507.00 | ΔGads = −6.1 | ||||||
| MW-CNT | 391.00 | ΔGads = −6.3 | ||||||
| rGO | OC | UV-Vis | 3–11 | 298 | 212.00 | - | Electro. | [239] |
| TC | 313.00 | |||||||
| GO | TC | UV-vis | 7.0 | 298 | 322.43 | ΔGads = −0.50 | π–π int. cation–π int | [211] |
| ΔHads = 10.52 | ||||||||
| rGO | TC | UV-vis | 3–10 | 298 | 558.66 | ΔGads = −24.3 | vdW, π–π int. cation–π int. | [240] |
| GO-MP | TC | UV-vis | - | 298 | 95.00 | - | π–π int. | [241] |
| MW-CNT 2% O | TC | UV-vis | 2–10 | 298 | 217.80 | ΔGads = −7.7 | Electro. π–π int. hydrophobic | [242] |
| MW-CNT 3.2% O | 269.25 | ΔGads = −6.2 | ||||||
| MW-CNT 4.7% O | 217.56 | ΔGads = −6.4 | ||||||
| MW-CNT 5.9% O | 210.43 | ΔGads = −6.6 | ||||||
| G-NS | TC | - | - | 298 | 78.60 | ΔGads = −11.9 | Electro. π–π int. | [136] |
| ΔHads = −13.0 | ||||||||
| GO | TC | HPLC | - | 293 | 844.00 | - | π–π int. Electro. | [215] |
| rGO | 308.00 | |||||||
| MW-CNT | 298.00 | |||||||
| GO-MP | TC | UV-vis | - | 298 | 115.30 | - | H-bond | [225] |
| rGO-MP | 302.40 | π–π int. | ||||||
| GO-MP | SDZ | 12.43 | H-bond Hydrophobic π–π int | |||||
| rGO-MP | 11.54 | |||||||
| rGO | TC | HPLC | - | 298 | 219.10 | ΔGads = −5.6 | π–π int. cation–π int. | [127] |
| SMZ | 174.42 | ΔGads = −5.7 | ||||||
| SW-CNT | SMD | HPLC | 5.0 | 298 | 16.30 | ΔGads = −10.0 | π–π int. | [213] |
| SMT | 312.00 | ΔGads = −5.9 | ||||||
| SMZ | 12.70 | ΔGads = −8.3 | ||||||
| SMX | 7.88 | ΔGads = −7.9 | ||||||
| GO | SMX | Florescence | 5.0 | 298 | 240.00 | ΔGads = −5.7 | Electro. π–π int. chemi ads. | [131] |
| G-NS | SMX | UV-vis | - | 298 | 103.00 | ΔGads = −0.4 | π–π int. | [225] |
| GO-NS | 122.00 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veclani, D.; Tolazzi, M.; Melchior, A. Molecular Interpretation of Pharmaceuticals’ Adsorption on Carbon Nanomaterials: Theory Meets Experiments. Processes 2020, 8, 642. https://doi.org/10.3390/pr8060642
Veclani D, Tolazzi M, Melchior A. Molecular Interpretation of Pharmaceuticals’ Adsorption on Carbon Nanomaterials: Theory Meets Experiments. Processes. 2020; 8(6):642. https://doi.org/10.3390/pr8060642
Chicago/Turabian StyleVeclani, Daniele, Marilena Tolazzi, and Andrea Melchior. 2020. "Molecular Interpretation of Pharmaceuticals’ Adsorption on Carbon Nanomaterials: Theory Meets Experiments" Processes 8, no. 6: 642. https://doi.org/10.3390/pr8060642
APA StyleVeclani, D., Tolazzi, M., & Melchior, A. (2020). Molecular Interpretation of Pharmaceuticals’ Adsorption on Carbon Nanomaterials: Theory Meets Experiments. Processes, 8(6), 642. https://doi.org/10.3390/pr8060642