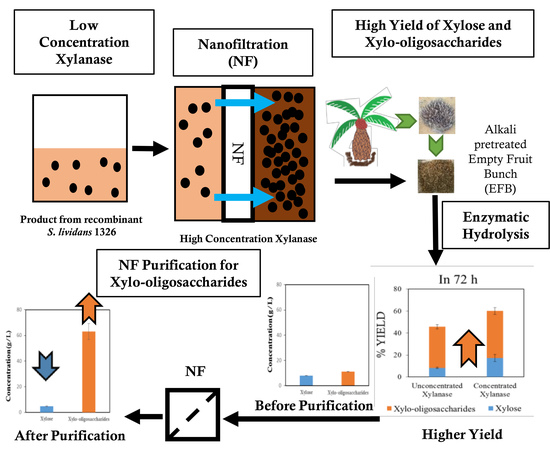
High Enzymatic Recovery and Purification of Xylooligosaccharides from Empty Fruit Bunch via Nanofiltration
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Microorganisms
2.2. Xylanase Production
2.3. Enzymatic Activity Assay and Protein Assay
2.4. Membrane Separation Process
2.5. Enzymatic Hydrolysis of Pretreated EFB
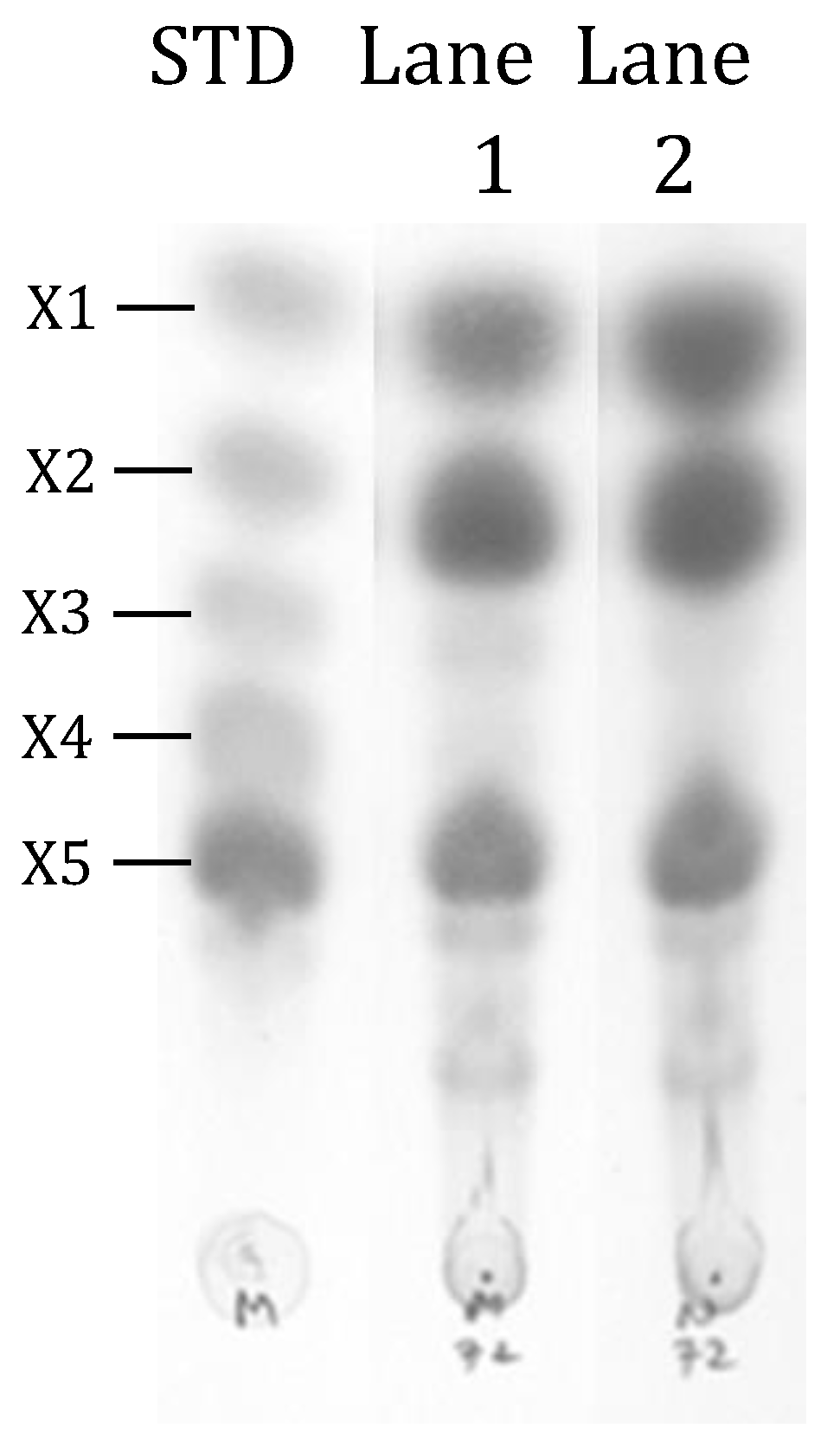
2.6. Thin-Layer Chromatography Analysis
2.7. Analytical Methods
3. Results and Discussion
3.1. Alkali Pretreatment
3.2. Characterization of XYN10Ks_480 Endoxylanase for Membrane Selections
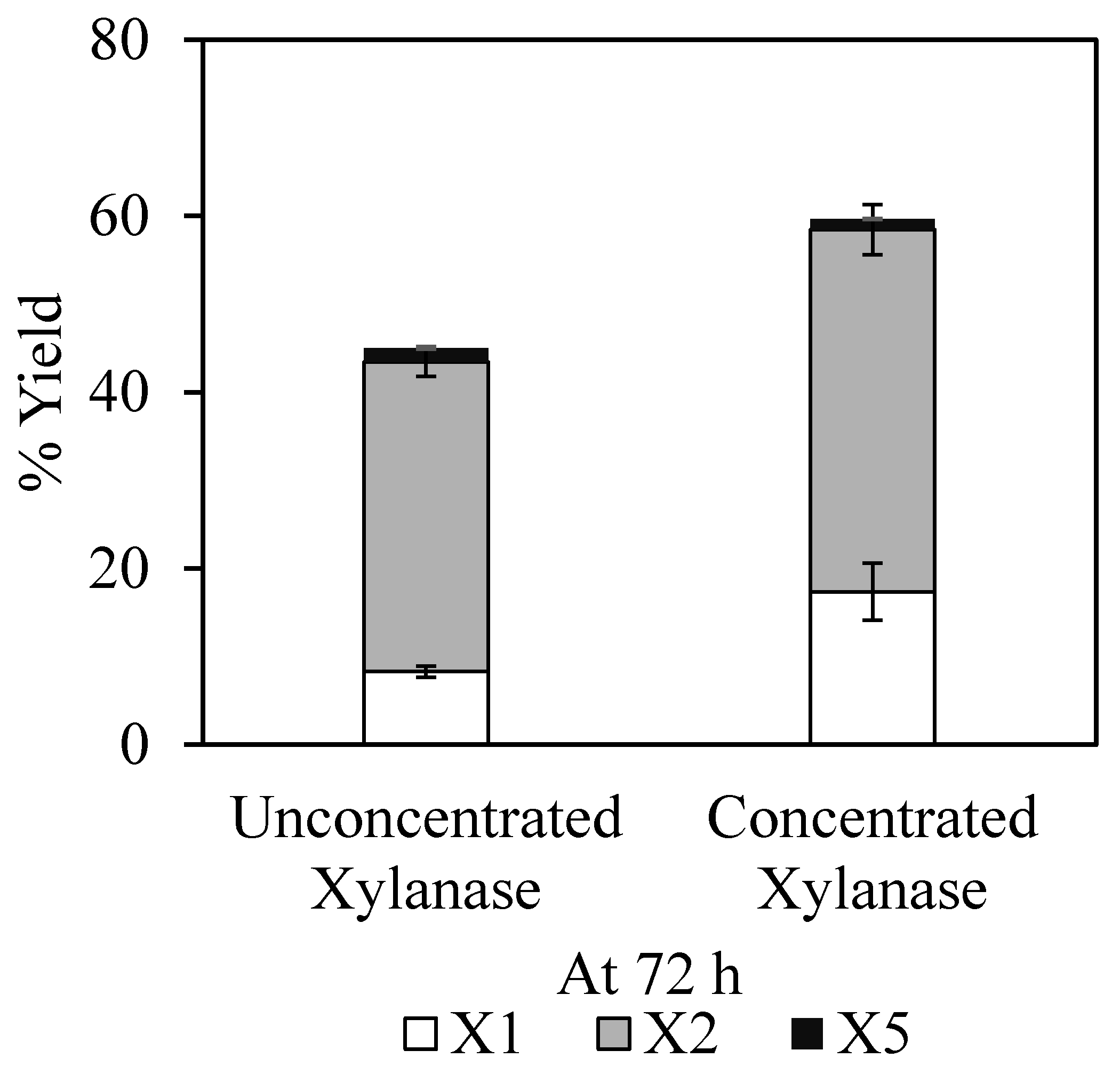
3.3. Enzymatic Hydrolysis of Alkali-Pretreated EFB with Concentrated or Unconcentrated XYN10Ks_480 Endoxylanase
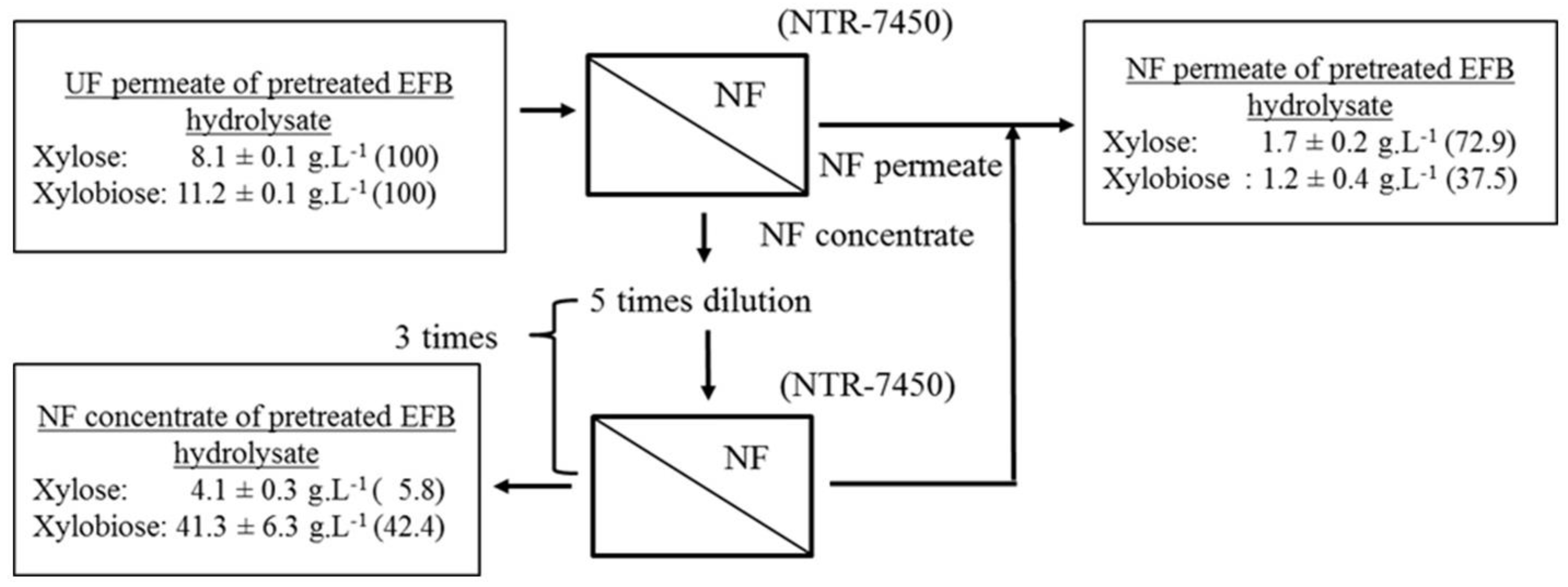
3.4. Concentration and Separation of Xylobiose by Nanofiltration
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Carvalho, A.F.A.; De Oliva Neto, P.; Da Silva, D.F.; Pastore, G.M. Xylo-oligosaccharides from lignocellulosic materials: Chemical structure, health benefits and production by chemical and enzymatic hydrolysis. Food Res. Int. 2013, 51, 75–85. [Google Scholar] [CrossRef]
- Hsu, C.-K.; Liao, J.-W.; Chung, Y.-C.; Hsieh, C.-P.; Chan, Y.-C. Xylooligosaccharides and Fructooligosaccharides Affect the Intestinal Microbiota and Precancerous Colonic Lesion Development in Rats. J. Nutr. 2004, 134, 1523–1528. [Google Scholar] [CrossRef] [PubMed]
- Moura, P.; Barata, R.; Carvalheiro, F.; Gírio, F.; Loureiro-Dias, M.C.; Esteves, M.P. In vitro fermentation of xylo-oligosaccharides from corn cobs autohydrolysis by Bifidobacterium and Lactobacillus strains. LWT 2007, 40, 963–972. [Google Scholar] [CrossRef]
- Samanta, A.K.; Jayapal, N.; Jayaram, C.; Roy, S.; Kolte, A.P.; Senani, S.; Sridhar, M. Xylooligosaccharides as prebiotics from agricultural by-products: Production and applications. Bioact. Carbohydr. Diet. Fibre 2015, 5, 62–71. [Google Scholar] [CrossRef]
- Amorim, C.; Silvério, S.C.; Prather, K.L.J.; Rodrigues, L.R. From lignocellulosic residues to market: Production and commercial potential of xylooligosaccharides. Biotechnol. Adv. 2019, 37, 107397. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Bowman, M.J.; Cotta, M.A.; Dien, B.S.; Iten, L.B.; Whitehead, T.R.; Rausch, K.D.; Tumbleson, M.E.; Singh, V. Miscanthus × giganteus xylooligosaccharides: Purification and fermentation. Carbohydr. Polym. 2016, 140, 96–103. [Google Scholar] [CrossRef]
- Ho, A.L.; Carvalheiro, F.; Duarte, L.C.; Roseiro, L.B.; Charalampopoulos, D.; Rastall, R.A. Production and purification of xylooligosaccharides from oil palm empty fruit bunch fibre by a non-isothermal process. Bioresour. Technol. 2014, 152, 526–529. [Google Scholar] [CrossRef]
- Aachary, A.A.; Prapulla, S.G. Xylooligosaccharides (XOS) as an Emerging Prebiotic: Microbial Synthesis, Utilization, Structural Characterization, Bioactive Properties, and Applications. Compr. Rev. Food Sci. Food Saf. 2011, 10, 2–16. [Google Scholar] [CrossRef]
- Dutta, P.D.; Neog, B.; Goswami, T. Xylanase enzyme production from Bacillus australimaris P5 for prebleaching of bamboo (Bambusa tulda) pulp. Mater. Chem. Phys. 2019, 243, 122227. [Google Scholar] [CrossRef]
- Rahmani, N.; Kahar, P.; Lisdiyanti, P.; Lee, J.; Yopi; Prasetya, B.; Ogino, C.; Kondo, A. GH-10 and GH-11 Endo-1,4-Β-xylanase enzymes from Kitasatospora sp. produce xylose and xylooligosaccharides from sugarcane bagasse with no xylose inhibition. Bioresour. Technol. 2019, 272, 315–325. [Google Scholar] [CrossRef]
- Rahmani, N.; Kahar, P.; Lisdiyanti, P.; Hermiati, E.; Lee, J.; Yopi; Prasetya, B.; Ogino, C.; Kondo, A. Xylanase and feruloyl esterase from actinomycetes cultures could enhance sugarcane bagasse hydrolysis in the production of fermentable sugars. Biosci. Biotechnol. Biochem. 2018, 82, 904–915. [Google Scholar] [CrossRef] [PubMed]
- Alvira, P.; Tomás-Pejó, E.; Ballesteros, M.; Negro, M.J. Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: A review. Bioresour. Technol. 2010, 101, 4851–4861. [Google Scholar] [CrossRef] [PubMed]
- Varkkey, H.; Tyson, A.; Choiruzzad, S.A.B. Palm oil intensification and expansion in Indonesia and Malaysia: Environmental and socio-political factors influencing policy. For. Policy Econ. 2018, 92, 148–159. [Google Scholar] [CrossRef]
- Shinoj, S.; Visvanathan, R.; Panigrahi, S.; Kochubabu, M. Oil palm fiber (OPF) and its composites: A review. Ind. Crops Prod. 2011, 33, 7–22. [Google Scholar] [CrossRef]
- Choi, W.I.; Park, J.Y.; Lee, J.P.; Oh, Y.K.; Park, Y.C.; Kim, J.S.; Park, J.M.; Kim, C.H.; Lee, J.S. Optimization of NaOH-catalyzed steam pretreatment of empty fruit bunch. Biotechnol. Biofuels 2013, 6, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Sugiharto, Y.E.C.; Harimawan, A.; Kresnowati, M.T.A.P.; Purwadi, R.; Mariyana, R.; Andry; Fitriana, H.N.; Hosen, H.F. Enzyme feeding strategies for better fed-batch enzymatic hydrolysis of empty fruit bunch. Bioresour. Technol. 2016, 207, 175–179. [Google Scholar] [CrossRef]
- He, Y.; Bagley, D.M.; Leung, K.T.; Liss, S.N.; Liao, B.Q. Recent advances in membrane technologies for biorefining and bioenergy production. Biotechnol. Adv. 2012, 30, 817–858. [Google Scholar] [CrossRef]
- Qi, B.; Luo, J.; Chen, G.; Chen, X.; Wan, Y. Application of ultrafiltration and nanofiltration for recycling cellulase and concentrating glucose from enzymatic hydrolyzate of steam exploded wheat straw. Bioresour. Technol. 2012, 104, 466–472. [Google Scholar] [CrossRef]
- Echavarría, A.P.; Torras, C.; Pagán, J.; Ibarz, A. Fruit Juice Processing and Membrane Technology Application. Food Eng. Rev. 2011, 3, 136–158. [Google Scholar] [CrossRef]
- Abels, C.; Carstensen, F.; Wessling, M. Membrane processes in biorefinery applications. J. Memb. Sci. 2013, 444, 285–317. [Google Scholar] [CrossRef]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Sasaki, K.; Okamoto, M.; Shirai, T.; Tsuge, Y.; Fujino, A.; Sasaki, D.; Morita, M.; Matsuda, F.; Kikuchi, J.; Kondo, A. Toward the complete utilization of rice straw: Methane fermentation and lignin recovery by a combinational process involving mechanical milling, supporting material and nanofiltration. Bioresour. Technol. 2016, 216, 830–837. [Google Scholar] [CrossRef] [PubMed]
- Pangsang, N.; Rattanapan, U.; Thanapimmetha, A.; Srinopphakhun, P.; Liu, C.G.; Zhao, X.Q.; Bai, F.W.; Sakdaronnarong, C. Chemical-free fractionation of palm empty fruit bunch and palm fiber by hot-compressed water technique for ethanol production. Energy Rep. 2019, 5, 337–348. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass: Laboratory Analytical Procedure (LAP); Issue Date: April 2008; Revision Date: July 2011 (Version 07-08-2011)—42618.pdf; Technical Report NREL/TP-510-42618; National Renewable Energy Laboratory: Golden, CO, USA, 2008; pp. 1–16.
- Akpinar, O.; Erdogan, K.; Bakir, U.; Yilmaz, L. Comparison of acid and enzymatic hydrolysis of tobacco stalk xylan for preparation of xylooligosaccharides. LWT 2010, 43, 119–125. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, Y.Y.; Kim, T.H. A review on alkaline pretreatment technology for bioconversion of lignocellulosic biomass. Bioresour. Technol. 2016, 199, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Voutilainen, S.; Ojamo, H.; Turunen, O. Stability and activity of Dictyoglomus thermophilum GH11 xylanase and its disulphide mutant at high pressure and temperature. Enzym. Microb. Technol. 2015, 70, 66–71. [Google Scholar] [CrossRef]
- Sasaki, K.; Tsuge, Y.; Kawaguchi, H.; Yasukawa, M.; Sasaki, D.; Sazuka, T.; Kamio, E.; Ogino, C.; Matsuyama, H.; Kondo, A. Sucrose purification and repeated ethanol production from sugars remaining in sweet sorghum juice subjected to a membrane separation process. Appl. Microbiol. Biotechnol. 2017, 101, 6007–6014. [Google Scholar] [CrossRef]
| Sample | Xylan | Glucan | Insoluble | Soluble | Ash | Others |
|---|---|---|---|---|---|---|
| Lignin | Lignin | |||||
| (%) | (%) | (%) | (%) | (%) | (%) | |
| Raw EFB | 23.4 ± 0.2 | 37.3 ± 0.0 | 21.2 ± 0.2 | 0.1 ± 0.0 | 0.7 ± 0.2 | 17.2 ± 0.3 |
| Alkali-pretreated EFB | 26.9 ± 1.1 | 45.0 ± 1.0 | 21.0 ± 0.4 | 0.1 ± 0.0 | 0.2 ± 0.1 | 7.5 ± 1.8 |
| Sample | Xylanase Activity (U.mL−1) | Protein (g.L−1) | Specific Activity (U.mg−1) |
|---|---|---|---|
| Recombinant Streptomyces lividans 1326 culture supernatant | 10.7 ± 0.5 | 1.5 ± 0.0 | 6.8 ± 0.2 |
| Membrane retentate | 75.3 ± 4.1 | 10.9 ± 0.2 | 6.9 ± 0.4 |
| Membrane permeate | 0 | 0.3 ± 0.0 | 0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wijaya, H.; Sasaki, K.; Kahar, P.; Rahmani, N.; Hermiati, E.; Yopi, Y.; Ogino, C.; Prasetya, B.; Kondo, A. High Enzymatic Recovery and Purification of Xylooligosaccharides from Empty Fruit Bunch via Nanofiltration. Processes 2020, 8, 619. https://doi.org/10.3390/pr8050619
Wijaya H, Sasaki K, Kahar P, Rahmani N, Hermiati E, Yopi Y, Ogino C, Prasetya B, Kondo A. High Enzymatic Recovery and Purification of Xylooligosaccharides from Empty Fruit Bunch via Nanofiltration. Processes. 2020; 8(5):619. https://doi.org/10.3390/pr8050619
Chicago/Turabian StyleWijaya, Hans, Kengo Sasaki, Prihardi Kahar, Nanik Rahmani, Euis Hermiati, Yopi Yopi, Chiaki Ogino, Bambang Prasetya, and Akihiko Kondo. 2020. "High Enzymatic Recovery and Purification of Xylooligosaccharides from Empty Fruit Bunch via Nanofiltration" Processes 8, no. 5: 619. https://doi.org/10.3390/pr8050619
APA StyleWijaya, H., Sasaki, K., Kahar, P., Rahmani, N., Hermiati, E., Yopi, Y., Ogino, C., Prasetya, B., & Kondo, A. (2020). High Enzymatic Recovery and Purification of Xylooligosaccharides from Empty Fruit Bunch via Nanofiltration. Processes, 8(5), 619. https://doi.org/10.3390/pr8050619