Metal Chlorides Grafted on SAPO-5 (MClx/SAPO-5) as Reusable and Superior Catalysts for Acylation of 2-Methylfuran Under Non-Microwave Instant Heating Condition
Abstract
1. Introduction
2. Experimental
2.1. Synthesis of 1-benzyl-2,3-dimethyl-1H-imidazol-3-ium hydroxide ([bzmIm]OH) Template Solution
2.2. Synthesis of SAPO-5 Molecular Sieve
2.3. Preparation of Metal Chlorides Grafted SAPO-5 (MClx/SAPO-5, M = Cu, Co, Sn, Fe, Zn) Solids
2.4. Characterization
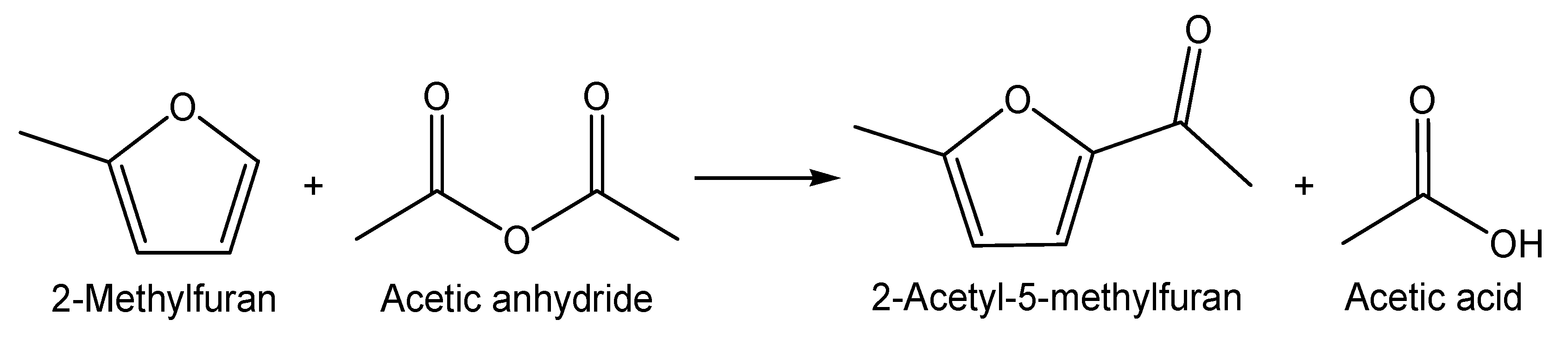
2.5. Catalytic Testing
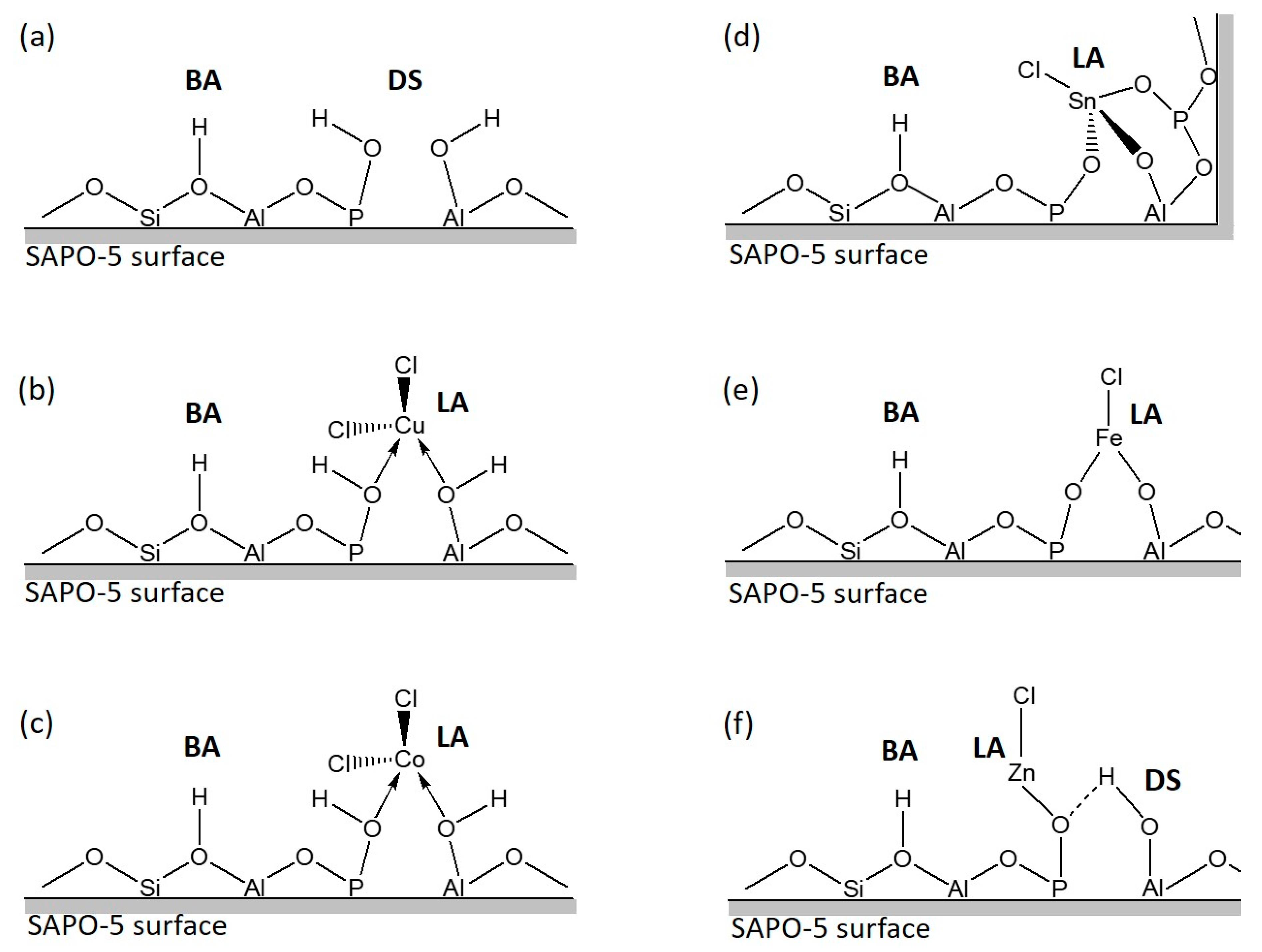
3. Results and Discussion
3.1. Characterization
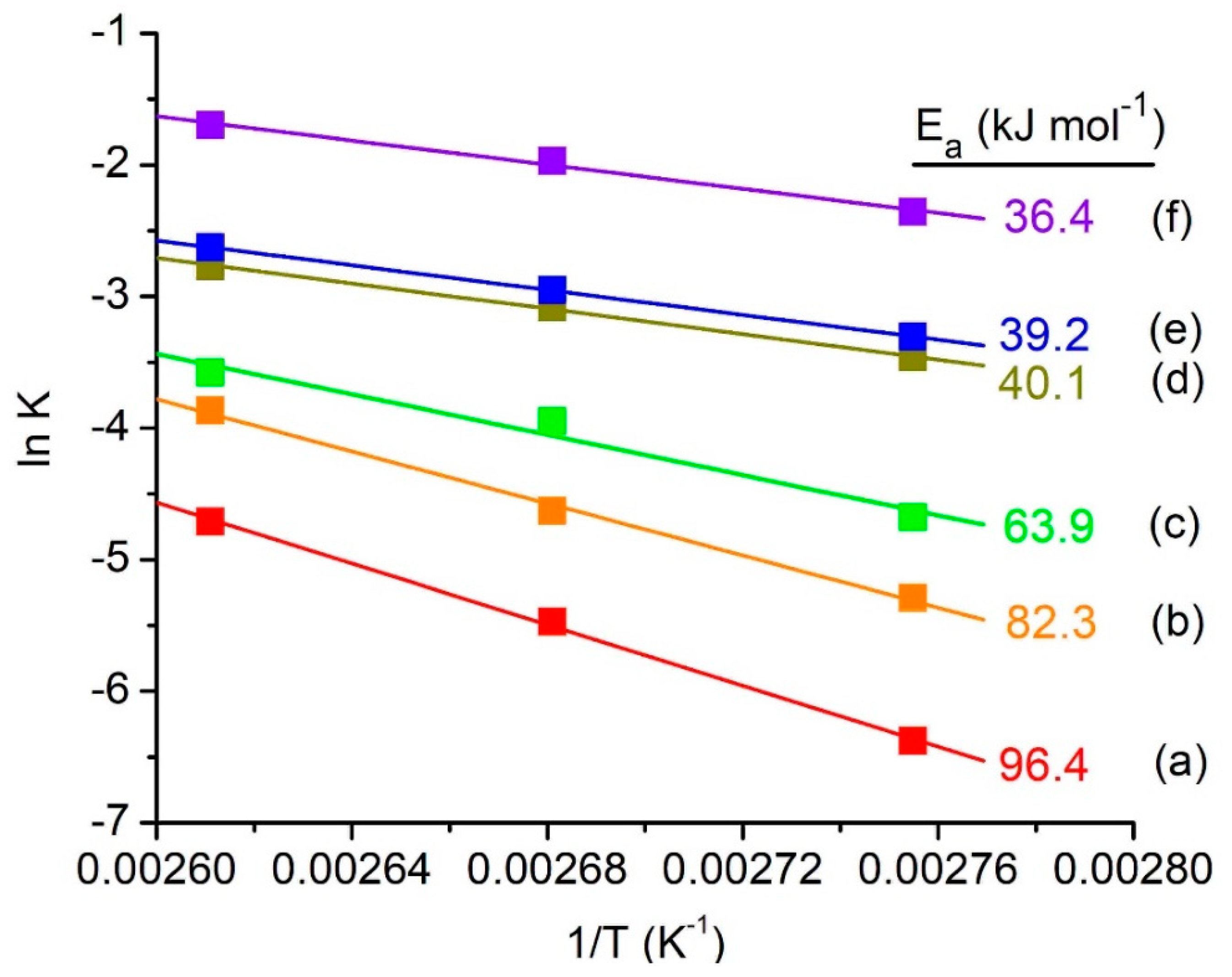
3.2. Catalytic Testing
3.2.1. Effect of Reaction Time and Temperature
3.2.2. Catalytic Comparative Study
3.2.3. Catalyst Reusability Test
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Look, S.A.; Burch, M.T.; Fenical, W.; Zheng, Q.; Clardy, J. Kallolide A, a new antiinflammatory diterpenoid, and related lactones from the Caribbean octocoral Pseudopterogorgia kallos (Bielschowsky). J. Org. Chem. 1985, 50, 5741–5746. [Google Scholar] [CrossRef]
- Schenkel, D.; Lemfack, M.C.; Piechulla, B.; Splivallo, R. A meta-analysis approach for assessing the diversity and specificity of belowground root and microbial volatiles. Front. Plant Sci. 2015, 6, 707. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, R.; Kumar, H.; Banerjee, M. Medicinal significance of furan derivatives: A Review. Int. J. Rev. Life Sci. 2012, 2, 7–16. [Google Scholar]
- Harrison, I.T.; Lewis, B.; Nelson, P.; Rooks, W.; Roszkowski, A.; Tomolonis, A.; Fried, J.H. Nonsteroidal antiinflammatory agents. I. 6-substituted 2-naphthylacetic acids. J. Med. Chem. 1970, 13, 203–205. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, H.; Chen, H.; Wan, Y.; Li, N.; Xu, Q.; He, J.; Chen, D.; Wang, L.; Lu, J. Controlling crystallite orientation of diketopyrrolopyrrole-based small molecules in thin films for highly reproducible multilevel memory device: Role of furan substitution. Adv. Funct. Mater. 2015, 25, 4246–4254. [Google Scholar] [CrossRef]
- Kruse, L.I.; Ladd, D.L.; Harrsch, P.B.; McCabe, F.L.; Mong, S.M.; Faucette, L.; Johnson, R. Synthesis, tubulin binding, antineoplastic evaluation, and structure-activity relationship of oncodazole analogs. J. Med. Chem. 1989, 32, 409–417. [Google Scholar] [CrossRef]
- Finan, P.; Fothergill, G. 506. Furans. Part II. Friedel-Crafts acylation of furan, 2-methylfuran, and 3-methylfuran. J. Chem. Soc. 1963, 2723–2727. [Google Scholar] [CrossRef]
- Spurlock, J.J. Hydantoins as anticonvulsants. I. 5-R-5-(2-Thienyl)-hydantoins1. J. Am. Chem. Soc. 1953, 75, 1115–1117. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Suzuki, K.; Singh, S.K. C-acylation of 2-methylfuran and thiophene using N-acylbenzotriazoles. Croat. Chem. Acta 2004, 77, 175–178. [Google Scholar] [CrossRef]
- Kresnawahjuesa, O.; Gorte, R.J.; White, D. The acylation of propene by acetic acid over H-[Fe] ZSM-5 and H-[Al] ZSM-5. J. Mol. Catal. A Chem. 2004, 212, 309–314. [Google Scholar] [CrossRef]
- Blaser, H.-U.; Indolese, A.; Schnyder, A.; Steiner, H.; Studer, M. Supported palladium catalysts for fine chemicals synthesis. J. Mol. Catal. A Chem. 2001, 173, 3–18. [Google Scholar] [CrossRef]
- Métivier, P. Catalysis for fine chemicals: An industrial perspective. In Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 2000; Volume 130, pp. 167–176. [Google Scholar]
- Ng, E.-P.; Awala, H.; Komaty, S.; Mintova, S. Microwave-Green synthesis of AlPO-n and SAPO-n (n = 5 and 18) nanosized crystals and their assembly in layers. Microporous Mesoporous Mater. 2019, 280, 256–263. [Google Scholar] [CrossRef]
- Wong, S.-F.; Deekomwong, K.; Wittayakun, J.; Ling, T.C.; Muraza, O.; Adam, F.; Ng, E.-P. Crystal growth study of KF nanozeolite and its catalytic behavior in Aldol condensation of benzaldehyde and heptanal enhanced by microwave heating. Mater. Chem. Phys. 2017, 196, 295–301. [Google Scholar] [CrossRef]
- Ng, E.-P.; Awala, H.; Ghoy, J.-P.; Vicente, A.; Ling, T.C.; Ng, Y.H.; Mintova, S.; Adam, F. Effects of ultrasonic irradiation on crystallization and structural properties of EMT-type zeolite nanocrystals. Mater. Chem. Phys. 2015, 159, 38–45. [Google Scholar] [CrossRef]
- Derakhshankhah, H.; Hajipour, M.J.; Barzegari, E.; Lotfabadi, A.; Ferdousi, M.; Saboury, A.A.; Ng, E.P.; Raoufi, M.; Awala, H.; Mintova, S. Zeolite nanoparticles inhibit Aβ–fibrinogen interaction and formation of a consequent abnormal structural clot. ACS Appl. Mater. Interfaces 2016, 8, 30768–30779. [Google Scholar] [CrossRef]
- Choo, M.-Y.; Oi, L.E.; Ling, T.C.; Ng, E.-P.; Lin, Y.-C.; Centi, G.; Juan, J.C. Deoxygenation of Triolein to green diesel in the H2-free condition: Effect of transition metal oxide supported on Zeolite Y. J. Anal. Appl. Pyrolysis 2020, 147, 104797. [Google Scholar] [CrossRef]
- Wang, D.; Jangjou, Y.; Liu, Y.; Sharma, M.K.; Luo, J.; Li, J.; Kamasamudram, K.; Epling, W.S. A comparison of hydrothermal aging effects on NH3-SCR of NOx over Cu-SSZ-13 and Cu-SAPO-34 catalysts. Appl. Catal. B Environ. 2015, 165, 438–445. [Google Scholar] [CrossRef]
- Leistner, K.; Mihai, O.; Wijayanti, K.; Kumar, A.; Kamasamudram, K.; Currier, N.W.; Yezerets, A.; Olsson, L. Comparison of Cu/BEA, Cu/SSZ-13 and Cu/SAPO-34 for ammonia-SCR reactions. Catal. Today 2015, 258, 49–55. [Google Scholar] [CrossRef]
- Tian, P.; Wei, Y.; Ye, M.; Liu, Z. Methanol to olefins (MTO): From fundamentals to commercialization. ACS Catal. 2015, 5, 1922–1938. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Z.; Wei, X.; Guo, F.; Li, P.; Guo, S. Synthesis of 2, 6-dimethylnaphthalene over SAPO-11, SAPO-5 and mordenite molecular sieves. Braz. J. Chem. Eng. 2017, 34, 295–306. [Google Scholar] [CrossRef]
- Wei, X.-L.; Lu, X.-H.; Zhang, T.-J.; Chu, X.; Zhou, D.; Nie, R.-F.; Xia, Q.-H. Synthesis and catalytic application of SAPO-5 by dry-gel conversion for the epoxidation of styrene with air. Microporous Mesoporous Mater. 2015, 214, 80–87. [Google Scholar] [CrossRef]
- Ahoba-Sam, C.; Erichsen, M.W.; Olsbye, U. Ethene and butene oligomerization over isostructural H-SAPO-5 and H-SSZ-24: Kinetics and mechanism. Chin. J. Catal. 2019, 40, 1766–1777. [Google Scholar] [CrossRef]
- Li, G.; Wang, B.; Chen, B.; Resasco, D.E. Role of water in cyclopentanone self-condensation reaction catalyzed by MCM-41 functionalized with sulfonic acid groups. J. Catal. 2019, 377, 245–254. [Google Scholar] [CrossRef]
- Grand, J.; Talapaneni, S.N.; Vicente, A.; Fernandez, C.; Dib, E.; Aleksandrov, H.A.; Vayssilov, G.N.; Retoux, R.; Boullay, P.; Gilson, J.-P. One-pot synthesis of silanol-free nanosized MFI zeolite. Nat. Mater. 2017, 16, 1010. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, W.; Chen, Y.; Wen, X.; Li, H.; Yuan, D.; Guo, Q.; Ren, S.; Pang, X.; Shen, B. Mild-acid-assisted thermal or hydrothermal dealumination of zeolite beta, its regulation to Al distribution and catalytic cracking performance to hydrocarbons. J. Catal. 2018, 362, 94–105. [Google Scholar] [CrossRef]
- Sun, X.; Wang, J.; Chen, J.; Zheng, J.; Shao, H.; Huang, C. Dehydration of fructose to 5-hydroxymethylfurfural over MeSAPOs synthesized from bauxite. Microporous Mesoporous Mater. 2018, 259, 238–243. [Google Scholar] [CrossRef]
- Ketzer, F.; Celante, D.; de Castilhos, F. Catalytic performance and ultrasonic-assisted impregnation effects on WO3/USY zeolites in esterification of oleic acid with methyl acetate. Microporous Mesoporous Mater. 2020, 291, 109704. [Google Scholar] [CrossRef]
- Kim, K.; Ryoo, R.; Jang, H.-D.; Choi, M. Spatial distribution, strength, and dealumination behavior of acid sites in nanocrystalline MFI zeolites and their catalytic consequences. J. Catal. 2012, 288, 115–123. [Google Scholar] [CrossRef]
- Freitas, E.F.; Araujo, A.A.; Paiva, M.F.; Dias, S.C.; Dias, J.A. Comparative acidity of BEA and Y zeolite composites with 12-tungstophosphoric and 12-tungstosilicic acids. Mol. Catal. 2018, 458, 152–160. [Google Scholar] [CrossRef]
- Chen, L.; Ping, Z.; Chuah, G.; Jaenicke, S.; Simon, G. A comparison of post-synthesis alumination and sol-gel synthesis of MCM-41 with high framework aluminum content. Microporous Mesoporous Mater. 1999, 27, 231–242. [Google Scholar] [CrossRef]
- Choudhary, V.R.; Jana, S.K. Benzylation of benzene and substituted benzenes by benzyl chloride over InCl3, GaCl3, FeCl3 and ZnCl2 supported on clays and Si-MCM-41. J. Mol. Catal. A Chem. 2002, 180, 267–276. [Google Scholar] [CrossRef]
- Hu, X.; Chuah, G.K.; Jaenicke, S. Room temperature synthesis of diphenylmethane over MCM-41 supported AlCl3 and other Lewis acids. Appl. Catal. A Gen. 2001, 217, 1–9. [Google Scholar] [CrossRef]
- Lili, Q.; Min, J.; Xinkui, W.; Min, H.; Tianxi, C. Isomerization of bridged tetrahydrodicyclopentadiene over AlCl3/MCM-41 catalyst. Chin. J. Catal. 2010, 31, 383–385. [Google Scholar]
- Sadjadi, S.; Lazzara, G.; Heravi, M.M.; Cavallaro, G. Pd supported on magnetic carbon coated halloysite as hydrogenation catalyst: Study of the contribution of carbon layer and magnetization to the catalytic activity. Appl. Clay Sci. 2019, 182, 105299. [Google Scholar] [CrossRef]
- Sadjadi, S.; Lazzara, G.; Malmir, M.; Heravi, M.M. Pd nanoparticles immobilized on the poly-dopamine decorated halloysite nanotubes hybridized with N-doped porous carbon monolayer: A versatile catalyst for promoting Pd catalyzed reactions. J. Catal. 2018, 366, 245–257. [Google Scholar] [CrossRef]
- Clark, J.H. Green chemistry: Challenges and opportunities. Green Chem. 1999, 1, 1–8. [Google Scholar] [CrossRef]
- Choo, M.-Y.; Juan, J.C.; Oi, L.E.; Ling, T.C.; Ng, E.-P.; Noorsaadah, A.R.; Centi, G.; Lee, K.T. The role of nanosized zeolite Y in the H2-free catalytic deoxygenation of triolein. Catal. Sci. Technol. 2019, 9, 772–782. [Google Scholar] [CrossRef]
- Choudhary, V.R.; Mantri, K.; Jana, S.K. Highly selective Si-MCM-41 supported InCl3, GaCl3, FeCl3 and ZnCl2 catalysts for low temperature esterification of tert-butanol by acetic anhydride. Microporous Mesoporous Mater. 2001, 47, 179–183. [Google Scholar] [CrossRef]
- International Zeolite Association. Database of Zeolite Structures. 2020. Available online: http://www.iza-structure.org/databases/ (accessed on 20 April 2020).
- Adam, F.; Appaturi, J.N.; Ng, E.-P. Halide aided synergistic ring opening mechanism of epoxides and their cycloaddition to CO2 using MCM-41-imidazolium bromide catalyst. J. Mol. Catal. A Chem. 2014, 386, 42–48. [Google Scholar] [CrossRef]
- Khoo, D.Y.; Awala, H.; Mintova, S.; Ng, E.-P. Synthesis of AlPO-5 with diol-substituted imidazolium-based organic template. Microporous Mesoporous Mater. 2014, 194, 200–207. [Google Scholar] [CrossRef]
- Geng, L.; Dong, H.; Liu, X.; Zhang, B. Efficient manipulation of continuous AFI-type aluminophosphate membranes with distinctive microstructures on macroporous α-Al2O3 substrates. Molecules 2018, 23, 1127. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wright, P.; Natarajan, S.; Thomas, J. Understanding the Brønsted acidity of SAPO-5, SAPO-17, SAPO-18 and SAPO-34 and their catalytic performance for methanol conversion to hydrocarbons. In Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 1994; Volume 84, pp. 1731–1738. [Google Scholar]
- Pfennig, B.W. Principles of Inorganic Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- Zerk, T.J.; Bernhardt, P.V. Redox-Coupled structural changes in copper chemistry: Implications for atom transfer catalysis. Coord. Chem. Rev. 2018, 375, 173–190. [Google Scholar] [CrossRef]
- Ginés-Molina, M.J.; Ahmad, N.H.; Mérida-Morales, S.; García-Sancho, C.; Mintova, S.; Eng-Poh, N.; Maireles-Torres, P. Selective conversion of glucose to 5-Hydroxymethylfurfural by using L-type zeolites with different morphologies. Catalysts 2019, 9, 1073. [Google Scholar] [CrossRef]
- Kumar, N.; Villegas, J.I.; Salmi, T.; Murzin, D.Y.; Heikkilä, T. Isomerization of n-butane to isobutane over Pt-SAPO-5, SAPO-5, Pt-H-mordenite and H-mordenite catalysts. Catal. Today 2005, 100, 355–361. [Google Scholar] [CrossRef]
- Obermayer, D.; Znidar, D.; Glotz, G.; Stadler, A.; Dallinger, D.; Kappe, C.O. Design and performance validation of a conductively heated sealed-vessel reactor for organic synthesis. J. Org. Chem. 2016, 81, 11788–11801. [Google Scholar] [CrossRef]
- Ahmad, N.H.; Daou, T.J.; Maireles-Torres, P.; Zaarour, M.; Mintova, S.; Ling, T.-C.; Ng, E.-P. Morphological effects on catalytic performance of LTL zeolites in acylation of 2-methylfuran enhanced by non-microwave instant heating. Mater. Chem. Phys. 2020, 244, 122688. [Google Scholar] [CrossRef]
- Ji, Y.; Pan, J.; Dauenhauer, P.; Gorte, R.J. Probing direct carbon-carbon acylation of furans and long-chain acids over H-ZSM-5. Appl. Catal. A Gen. 2019, 577, 107–112. [Google Scholar] [CrossRef]
- Wang, Z.; Li, H.; Zhao, W.; Yang, S. Low-Temperature and solvent-free production of biomass-derived diesel-range C17 precursor via one-pot cascade acylation-alkylation over Sn4+-montmorillonite. J. Ind. Eng. Chem. 2018, 66, 325–332. [Google Scholar] [CrossRef]
- Xiong, Y.; Chen, W.; Zeng, A. Optimization for catalytic performances of Hβ zeolite in the acylation of 2-methylfuran by surface modification and solvents effect. Res. Chem. Intermed. 2017, 43, 1557–1574. [Google Scholar] [CrossRef]
- Koehle, M.; Zhang, Z.; Goulas, K.A.; Caratzoulas, S.; Vlachos, D.G.; Lobo, R.F. Acylation of methylfuran with Brønsted and Lewis acid zeolites. Appl. Catal. A Gen. 2018, 564, 90–101. [Google Scholar] [CrossRef]
- Ghrear, T.M.A.; Wong, K.L.; Tan, S.H.; Ling, T.C.; Awala, H.; Ng, E.-P. Organotemplate-Free Cs-ABW nanozeolite as highly reactive and recyclable catalyst for Henry reaction between benzaldehyde and nitroethane. Turk. J. Chem. 2019, 43, 568–581. [Google Scholar] [CrossRef]
- Rothenberg, G. Catalysis: Concepts and Green Applications; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Liu, F.; Wang, T.; Zheng, Y.; Wang, J. Synergistic effect of Brønsted and Lewis acid sites for the synthesis of polyoxymethylene dimethyl ethers over highly efficient SO42−/TiO2 catalysts. J. Catal. 2017, 355, 17–25. [Google Scholar] [CrossRef]





| Element | Samples (wt%) | |||||
|---|---|---|---|---|---|---|
| SAPO-5 | CuClx/SAPO-5 | CoClx/SAPO-5 | SnClx/SAPO-5 | FeClx/SAPO-5 | ZnClx/SAPO-5 | |
| C | 18.47 | 42.15 | 39.86 | 5.14 | 17.14 | 23.10 |
| O | 39.63 | 21.36 | 20.20 | 35.92 | 34.01 | 30.90 |
| Al | 16.90 | 8.91 | 8.42 | 15.20 | 14.65 | 13.25 |
| Si | 2.16 | 1.11 | 1.05 | 1.93 | 1.81 | 1.55 |
| P | 16.36 | 9.05 | 8.56 | 14.87 | 13.91 | 12.82 |
| Cl | - | 5.76 | 5.45 | 5.96 | 7.61 | 5.06 |
| Cu | - | 5.34 | - | - | - | - |
| Pt | 6.49 | 6.32 | 5.98 | 4.73 | 3.31 | 5.13 |
| Co | - | - | 5.05 | - | - | - |
| Sn | - | - | - | 16.24 | - | - |
| Fe | - | - | - | - | 7.54 | - |
| Zn | - | - | - | - | - | 7.77 |
| Total | 100 | 100 | 100 | 100 | 100 | 100 |
| Sample | Molecular Formula | Si/(Al + P + Si) Ratio | Cl/M Ratio a |
|---|---|---|---|
| SAPO-5 | Si1.485Al12.093P10.196O47.858 | 0.062 | - |
| CuClx/SAPO-5 | Cu3.019Cl5.826Si1.419Al11.843P10.486O47.926 | 0.060 | 1.93 (2) |
| CoClx/SAPO-5 | Co2.957Cl5.825Si1.455Al12.012P10.281O47.817 | 0.061 | 1.97 (2) |
| SnClx/SAPO-5 | Sn2.916Cl3.576Si1.464Al11.993P10.221O47.827 | 0.062 | 1.23 (4) |
| FeClx/SAPO-5 | Fe3.039Cl4.825Si1.455Al12.210P10.098O47.838 | 0.061 | 1.59 (3) |
| ZnClx/SAPO-5 | Zn2.945Cl3.535Si1.369Al12.159P10.252O47.865 | 0.059 | 1.20 (2) |
| Samples | d100 Spacing (Å) | ao (Å) a | SBET (m2/g) b | D (nm) c | Vtot (cm3/g) d |
|---|---|---|---|---|---|
| SAPO-5 | 11.95 | 13.80 | 257 | 37.9 | 0.35 |
| CuClx/SAPO-5 | 11.84 | 13.67 | 130 | 26.7 | 0.16 |
| CoClx/SAPO-5 | 11.85 | 13.68 | 122 | 24.7 | 0.07 |
| SnClx/SAPO-5 | 11.86 | 13.69 | 107 | 23.3 | 0.09 |
| FeClx/SAPO-5 | 11.85 | 13.68 | 115 | 26.1 | 0.12 |
| ZnClx/SAPO-5 | 11.82 | 13.65 | 89 | 19.3 | 0.04 |
| Samples | Py-FTIR Acidity (µmol/g) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lewis Acid Sites (L) | Brönsted Acid Sites (B) | Total Acid Sites (L + B) | L/B Ratio | |||||||||
| 25 °C | 150 °C | 300 °C | 25 °C | 150 °C | 300 °C | 25 °C | 150 °C | 300 °C | 25 °C | 150 °C | 300 °C | |
| SAPO-5 | 432.4 | 71.9 | 8.6 | 33.6 | 26.2 | 24.6 | 466.0 | 98.1 | 33.2 | 12.9 | 2.7 | 0.3 |
| CuClx/SAPO-5 | 370.6 | 126.6 | 38.9 | 34.0 | 28.1 | 24.6 | 404.6 | 154.7 | 63.5 | 10.9 | 4.5 | 1.6 |
| CoClx/SAPO-5 | 322.5 | 137.4 | 100.9 | 33.8 | 27.2 | 25.1 | 356.3 | 164.6 | 126.0 | 9.5 | 5.1 | 4.0 |
| SnClx/SAPO-5 | 320.9 | 167.3 | 148.9 | 32.3 | 25.4 | 23.7 | 353.2 | 192.7 | 172.6 | 9.9 | 6.6 | 6.3 |
| FeClx/SAPO-5 | 534.1 | 290.8 | 219.1 | 31.4 | 26.2 | 25.2 | 565.5 | 317.0 | 244.3 | 17.0 | 11.1 | 8.7 |
| ZnClx/SAPO-5 | 496.8 | 444.9 | 83.5 | 31.2 | 27.7 | 22.8 | 528.0 | 472.6 | 106.3 | 15.9 | 16.1 | 3.7 |
| Catalyst | Conversion (%) |
|---|---|
| SAPO-5 | 45.1 |
| CuClx/SAPO-5 | 66.7 |
| CoClx/SAPO-5 | 72.3 |
| SnClx/SALP-5 | 81.5 |
| FeClx/SAPO-5 | 87.8 |
| ZnClx/SAPO-5 | 94.5 |
| H2SO4 | 68.4 |
| CH3COOH | 32.7 |
| HNO3 | 56.2 |
| ZnCl2 | 75.7 |
| FeCl3 | 68.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Auwal, I.A.; Wong, K.-L.; Ling, T.C.; Ooi, B.S.; Ng, E.-P. Metal Chlorides Grafted on SAPO-5 (MClx/SAPO-5) as Reusable and Superior Catalysts for Acylation of 2-Methylfuran Under Non-Microwave Instant Heating Condition. Processes 2020, 8, 603. https://doi.org/10.3390/pr8050603
Auwal IA, Wong K-L, Ling TC, Ooi BS, Ng E-P. Metal Chlorides Grafted on SAPO-5 (MClx/SAPO-5) as Reusable and Superior Catalysts for Acylation of 2-Methylfuran Under Non-Microwave Instant Heating Condition. Processes. 2020; 8(5):603. https://doi.org/10.3390/pr8050603
Chicago/Turabian StyleAuwal, Ismail Alhassan, Ka-Lun Wong, Tau Chuan Ling, Boon Seng Ooi, and Eng-Poh Ng. 2020. "Metal Chlorides Grafted on SAPO-5 (MClx/SAPO-5) as Reusable and Superior Catalysts for Acylation of 2-Methylfuran Under Non-Microwave Instant Heating Condition" Processes 8, no. 5: 603. https://doi.org/10.3390/pr8050603
APA StyleAuwal, I. A., Wong, K.-L., Ling, T. C., Ooi, B. S., & Ng, E.-P. (2020). Metal Chlorides Grafted on SAPO-5 (MClx/SAPO-5) as Reusable and Superior Catalysts for Acylation of 2-Methylfuran Under Non-Microwave Instant Heating Condition. Processes, 8(5), 603. https://doi.org/10.3390/pr8050603








