Optimizing Yield and Quality of Bio-Oil: A Comparative Study of Acacia tortilis and Pine Dust
Abstract
:1. Introduction
1.1. Socio-Economic Background
1.2. Technical Background
1.3. The State of Research
1.4. The Purpose and Significance of This Research
2. Materials and Methods
2.1. Methods of Feedstock Preparation and Characterization
2.2. Pyrolysis
2.3. Characterization of Products
3. Results
3.1. Characterization of Feedstocks
3.2. Optimum Pyrolysis Conditions
3.2.1. Optimum Pyrolysis Temperature
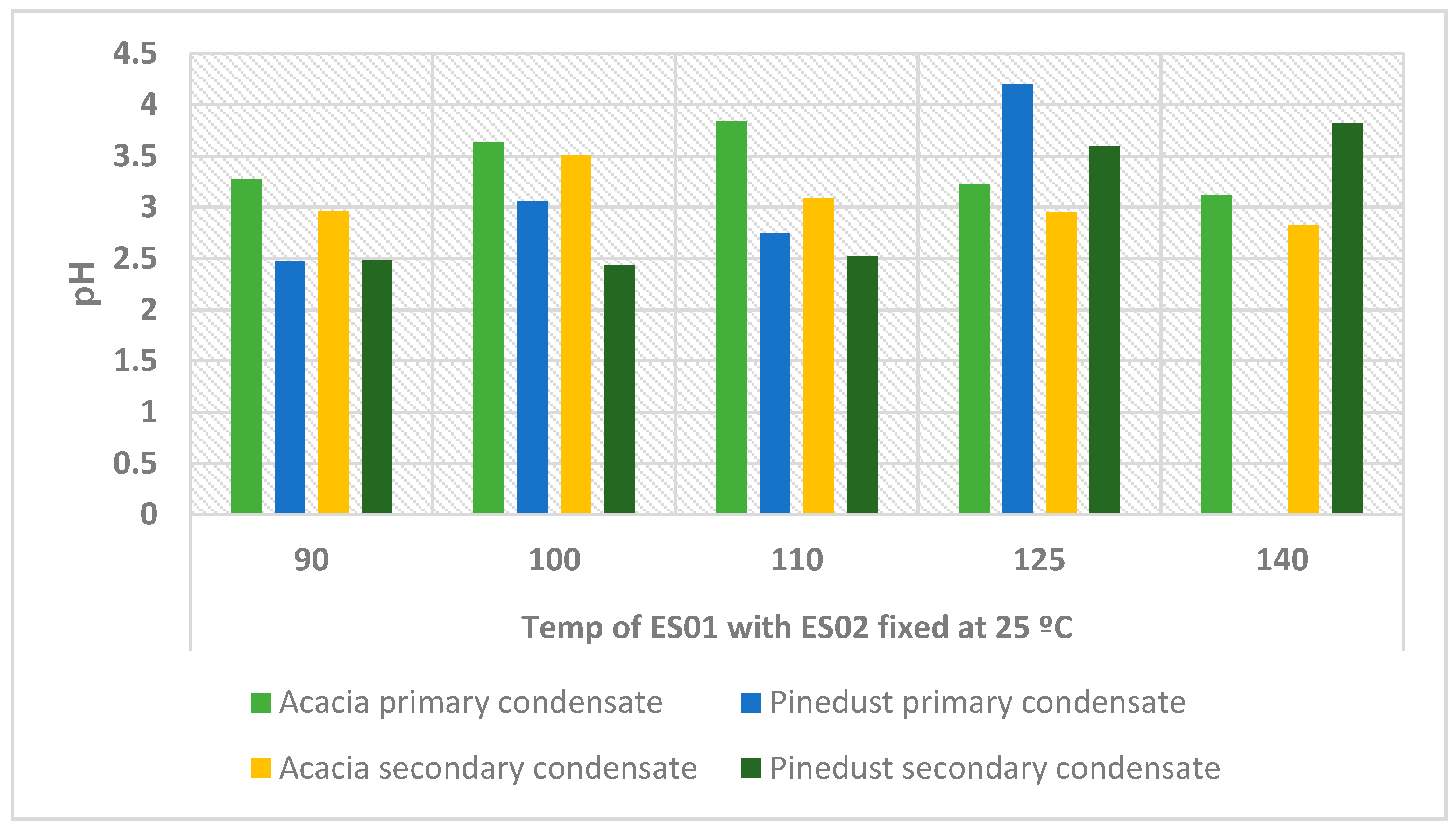
3.2.2. The Effect of Fractionation on pH
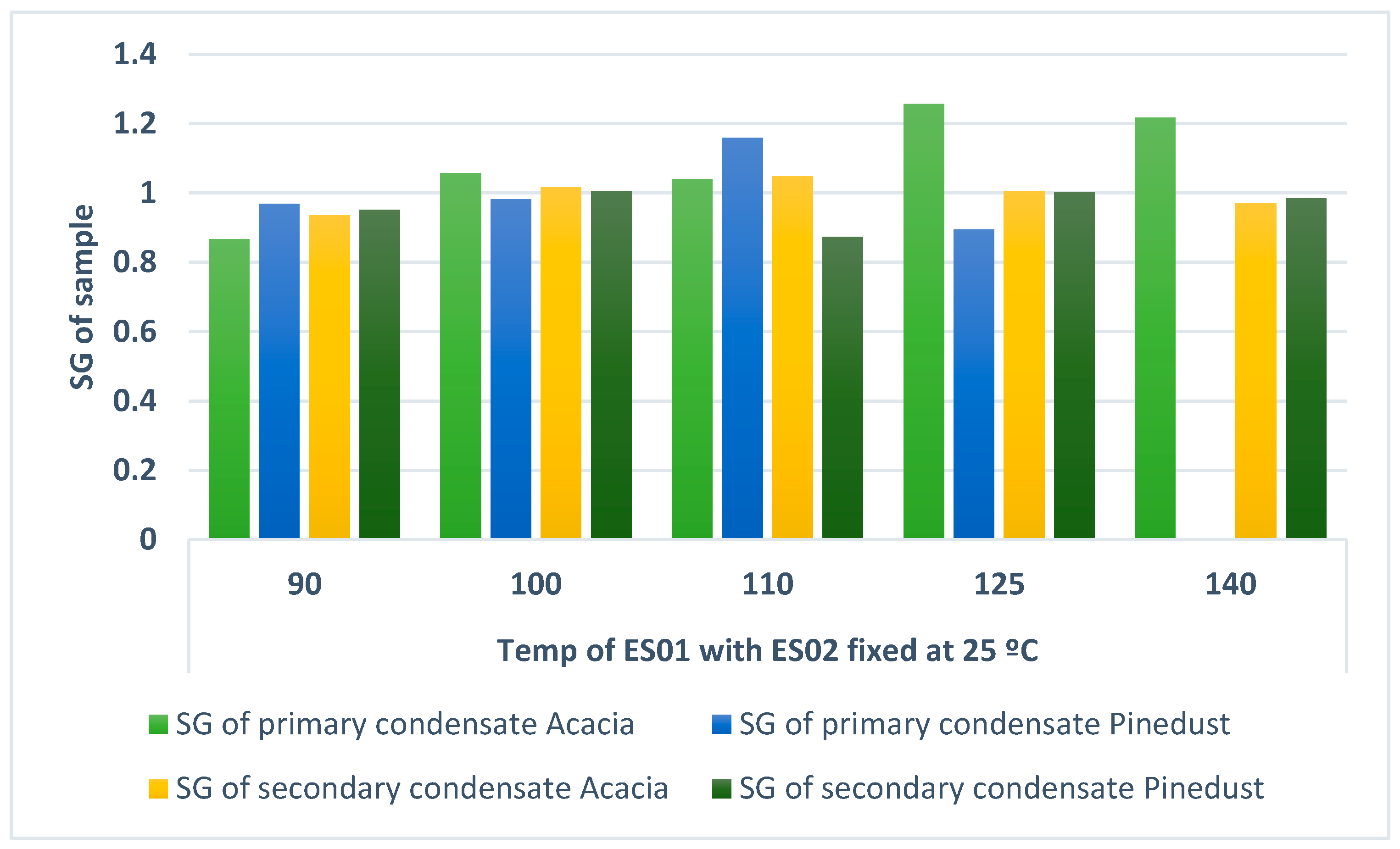
3.2.3. The Effect of Fractionation Temperature on SG, Heavy Oil Yields and Quality
3.2.4. Properties of the Bio-Oils Obtained at Various Condenser temperatures
Physico-Chemical Properties of the Heavier (Choice) Bio-Oil
The Chemical Composition of the Bio-Oils
3.2.5. Uncertainty Analysis
4. Overall Discussion
5. Conclusions and Recommendations
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Deenanath, E.D.; Iyuke, S.; Rumbold, K. The bioethanol industry in sub-Saharan Africa: History, challenges, and prospects. J. Biomed. Biotechnol. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Neupane, B.; Rubin, J. Implications of U.S. biofuels policy for sustainable transportation energy in Maine and the Northeast. Renew. Sustain. Energy Rev. 2016, 70, 729–735. [Google Scholar] [CrossRef] [Green Version]
- Gooty, A.T. Fractional Condensation of Bio-Oil Vapors; Western University: London, UK, 2012. [Google Scholar]
- Batidzirai, B.; Smeets, E.M.W.; Faaij, A.P.C. Bioenergy for Sustainable Development in Africa; Springer Science & Business Media: Berlin, Germany, 2012; pp. 117–130. [Google Scholar] [CrossRef]
- Walker, G.M. Bioethanol: Science and Technology of Fuel Alcohol; Bookboon: London, UK, 2012. [Google Scholar]
- Charis, G.; Danha, G.; Muzenda, E. The socio-economic implication of 2nd generation biofuels in Southern Africa: A critical review. In Proceedings of the International Conference on Industrial Engineering and Operations Management, Paris, France, 26–27 July 2018. [Google Scholar]
- Gasparatos, A.; von Maltitz, G.P.; Johnson, F.X.; Lee, L.; Mathai, M.; De Oliveira, J.P.; Willis, K.J. Biofuels in sub-Sahara Africa: Drivers, impacts and priority policy areas. Renew. Sustain. Energy Rev. 2015, 45, 879–901. [Google Scholar] [CrossRef]
- Amigun, B.; Kaviti, J.; Stafford, W. Biofuels and sustainability in Africa. Renew. Sustain. Energy Rev. 2011, 15, 1360–1372. [Google Scholar] [CrossRef]
- Von Maltitz, G.P.; Setzkorn, K.A. A typology of Southern African biofuel feedstock production projects. Biomass Bioenergy 2013, 59, 33–49. [Google Scholar] [CrossRef]
- Blimpo, M.P.; Cosgrove-Davies, M. Electricity Access in Sub-Saharan Africa: Uptake, Reliability, and Complementary Factors for Economic Impact; The World Bank: Washington, DC, USA, 2019. [Google Scholar]
- Kass, M. Evaluation of Bio-oils for Use in Marine Engines; The Bioenergy Technology Office of the U.S. Department of Energy: Oak Ridge, TN, USA, 2019.
- Charis, G.; Danha, G.; Muzenda, E. Waste valorisation opportunities for bush encroacher biomass in savannah ecosystems: A comparative case analysis of Botswana and Namibia. Procedia Manuf. 2019, 35, 974–979. [Google Scholar] [CrossRef]
- Charis, G.; Danha, G.; Muzenda, E. A review of timber waste utilization: Challenges and opportunities in Zimbabwe. Procedia Manuf. 2019, 35, 419–429. [Google Scholar] [CrossRef]
- Montoya, J.I.; Valdés, C.; Chejne, F.; Gómez, C.A.; Blanco, A.; Marrugo, G.; Osorio, J.; Castillo, E.; Aristóbulo, J.; Acero, J. Bio-oil production from Colombian bagasse by fast pyrolysis in a fluidized bed: An experimental study. J. Anal. Appl. Pyrolysis 2015, 112, 379–387. [Google Scholar] [CrossRef]
- Bridgwater, A.V. Review of fast pyrolysis of biomass and product upgrading. Biomass Bioenergy 2011, 38, 68–94. [Google Scholar] [CrossRef]
- Pratap, A.; Chouhan, S. Critical Analysis of Process Parameters for Bio-oil Production via Pyrolysis of Biomass: A Review. Recent Patents Eng. 2013. [Google Scholar] [CrossRef]
- Badger, P.; Badger, S.; Puettmann, M.; Steele, P.; Cooper, J. Techno-Economic Analysis: Preliminary Assessment of Pyrolysis Oil Production Costs and Material Energy Balance Associated with a Transportable Fast Pyrolysis System. Bioresources 2011, 6, 34–47. [Google Scholar]
- Fonts, I.; Juan, A.; Gea, G.; Murillo, M.B.; Sa, L. Sewage Sludge Pyrolysis in Fluidized Bed, 1: Influence of Operational Conditions on the Product Distribution. Ind. Eng. Chem. Res. 2008, 47, 5376–5385. [Google Scholar] [CrossRef]
- Sirijanusorn, S.; Sriprateep, K.; Pattiya, A. Bioresource Technology Pyrolysis of cassava rhizome in a counter-rotating twin screw reactor unit. Bioresour. Technol. 2013, 139, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Guedes, R.E.; Luna, A.S.; Torres, A.R. Operating parameters for bio-oil production in biomass pyrolysis: A review. J. Anal. Appl. Pyrolysis 2018, 129, 134–149. [Google Scholar] [CrossRef]
- Ellens, C.J.; Brown, R.C. Optimization of a free-fall reactor for the production of fast pyrolysis bio-oil. Bioresour. Technol. 2012, 103, 374–380. [Google Scholar] [CrossRef]
- Bridgwater, A.V.; Meier, D.; Radlein, D. An overview of fast pyrolysis of biomass. Org. Geochem. 1999, 30, 1479–1493. [Google Scholar] [CrossRef]
- Uddin, M.N.; Techato, K.; Taweekun, J.; Rahman, M.M.; Rasul, M.G.; Mahlia, T.M.I.; Ashrafur, S.M. An overview of recent developments in biomass pyrolysis technologies. Energies 2018, 11, 3115. [Google Scholar] [CrossRef] [Green Version]
- Papari, S.; Hawboldt, K. A review on the condensing systems for biomass pyrolysis process. Fuel Process. Technol. 2018. [Google Scholar] [CrossRef]
- Chen, T.; Deng, C.; Liu, R. Effect of Selective Condensation on the Characterization of Bio-oil from Pine Sawdust Fast Pyrolysis Using a Fluidized-Bed Reactor. Energy Fuels 2010, 24. [Google Scholar] [CrossRef]
- Oasmaa, A.; Sipilä, K.; Solantausta, Y.; Kuoppala, E. Quality Improvement of Pyrolysis Liquid: Effect of Light Volatiles on the Stability of Pyrolysis Liquids. Energy Fuels 2005, 19, 2556–2561. [Google Scholar] [CrossRef]
- Charis, G.; Danha, G.; Muzenda, E. Thermal and chemical characterization of lignocellulosic wastes for energy uses. In Wastes: Solutions, Treatments and Opportunities, Proceedings of the 5th International Conference Wastes 2019, Lisbon, Portugal, 4–6 September 2019; CRC Press: Boca Raton, FL, USA, 2018; pp. 2–7. [Google Scholar]
- Charis, G.; Danha, G.; Muzenda, E. Selective bio-oil fraction optimization and characterization for waste lignocellulosic feedstocks. 2019. Unpublished. [Google Scholar]
- Olarte, M.V.; Olarte, M.V.; Christensen, E.D.; Padmaperuma, A.B.; Connatser, R.M.; Stankovikj, F.; Meier, D.; Paasikallio, V. Standardization of chemical analytical techniques for pyrolysis. Biofuels Bioprod. Biorefin. 2016, 10, 496–507. [Google Scholar]
- Ngo, T.; Kim, J.; Kim, S. Fast pyrolysis of palm kernel cake using a fluidized bed reactor: Design of experiment and characteristics of bio-oil. J. Ind. Eng. Chem. 2013, 19, 137–143. [Google Scholar] [CrossRef]
- Lira, C.S.; Berruti, F.M.; Palmisano, P.; Berruti, F.; Briens, C.; Pécora, A.A.B. Fast pyrolysis of Amazon tucumã (Astrocaryum aculeatum) seeds in a bubbling fluidized bed reactor. J. Anal. Appl. Pyrolysis 2013, 99, 23–31. [Google Scholar] [CrossRef]
- Pauls, R.E. A Review of Chromatographic Characterization Techniques for Biodiesel and Biodiesel Blends. J. Chromatogr. Sci. 2011, 49, 384–396. [Google Scholar] [CrossRef] [Green Version]
- Mccormick, R.L.; Ratcliff, M.; Moens, L.; Lawrence, R. Several factors affecting the stability of biodiesel in standard accelerated tests. Fuel Process. Technol. 2007, 651–657. [Google Scholar] [CrossRef]
- Reza, S.; Ahmed, A.; Caesarendra, W.; Abu Bakar, M.S.; Shams, S.; Saidur, R.; Aslfattahi, N.; Azad, A.K. Acacia Holosericea: An Invasive Species for Bio-char, Bio-oil, and Biogas Production. Bioengineering 2019, 6, 33. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, A.; Bakar, M.S.A.; Azad, A.K.; Sukri, R.S.; Mahlia, T.M.I. Potential thermochemical conversion of bioenergy from Acacia species in Brunei Darussalam: A review. Renew. Sustain. Energy Rev. 2018, 82, 3060–3076. [Google Scholar] [CrossRef]
- Chaula, Z.; Said, M.; John, G. Thermal Characterization of Pine Sawdust as Energy Source Feedstock. J. Energy Technol. Policy 2014, 4, 57–65. [Google Scholar]
- Olatunji, O.; Akinlabi, S.; Oluseyi, A.; Peter, M.; Madushele, N. Experimental investigation of thermal properties of Lignocellulosic biomass: A review. IOP Conf. Ser. Mater. Sci. Eng. 2018, 413. [Google Scholar] [CrossRef]
- Mishra, R.K.; Mohanty, K. Thermal and catalytic pyrolysis of pine sawdust (Pinus ponderosa) and Gulmohar seed (Delonix regia) towards production of fuel and chemicals. Mater. Sci. Energy Technol. 2019, 2, 139–149. [Google Scholar] [CrossRef]
- Salehi, E.; Abedi, J.; Harding, T. Bio-oil from sawdust: Pyrolysis of sawdust in a fixed-bed system. Energy Fuels 2009, 23, 3767–3772. [Google Scholar] [CrossRef]
- Ahmed, A.; Bakar, M.S.A.; Azad, A.K.; Sukri, R.S.; Phusunti, N. Intermediate pyrolysis of Acacia cincinnata and Acacia holosericea species for bio-oil and biochar production. Energy Convers. Manag. 2018, 176, 393–408. [Google Scholar] [CrossRef]
- Uzun, B.B.; Pütün, A.E.; Pütün, E. Fast pyrolysis of soybean cake: Product yields and compositions. Bioresour. Technol. 2006, 97, 569–576. [Google Scholar] [CrossRef]
- Bridgwater, T. Challenges and Opportunities in Fast Pyrolysis of Biomass. Johns. Matthey Technol. Rev. 2018, 62, 118–130. [Google Scholar] [CrossRef]
- Gauthier-maradei, P.; Manrique, M.; Gauthier, P.; Mantilla, S.V. Methodology for extraction of phenolic compounds of bio-oil from agricultural biomass wastes. Waste Biomass Valorization 2015, 6, 371–383. [Google Scholar] [CrossRef]
- Balat, M. Bio-oil production from pyrolysis of black locust (Robinia pseudoacacia) wood. Energy Explor. Exploit. 2010, 28, 173–186. [Google Scholar] [CrossRef]
- Lyu, G.; Wu, S.; Zhang, H. Estimation and comparison of bio-oil components from different pyrolysis conditions. Front. Energy Res. 2015, 3, 28. [Google Scholar] [CrossRef] [Green Version]
- Muda, N.A.; Yoshida, H.; Ishak, H.; Ismail, M.H.S.; Izhar, S. Conversion of oil palm trunk into bio-oil via treatment with subcritical water. J. Wood Chem. Technol. 2019, 39, 255–269. [Google Scholar] [CrossRef]
- Kim, J. Bioresource Technology Production, separation and applications of phenolic-rich bio-oil—A review. Bioresour. Technol. 2015, 178, 90–98. [Google Scholar] [CrossRef]
- Fahmy, T.Y.A.; Fahmy, Y.; Mobarak, F.; El-Sakhawy, M.; Abou-Zeid, R.E. Biomass pyrolysis: Past, present, and future. Environ. Dev. Sustain. 2018, 1–16. [Google Scholar] [CrossRef]
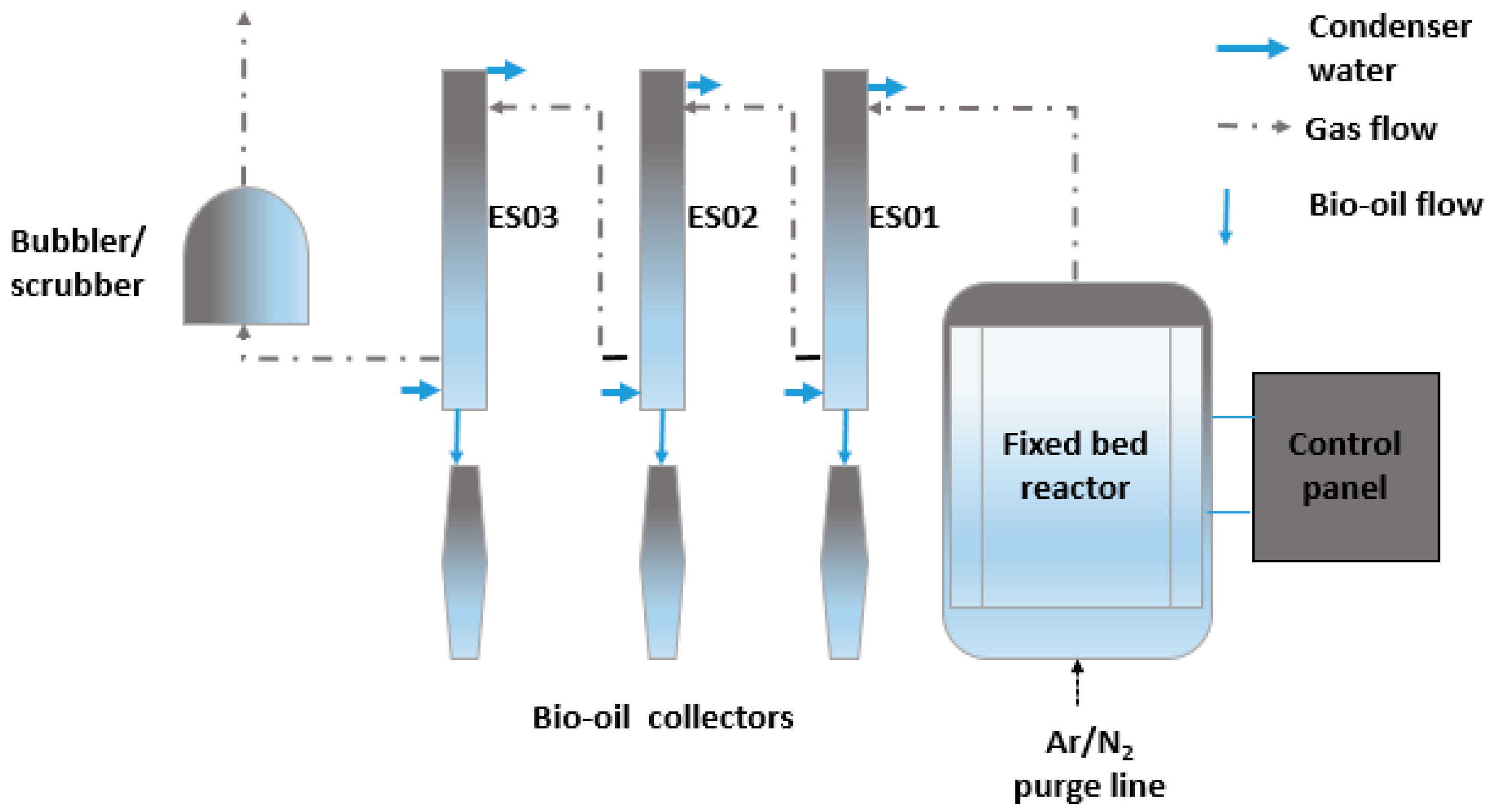


| Pyrolysis Reactor | Description and Operation Philosophy | Operation Complexity and Max Oil Yield | Scale Up | Inert Gas Flow Rate | Particle Size | R&D Highest Status |
|---|---|---|---|---|---|---|
| Fixed bed | Biomass is placed immobile, above the inert gas distributor plate. Char remains in the reactor while oil and gas are collected downstream | Medium; up to 75 wt % oil | Hard | Low | Large | Few at pilot scale; Multiple lab scale |
| Bubbling fluidized bed (BFB) | Comprises reactor section with a continuous feed of biomass and high flow of inert gas to fluidize the particles. Char and sand are collected using cyclones. | Medium; up to 75 wt % oil | Easy | High | Small | Multiple demo and lab-scale plants |
| Circulating fluidized bed (CFB) | Similar to BFB, but collected char and sand are recycled through a combustor, which supplies hot sand to the fluidized bed. | High; up to 75 wt % oil | Hard | High | Medium | Multiple pilot and lab-scale plants |
| Ablative | Heat transfer to the biomass is direct from the walls of the reactor; no fluidizing gas. Biomass melts and vaporizes rapidly to form pyrolysis vapors. | High; up to 75 wt % oil | Hard | Low | Large | Few at pilot scale |
| Rotating cone | Heat transferred by reactor wall and hot sand, introduced into the rotating cone along with the biomass. The hot pyrolysis vapor is recovered from the bottom of the cone. | Medium; up to 70 wt % oil | Medium | Low | Medium | Demo/industrial scale |
| Screw/auger | Heat is mainly transferred by the wall surfaces. The biomass is moved along a heated cylindrical reaction zone by a screw | Low; up to 70 wt % oil | Easy | Low | Medium | Multiple pilot and lab-scale |
| Biomass | Ultimate Composition (%) | Proximate Composition (Dry Basis) (%) | HHV (MJ/kg) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | H | N | S | O * | Ash | * FC | VM | MC | ||
| A. tortilis | 41.47 | 5.15 | 1.23 | nd | 52.15 | 3.90 | 19.59 | 76.51 | 3.72 | 17.27 |
| Pine dust | 45.76 | 5.54 | 0.039 | nd | 48.66 | 0.83 | 20.00 | 79.16 | 6.50 | 17.57 |
| Temp of ES01 | 90 °C | 100 °C | 110 °C | 125 °C | 140 °C | |
|---|---|---|---|---|---|---|
| Acacia tortilis | Heavy oil yield | 6.0% ± 0.2% | 5.4% ± 0.1% | 4.9% ± 0.1% | 2.8% ± 0.1% | 3.9% ± 0.1% |
| Total oil yield | 38.8% ± 1.0% | 41.9% ± 1.0% | 40.8% ± 1.0% | 36.5% ± 0.9% | 37.5% ± 1.0% | |
| Pine dust | Heavy oil yield | 15.4% ± 0.4% | 10.7% ± 0.3% | 12.7% ± 0.3% | 7.2% ± 0.2% | 0.2% |
| Total oil yield | 34.7% ± 0.9% | 46.1% ± 1.2% | 44.8% ± 1.1% | 44.4% ± 1.1% | 33.5% ± 0.9% |
| Viscosity (mPa·s) at 25 °C | HHV (MJ/kg) | SG | |||||
|---|---|---|---|---|---|---|---|
| Primary condenser (ES01) Temp °C | 90 °C | A. tortilis oil | Pine dust oil | A. tortilis oil | Pine dust oil | A. tortilis oil | Pine dust oil |
| 2217.6 | 3043 | 4.310 | 5.227 | 0.867 | 0.968 | ||
| 100 °C | 4804.8 | 13,951 | 21.412 | 9.235 | 1.057 | 0.982 | |
| 110 °C | Too little | 10,151 | 23.610 | 15.780 | 1.040 | 1.159 | |
| 125 °C | Too little | 2142 | 26.191 | 0.6338 | 1.257 | 0.894 | |
| 140 °C | Too little | Very little | 36.809 | Very little | 1.217 | Very little | |
| Conventional diesel | 2178 | 43–45 | 0.844 | ||||
| Light fuel oil (LFO) | - | 42–44 | 0.85–0.910 | ||||
| Heavy fuel oil (HFO) | >17,800 at 50 °C | 40 | 0.940–0.989 | ||||
| Compound | Compound Class | Molecular Weight (g/mol) | Area % | Retention Time (min) | |
|---|---|---|---|---|---|
| Primary condensate | Cresol | Methylphenol | 138.16 | 7.759 | 8.23 |
| Phenol, 4-ethyl-2-methoxy- | Phenol | 152.19 | 5.325 | 10.31 | |
| Phenol, 2,6-dimethoxy- | Phenol | 154.16 | 5.120 | 11.83 | |
| Phenol, 2-methoxy- | Phenol | 164.20 | 4.847 | 5.89 | |
| Benzene, 1,3-bis(1,1-dimethylethyl)- | Hydrocarbon | 190.33 | 4.390 | 10.09 | |
| Phenol, 2,4-bis(1,1-dimethylethyl)- | Phenol | 278.50 | 4.254 | 16.28 | |
| 5-tert-Butylpyrogallol | Phenol | 182.22 | 3.302 | 16.16 | |
| Heneicosane | Alkane hydrocarbon | 296.583 | 3.3148 | 18.57 | |
| Others | |||||
| Benzoic acid | Carboxylic acid | 122.12 | 0,6791 | 8.48 | |
| Cyclopenten-1-one, 2-hydroxy-3-methyl- | Ketone | 68.12 | 2,1704 | 4.73 | |
| Octane, 3-ethyl- | Alkyl hydrocarbon | 142.28 | 1.073 | 5.54 | |
| Furaldehyde phenylhydrazone OR Furfuraldehyde | Aldehyde | 96.0841 | 0.1729 | 19.53 | |
| Secondary condensate | Phenol, 2-methoxy- | Phenol | 164.20 | 15.638 | 6.06 |
| Phenol, 2,6-dimethoxy- | Phenol | 154.16 | 12.377 | 12.38 | |
| 2-Cyclopenten-1-one, 2-hydroxy-3-methyl- | Ketone | 112.13 | 7.533 | 5.01 | |
| Hydroquinone mono-trimethylsilyl ether | Phenol | 110.03 | 6.052 | 16.29 | |
| 4-Methoxy-2-methyl-1-(methylthio)benzene | Hydrocarbon (phenylpropanes) | 168.26 | 5.103 | 14.40 | |
| Benzene, 1,3-bis(1,1-dimethylethyl)- | Hydrocarbon | 190.32 | 3.960 | 10.12 | |
| 2-Cyclopenten-1-one, 3-ethyl-2-hydroxy- | Ketone | 126.15 | 3.764 | 6.83 | |
| Cyclohexanol, 2,2-dichloro-1-methyl- | Alcohol | 183.03 | 2.138 | 4.49 | |
| Others | |||||
| Trans 2-(2-Pentenyl)furan | Furan | 136.19 | 0.2457 | 7.36 | |
| 3,4-dimethylcyclohexanol | Alcohol | 128.21 | 0.3821 | 4.66 |
| Compound | Compound Class | Molecular Weight (g/mol) | Area % | Retention Time (min) | |
|---|---|---|---|---|---|
| Primary condensate | Phenol, 2-methoxy- | Phenol | 164.20 | 11.066 | 6.01 |
| Cresol | Methylphenol | 138.16 | 7.154 | 8.26 | |
| Benzene, 1,3-bis(1,1-dimethylethyl)- | Hydrocarbons | 190.33 | 4.935 | 10.10 | |
| 1,2-Cyclopentanedione, 3-methyl- | Ketone | 183.07 | 4.851 | 4.77 | |
| Hexadecane | Hydrocarbon | 4.1684 | 18.80 | ||
| Phenol, 2,4-bis(1,1-dimethylethyl)- | Phenol | 278.5 | 4.047 | 16.27 | |
| Homovanillyl alcohol | Alcohol | 168.19 | 3.8349 | 16.04 | |
| Phenol, 4-ethyl-2-methoxy- | 152.19 | 3.6806 | 10.32 | ||
| Others | |||||
| trans-Isoeugenol | Phenol | 164.20 | 2.544 | 13.37 | |
| Tridecane, 7-hexyl- | Hydrocarbon | 268.5 | 1.6788 | 22.42 | |
| Propanal, 2-propenylhydrazone | Aromatic aldehyde | 126.15 | |||
| Methoxyacetic acid, nonyl ester | Monocarboxylic acid and ether | 216.32 | 0.2737 | 7.23 | |
| Secondary condensate | Phenol, 2-methoxy- | Phenol | 164.20 | 14.526 | 5.99 |
| Cresol | Methylphenol | 138.16 | 14.093 | 8.38 | |
| Phenol, 4-ethyl-2-methoxy- | Phenol | 152.19 | 9.343 | 10.42 | |
| 2-Cyclopenten-1-one, 2-hydroxy-3-methyl- | Ketone | 112.13 | 7.509 | 4.93 | |
| Phenol, 2-methoxy-4-(1-propenyl)-, (Z)- | Phenol | 164.20 | 4.767 | 14.44 | |
| Phenol, 2-methoxy-4-propyl- | Phenol | 166.22 | 2.871 | 12.53 | |
| Benzene, 1,3-bis(1,1-dimethylethyl)- | Hydrocarbon | 190.32 | 2.281 | 10.10 | |
| Eugenol | Guaiacol (phenol) | 164.20 | 2.276 | 12.23 | |
| Others | |||||
| Propanoic acid, 2-methyl-, 2,2-dimethyl-1-(2-hydroxy-1-methylethyl)propyl ester | Carboxylic acid and ester | 244.37 | 0.7445 | 5.76 | |
| Maltol | Pyranones (ketones) | 126.11 | 1.47 | 6.47 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Charis, G.; Danha, G.; Muzenda, E. Optimizing Yield and Quality of Bio-Oil: A Comparative Study of Acacia tortilis and Pine Dust. Processes 2020, 8, 551. https://doi.org/10.3390/pr8050551
Charis G, Danha G, Muzenda E. Optimizing Yield and Quality of Bio-Oil: A Comparative Study of Acacia tortilis and Pine Dust. Processes. 2020; 8(5):551. https://doi.org/10.3390/pr8050551
Chicago/Turabian StyleCharis, Gratitude, Gwiranai Danha, and Edison Muzenda. 2020. "Optimizing Yield and Quality of Bio-Oil: A Comparative Study of Acacia tortilis and Pine Dust" Processes 8, no. 5: 551. https://doi.org/10.3390/pr8050551
APA StyleCharis, G., Danha, G., & Muzenda, E. (2020). Optimizing Yield and Quality of Bio-Oil: A Comparative Study of Acacia tortilis and Pine Dust. Processes, 8(5), 551. https://doi.org/10.3390/pr8050551





