Denitrification Kinetics of Nitrate by a Heterotrophic Culture in Batch and Fixed-Biofilm Reactors
Abstract
1. Introduction
2. Kinetic Model
2.1. Batch Kinetic Model
2.2. Determination of Biokinetic Parameters
2.3. Biofilm Kinetic Model
2.4. Model Numerical Solution
3. Materials and Methods
3.1. Inoculum and Culture Medium
3.2. Supporting Media
3.3. Batch Tests
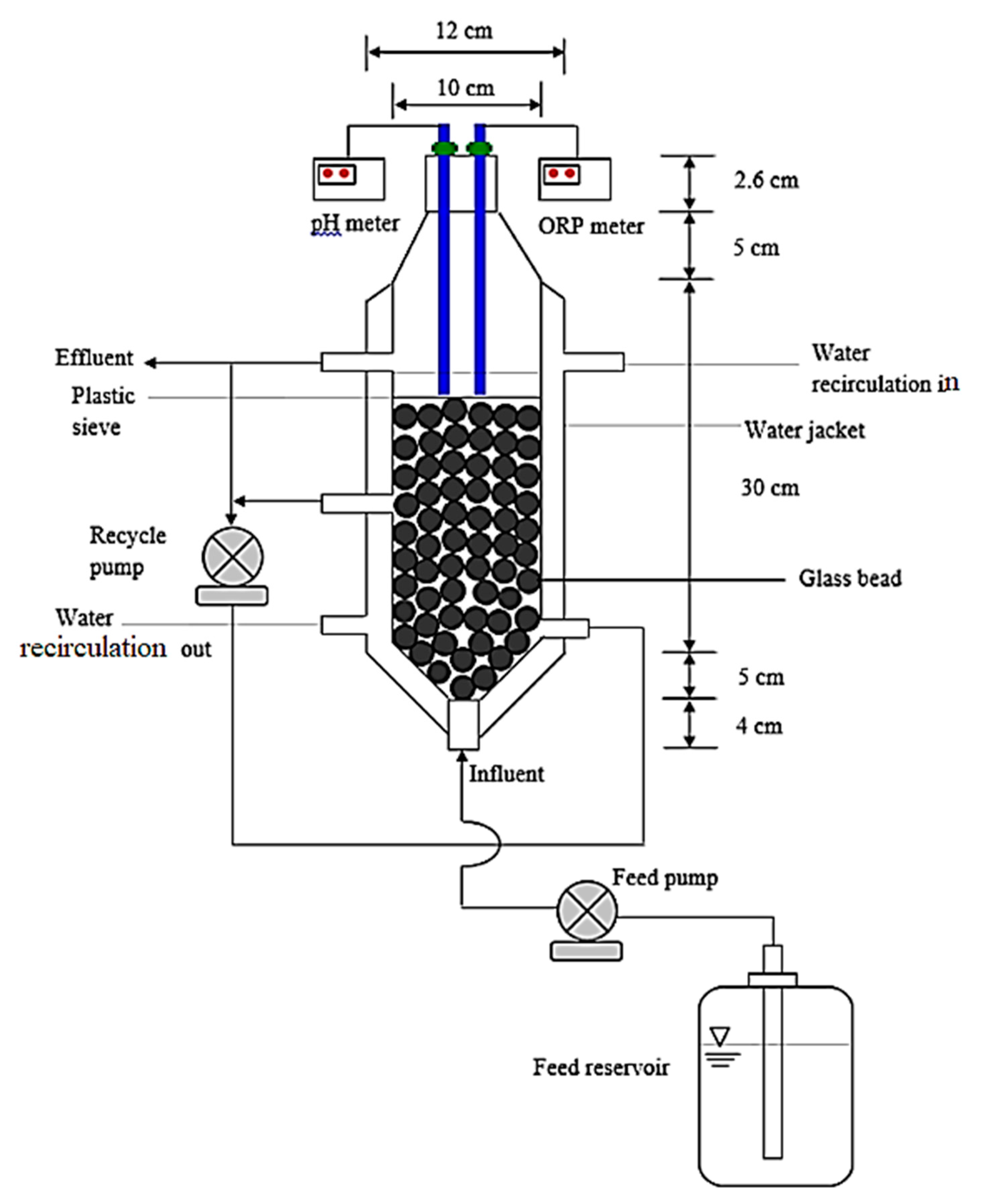
3.4. Continuous-Flow Bioreactor
3.5. Analytical Methods
4. Results and Discussion
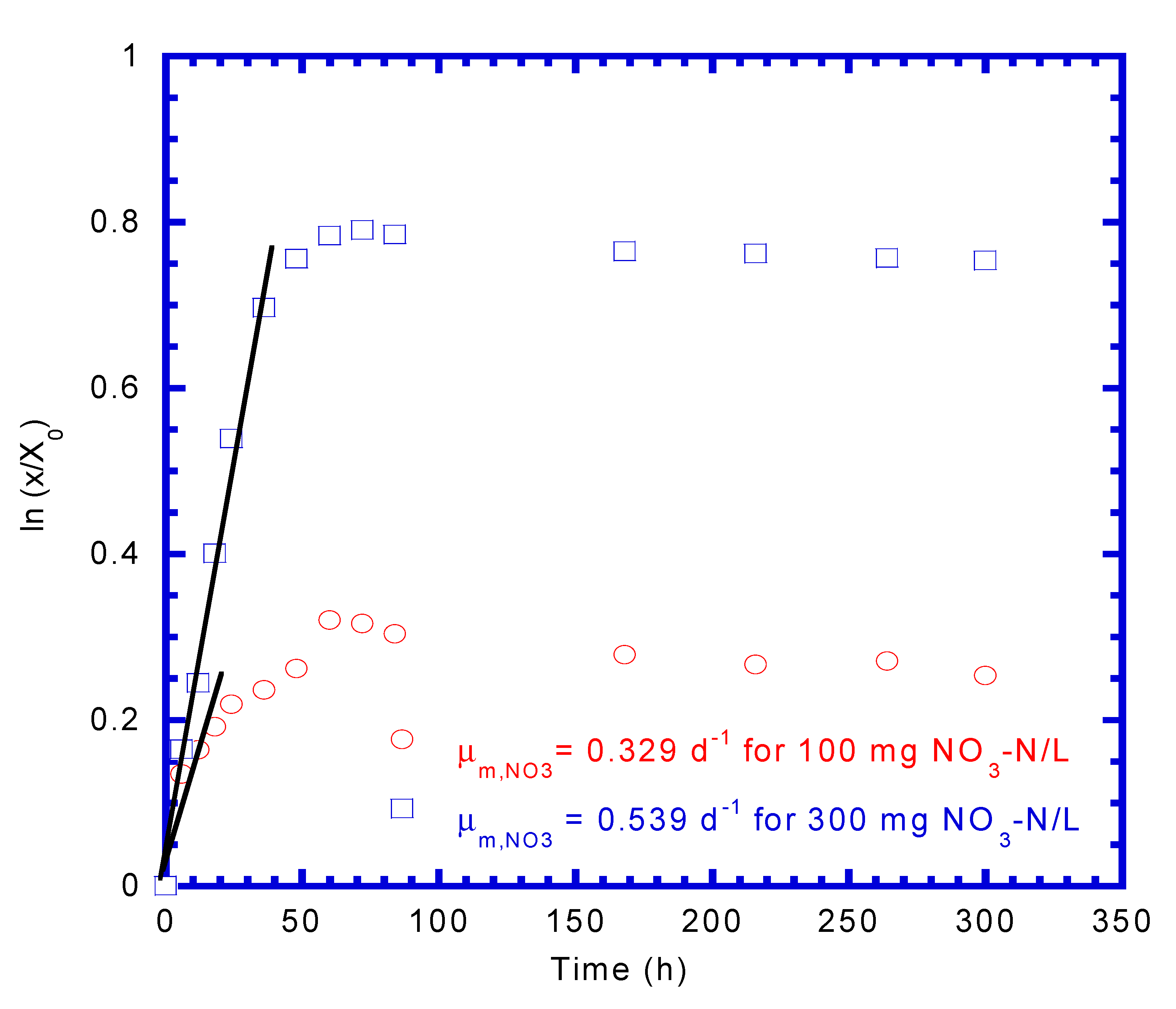
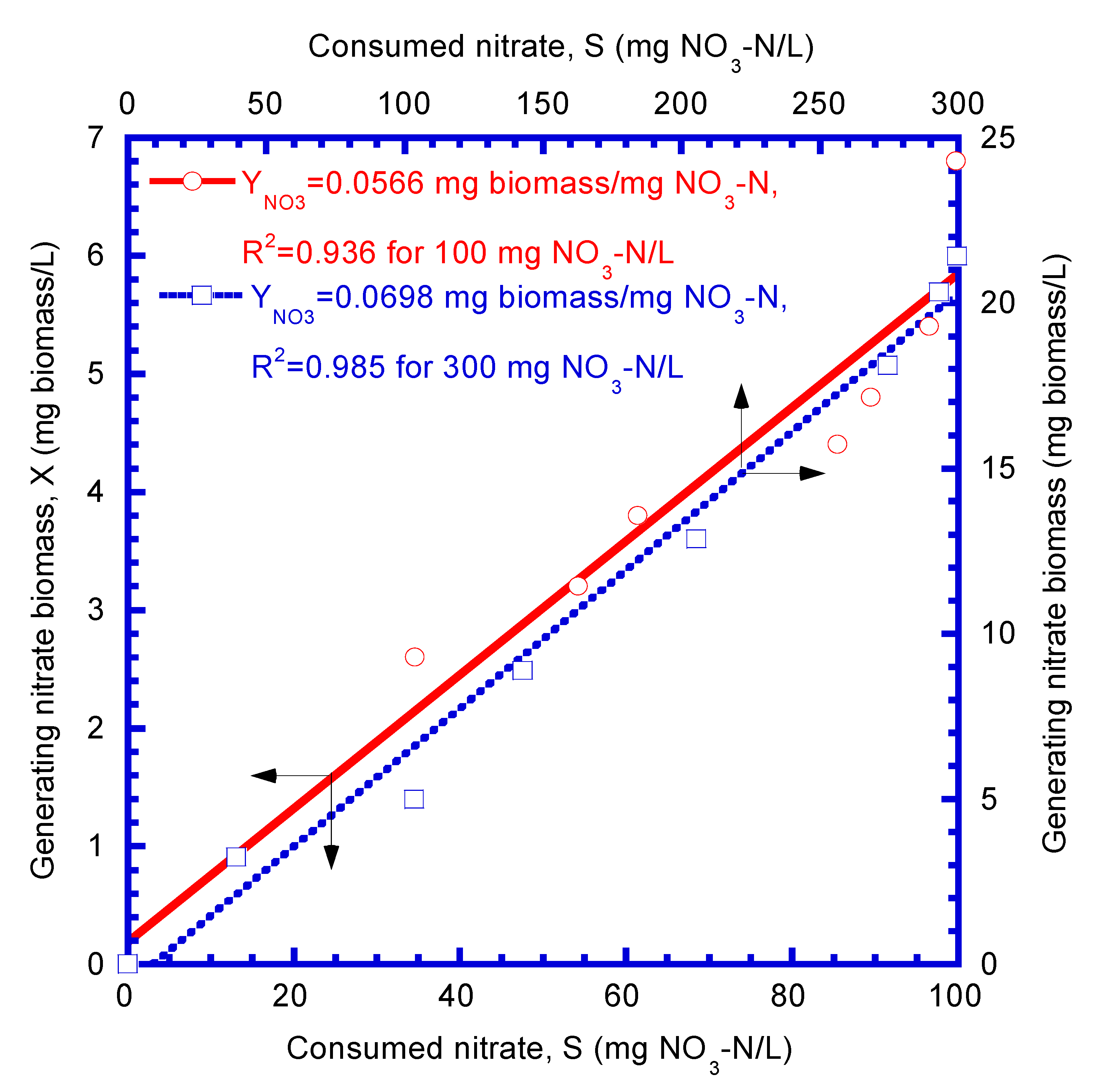
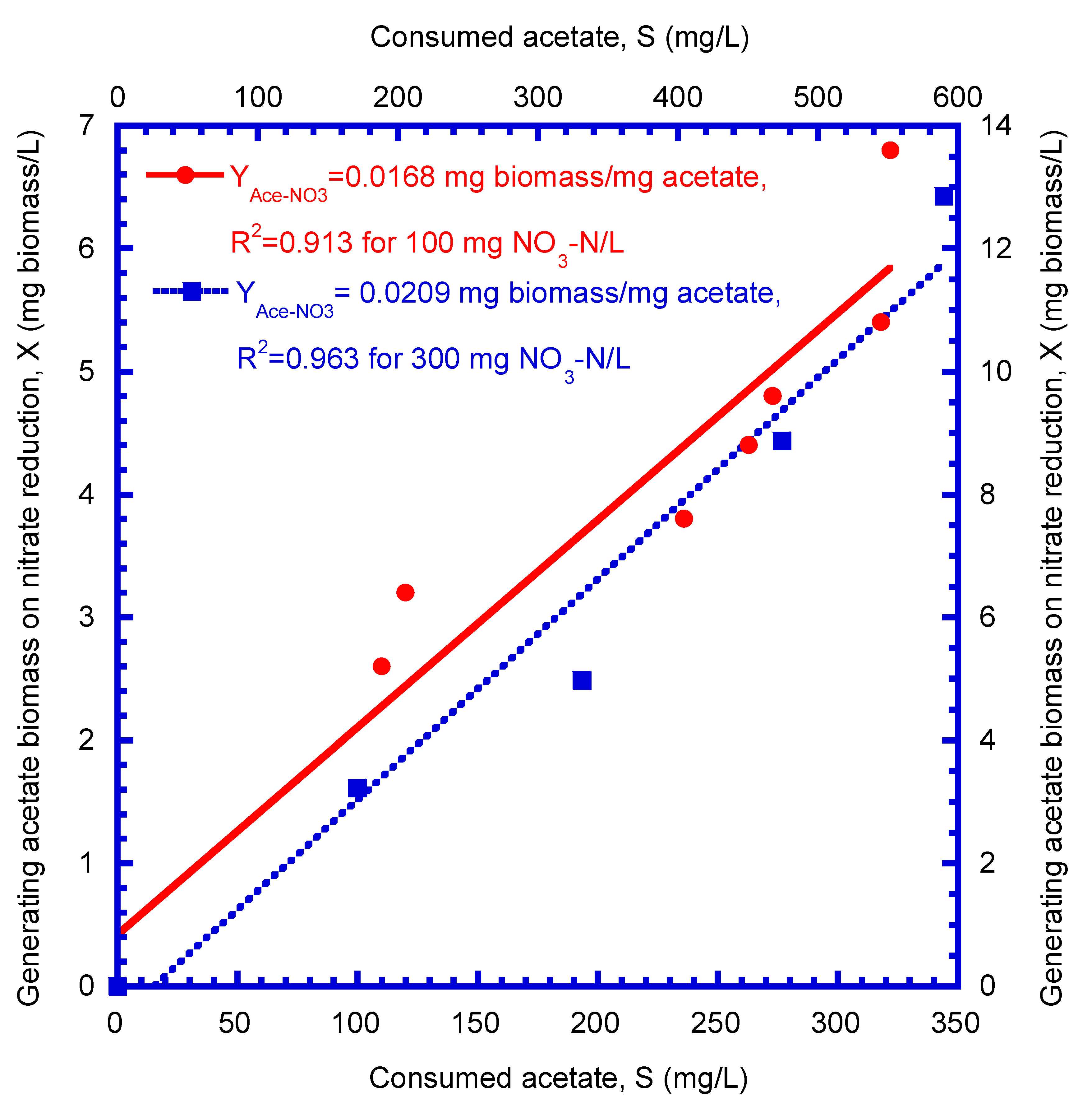
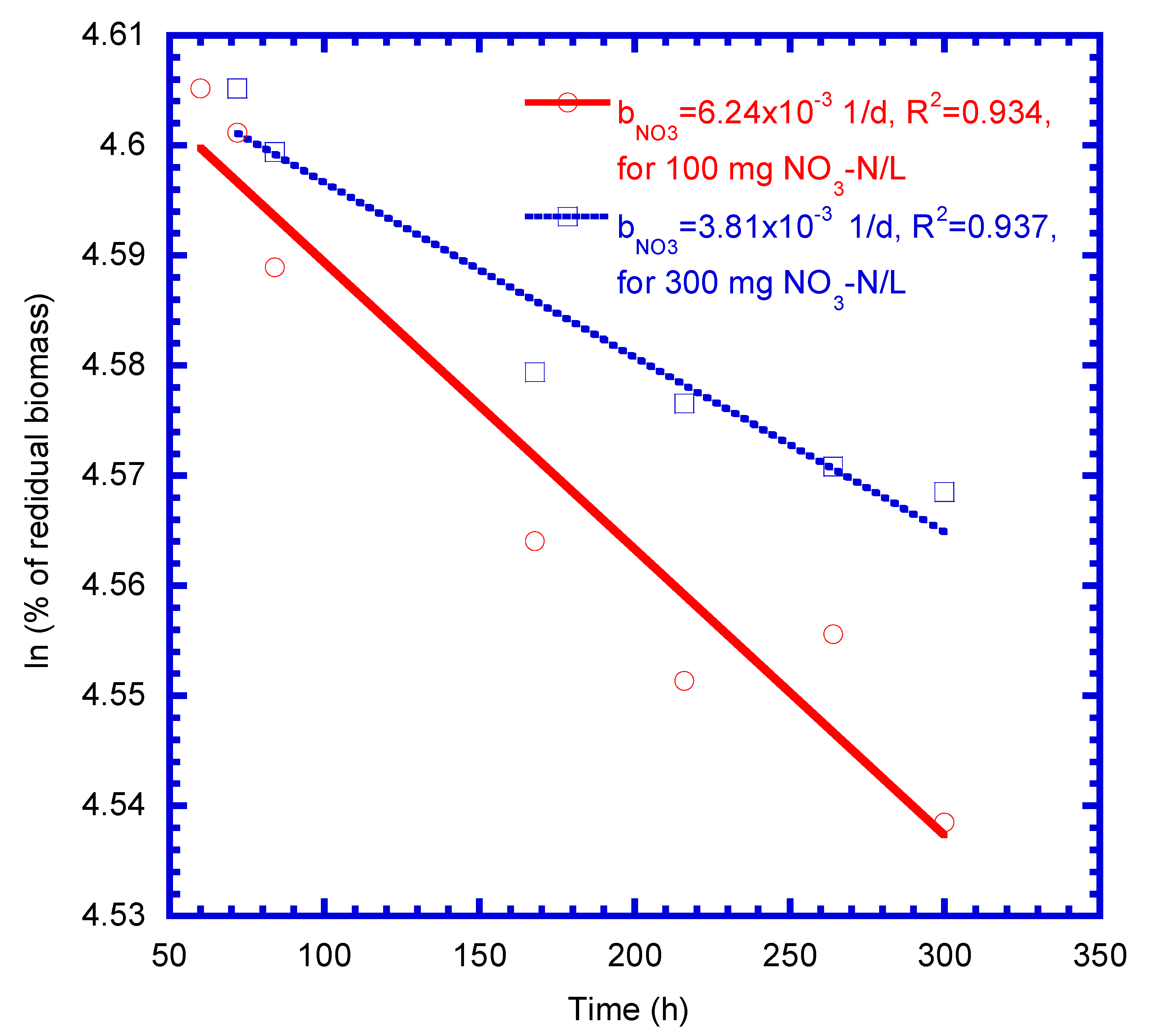
4.1. Determination of Biokinetic Parameters
4.2. Determination of Mass Transfer Coefficients
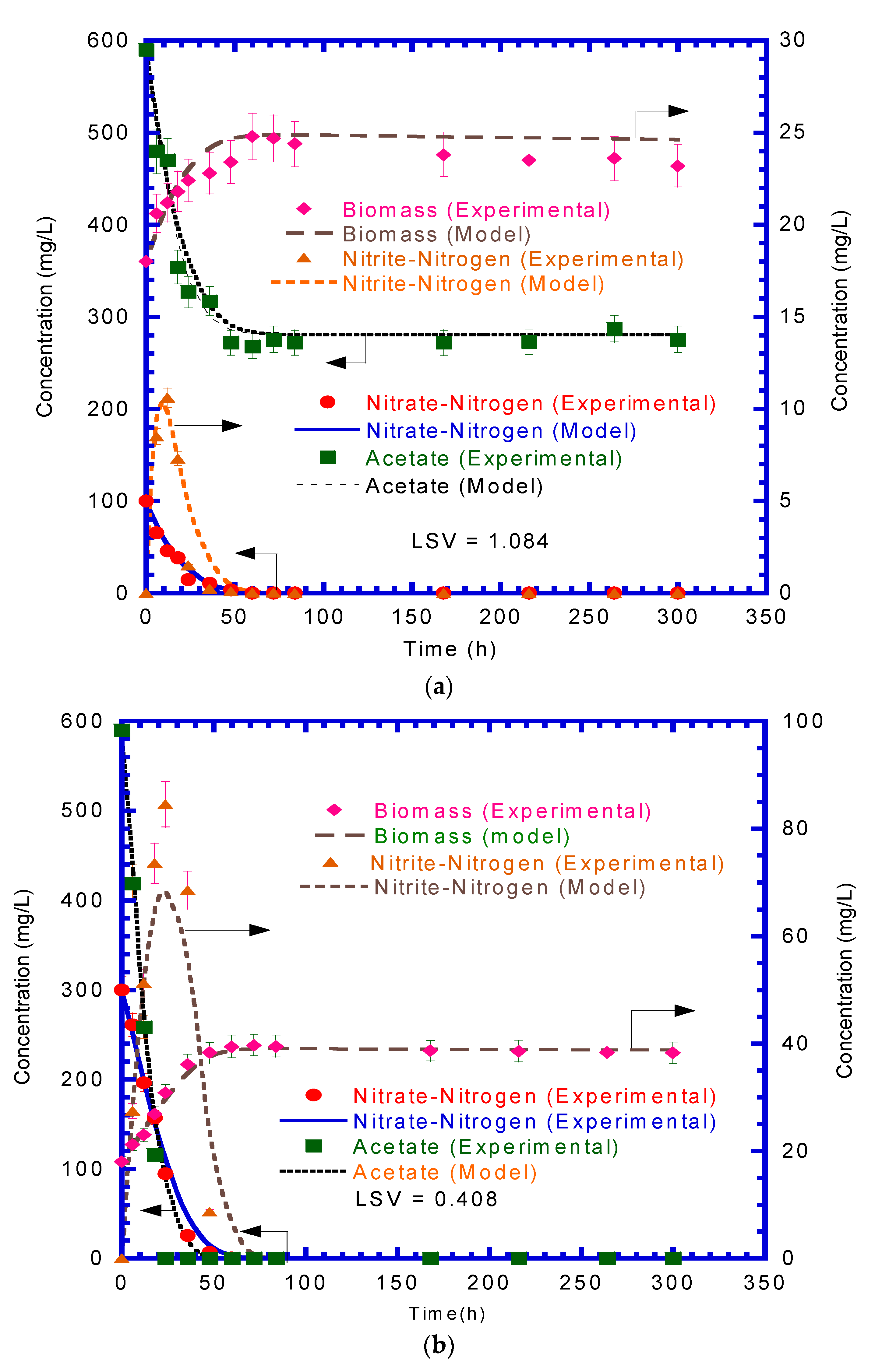
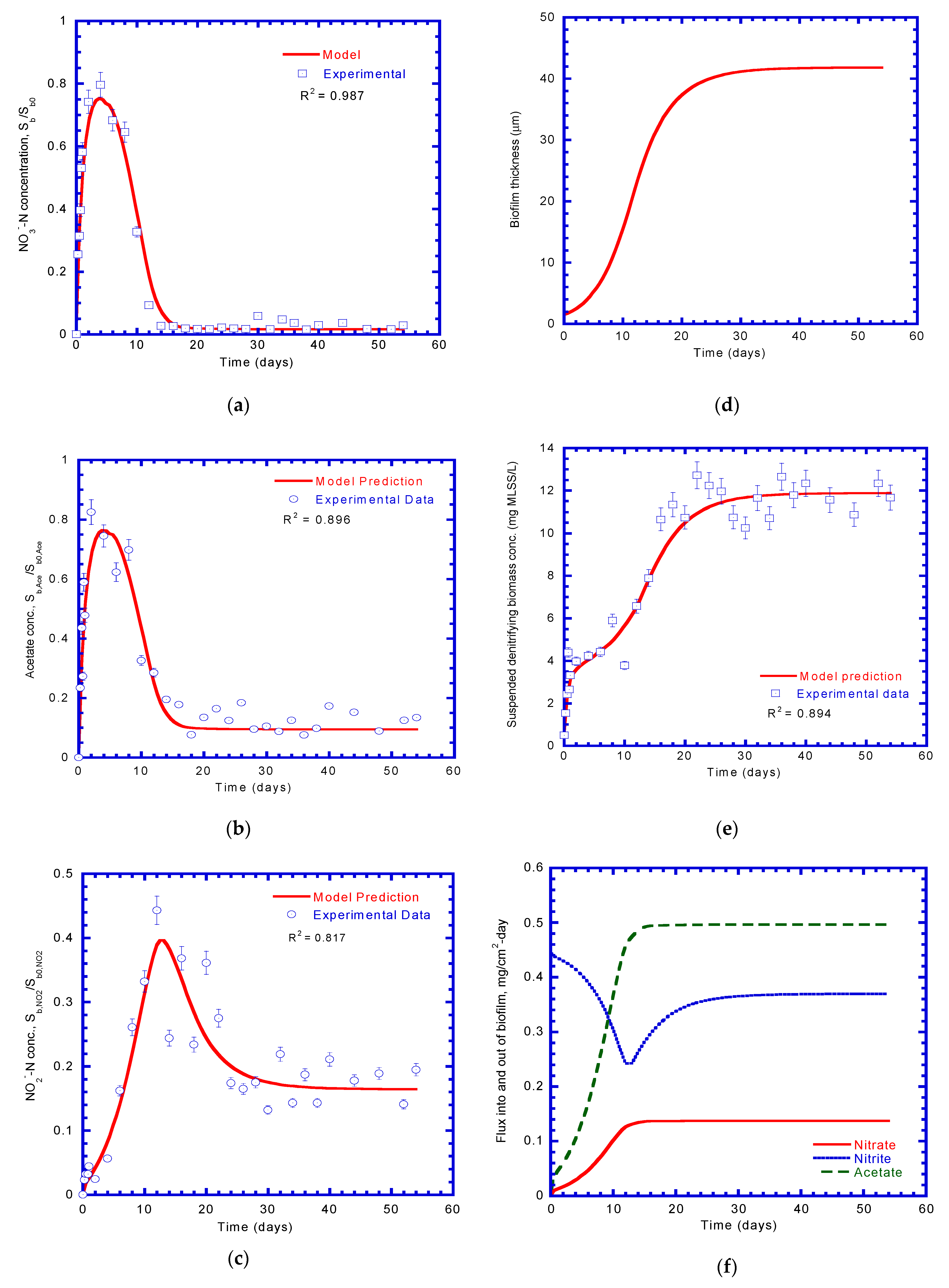
4.3. Nitrate Reduction with Acetate Degradation
4.4. Growth of Denitrifying Biomass
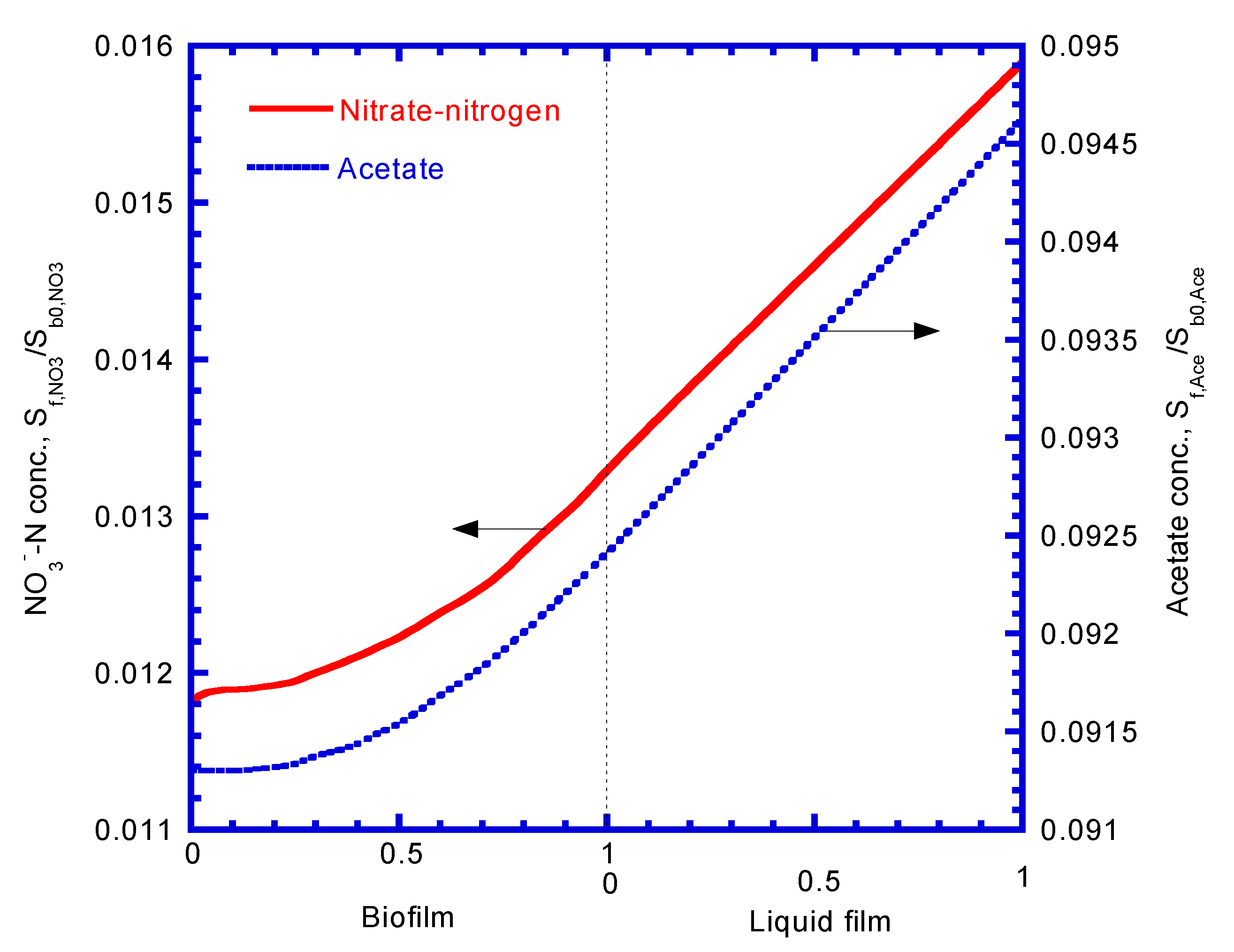
4.5. Concentration Profiles of Nitrate and Acetate
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Karanasios, K.A.; Vasiliadou, I.A.; Pavlou, S.; Vayenas, D.V. Hydrogenotrophic denitrification of potable water: A review. J. Hazard. Mater. 2010, 180, 20–37. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Huang, M.; Bao, S.; Tang, W.; Fang, T. Nitrate removal from low carbon-to-nitrogen ratio wastewater by combining iron-based chemical reduction and autotrophic denitrification. Bioresour. Technol. 2020, 301, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Nava, Y.; Marañón, E.; Soons, J.; Castrillón, L. Denitrification of wastewater containing high nitrate and calcium concentrations. Bioresour. Technol. 2008, 99, 7976–7981. [Google Scholar] [CrossRef] [PubMed]
- Saliling, W.J.B.; Westerman, P.W.; Losordo, T.M. Wood chips and wheat straw as alternative biofilter media for denitrification reactors treating aquaculture and other wastewaters with high nitrate concentrations. Aquacult. Eng. 2007, 37, 222–233. [Google Scholar] [CrossRef]
- Honda, H.; Watanabe, Y.; Kikuchi, K.; Iwata, N.; Takeda, S.; Uemoto, H.; Furuta, T.; Kiyono, M. High density rearing of Japanese flounder, Paralichthys olivaceus with a closed seawater recirculation system equipped with a denitrification unit. Aquacult. Sci. 2010, 41, 19–26. [Google Scholar]
- Ayyasamy, P.M.; Rajakumar, S.; Sathishkumar, M.; Swaminathan, K.; Shanthi, K.; Lakshmanaperumalsamy, P.; Lee, S. Nitrate removal from synthetic medium and groundwater with aquatic macrophytes. Desalination 2009, 242, 286–296. [Google Scholar] [CrossRef]
- Jang, C.S.; Chen, S.K. Integrating indicator-based geostatistical estimation and aquifer vulnerability of nitrate-N for establishing groundwater protection zones. J. Hydrol. 2015, 523, 441–451. [Google Scholar] [CrossRef]
- Wang, Q.; Feng, C.; Zhao, Y.; Hao, C. Denitrification of nitrate contaminated groundwater with a fiber-based biofilm reactor. Bioresour. Technol. 2009, 100, 2223–2227. [Google Scholar] [CrossRef]
- Rezvani, F.; Sarrafzadeh, M.H.; Ebrahimi, S.; Oh, H.M. Nitrate removal from drinking water with a forcus on biological methods: A review. Environ. Sci. Pollut. Res. 2019, 26, 1124–1141. [Google Scholar] [CrossRef] [PubMed]
- Ersever, I.; Ravindran, V.; Pirbazari, M. Biological denitrification of reverse osmosis brine concentrates: I. Batch reactor and chemostat studies. J. Environ. Eng. Sci. 2007, 6, 503–518. [Google Scholar] [CrossRef]
- Tang, K.; Baskaran, V.; Nemati, M. Bacteria of the sulphur cycle: An overview of microbiology, biokinetics and their role in petroleum and mining industries. Biochem. Eng. J. 2009, 44, 73–94. [Google Scholar] [CrossRef]
- Biradar, P.M.; Roy, S.B.; D’Souza, S.F.; Pandit, A.B. Excess cell mass as an internal carbon source for biological denitrification. Bioresour. Technol. 2010, 101, 1787–1791. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, S.; Dörsch, P.; Bakken, R. Autoxidation and acetylene-accelerated oxidation of NO in a 2-phase system: Implications for the expression of denitrification in ex situ experiments. Soil Biology Biochem. 2013, 57, 606–614. [Google Scholar] [CrossRef]
- Vasiliadou, I.A.; Pavlou, S.; Vayenas, D.V. A kinetic study of hydrogenotrophic denitrification. Process. Biochem. 2006, 41, 1401–1408. [Google Scholar] [CrossRef]
- Soto, O.; Aspé, E.; Roeckel, M. Kinetics of cross-inhibited denitrification of a high load wastewater. Enzym. Microb. Technol. 2007, 40, 1627–1634. [Google Scholar] [CrossRef]
- An, S.K.; Stone, H.; Nemati, M. Biological removal of nitrite by an oil reservoir culture capable of autotrophic and heterotrophic activities: Kinetic evaluation and modeling of heterotrophic process. J. Hazard. Mater. 2011, 190, 686–693. [Google Scholar] [CrossRef]
- Gear, C.W. The automatic integration of ordinary differential equations. Commun. ACM 1971, 14, 176–179. [Google Scholar] [CrossRef]
- Skoneczny, S.; Cioch-Skoneczny, M. Mathematical modeling and approximate solutions for microbiological processes in bioifilm through homotopy-based methods. Chem. Eng. Res. Des. 2018, 139, 309–320. [Google Scholar] [CrossRef]
- Gu, W.; Wang, L.; Liu, Y.; Liang, P.; Zhang, X.; Li, Y.; Huang, X. Anammox bacteria enrichment and denitrification in moving bed biofilm reactors packed with different buoyant carriers: Performances and mechanisms. Sci. Total Environ. 2020, 719, 1–11. [Google Scholar] [CrossRef]
- APHA. Standard Method for the Examination of Water and Wastewater, 21st ed.; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Koutinas, M.; Kiparissides, A.; Lam, M.C.; Silva-Rocha, R.; Godinho, M.; de Lorenzo, V.; dos Santos, V.A.P.M.; Pistikopoulos, E.N.; Mantalaris, A. Improving the prediction of Pseudomonas putida mt-2 growth kinetics with the use of a gene expression regulation model of the TOL plasmid. Biochem. Eng. J. 2011, 55, 108–118. [Google Scholar] [CrossRef]
- Abuhamed, T.; Bayraktar, E.; Mehmetoğlu, T.; Mehmetoğlu, Ü. Kinetics model for growth of Pseudomonas putida F1 during benzene, toluene and phenol biodegradation. Process. Biochem. 2004, 39, 983–988. [Google Scholar] [CrossRef]
- Sivarajan, P.; Arutchelvan, V.; Nagarajan, S. Biodegradation kinetics of 2-chlorophenol with starch water as co-substrate using anaerobic batch reactor. Inter. J. Eng. Res. Technol. 2016, 5, 141–145. [Google Scholar] [CrossRef]
- Williamson, K.; McCarty, P.L. Verification studies of the biofilm model for bacterial substrate utilization. J. Water Pollut. Control. Fed. 1976, 48, 281–296. [Google Scholar]
- Wilke, C.E.; Chang, P. Correlation of diffusion coefficients in dilute solutions. Aiche J. 1955, 1, 264–270. [Google Scholar] [CrossRef]
- Perry, R.H.; Chilton, C.H. Chemical Engineers Handbook, 5th ed.; McGraw-Hill: New York, NY, USA, 1973; pp. 3–229. [Google Scholar]
- Cussler, E.L. Diffusion, Mass Transfer in Fluid Systems, 2nd ed.; Cambridge University Press: New York, NY, USA, 1997. [Google Scholar]
- Speitel, G.E., Jr.; DiGiano, F.A. Biofilm shearing under dynamic conditions. J. Environ. Eng. ASCE 1987, 113, 464–475. [Google Scholar] [CrossRef]
- Lazarova, V.; Capdeville, B.; Nikolov, L. Influence of seeding conditions on nitrite accumulation in a denitrifying bed reactor. Water Res. 1994, 28, 1189–1197. [Google Scholar] [CrossRef]
- Jiang, F.; Leung, D.H.W.; Li, S.; Chen, G.H.; Okabe, S.; van Loosdrecht, M.C.M. A biofilm model for prediction of pollutant transformation in sewers. Water Res. 2009, 43, 3187–3198. [Google Scholar] [CrossRef]

| Symbol | Parameter Description (Unit) | Nitrate Concentration (mg/L) | Average | |
|---|---|---|---|---|
| 100 | 300 | |||
| Maximum specific growth rate of nitrate reduction (1/d) | 0.329 | 0.539 | 0.434 | |
| Maximum specific growth rate of nitrite reduction (1/d) | 0.120 | 0.122 | 0.121 | |
| Nitrate biomass yield (mg biomass/mg NO3–N) | 0.0566 | 0.0698 | 0.0632 | |
| Nitrite biomass yield (mg biomass/mg NO2–N) | 0.0217 | 0.0225 | 0.0221 | |
| Acetate biomass yield on nitrate reduction (mg biomass/mg acetate) | 0.0168 | 0.0209 | 0.0189 | |
| Acetate biomass yield on nitrite reduction (mg biomass/mg acetate) | 0.0847 | 0.0794 | 0.0821 | |
| Monod half saturation constant of nitrate (mg NO3–N/L) | 266.2 | 186.5 | 226.35 | |
| Monod half saturation constant of nitrite (mg NO2–N/L) | 4.14 | 4.32 | 4.23 | |
| Decay coefficient of nitrate biomass (1/d) | 6.24 × 10−3 | 3.81 × 10−3 | 5.03 × 10–3 | |
| Decay coefficient of nitrite biomass (1/d) | 6.4 × 10–4 | 2.6 × 10–4 | 4.5 × 10–4 | |
| Parameter | Value |
|---|---|
| Measured | |
| Acetate biomass yield on nitrate reduction, (mg biomass/mg acetate) | 0.0189 |
| Acetate concentration in the feed, (mg/L) | 590 |
| Concentration of suspended nitrifying biomass in the reactor, (mg biomass/L) | 0.5 |
| Decay coefficient of nitrate biomass, (1/d) | 5.03 × 10–3 |
| Density of nitrifying biofilm, (mg biomass/L) | 10.64 |
| Effective working volume, (mL) | 1.6 × 103 |
| Influent flow rate, (mL/d) | 640 |
| Maximum specific growth rate of nitrate reduction, (1/d) | 0.434 |
| Nitrate biomass yield, (mg biomass/mg NO3-N) | 0.0632 |
| Nitrate-nitrogen concentration in the feed, (mg/L) | 150 |
| Reactor porosity (dimensionless) | 0.43 |
| Fitted | |
| Acetate biomass yield on nitrite reduction, (mg biomass/mg acetate) | 0.0821 |
| Decay coefficient of nitrite biomass, (1/d) | 4.5 × 10–4 |
| Maximum specific growth rate of nitrite reduction, (1/d) | 0.121 |
| Monod half-saturation constant of nitrite, (mg NO2-N/L) | 4.23 |
| Nitrite biomass yield, (mg biomass/mg NO2-N) | 0.0221 |
| Calculated | |
| Diffusion coefficient of acetate, (cm2/d) | 1.051 |
| Diffusion coefficient of nitrate, (cm2/d) | 1.326 |
| Diffusion coefficient of nitrite, (cm2/d) | 1.509 |
| Liquid film transfer coefficient of acetate, (cm/d) | 299.14 |
| Liquid film transfer coefficient of nitrate, (cm/d) | 349.41 |
| Liquid film transfer coefficient of nitrite, (cm/d) | 381.07 |
| Monod half-saturation constant of nitrate, (mg NO3-N/L) | 226.35 |
| Specific shear-loss coefficient of denitrifying biomass, (1/d) | 2.71 × 10–2 |
| Total surface area of glass beads, (cm2) | 1.829 × 104 |
| Assumed | |
| Initial denitrifying biofilm thickness, (µm) | 1.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.-H.; Gu, Y.-J. Denitrification Kinetics of Nitrate by a Heterotrophic Culture in Batch and Fixed-Biofilm Reactors. Processes 2020, 8, 547. https://doi.org/10.3390/pr8050547
Lin Y-H, Gu Y-J. Denitrification Kinetics of Nitrate by a Heterotrophic Culture in Batch and Fixed-Biofilm Reactors. Processes. 2020; 8(5):547. https://doi.org/10.3390/pr8050547
Chicago/Turabian StyleLin, Yen-Hui, and Yi-Jie Gu. 2020. "Denitrification Kinetics of Nitrate by a Heterotrophic Culture in Batch and Fixed-Biofilm Reactors" Processes 8, no. 5: 547. https://doi.org/10.3390/pr8050547
APA StyleLin, Y.-H., & Gu, Y.-J. (2020). Denitrification Kinetics of Nitrate by a Heterotrophic Culture in Batch and Fixed-Biofilm Reactors. Processes, 8(5), 547. https://doi.org/10.3390/pr8050547






