Review of Evidence Available on Hesperidin-Rich Products as Potential Tools against COVID-19 and Hydrodynamic Cavitation-Based Extraction as a Method of Increasing Their Production
Abstract
1. Introduction
2. Sources and Bioactive Properties of Hesperidin and Other Citrus Flavonoids
2.1. Sources of Hesperidin
2.2. Safety and Broad Spectrum Activities
2.3. Antiviral Activity
2.4. The Bioavailability Issue
3. Early Evidence of Potential Activity against SARS-CoV-2
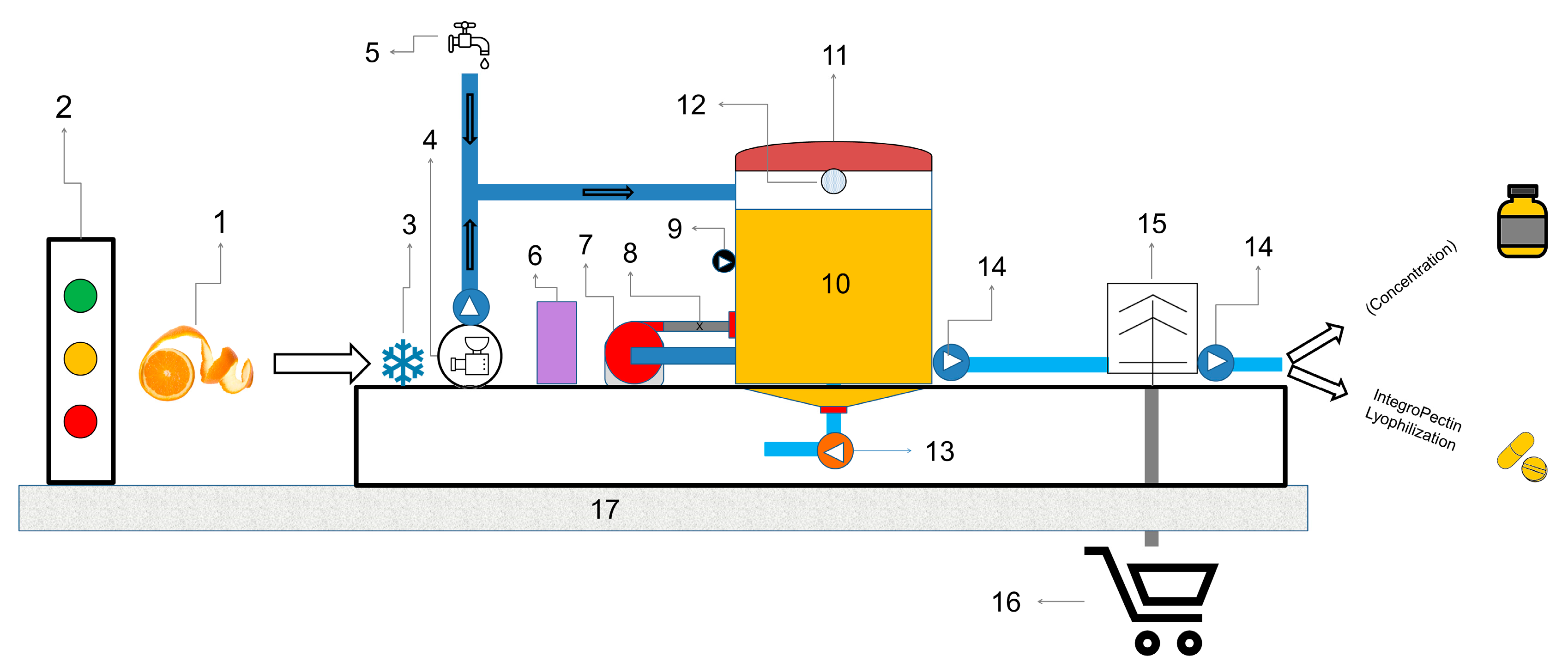
4. Extraction of Hesperidin and Other Citrus Bioactive Compounds
4.1. Extraction Methods
4.2. Properties of the IntegroPectin
5. Discussion and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bialek, S.; Boundy, E.; Bowen, V.; Chow, N.; Cohn, A.; Dowling, N.; Ellington, S.; Gierke, R.; Hall, A.; MacNeil, J.; et al. Severe Outcomes Among Patients with Coronavirus Disease 2019 (COVID-19)—United States, February 12–March 16, 2020. MMWR. Morb. Mortal. Wkly. Rep. 2020, 69, 343–346. [Google Scholar] [CrossRef]
- Wu, J.T.; Leung, K.; Bushman, M.; Kishore, N.; Niehus, R.; de Salazar, P.M.; Cowling, B.J.; Lipsitch, M.; Leung, G.M. Estimating clinical severity of COVID-19 from the transmission dynamics in Wuhan, China. Nat. Med. 2020, 26, 506–510. [Google Scholar] [CrossRef] [PubMed]
- Emanuel, E.J.; Persad, G.; Upshur, R.; Thome, B.; Parker, M.; Glickman, A.; Zhang, C.; Boyle, C.; Smith, M.; Phillips, J.P. Fair Allocation of Scarce Medical Resources in the Time of Covid-19. N. Engl. J. Med. 2020. [Google Scholar] [CrossRef]
- Thunström, L.; Newbold, S.C.; Finnoff, D.; Ashworth, M.; Shogren, J.F. The benefits and costs of flattening the curve for COVID-19. SSRN Electron. J. 2020, 1–17. [Google Scholar] [CrossRef]
- Walls, A.C.; Park, Y.-J.; Tortorici, M.A.; Wall, A.; McGuire, A.T.; Veesler, D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell 2020, 181, 281–292.e6. [Google Scholar] [CrossRef]
- Yan, R.; Zhang, Y.; Li, Y.; Xia, L.; Guo, Y.; Zhou, Q. Structural basis for the recognition of the SARS-CoV-2 by full-length human ACE2. Science 2020, 367, 1444–1448. [Google Scholar] [CrossRef]
- Neuman, B. What the Coronavirus Does to Your Body that Makes It so Deadly. Available online: https://theconversation.com/what-the-coronavirus-does-to-your-body-that-makes-it-so-deadly-133856 (accessed on 6 April 2020).
- Li, H.; Liu, S.-M.; Yu, X.-H.; Tang, S.-L.; Tang, C.-K. Coronavirus disease 2019 (COVID-19): Current status and future perspective. Int. J. Antimicrob. Agents 2020, 105951. [Google Scholar] [CrossRef]
- Xu, Y.H.; Dong, J.H.; An, W.M.; Lv, X.Y.; Yin, X.P.; Zhang, J.Z.; Dong, L.; Ma, X.; Zhang, H.J.; Gao, B.L. Clinical and computed tomographic imaging features of novel coronavirus pneumonia caused by SARS-CoV-2. J. Infect. 2020, 80, 394–400. [Google Scholar] [CrossRef]
- Ma, L.; Xie, W.; Li, D.; Shi, L.; Mao, Y.; Xiong, Y.; Zhang, Y.; Zhang, M. Effect of SARS-CoV-2 infection upon male gonadal function: A single center-based study. medRxiv 2020. [Google Scholar] [CrossRef]
- Shang, W.; Yang, Y.; Rao, Y.; Rao, X. The outbreak of SARS-CoV-2 pneumonia calls for viral vaccines. npj Vaccines 2020, 5, 18. [Google Scholar] [CrossRef]
- Wu, F.; Wang, A.; Liu, M.; Wang, Q.; Chen, J.; Xia, S.; Ling, Y.; Zhang, Y.; Xun, J.; Lu, L.; et al. Neutralizing antibody responses to SARS-CoV-2 in a COVID-19 recovered patient cohort and their implications. medRxiv 2020. [Google Scholar] [CrossRef]
- Kupferschmidt, K.; Cohen, J. Race to find COVID-19 treatments accelerates. Science 2020, 367, 1412–1413. [Google Scholar] [CrossRef] [PubMed]
- Sarfraz, I.; Rasul, A.; Hussain, G.; Adem, S.; Ali, M. Natural Immune Boosters as First-Line Armours to Combat Viral Infection-COVID19: Myth or Science? Preprints 2020. [Google Scholar] [CrossRef]
- Galanakis, C.M. The Food Systems in the Era of the Coronavirus (COVID-19) Pandemic Crisis. Foods 2020, 9, 523. [Google Scholar] [CrossRef]
- Garg, A.; Garg, S.; Zaneveld, L.J.D.; Singla, A.K. Chemistry and pharmacology of the Citrus bioflavonoid hesperidin. Phyther. Res. 2001, 15, 655–669. [Google Scholar] [CrossRef] [PubMed]
- Abad-García, B.; Berrueta, L.A.; Garmóngarmón-Lobato, S.; Urkaregi, A.; Gallo, B.; Vicente, F. Chemometric Characterization of Fruit Juices from Spanish Cultivars According to Their Phenolic Compound Contents: I. Citrus Fruits. J. Agric. Food Chem. 2012, 60, 3635–3644. [Google Scholar] [CrossRef]
- Rouseff, R.L.; Martin, S.F. Quantitative Survey of Narirutin, Naringin, Hesperidin, and Neohesperidin in Citrus. J. Agric. Food Chem. 1987, 35, 1027–1030. [Google Scholar] [CrossRef]
- Barreca, D.; Gattuso, G.; Bellocco, E.; Calderaro, A.; Trombetta, D.; Smeriglio, A.; Laganà, G.; Daglia, M.; Meneghini, S.; Nabavi, S.M. Flavanones: Citrus phytochemical with health-promoting properties. BioFactors 2017, 43, 495–506. [Google Scholar] [CrossRef]
- Barthe, G.A.; Jourdan, P.S.; McIntosh, C.A.; Mansell, R.L. Radioimmunoassay for the quantitative determination of hesperidin and analysis of its distribution in Citrus sinensis. Phytochemistry 1988, 27, 249–254. [Google Scholar] [CrossRef]
- du Preez, B.V.P.; de Beer, D.; Joubert, E. By-product of honeybush (Cyclopia maculata) tea processing as source of hesperidin-enriched nutraceutical extract. Ind. Crops Prod. 2016, 87, 132–141. [Google Scholar] [CrossRef]
- Omidbaigi, R.; Faghih Nasiri, M. Quantitative distribution of hesperidin in Citrus species, during fruit maturation and optimal harvest time. Nat. Prod. Radiance 2004, 3, 12–15. [Google Scholar]
- Bocco, A.; Cuvelier, M.E.; Richard, H.; Berset, C. Antioxidant Activity and Phenolic Composition of Citrus Peel and Seed Extracts. J. Agric. Food Chem. 1998, 46, 2123–2129. [Google Scholar] [CrossRef]
- Mouly, P.; Gaydou, E.M.; Auffray, A. Simultaneous Separation of Flavanone Glycosides and Polymethoxylated Flavones in Citrus Juices Using Liquid Chromatography. J. Chromatogr. A 1998, 800, 171–179. [Google Scholar] [CrossRef]
- Hejniak, J.; Baranowska, I.; Stencel, S.; Bajkacz, S. Separation and Determination of Selected Polyphenols from Medicinal Plants. J. Chromatogr. Sci. 2019, 57, 17–26. [Google Scholar] [CrossRef]
- Areias, F.M.; Valentão, P.; Andrade, P.B.; Ferreres, F.; Seabra, R.M. Phenolic fingerprint of peppermint leaves. Food Chem. 2001, 73, 307–311. [Google Scholar] [CrossRef]
- Guédon, D.J.; Pasquier, B.P. Analysis and Distribution of Flavonoid Glycosides and Rosmarinic Acid in 40 Mentha X piperita Clones. J. Agric. Food Chem. 1994, 42, 679–684. [Google Scholar] [CrossRef]
- Gerozanno, N.; Blacno, S.; Robin, J.H. Contenido de glicósidos de flavonoides en frutos inmaduros en Citrus aurantium y Citrus sinensis del noroeste argentino. Inf. Tecnológica 2002, 13, 49–53. [Google Scholar]
- Ganeshpurkar, A.; Saluja, A. The pharmacological potential of hesperidin. Indian J. Biochem. Biophys. 2019, 56, 287–300. [Google Scholar]
- Smith, C.J. Non-hormonal control of vaso-motor flushing in menopausal patients. Chic. Med. 1964, 67, 193–195. [Google Scholar] [PubMed]
- Meyer, O.C. Safety and security of Daflon 500 mg in venous insufficiency and in hemorrhoidal disease. Angiology 1994, 45, 579–584. [Google Scholar] [CrossRef]
- Milenkovic, D.; Deval, C.; Dubray, C.; Mazur, A.; Morand, C. Hesperidin displays relevant role in the nutrigenomic effect of orange juice on blood leukocytes in human volunteers: A randomized controlled Cross-Over study. PLoS ONE 2011, 6, e26669. [Google Scholar] [CrossRef] [PubMed]
- Pugin, J.; Verghese, G.; Widmer, M.C.; Matthay, M.A. The alveolar space is the site of intense inflammatory and profibrotic reactions in the early phase of acute respiratory distress syndrome. Crit. Care Med. 1999, 27, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A. Cellular and molecular mechanisms of fibrosis. J. Pathol. 2008, 214, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.; Im, M.; Gu, M.J.; Ma, J.Y. Citrus unshiu peel extract alleviates cancer-induced weight loss in mice bearing CT-26 adenocarcinoma. Sci. Rep. 2016, 6, 24214. [Google Scholar] [CrossRef]
- Ahmadi, A.; Shadboorestan, A. Oxidative stress and cancer; the role of hesperidin, a citrus natural bioflavonoid, as a cancer chemoprotective agent. Nutr. Cancer 2016, 68, 29–39. [Google Scholar] [CrossRef]
- Maekawa, S.; Sato, K.; Fujita, K.; Daigaku, R.; Tawarayama, H.; Murayama, N.; Moritoh, S.; Yabana, T.; Shiga, Y.; Omodaka, K.; et al. The neuroprotective effect of hesperidin in NMDA-induced retinal injury acts by suppressing oxidative stress and excessive calpain activation. Sci. Rep. 2017, 7, 6885. [Google Scholar] [CrossRef]
- Liu, W.Y.; Liou, S.S.; Hong, T.Y.; Liu, I.M. Protective effects of hesperidin (Citrus flavonone) on high glucose induced oxidative stress and apoptosis in a cellular model for diabetic retinopathy. Nutrients 2017, 9, 1312. [Google Scholar] [CrossRef]
- Dong, W.; Wei, X.; Zhang, F.; Hao, J.; Huang, F.; Zhang, C.; Liang, W. A dual character of flavonoids in influenza A virus replication and spread through modulating cell-autonomous immunity by MAPK signaling pathways. Sci. Rep. 2014, 4, 7237. [Google Scholar] [CrossRef]
- Aparicio, S. A systematic computational study on flavonoids. Int. J. Mol. Sci. 2010, 11, 2017–2038. [Google Scholar] [CrossRef]
- Yonekawa, M.; Shimizu, M.; Kaneko, A.; Matsumura, J.; Takahashi, H. Suppression of R5-type of HIV-1 in CD4 + NKT cells by Vδ1 + T cells activated by flavonoid glycosides, hesperidin and linarin. Sci. Rep. 2019, 9, 7506. [Google Scholar] [CrossRef]
- De Clercq, E. Potential antivirals and antiviral strategies against SARS coronavirus infections. Expert Rev. Anti. Infect. Ther. 2006, 4, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.W.; Tsai, F.J.; Tsai, C.H.; Lai, C.C.; Wan, L.; Ho, T.Y.; Hsieh, C.C.; Chao, P.D.L. Anti-SARS coronavirus 3C-like protease effects of Isatis indigotica root and plant-derived phenolic compounds. Antiviral Res. 2005, 68, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Keyaerts, E.; Vijgen, L.; Maes, P.; Neyts, J.; Ranst, M. Van In vitro inhibition of severe acute respiratory syndrome coronavirus by chloroquine. Biochem. Biophys. Res. Commun. 2004, 323, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; He, Y.; Zhou, Y.; Liu, S.; Zheng, B.J.; Jiang, S. The spike protein of SARS-CoV—A target for vaccine and therapeutic development. Nat. Rev. Microbiol. 2009, 7, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Kanaze, F.; Kokkalu, E.; Niopas, I.; Georgarakis, M.; Stergious, A.; Bikiaris, D. Thermal analysis study of flavonoid solid dispersions having enhanced solubility. J. Therm. Anal. Calorim. 2006, 83, 283–290. [Google Scholar] [CrossRef]
- de la Rosa, J.D.P.; Ruiz-Palomino, P.; Arriola-Guevara, E.; García-Fajardo, J.; Sandoval, G.; Guatemala-Morales, G.M. A green process for the extraction and purification of hesperidin from mexican lime peel (Citrus aurantifolia Swingle) that is extendible to the citrus genus. Processes 2018, 6, 266. [Google Scholar] [CrossRef]
- Sansone, F.; Rossi, A.; Gaudio, P.; Simone, F.; Aquino, R.P.; Lauro, M.R. Hesperidin gastroresistant microparticles by spray-drying: Preparation, characterization, and dissolution profiles. AAPS PharmSciTech 2009, 10, 391–401. [Google Scholar] [CrossRef]
- Yamada, M.; Tanabe, F.; Arai, N.; Mitsuzumi, H.; Miwa, Y.; Kubota, M.; Chaen, H.; Kibata, M. Bioavailability of glucosyl hesperidin in rats. Biosci. Biotechnol. Biochem. 2006, 70, 1386–1394. [Google Scholar] [CrossRef]
- Cao, R.; Yang, X.; Strappe, P.; Blanchard, C.; Zhou, Z. Natural products derived from tea on the solubility of hesperidin by LC-TOF/MS and NMR. Int. J. Food Prop. 2017, 20, S270–S278. [Google Scholar] [CrossRef]
- Guo, J.; Lu, S.; Liu, Z.; Tang, W.; Tu, K. Solubilization of hesperidin with octenyl succinic anhydride modified sweet potato starch. Food Chem. 2019, 285, 180–185. [Google Scholar] [CrossRef]
- Fahrurroji, A.; Thendriani, D.; Riza, H. Hesperidin Hydrogel Formulation Using Pectin-Chitosan Polymer Combination. Int. J. Pharm. Pharm. Sci. 2017, 9, 98. [Google Scholar] [CrossRef]
- Ahn, S.; Halake, K.; Lee, J. Antioxidant and ion-induced gelation functions of pectins enabled by polyphenol conjugation. Int. J. Biol. Macromol. 2017, 101, 776–782. [Google Scholar] [CrossRef]
- Minzanova, S.T.; Mironov, V.F.; Arkhipova, D.M.; Khabibullina, A.V.; Mironova, L.G.; Zakirova, Y.M.; Milyukov, V.A. Biological activity and pharmacological application of pectic polysaccharides: A review. Polymers 2018, 10, 1407. [Google Scholar] [CrossRef] [PubMed]
- Waheeb, H.M.; Sulaiman, G.M.; Jabir, M.S. Effect of hesperidin conjugated with golden nanoparticles on phagocytic activity: In vitro study. AIP Conf. Proc. 2020, 2213, 020217. [Google Scholar] [CrossRef]
- Nectoux, A.M.; Abe, C.; Huang, S.W.; Ohno, N.; Tabata, J.; Miyata, Y.; Tanaka, K.; Tanaka, T.; Yamamura, H.; Matsui, T. Absorption and Metabolic Behavior of Hesperidin (Rutinosylated Hesperetin) after Single Oral Administration to Sprague-Dawley Rats. J. Agric. Food Chem. 2019, 67, 9812–9819. [Google Scholar] [CrossRef] [PubMed]
- Trotta, V.; Scalia, S. Pulmonary delivery systems for polyphenols. Drug Dev. Ind. Pharm. 2017, 43, 1043–1052. [Google Scholar] [CrossRef] [PubMed]
- Utomo, R.Y.; Ikawati, M.; Meiyanto, E. Revealing the Potency of Citrus and Galangal Constituents to Halt SARS-CoV-2 Infection. Preprints 2020, 2020030214. [Google Scholar] [CrossRef]
- Wu, C.; Liu, Y.; Yang, Y.; Zhang, P.; Zhong, W.; Wang, Y.; Wang, Q.; Xu, Y.; Li, M.; Li, X.; et al. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm. Sin. B 2020. [Google Scholar] [CrossRef]
- Chen, Y.W.; Yiu, C.-P.B.; Wong, K.-Y. Prediction of the SARS-CoV-2 (2019-nCoV) 3C-like protease (3CLpro) structure: Virtual screening reveals velpatasvir, ledipasvir, and other drug repurposing candidates. F1000Research 2020, 9, 129. [Google Scholar] [CrossRef]
- Adem, S.; Eyupoglu, V.; Sarfraz, I.; Rasul, A.; Ali, M. Identification of Potent COVID-19 Main Protease (Mpro) Inhibitors from Natural Polyphenols: An in Silico Strategy Unveils a Hope against CORONA. Preprints 2020, 2020030333. [Google Scholar] [CrossRef]
- Joshi, R.S.; Jagdale, S.S.; Bansode, S.B.; Shankar, S.S.; Tellis, M.B.; Pandya, V.K.; Chugh, A.; Giri, A.P.; Kulkarni, M.J. Discovery of Potential Multi-Target-Directed Ligands by Targeting Host-specific SARS-CoV-2 Structurally Conserved Main Protease. J. Biomol. Struct. Dyn. 2020. [Google Scholar] [CrossRef] [PubMed]
- Tallei, T.E.; Tumilaar, S.G.; Niode, N.J.; Fatimawali, F.; Kepel, B.J.; Idroes, R.; Effendi, Y. Potential of Plant Bioactive Compounds as SARS-CoV-2 Main Protease (Mpro) and Spike (S) Glycoprotein Inhibitors: A Molecular Docking Study. Preprints 2020, 2020040102. [Google Scholar] [CrossRef]
- Chen, H.; Du, Q. Potential natural compounds for preventing SARS-CoV-2 (2019-nCoV) infection. Preprints 2020, 2020010358. [Google Scholar] [CrossRef]
- Cheng, L.; Zheng, W.; Li, M.; Huang, J.; Bao, S. Citrus fruits are rich in flavonoids for immunoregulation and potential targeting ACE2. Preprints 2020, 2020020313. [Google Scholar] [CrossRef]
- Su, W.-W.; Wang, Y.-G.; Li, P.-B.; Wu, H.; Zeng, X.; Shi, R.; Zheng, Y.-Y.; Li, P.-L.; Peng, W.; Su, W.-W.; et al. The potential application of the traditional Chinese herb Exocarpium Citri grandis in the prevention and treatment of COVID-19. Tradit. Med. Res. 2020, 5, 160–166. [Google Scholar] [CrossRef]
- Xu, Z.; Peng, C.; Shi, Y.; Zhu, Z.; Mu, K.; Wang, X.; Zhu, W. Nelfinavir was predicted to be a potential inhibitor of 2019-nCov main protease by an integrative approach combining homology modelling, molecular docking and binding free energy calculation. bioRxiv 2020. [Google Scholar] [CrossRef]
- Basu, A.; Sarkar, A.; Maulik, U. Computational approach for the design of potential spike protein binding natural compounds in SARS- CoV2. OSF Prepr. 2020. [Google Scholar] [CrossRef]
- Yang, R.; Liu, H.; Bai, C.; Wang, Y.; Zhang, X.; Guo, R.; Wu, S.; Wang, J.; Leung, E.; Chang, H.; et al. Chemical composition and pharmacological mechanism of Qingfei Paidu Decoction and Ma Xing Shi Gan Decoction against Coronavirus Disease 2019 (COVID-19): In silico and experimental study. Pharmacol. Res. 2020, 104820. [Google Scholar] [CrossRef] [PubMed]
- Dugo, G.; Di Giacomo, A. Citrus: The Genus Citrus; CRC Press: Boca Raton, FL, USA, 2002; ISBN 9780203216613. [Google Scholar]
- Hilali, S.; Fabiano-Tixier, A.S.; Ruiz, K.; Hejjaj, A.; Ait Nouh, F.; Idlimam, A.; Bily, A.; Mandi, L.; Chemat, F. Green Extraction of Essential Oils, Polyphenols, and Pectins from Orange Peel Employing Solar Energy: Toward a Zero-Waste Biorefinery. ACS Sustain. Chem. Eng. 2019, 7, 11815–11822. [Google Scholar] [CrossRef]
- Meneguzzo, F.; Zabini, F.; Albanese, L.; Crisci, A. Novel Affordable, Reliable and Efficient Technologies to Help Addressing the Water-Energy-Food Nexus. Eur. J. Sustain. Dev. 2019, 8, 1–17. [Google Scholar] [CrossRef]
- Panda, D.; Saharan, V.K.; Manickam, S. Controlled Hydrodynamic Cavitation: A Review of Recent Advances and Perspectives for Greener Processing. Processes 2020, 8, 220. [Google Scholar] [CrossRef]
- Holkar, C.R.; Jadhav, A.J.; Pinjari, D.V.; Pandit, A.B. Cavitationally Driven Transformations: A Technique of Process Intensification. Ind. Eng. Chem. Res. 2019, 58, 5797–5819. [Google Scholar] [CrossRef]
- Albanese, L.; Meneguzzo, F. Hydrodynamic Cavitation Technologies: A Pathway to More Sustainable, Healthier Beverages, and Food Supply Chains. In Processing and Sustainability of Beverages; Grumezescu, A.M., Holban, A.M., Eds.; Woodhead Publishing: Kidlington, UK, 2019; pp. 319–372. ISBN 978-0-12-815259-1. [Google Scholar]
- Albanese, L.; Bonetti, A.; D’Acqui, L.P.; Meneguzzo, F.; Zabini, F. Affordable Production of Antioxidant Aqueous Solutions by Hydrodynamic Cavitation Processing of Silver Fir (Abies Alba Mill.) Needles. Foods 2019, 8, 65. [Google Scholar] [CrossRef]
- Albanese, L.; Meneguzzo, F. Hydrodynamic Cavitation-Assisted Processing of Vegetable Beverages: Review and the Case of Beer-Brewing. In Production and Management of Beverages; Grumezescu, A.M., Holban, A.M., Eds.; Woodhead Publishing: Kidlington, UK, 2019; pp. 211–257. ISBN 978-0-12-815260-7. [Google Scholar]
- Meneguzzo, F.; Brunetti, C.; Fidalgo, A.; Ciriminna, R.; Delisi, R.; Albanese, L.; Zabini, F.; Gori, A.; dos Nascimento, L.B.; Carlo, A.; et al. Real-Scale Integral Valorization of Waste Orange Peel via Hydrodynamic Cavitation. Processes 2019, 7, 581. [Google Scholar] [CrossRef]
- Nuzzo, D.; Cristaldi, L.; Sciortino, M.; Albanese, L.; Scurria, A.; Zabini, F.; Lino, C.; Pagliaro, M.; Meneguzzo, F.; Di Carlo, M.; et al. Exceptional Antioxidant, Non-Cytotoxic Activity of Integral Lemon Pectin from Hydrodynamic Cavitation. ChemistrySelect 2020. [Google Scholar] [CrossRef]
- Presentato, A.; Scurria, A.; Albanese, L.; Lino, C.; Sciortino, M.; Pagliaro, M.; Zabini, F.; Meneguzzo, F.; Alduina, R.; Nuzzo, D.; et al. Superior antibacterial activity of integral lemon pectin from hydrodynamic cavitation. ChemistryOpen 2020. [Google Scholar] [CrossRef]
- Albanese, L.; Ciriminna, R.; Meneguzzo, F.; Pagliaro, M. Energy efficient inactivation of Saccharomyces cerevisiae via controlled hydrodynamic cavitation. Energy Sci. Eng. 2015, 3, 221–238. [Google Scholar] [CrossRef]
- Ciriminna, R.; Albanese, L.; Di Stefano, V.; Delisi, R.; Avellone, G.; Meneguzzo, F.; Pagliaro, M. Beer produced via hydrodynamic cavitation retains higher amounts of xanthohumol and other hops prenylflavonoids. LWT Food Sci. Technol. 2018, 91, 160–167. [Google Scholar] [CrossRef]
- Albanese, L.; Ciriminna, R.; Meneguzzo, F.; Pagliaro, M. Beer-brewing powered by controlled hydrodynamic cavitation: Theory and real-scale experiments. J. Clean. Prod. 2017, 142, 1457–1470. [Google Scholar] [CrossRef]
- Albanese, L.; Ciriminna, R.; Meneguzzo, F.; Pagliaro, M. Gluten reduction in beer by hydrodynamic cavitation assisted brewing of barley malts. LWT Food Sci. Technol. 2017, 82, 342–353. [Google Scholar] [CrossRef]
- Albanese, L.; Ciriminna, R.; Meneguzzo, F.; Pagliaro, M. Innovative beer-brewing of typical, old and healthy wheat varieties to boost their spreading. J. Clean. Prod. 2018, 171, 297–311. [Google Scholar] [CrossRef]
- CAVIBEER|CNR & Bysea S.r.l. Cavibeer. Available online: http://www.cavibeer.com/ (accessed on 25 July 2019).
- Meneguzzo, F.; Albanese, L.; Zabini, F. Hydrodynamic cavitation in beer and other beverages processing. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2020; p. 26. ISBN 9780081005965. [Google Scholar]
- Albanese, L.; Baronti, S.; Liguori, F.; Meneguzzo, F.; Barbaro, P.; Vaccari, F.P. Hydrodynamic cavitation as an energy efficient process to increase biochar surface area and porosity: A case study. J. Clean. Prod. 2019, 210, 159–169. [Google Scholar] [CrossRef]
- Carpenter, J.; George, S.; Saharan, V.K. Low pressure hydrodynamic cavitating device for producing highly stable oil in water emulsion: Effect of geometry and cavitation number. Chem. Eng. Process. Process Intensif. 2017, 116, 97–104. [Google Scholar] [CrossRef]
- Ciriminna, R.; Chavarría-Hernández, N.; Inés Rodríguez Hernández, A.; Pagliaro, M. Pectin: A new perspective from the biorefinery standpoint. Biofuels Bioprod. Biorefining 2015, 9, 368–377. [Google Scholar] [CrossRef]
- Freeze Drying of Pharmaceutical Products; Fissore, D., Pisano, R., Barresi, A., Eds.; CRC Press: Boca Raton, FL, USA, 2019; ISBN 9780429022074. [Google Scholar]
- Capozzi, L.C.; Trout, B.L.; Pisano, R. From Batch to Continuous: Freeze-Drying of Suspended Vials for Pharmaceuticals in Unit-Doses. Ind. Eng. Chem. Res. 2019, 58, 1635–1649. [Google Scholar] [CrossRef]
- Abbasi, E.; Saadat, S.; Karimi Jashni, A.; Hadi Shafaei, M. A Novel Method for Optimization of Slit Venturi Dimensions Through CFD Simulation and RSM Design. Ultrason. Sonochem. 2020, 67, 105088. [Google Scholar] [CrossRef]
- Mane, M.B.; Bhandari, V.M.; Balapure, K.; Ranade, V.V. A novel hybrid cavitation process for enhancing and altering rate of disinfection by use of natural oils derived from plants. Ultrason. Sonochem. 2020, 61, 104820. [Google Scholar] [CrossRef]
- Wang, M.; Cao, R.; Zhang, L.; Yang, X.; Liu, J.; Xu, M.; Shi, Z.; Hu, Z.; Zhong, W.; Xiao, G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020, 30, 269–271. [Google Scholar] [CrossRef]
- Liu, J.; Cao, R.; Xu, M.; Wang, X.; Zhang, H.; Hu, H.; Li, Y.; Hu, Z.; Zhong, W.; Wang, M. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020, 6, 16. [Google Scholar] [CrossRef] [PubMed]
- Schrezenmeier, E.; Dörner, T. Mechanisms of action of hydroxychloroquine and chloroquine: Implications for rheumatology. Nat. Rev. Rheumatol. 2020, 16, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Ochsendorf, F.R.; Runne, U. Chloroquine and hydroxychloroquine: Side effect profile of important therapeutic drugs. Hautarzt 1991, 42, 140–146. [Google Scholar] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meneguzzo, F.; Ciriminna, R.; Zabini, F.; Pagliaro, M. Review of Evidence Available on Hesperidin-Rich Products as Potential Tools against COVID-19 and Hydrodynamic Cavitation-Based Extraction as a Method of Increasing Their Production. Processes 2020, 8, 549. https://doi.org/10.3390/pr8050549
Meneguzzo F, Ciriminna R, Zabini F, Pagliaro M. Review of Evidence Available on Hesperidin-Rich Products as Potential Tools against COVID-19 and Hydrodynamic Cavitation-Based Extraction as a Method of Increasing Their Production. Processes. 2020; 8(5):549. https://doi.org/10.3390/pr8050549
Chicago/Turabian StyleMeneguzzo, Francesco, Rosaria Ciriminna, Federica Zabini, and Mario Pagliaro. 2020. "Review of Evidence Available on Hesperidin-Rich Products as Potential Tools against COVID-19 and Hydrodynamic Cavitation-Based Extraction as a Method of Increasing Their Production" Processes 8, no. 5: 549. https://doi.org/10.3390/pr8050549
APA StyleMeneguzzo, F., Ciriminna, R., Zabini, F., & Pagliaro, M. (2020). Review of Evidence Available on Hesperidin-Rich Products as Potential Tools against COVID-19 and Hydrodynamic Cavitation-Based Extraction as a Method of Increasing Their Production. Processes, 8(5), 549. https://doi.org/10.3390/pr8050549