Abstract
Bee pollens are rich source of essential amino acids and are often considered as complete food for human beings. Herein, we exploited the potential reducing abilities of Bee pollens extract for the eco-friendly preparation of silver nanoparticles (AgNPs-G). The resulting NPs were characterized using a combination of microscopic and spectroscopic techniques. The analyses confirm the formation of spherical Ag NPs. AgNPs-G obtained from the aqueous extract of bee pollens was used to study their antibacterial properties against Gram-positive and Gram-negative microbes using the Minimum Inhibitory Concentration 50 (MIC50) method. The antibacterial properties of AgNPs-G were compared to the properties of chemically synthesized Ag NPs (AgNPs-C) using sodium borohydride as a reducing agent. The green synthesized nanoparticles (AgNPs-G) exhibited a better antibacterial activity against most of the studied strains when compared to the chemically synthesized Ag NPs (AgNPs-C). In addition, the anti-cancer activity of Ag NPs was also studied against human liver and breast carcinoma cell lines by applying MTT-assay. The Ag NPs demonstrated considerable anticancer activity against the studied cell lines and exhibited high IC50 values in both MCF-7 and HepG2 cell lines.
1. Introduction
Recently, the trend of exploiting the antioxidant properties of extracts of various natural products for the preparation of nanomaterials has gained considerable popularity [1]. Among the different nanoparticles, silver has gained the significant consideration of scientists and technologists because of its amazing optical and electronic properties [2]. Particularly, Ag NPs-based materials have garnered several potential biomedical applications due to their strong biocompatibility, high efficiency, easy availability and low cost [3]. In addition, these types of NPs also possess strong bactericidal and inhibitory properties against various microbes [4]. Rampant applications of Ag NPs in different fields have significantly enhanced the demand for their preparation.
Different types of chemical and physical methods have been applied for the preparation of Ag NPs. [5]. Physical methods like physical vapor deposition and ball milling usually need sophisticated instruments, high pressure, temperature, etc. In contrast, the chemical methods involve wet chemical processes such as the colloidal synthesis of nanoparticles, which requires hazardous chemicals and stabilizing agents [6]. Although, using these processes, high-quality NPs with controlled size and morphology can be obtained, they are often not suitable for biological applications [7,8]. Therefore, this motivates to researchers to search for other eco-friendly alternatives for the preparation of biocompatible Ag NPs [9].
Recently, applications of green chemistry principles to obtain biocompatible nanomaterials have gained significant attention. Using these principles, various types of metallic and metal oxide nanoparticles can be conveniently prepared by the proper choice of reductants, solvents and stabilizers [10]. Green chemical synthesis usually involves physiological and environmental friendly reaction conditions including low-temperature electrochemical and microwave-based methods, ionic liquids, supercritical liquids, and sonochemical preparations. [11]. Notably, the extracts of various natural products such as plants, micro and marine organisms have been proven to be effective as reducing agents during the eco-friendly preparation of metallic NPs including Ag [12]. The benefits of using these materials include minimizing the environmental threat, eliminating the usage of toxic chemicals and minimal production of hazardous waste [13].
In addition, the nanoparticles prepared by the application of natural product extract have often demonstrated enhanced bioactivity, due to the occurrence of phytoconstituents as stabilizing ligands on their surfaces. These ligands not only enhance the bioavailability of the material but also promote the attachment of the synthesized NPs to different biomolecules and bio-surfaces, which ultimately helps to penetrate the bacterial cell walls [14]. Moreover, the phytochemicals of natural products often possess their own bioactivity, which may also contribute to the biological activity of the green synthesized NPs. For example, we have successfully demonstrated the enhancement of the bioavailability and bioactivity of green synthesized Ag NPs prepared from different plant extracts [15,16].
Products from honey bee like pollen, bee wax, honey, royal jelly are considered to be promising sources of antioxidants as they are rich in flavonoids like quercetin, kaempferol, naringenin as well as phenolic compounds, including derivatives of cinnamic and benzoic acid [17,18]. The pharmacological effect of bee products has been largely attributed to the species of the bee, geographic location, plant species, seasonality as well as the extraction process [19]. Bee pollen, also referred as “the life-giving dust”, is an established high-energy food. This product is a combination of salivary gland secretion of the insect and plants’ flowers pollens, which are collected by the bees. Anaerobic fermentation of the pollen in the honeycomb leads to formation of lactic acid, a preservative and the end product has been proven to be a rich source of nutrients for bee larvae as well as humans [20,21]. Clinically, bee pollens have been shown to mitigate the side effects of chemotherapeutic drugs such as cisplatin, protect against pro-oxidant toxicity like aflatoxin-induced oxidation, and also counter hyperpigmentation when used in cosmetics [22,23]. Extensive research on the properties of bee pollen extracts from different species has revealed the presence of various phytochemicals including phenols, polyphenols, flavonol and phenylpropanoids, which may have been responsible for their antioxidant properties [24,25,26].
In this study (Figure 1), we applied the bee pollens’ aqueous extract as a bio reductant during the green preparation of Ag NPs (AgNPs-G). The preparation of Ag NPs was confirmed by the combination of different types of microscopic and spectroscopic techniques. The antibacterial properties of as-prepared Ag NPs were tested against different Gram-positive and negative bacteria. In addition, the anticancer properties of these NPs were also investigated against two different cell lines including MCF-7 and HepG2 cell lines.
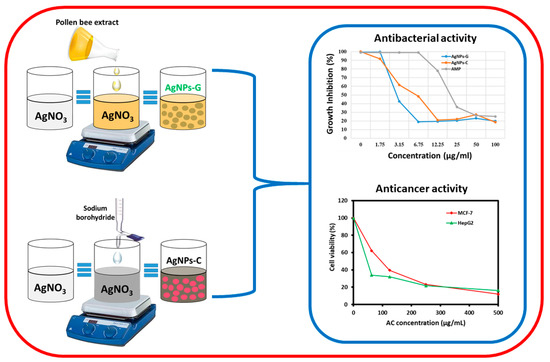
Figure 1.
Graphical representation of green synthesized silver nanoparticles using pollen bee extract (AgNPs-G), chemical synthesized silver nanoparticles using sodium borohydride (AgNPs-C) and evaluation of their antibacterial and anticancer activity.
2. Materials and Methods
2.1. Preparation of Bee Pollen Extract
Commercial bee pollen (First Elite 100% natural bee pollen) was procured from the Wadi-Al-Nahil local market, Riyadh, KSA. The extraction of bee pollen grain involved treating 1250 g of pollen grains with analytical grade 95% ethanol for 48 h at room temperature. The stirring with ethanol was continued with cycles of decantation and addition until exhaustion. The filtrate aliquots were mixed and filtered together to remove particulates and the resulting solution was subjected to evaporation on a rotary evaporator at 45 °C under reduced pressure to obtain a dark yellow gummy extract (yield—383 g, 30.6% w/w). About 80 g of the gummy mixture was dissolved in a mixture of 1 L distilled water and 30% methanol. This hydro-methanolic solution was subsequently subjected to fractionation using petroleum ether (3 × 0.5 L), dichloromethane (3 × 0.5 L), ethyl acetate (4 × 0.5 L), and n-butanol saturated with water (4 × 0.5 L). All the solvents used were distilled prior to use. Solvent-free residue was generated by concentrating each fraction under pressure, including pollen grain petroleum ether (PGp 25 g), dichloromethane (PGc 15.0 g), ethyl acetate (PGe 10.0 g), and n-butanol (PGb 10.0 g). The aqueous layer was separately concentrated to PGa (19.0 g) for further experiments involving generation of silver nanoparticles (Ag NPs).
2.2. Preparations of Silver Nanoparticles Using Bee Pollen Extract (AgNPs-G)
The aqueous extract of bee pollen grain (PGa) was used as reductant. An amount of 1 mL of aqueous bee pollen extract was poured into silver nitrate solution (84.935 mg) in a 100-mL flask. The mixture was allowed to stir for ~2 h at 90 °C. The change in color to dark brown was noted which was indicative of generation of Ag NPs. Confirmation was done using UV absorption spectroscopy between 380 and 450 nm. Chemically synthesized Ag NPs (AgNPs-C) were prepared using a reported method [27]. Briefly, NaBH4 was added slowly to the aqueous AgNO3 solution under gentle stirring at room temperature. After some time, the color of the solution changed and a black precipitate was obtained, which was isolated by centrifuge. The AgNPs-C was identified by UV spectroscopy (Figure S1) and an XRD analysis (Figure S2) is provided in the Supplementary Information.
2.3. Assessing Roles of the Prepared Bee Pollen Extract Ag NPs
A Perkin Elmer 1000 Fourier-transform infrared spectroscopy (FTIR) instrument was used to study both reducing and stabilizing properties of the prepared bee pollen extract. The sample for analysis was subjected to repeat washing prior to FTIR analysis to remove the unbound chemical constituents of the pollen extract. Peaks at specific positions were studied to determine the success of binding of the phytomolecules from the bee pollen aqueous extract to the prepared silver nanoparticles.
2.4. Assessing Size and Morphology of the Ag NPs
The size and morphology of the Ag NPs was analyzed by using transmission electron microscopy (TEM). A TEM analysis was carried out by using a JEM 1101 transmission electron microscope (JEOL, Tokyo, Japan).
2.5. UV-Vis Analysis
Optical measurements were carried out by using a UV-visible spectrophotometer (Perkin Elmer lambda 35, Waltham, MA, USA).
2.6. Antibacterial Activity Screening of the Nanoparticles (NPs)
The screening of the antibacterial potency of Ag NPs was performed using the classical agar disk diffusion and minimum inhibitory concentration (MIC50) methods. Four American Type Culture Collection (ATCC) bacterial strains, namely Escherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa and Bacillus subtilis were procured from the Department of Microbiology, College of Pharmacy, King Saud University. All the strains were sub-cultured in freshly prepared nutrient broth and post observation of growth was standardized as per 0.5 McFarland turbidity standards. The inoculum was further plated on nutrient agar plates using the spread plate technique and a 6-mm sterile borer was used to bore 4 wells in the ready plate. To each of the wells, 100 µL of 100 µg/mL each of Ag NPs and ampicillin was added and the fourth well remained as the negative control. The plates were incubated at 37 °C and the zone of inhibition was noted for each of the added agents.
To determine the MIC50, the microdilution technique was used, which involves the use of an agar dilution process, as described in a previous report, with slight modifications [28]. Samples of Ag NPs and ampicillin were diluted using sterile nutrient broth from 100 µg/mL to 3.72 µg/mL in a 96-well plate. Each of the dilutions was inoculated with 10 µL of the bacterial culture containing approximately 1.0 × 106 CFU/mL. Inoculation for each strain was done in triplicates followed by incubation at 37 °C for a period of 16 h. Growth control and negative control were also set up in parallel and incubated without the addition of the inoculum. The level of growth in each of the test groups was compared to the growth control and the MIC was expressed as the highest dilution that exhibited growth inhibition, judged by the lack of turbidity in the sample.
2.7. Cytotoxic Activity Ag NPs
The cell lines and cell cultures utilized to study the anticancer activity of the as-prepared Ag NPs included a human liver carcinoma cell line (HepG2) and a breast cancer cell line (MCF-7). Both were cultured in Dulbecco’s Modified Eagle Medium (DMEM) with 7% fetal bovine serum (FBS) and 1% each of streptomycin and penicillin. The culturing was done at 37 °C in 5% CO2 supply incubator. Monolayer sub-culturing was done at routine intervals and for each round of experiment, the exponentially growing cells were utilized.
Anticancer activity was assessed by the MTT (3-[4,5-dimethylthiazole-2-y]-2,5-diphenyltetrazolium bromide) colorimetric assay [29]. The activity of the Ag NPs was evaluated on both cell lines and the IC50 was evaluated by the cytotoxicity test. Each cell suspension of about 5 × 104 cells were seeded in a 96-well flat plate containing DMEM with 5% FBS and different concentrations of the NPs: 31 µg/mL, 62 µg/mL, 125 µg/mL, 250 µg/mL and 500 µg/mL. This mixture was incubated at 37 °C for a period of 24 h in a 5% CO2 incubator. Post incubation, the cells were subjected to a wash serum free medium and a 100 mL of 5 mg/mL of MTT solution was added followed by incubation for 4–5 h. Post MTT incubation, the cells were again washed with phosphate buffer saline, and 100 µL of dimethyl sulfoxide (DMSO) was added to solubilize the unbound formazan. The plates were read at 570 nm absorbance in a plate reader (Biotech, USA) and the entire experiment was run in triplicates. The IC50 was determined by the color intensity of the generated formazan dye proportionally to the number of viable cells in the well.
3. Results and Discussions
3.1. UV-Vis Analysis
Ag NPs were prepared under eco-friendly conditions by the reduction of AgNO3 in water using the bee pollen aqueous extract as a reducing agent. This environmentally friendly preparation was carried out without using any harmful reducing or stabilizing agents, since the bee pollen extract not only reduced but also functionalized the surface of the resultant Ag NPs. On adding the pollen extract to AgNO3 solution, the color of the reaction mixture slowly turned to dark brown which points towards the effective synthesis of Ag NPs. Initially, the formation of Ag NPs was confirmed by a UV analysis (Figure 2). Ag NPs commonly show absorption in the visible range of the spectrum from 380 to 450 nm, depending on the quality of NPs (size and shape). In this case, a broad absorption peak appeared at ~440 nm, as shown in Figure 2, which confirms the formation of Ag NPs.
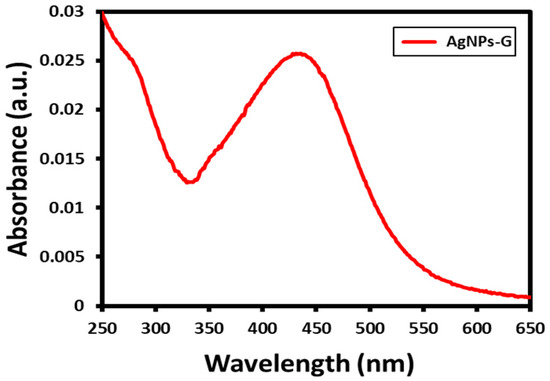
Figure 2.
UV absorption spectroscopy analysis of the prepared AgNPs-G.
3.2. XRD Analysis
The formation and crystallinity of the as-prepared Ag NPs was also confirmed by using the powder diffraction technique. The XRD spectrum in Figure 3 indicates the presence of a face-centered cubic (fcc) structure for the Ag NPs. Particularly, the presence of distinct peaks at various positions, including at 37.75° (111), 44.41° (200), 63.83° (220), 76.95° (311), and 81.63° (222) clearly confirms the formation of Ag NPs. Among all the other diffraction peaks, the peak at (111) is the most intense peak, which may correspond to the direction of the growth of nanocrystals. In addition to the characteristic’s peaks of Ag NPs, the XRD pattern also contains various additional peaks. These peaks can be attributed to the inorganic residual moieties that may be present in the bee pollen grain extract. The residual compounds may have been bound to the surface of the NPs and thus, contributed to the additional reflections in the XRD pattern of Ag-NPs synthesized by bee pollen grains extract.
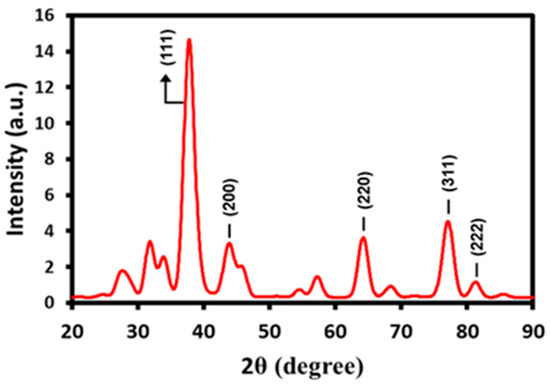
Figure 3.
XRD pattern of prepared AgNPs-G indicated by presence of face-centered cubic (fcc) structure.
3.3. FT-IR Analysis
Furthermore, FT-IR was also used to investigate the dual role of bee pollen grains as a reductant and stabilizer. For this purpose, the FT-IR sample was washed repeatedly prior to the measurement to remove the unbound chemical constituents of pollen extract. Figure 4 demonstrates the FT-IR spectrum of bee pollen grains-mediated synthesized Ag NPs. A careful analysis of the spectrum indicates the presence of hydroxyl rich phytomolecules such as flavonoids and polyphenols, which are known to demonstrate reducing and stabilizing abilities. These phytomolecules may have reduced the silver precursor to produce Ag NPs. The FT-IR spectrum of green synthesized Ag NPs exhibits various peaks at different positions, such as the peaks at 3430 cm−1 and 2955 cm−1, which are related to the presence of hydrogen-bonded OH groups and C-H groups, respectively. Other peaks representing the C−H, C−C, and C−O stretching of the aromatics are present at 1743, 1620 and 1407 cm−1, respectively. Moreover, peaks indicating the occurrence of C−O stretching of carboxylic acids, alcohols and ether and ester groups are located at 1260 and 1074 cm−1. Furthermore, the peaks around 1743 cm−1 can be attributed to the C=O group of aldehydes, ketone, or COOH groups. The presence of these peaks in the IR spectrum of Ag NPs confirms the successful binding of the phytomolecules of bee pollen extracts to the surface of nanoparticles as stabilizing ligands.
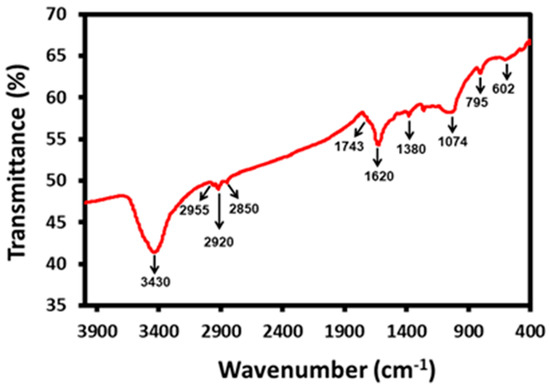
Figure 4.
FTIR spectrum of the prepared AgNPs-G.
3.4. TEM Analysis
The size and morphology of the bee pollen grain extract-synthesized Ag NPs were determined using a TEM analysis. Figure 5 demonstrates the formation of spherical shape Ag NPs, with an average size of ~10–30 nm. Mostly, the NPs are well dispersed, whereas some of them are slightly agglomerated. The few large particles seen in Figure 5b (~44 nm) could be due to the agglomeration of smaller particles; however, the majority of the particles in the TEM images are less than 30 nm in size.
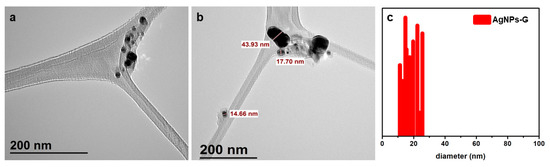
Figure 5.
(a,b) TEM images of the green synthesized AgNPs-G using the aqueous extract of bee pollen grains, (c) particle size distribution of AgNPs-G.
3.5. Antibacterial Activity of the Ag NPs
The antibacterial properties of Ag NPs were measured using the disk diffusion method involving both Gram positive and negative organisms such as B. subtilis, S. aureus, P. aeruginosa and E. coli. The activity of AgNPs-G was compared with the commercially available ampicillin and chemically synthesized Ag NPs (AgNPs-C). For the Gram-positive organisms, AgNPs-G exhibited a comparable zone of inhibition corresponding to known antibiotic ampicillin at 18 mm and 17 mm for B. subtilis and S. aureus. Indeed, in the case of B. subtilis, the AgNPs-G demonstrated an even higher effect as compared to ampicillin at 14 mm. Moreover, in the case of Gram-negative organisms, the effect of AgNPs-G was highest against P. aeruginosa at 18 mm, while E.coli exhibited high inhibition with ampicillin at 22 mm. However, the chemically synthesized Ag NPs (AgNPs-C) exhibited much lower antibacterial activities when compare to both AgNPs-G and ampicillin. The results are summarized in Table 1.

Table 1.
Antibacterial activity study outcome of AgNPs-G.
The MIC50 study was also done by the microdilution technique involving agar dilution. In the case of B. subtilis and S. aureus (Gram-positive bacteria), AgNP-G exhibited the lowest MIC50 at 3.15 µg/mL, while for the P. aeruginosa (Gram-negative), the MIC50 was detected to be 6.75 µg/mL. In all the cases, AgNP-G was found to have the lowest MIC50 in comparison to ampicillin. The graphical presentation of the MIC50 outcome is highlighted in Figure 6.
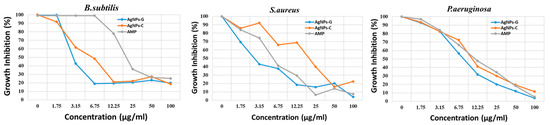
Figure 6.
Antimicrobial activity of B.subtilis, S.aureus and P.aeruginosa by MIC50 method for AgNPs-G in comparison to AgNPs-C and ampicillin (AMP).
3.6. Anticancer Cytotoxic Activity of AgNPs-G by MTT Assay
The anticancer activity assessment of AgNPs-G was done by utilizing a human liver cancer and breast cancer cell line, HepG2 and MCF-7, by the MTT assay. The outcome of the assay for AgNPs-G is shown in Figure 7. In the case of AgNPs-G, a less than 25% viability was noted for both MCF-7 and HepG2 at a concentration of 500 µg/mL which is better in comparison to that of the AgNPs-C (AP) wherein a viability of over 25% was seen at the same concentration.
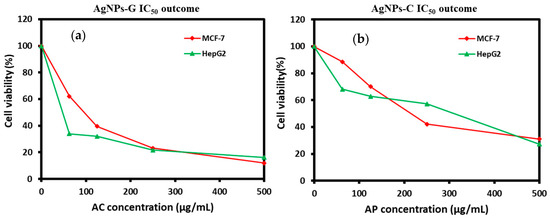
Figure 7.
Viability outcome by MTT assay for (a) AgNPs-G and (b) AgNPs-C.
The IC50 analysis for AgNPs-G and AgNPs-C was also done on both the cell lines as part of the anticancer activity assessment. The results are shown in Table 2. The AgNPs-G are exhibited a low IC50 for both cell lines HepG2 and MCF-7 at 47.6 +/− 1.5 µg/mL and 96 +/− 3.5 µg/mL in comparison to Ag NPs.

Table 2.
IC50 outcome for GNPs and Ag NPs for anticancer activity study.
3.7. Discussion
Pollen produced by the anther of the plant is collected by bees mixed with their salivary secretion and nectar and stored in bee colonies wherein it serves as a principle food source, also termed “bee-collected pollen” [30]. Beekeeping is a major growing business in Saudi Arabia and the knowledge on floral resources helps beekeepers maintain colony strength to harvest high-quality as well as high-yield bee products including honey [31]. Providing a map of nectar and pollen sources to beekeepers in their respective areas plays a crucial role in devising a plan for colony management and identifying alternate back-up sources to move colonies during certain periods as per need and emergency [32]. Honeybee pollen, which is a result of the agglutination of floral pollens and salivary secretion of the bees, has proven to be an excellent food supplement in humans, depending on the geography of origin, as it highly influences the chemical composition. An increasing number of studies have focused on identifying the pharmacological benefits of each pollen variety in the respective geographical location to identify the utility of bee products for humans beyond food supplementation. Phenols and flavonoids have been widely studied for their antimicrobial and antioxidant activities. Bee pollen consumption has been linked to many benefits, like tackling anemia, depression, impaired immune function, memory loss, ageing, and impotency [33].
Our study focused on identifying the antibacterial and anticancer activity of Ag NPs prepared by using a commercial pollen variety from Riyadh, Saudi Arabia. Bee pollen extract was prepared using analytical-grade solvents and a series of filtration and concentration steps. Furthermore, the aqueous part of the filtrate was utilized for the preparation of AgNPs-G, the characteristics and success of which were assessed using different analytical techniques, including UV absorption spectroscopy, XRD pattern, FTIR and TEM. The as-prepared NPs were then tested for antibacterial activity using the traditional disk diffusion method and MIC50, while anticancer activity was determined using human cancer cell lines and IC50.
An antibacterial activity assessment of AgNPs-G was performed in comparison to chemically synthesized Ag NPs (AgNP-C) and ampicillin using ATCC strains of Gram positive organisms like B.subtilis and S.aureus as well as Gram negative organisms like P.aeruginosa and E.coli. Many studies have assessed the effect of methanolic and ethanolic bee pollen extract on different organisms, and one such study which assessed the antibacterial activity against S.aureus detected the highest zone of inhibition in the case of 70% methanol extract of 15% pollen [34]. Another study which evaluated the biological activity of Helianthus annuus L. bee pollen against Gram positive and negative organisms detected a good zone of inhibition against P.aeruginosa using both dried and frozen extracts [35]. In this study, the Gram negative and positive organisms exhibited comparable resistance towards the AgNPs-G except for E.coli, which exhibited maximum inhibition against the positive-control ampicillin. The as-prepared nanoparticles exhibited a better inhibition activity in comparison to the positive control for B.subtilis and P.aeruginosa. A study on antibacterial and antioxidant activity of bee products on different Gram positive and negative organisms detected S.aureus and E.coli to be more resistant to the tested bee products [36]. In our case, the MIC50 study for each of the AgNPs-G, AgNPs-C and positive control also detected the least for AgNP-G against the ATCC test organisms studied. The results indicate that the as-prepared AgNPs-G obtained from commercial pollen is a promising candidate for use in food packaging as well as the pharmaceutical industry.
The anticancer activity of bee pollens is attributed to different peptides which induce in vitro apoptosis in many transformed human cancer cell lines, including those from the lung, liver, prostate, and bladder. [37]. Previous studies have detected the steroid fraction of chloroform extract from Brassica campestris bee pollen to exhibit strong cytotoxic activity on human prostate cancer cells by the stimulation of TNF-alpha secretion and apoptosis induction [38]. A study on the anticancer activity of Ag NPs of stingless bee propolis exhibited significant activity against A549 human lung cancer cells and the IC50 value was identified to be 38 µg/mL. Nanoparticles associated with drug delivery systems have been found to be promising in cancer because of their ability to cross the biological barrier and achieve therapeutic concentration in tumors even in low dosage of drug administration and Ag NPs have gained widespread attention in this space [39]. Ag NPs have shown great potential in reducing angiogenesis and oxidative stress. A study that assessed the potential of Ag NPs prepared by using aqueous extract of rapeseed flower pollens against different cancer cell lines detected cytotoxic activity towards MDA-MB-231 and MCF-7 cells [40]. Another study which documented the effect of Ag NPS obtained by using honeybee extract detected 60% inhibition against colon cancer by down regulating the expression of Bcl2 and the surviving gene [41].
In our study AgNPs-G were generated and their anticancer activity was assessed using human a liver carcinoma cell line (HepG2) and a breast cancer cell line (MCF-7). The IC50 studies done using AgNPs-G, AgNPs-C. AgNPs-G showed them to be active at low concentrations of 47.6 +/− 1.5 µg/mL against HepG2 and 96 +/− 3.5 µg/mL against MCF-7 in comparison to Ag NPs which showed a high concentration at 310 +/− 5.5 µg/mL and 214 +/− 5 µg/mL. A viability study by MTT assay detected a greater reduction by AgNPs-G as compared to AgNPs-C. The prepared nanoparticles were found to be effective against the studied cancer cell lines albeit at IC50 higher than the positive control, indicating their potential use against human cancer.
4. Conclusions
Bee pollens are considered as high-energy food, which is obtained from the plant flower pollens collected by the bees. It is an apitherapeutic product containing ~22.7% proteins, which includes essential amino acids, lipids inclusive of essential fatty acids and phenolic compounds. In this study, eco-friendly synthesis of Ag NPs was reported by an aqueous extract of bee pollen, which contains a variety of potential reducing agents, including polyphenols and flavonoids. Spherically shaped, highly crystalline Ag NPs (AgNPs-G) were prepared in the absence of any hazardous chemical or capping agents. The biosynthesized Ag NPs demonstrated excellent antioxidant properties, which were confirmed by testing the antibacterial and anticancer properties of nanoparticles against various bacterial strains and cell lines. Indeed, in many cases, the AgNPs-G exhibited superior antioxidant properties when compared to the chemically synthesized AgNPs-C and commercially available ampicillin.
Supplementary Materials
The following are available online at https://www.mdpi.com/2227-9717/8/5/524/s1, Figure S1: UV absorption spectroscopy analysis of the prepared AgNPs-C, Figure S2: XRD spectrum of prepared AgNPs-C.
Author Contributions
H.M.A.-Y., M.A., A.S.A. and R.S. designed the project. M.S.A., A.M., M.K., M.R.S., and M.S.O. helped to draft the manuscript. H.M.A.-Y., and R.S. carried out the preparation of extract and characterization. M.R.S. and M.K. carried out the interpretation of some part of results. A.S.A. and R.S. carried out the antimicrobial and anticancer activity. M.R.H. and M.R.H.S. provided scientific guidance for successful completion of the project and also helped to draft the manuscript. All authors read and approved the final manuscript.
Funding
This research and APC was funded by Deanship of Scientific Research, King Saud University, Research group project No. RG-1440-070.
Acknowledgments
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding this work through the research group project No. RG-1440-070.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Adil, S.F.; Assal, M.E.; Khan, M.; Al-Warthan, A.; Siddiqui, M.R.H.; Liz-Marzán, L.M. Biogenic synthesis of metallic nanoparticles and prospects toward green chemistry. Dalton Trans. 2015, 44, 9709–9717. [Google Scholar] [CrossRef] [PubMed]
- Syafiuddin, A.; Salmiati; Salim, M.R.; Kueh, A.; Hadibarata, T.; Nur, H. A Review of Silver Nanoparticles: Research Trends, Global Consumption, Synthesis, Properties, and Future Challenges. J. Chin. Chem. Soc. 2017, 64, 732–756. [Google Scholar] [CrossRef]
- Marin, S.; Vlasceanu, G.M.; Tiplea, R.E.; Bucur, I.R.; Lemnaru, M.; Marin, M.M.; Grumezescu, A.M. Applications and toxicity of silver nanoparticles: A recent review. Curr. Top. Med. Chem. 2015, 15, 1596–1604. [Google Scholar] [CrossRef] [PubMed]
- Lima, R.; Seabra, A.B.; Durán, N. Silver nanoparticles: A brief review of cytotoxicity and genotoxicity of chemically and biogenically synthesized nanoparticles. J. Appl. Toxicol. 2012, 32, 867–879. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.S.; Cheong, K.Y. Advances of Ag, Cu, and Ag–Cu alloy nanoparticles synthesized via chemical reduction route. J. Nanoparticle Res. 2013, 15, 15. [Google Scholar] [CrossRef]
- Khan, M.; Shaik, M.R.; Adil, S.F.; Khan, S.T.; Al-Warthan, A.A.; Siddiqui, M.R.H.; Tahir, M.N.; Tremel, W.; Siddiqui, R.H. Plant extracts as green reductants for the synthesis of silver nanoparticles: Lessons from chemical synthesis. Dalton Trans. 2018, 47, 11988–12010. [Google Scholar] [CrossRef] [PubMed]
- Pillai, Z.S.; Kamat, P.V. What Factors Control the Size and Shape of Silver Nanoparticles in the Citrate Ion Reduction Method? J. Phys. Chem. B 2004, 108, 945–951. [Google Scholar] [CrossRef]
- Beyene, H.D.; Werkneh, A.A.; Bezabh, H.K.; Ambaye, T.G. Synthesis paradigm and applications of silver nanoparticles (AgNPs), a review. Sustain. Mater. Technol. 2017, 13, 18–23. [Google Scholar] [CrossRef]
- Koduru, J.R.; Kailasa, S.K.; Bhamore, J.R.; Kim, K.-H.; Dutta, T.; Vellingiri, K. Phytochemical-assisted synthetic approaches for silver nanoparticles antimicrobial applications: A review. Adv. Colloid Interface Sci. 2018, 256, 326–339. [Google Scholar] [CrossRef]
- Duan, H.; Wang, D.; Li, Y. Green chemistry for nanoparticle synthesis. Chem. Soc. Rev. 2015, 44, 5778–5792. [Google Scholar] [CrossRef]
- Rauwel, P.; Rauwel, E.; Ferdov, S.; Singh, M.P. Silver Nanoparticles: Synthesis, Properties, and Applications. Adv. Mater. Sci. Eng. 2015, 2015, 1–2. [Google Scholar] [CrossRef]
- Hulkoti, N.I.; Taranath, T.C. Biosynthesis of nanoparticles using microbes—A review. Colloids Surf. B Biointerfaces 2014, 121, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Park, Y. A new paradigm shift for the green synthesis of antibacterial silver nanoparticles utilizing plant extracts. Toxicol. Res. 2014, 30, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Kanchic, S.; Bisetty, K. Biogenic synthesis of nanoparticles: A review. Arab. J. Chem. 2019, 12, 3576–3600. [Google Scholar] [CrossRef]
- Alkhathlan, H.Z.; Khan, M.; Khan, S.T.; Khan, M.; Adil, S.F.; Musarrat, J.; Al-Warthan, A.; Siddiqui, M.R.H.; Al-Khedhairy, A. Antibacterial properties of silver nanoparticles synthesized using Pulicaria glutinosa plant extract as a green bioreductant. Int. J. Nanomed. 2014, 9, 3551–3565. [Google Scholar] [CrossRef] [PubMed]
- Shaik, M.R.; AlBalawi, G.H.; Khan, S.T.; Khan, M.; Adil, S.F.; Kuniyil, M.; Kuniyil, M.; Siddiqui, M.R.H.; Alkhathlan, H.Z.; Khan, M. “Miswak” Based Green Synthesis of Silver Nanoparticles: Evaluation and Comparison of Their Microbicidal Activities with the Chemical Synthesis. Molecules 2016, 21, 1478. [Google Scholar] [CrossRef] [PubMed]
- Mohdaly, A.A.; Mahmoud, A.A.; Roby, M.; Smetanska, I.; Hassanien, M.F.R. Phenolic Extract from Propolis and Bee Pollen: Composition, Antioxidant and Antibacterial Activities. J. Food Biochem. 2015, 39, 538–547. [Google Scholar] [CrossRef]
- Rzepecka-Stojko, A.; Stojko, J.; Kurek-Górecka, A.; Górecki, M.; Kabała-Dzik, A.; Kubina, R.; Moździerz, A.; Buszman, E. Polyphenols from Bee Pollen: Structure, Absorption, Metabolism and Biological Activity. Molecules 2015, 20, 21732–21749. [Google Scholar] [CrossRef]
- Kocot, J.; Kiełczykowska, M.; Luchowska-Kocot, D.; Kurzepa, J.; Musik, I. Antioxidant Potential of Propolis, Bee Pollen, and Royal Jelly: Possible Medical Application. Oxidative Med. Cell. Longev. 2018, 2018, 7074209. [Google Scholar] [CrossRef]
- Komosińska-Vassev, K.; Olczyk, P.; Kaźmierczak, J.; Mencner, Ł.; Olczyk, K. Bee Pollen: Chemical Composition and Therapeutic Application. Evid. -Based Complement. Altern. Med. 2015, 2015, 297425. [Google Scholar] [CrossRef]
- Pascoal, A.; Rodrigues, S.; Teixeira, A.; Feás, X.; Estevinho, L.M.; Estevinho, L.M. Biological activities of commercial bee pollens: Antimicrobial, antimutagenic, antioxidant and anti-inflammatory. Food Chem. Toxicol. 2014, 63, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Tohamy, A.A.; Abdella, E.M.; Ahmed, R.R.; Ahmed, Y.K. Assessment of anti-mutagenic, anti-histopathologic and antioxidant capacities of Egyptian bee pollen and propolis extracts. Cytotechnology 2013, 66, 283–297. [Google Scholar] [CrossRef] [PubMed]
- El-Bialy, B.E.; Abdeen, E.; El-Borai, N.B.; El-Diasty, E.M. Experimental Studies on Some Immunotoxicological Aspects of Aflatoxins Containing Diet and Protective Effect of Bee Pollen Dietary Supplement. Pak. J. Boil. Sci. 2016, 19, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Kalaycıoğlu, Z.; Kaygusuz, H.; Döker, S.; Kolaylı, S.; Erim, F.B. Characterization of Turkish honeybee pollens by principal component analysis based on their individual organic acids, sugars, minerals, and antioxidant activities. LWT 2017, 84, 402–408. [Google Scholar] [CrossRef]
- Leja, M.; Mareczek, A.; Wyżgolik, G.; Klepacz-Baniak, J.; Czekońska, K. Antioxidative properties of bee pollen in selected plant species. Food Chem. 2007, 100, 237–240. [Google Scholar] [CrossRef]
- Sousa, C.; Moita, E.; Valentão, P.; Fernandes, F.; Monteiro, P.; Andrade, P.B. Effects of Colored and Noncolored Phenolics ofEchium plantagineumL. Bee Pollen in Caco-2 Cells under Oxidative Stress Induced bytert-Butyl Hydroperoxide. J. Agric. Food Chem. 2015, 63, 2083–2091. [Google Scholar] [CrossRef] [PubMed]
- Mehr, F.P.; Khanjani, M.; Vatani, P. Synthesis of Nano-Ag particles using Sodium Borohydride. Orient. J. Chem. 2015, 31, 1831–1833. [Google Scholar] [CrossRef]
- Al Akeel, R.; Mateen, A.; Syed, R.; Alyousef, A.A.; Shaik, M.R. Screening, Purification and Characterization of Anionic Antimicrobial Proteins from Foeniculum Vulgare. Molecules 2017, 22, 602. [Google Scholar] [CrossRef] [PubMed]
- Abuderman, A.A.; Syed, R.; Alyousef, A.A.; Alqahtani, M.S.; Shams, S.; Malik, A. Green Synthesized Silver Nanoparticles of Myrtus communis L (AgMC) Extract Inhibits Cancer Hallmarks via Targeting Aldose Reductase (AR) and Associated Signaling Network. Processes 2019, 7, 860. [Google Scholar] [CrossRef]
- Khider, M.; Elbanna, K.; Mahmoud, A.; Owayss, A.A. Egyptian honeybee pollen as antimicrobial, antioxidant agents, and dietary food supplements. Food Sci. Biotechnol. 2013, 22, 1–9. [Google Scholar] [CrossRef]
- Goodwillie, C. Wind pollination and reproductive assurance inLinanthus parviflorus(Polemoniaceae), a self-incompatible annual. Am. J. Bot. 1999, 86, 948–954. [Google Scholar] [CrossRef] [PubMed]
- Taha, E.-K.A. A study on nectar and pollen sources for honeybee, Apis mellifera L. in Al-Ahsa Saudi Arabia. J. Entomol. Zool. Stud. 2015, 3, 272–277. [Google Scholar]
- Llnskens, H.F.; Jorde, W. Pollen as food and medicine—A review. Econ. Bot. 1997, 51, 78–86. [Google Scholar] [CrossRef]
- Mărgăoan, R.; Mărghitaş, L.A.; Dezmirean, D.S.; Gherman, B.; Chirila, F.; Zacharias, I.; Bobis, O. Antimicrobial Activity of Bee Pollen Ethanolic and Methanolic Extracts on Staphylococcus aureus Bacterial Strain. Bull. Univ. Agric. Sci. Veter- Med. Cluj-Napoca. Anim. Sci. Biotechnol. 2015, 72, 78–80. [Google Scholar] [CrossRef]
- Fatrcová-Šramková, K.; Nôžková, J.; Máriássyová, M.; Kačániová, M. Biologically active antimicrobial and antioxidant substances in theHelianthus annuusL. bee pollen. J. Environ. Sci. Health Part B 2015, 51, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Karadal, F.; Onmaz, N.E.; Abay, S.; Yildirim, Y.; Al, S.; Tatyuz, I.; Akcay, A. A study of antibacterial and antioxidant activities of bee products: Propolis, pollen and honey samples. Ethiop. J. Health Dev. 2018, 32. [Google Scholar]
- Premratanachai, P.; Chanchao, C.; Amano, K. Review of the anticancer activities of bee products. Asian Pac. J. Trop. Biomed. 2014, 4, 337–344. [Google Scholar] [CrossRef]
- Wu, Y.-D.; Lou, Y.-J. A steroid fraction of chloroform extract from bee pollen ofBrassica campestrisinduces apoptosis in human prostate cancer PC-3 cells. Phytother. Res. 2007, 21, 1087–1091. [Google Scholar] [CrossRef]
- Kothai, S.; Varthamanan, S.J. Anti cancer activity of silver nano particles bio- synthesized using stingless bee propolis (Tetragonula iridipennis) of Tamilnadu. Asian J. Biomed. Pharm. Sci. 2015, 4, 30–37. [Google Scholar] [CrossRef]
- Hajebi, S.; Homayouni-Tabrizi, M.; Moghaddam, M.N.; Shahraki, F.; Yadamani, S. Rapeseed flower pollen bio-green synthesized silver nanoparticles: A promising antioxidant, anticancer and antiangiogenic compound. JBIC J. Boil. Inorg. Chem. 2019, 24, 395–404. [Google Scholar] [CrossRef]
- El-Deeb, N.M.; El-Sherbiny, I.M.; El-Aassara, M.R.; E Hafez, E. Novel Trend in Colon Cancer Therapy Using Silver Nanoparticles Synthesized by Honey Bee. J. Nanomed. Nanotechnol. 2015, 6, 265. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).