RETRACTED: A Review on Insights for Green Production of Unconventional Protein and Energy Sources Derived from the Larval Biomass of Black Soldier Fly
Abstract
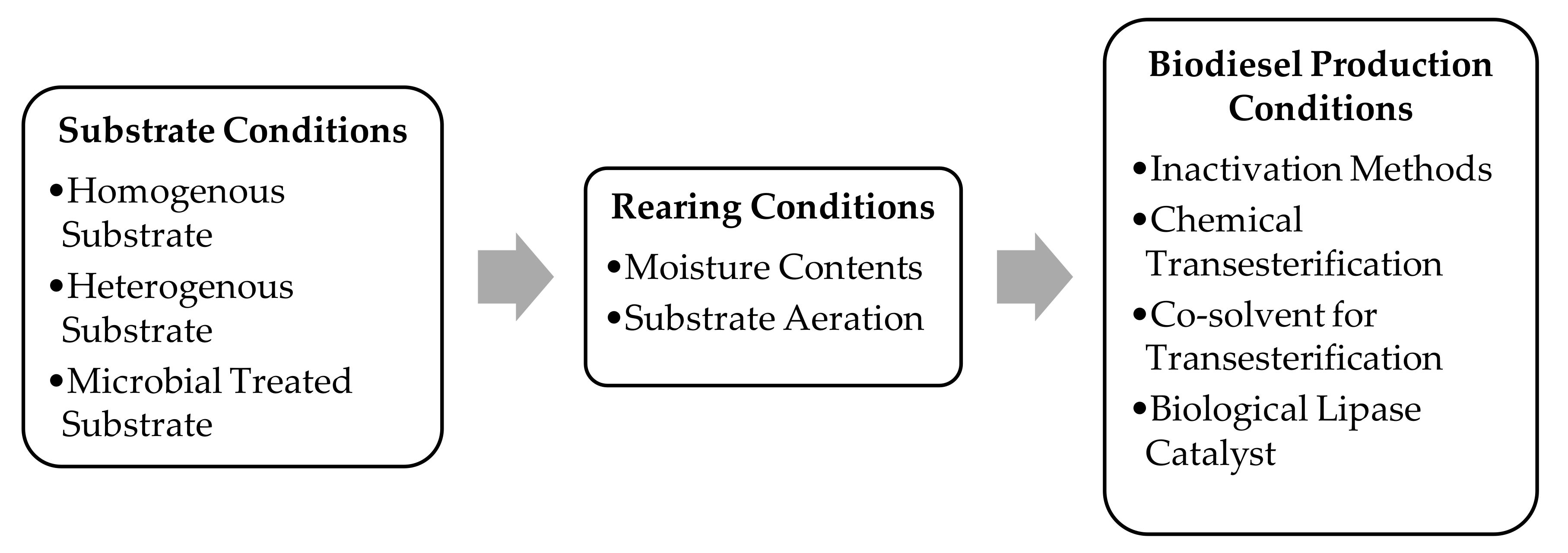
1. Introduction
2. Homogenous Substrate
3. Heterogenous Substrate
4. Microbial-Treated Substrates
5. Substrate Moisture Contents
6. Substrate Aeration
7. Inactivation Methods
8. BSFL-Based Biodiesel
9. Transesterification of Larval Lipids
10. Co-Solvent for Transesterification of Larval Lipids
11. Biological Lipase Catalyst for Transesterification of Larval Lipids
12. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Barnwal, B.K.; Sharma, M.P. Prospects of biodiesel production from vegetable oils in India. Renew. Sustain. Energy Rev. 2005, 9, 363–378. [Google Scholar] [CrossRef]
- Boocock, D.G.B.; Konar, S.K.; Mao, V.; Sidi, H. Fast one phase oil-rich processes for preparation of vegetable oil methyl esters. Biomass Bioenergy 1996, 11, 43–50. [Google Scholar] [CrossRef]
- Atabani, A.E.; Silitonga, A.S.; Badruddin, I.A.; Mahlia, T.M.I.; Masjuki, H.H.; Mekhilef, S. A comprehensive review on biodiesel as an alternative energy resource and its characteristics. Renew. Sustain. Energy Rev. 2012, 16, 2070–2093. [Google Scholar] [CrossRef]
- Sitepu, I.R.; Sectric, R.; Ignatia, L.; Levin, D.; German, J.B.; Gillies, L.A.; Almada, L.A.G.; Boundy-Mills, K.L. Manipulation of culture conditions alters lipid content and fatty acid profiles of a wide variety of known and new oleaginous yeast species. Bioresour. Technol. 2013, 144, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Xin, L.; Hong-ying, H.; Ke, G.; Ying-xue, S. Effects of different nitrogen and phosphorus concentrations on the growth, nutrient uptake, and lipid accumulation of a freshwater microalga Scenedesmus sp. Bioresour. Technol. 2010, 101, 5494–5500. [Google Scholar] [CrossRef] [PubMed]
- Pinzi, S.; Leiva, D.; López-García, I.; Redel-Macías, M.D.; Dorado, M.P. Latest trends in feedstocks for biodiesel production. Biofuels Bioprod. Biorefining 2014, 8, 126–143. [Google Scholar] [CrossRef]
- Manzano-Agugliaro, F.; Sanchez-Muros, M.J.; Barroso, F.G.; Martínez-Sánchez, A.; Rojo, S.; Pérez-Bañón, C. Insects for biodiesel production. Renew. Sustain. Energy Rev. 2012, 16, 3744–3753. [Google Scholar] [CrossRef]
- Leong, S.Y.; Kutty, S.R.M.; Malakahmad, A.; Tan, C.K. Feasibility study of biodiesel production using lipids of Hermetia illucens larva fed with organic waste. Waste Manag. 2016, 47 Pt A, 84–90. [Google Scholar] [CrossRef]
- Lalander, C.; Diener, S.; Zurbrügg, C.; Vinnerås, B. Effects of feedstock on larval development and process efficiency in waste treatment with black soldier fly (Hermetia illucens). J. Clean. Prod. 2019, 208, 211–219. [Google Scholar] [CrossRef]
- Li, Q.; Zheng, L.; Qiu, N.; Cai, H.; Tomberlin, J.K.; Yu, Z. Bioconversion of dairy manure by black soldier fly (Diptera: Stratiomyidae) for biodiesel and sugar production. Waste Manag. 2011, 31, 1316–1320. [Google Scholar] [CrossRef]
- Newton, L.; Sheppard, C.; Watson, D.W.; Burtle, G.; Dove, R. Using the Black Soldier Fly, Hermetia Illucens, as a Value-Added Tool for the Management of Swine Manure; North Carolina State University: Raleigh, NC, USA, 2005. [Google Scholar]
- Arango Gutiérrez, G.P.; Vergara Ruiz, R.A.; Me-jía Vélez, H. Analisis composicional, microbiológico y digestibilidad de la proteína de la harina de larvas de Hermetia illuscens L (Diptera: Stratiomyiidae) en Angelópolis-An-tioquia, Colombia. Revista Facultad Nacional de Agronomía-Medellín 2004, 57, 2491–2500. [Google Scholar]
- Li, W.; Li, M.; Zheng, L.; Liu, Y.; Zhang, Y.; Yu, Z.; Ma, Z.; Li, Q. Simultaneous utilization of glucose and xylose for lipid accumulation in black soldier fly. Biotechnol. Biofuels 2015, 8, 117. [Google Scholar] [CrossRef]
- Li, W.; Li, Q.; Wang, Y.; Zheng, L.; Zhang, Y.; Yu, Z.; Chen, H.; Zhang, J. Efficient bioconversion of organic wastes to value-added chemicals by soaking, black soldier fly (Hermetia illucens L.) and anaerobic fermentation. J. Environ. Manag. 2018, 227, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Rehman, K.U.; Cai, M.; Xiao, X.; Zheng, L.; Wang, H.; Soomro, A.A.; Zhou, Y.; Li, W.; Yu, Z.; Zhang, J. Cellulose decomposition and larval biomass production from the co-digestion of dairy manure and chicken manure by mini-livestock (Hermetia illucens L.). J. Environ. Manag. 2017, 196, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Rehman, K.U.; Rehman, R.U.; Somroo, A.A.; Cai, M.; Zheng, L.; Xiao, X.; Rehman, A.U.; Rehman, A.; Tomberlin, J.K.; Yu, Z.; et al. Enhanced bioconversion of dairy and chicken manure by the interaction of exogenous bacteria and black soldier fly larvae. J. Environ. Manag. 2019, 237, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Somroo, A.A.; Rehman, K.U.; Zheng, L.; Cai, M.; Xiao, X.; Hu, S.; Mathys, A.; Gold, M.; Yu, Z.; Zhang, J. Influence of Lactobacillus buchneri on soybean curd residue co-conversion by black soldier fly larvae (Hermetia illucens) for food and feedstock production. Waste Manag. 2019, 86, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Hou, Y.; Li, W.; Yang, S.; Li, Q.; Yu, Z. Biodiesel production from rice straw and restaurant waste employing black soldier fly assisted by microbes. Energy 2012, 47, 225–229. [Google Scholar] [CrossRef]
- Barry, T. Evaluation of the economic, social, and biological feasibility of bioconverting food wastes with the black soldier fly (hermetia illucens). Ph.D. Thesis, University of North Texas, Denton, TX, USA, 2014. [Google Scholar]
- Palma, L.; Ceballos, S.J.; Johnson, P.C.; Niemeier, D.; Pitesky, M.; VanderGheynst, J.S. Cultivation of black soldier fly larvae on almond byproducts: Impacts of aeration and moisture on larvae growth and composition. J. Sci. Food Agric. 2018, 98, 5893–5900. [Google Scholar] [CrossRef] [PubMed]
- Cammack, J.A.; Tomberlin, J.K. The impact of diet protein and carbohydrate on select life-history traits of the black soldier fly Hermetia illucens (L.) (Diptera: Stratiomyidae). Insects 2017, 8, 56. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.Y.K.; Chiu, S.L.H.; Lo, I.M.C. Effects of moisture content of food waste on residue separation, larval growth and larval survival in black soldier fly bioconversion. Waste Manag. 2017, 67, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, X.; Wang, H.; Yang, Q.; Ur Rehman, K.; Li, W.; Cai, M.; Li, Q.; Mazza, L.; Zhang, J.; et al. Dynamic changes of nutrient composition throughout the entire life cycle of black soldier fly. PLoS ONE 2017, 12, e0182601. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.-Y.; Rosli, S.-S.; Uemura, Y.; Ho, Y.C.; Leejeerajumnean, A.; Kiatkittipong, W.; Cheng, C.-K.; Lam, M.-K.; Lim, J.-W. Potential protein and biodiesel sources from black soldier fly larvae: Insights of larval harvesting instar and fermented feeding medium. Energies 2019, 12, 1570. [Google Scholar] [CrossRef]
- Larouche, J.; Deschamps, M.-H.; Saucier, L.; Lebeuf, Y.; Doyen, A.; Vandenberg, G.W. Effects of killing methods on lipid oxidation, colour and microbial load of black soldier fly (hermetia illucens) larvae. Animals 2019, 9, 182. [Google Scholar] [CrossRef] [PubMed]
- Caligiani, A.; Marseglia, A.; Sorci, A.; Bonzanini, F.; Lolli, V.; Maistrello, L.; Sforza, S. Influence of the killing method of the black soldier fly on its lipid composition. Food Res. Int. 2019, 116, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Surendra, K.C.; Olivier, R.; Tomberlin, J.K.; Jha, R.; Khanal, S.K. Bioconversion of organic wastes into biodiesel and animal feed via insect farming. Renew. Energy 2016, 98, 197–202. [Google Scholar] [CrossRef]
- Li, Q.; Zheng, L.; Cai, H.; Garza, E.; Yu, Z.; Zhou, S. From organic waste to biodiesel: Black soldier fly, Hermetia illucens, makes it feasible. Fuel 2011, 90, 1545–1548. [Google Scholar] [CrossRef]
- Zheng, L.; Li, Q.; Zhang, J.; Yu, Z. Double the biodiesel yield: Rearing black soldier fly larvae, Hermetia illucens, on solid residual fraction of restaurant waste after grease extraction for biodiesel production. Renew. Energy 2012, 41, 75–79. [Google Scholar] [CrossRef]
- Nguyen, H.C.; Liang, S.-H.; Li, S.-Y.; Su, C.-H.; Chien, C.-C.; Chen, Y.-J.; Huong, D.T.M. Direct transesterification of black soldier fly larvae (Hermetia illucens) for biodiesel production. J. Taiwan Inst. Chem. Eng. 2018, 85, 165–169. [Google Scholar] [CrossRef]
- Nguyen, H.C.; Liang, S.-H.; Doan, T.T.; Su, C.-H.; Yang, P.-C. Lipase-catalyzed synthesis of biodiesel from black soldier fly (Hermetica illucens): Optimization by using response surface methodology. Energy Convers. Manag. 2017, 145, 335–342. [Google Scholar] [CrossRef]
- Nguyen, H.C.; Liang, S.-H.; Chen, S.-S.; Su, C.-H.; Lin, J.-H.; Chien, C.-C. Enzymatic production of biodiesel from insect fat using methyl acetate as an acyl acceptor: Optimization by using response surface methodology. Energy Convers. Manag. 2018, 158, 168–175. [Google Scholar] [CrossRef]
- Lim, J.W.; Mohd-Noor, S.N.; Wong, C.Y.; Lam, M.K.; Goh, P.S.; Beniers, J.J.A.; Oh, W.D.; Jumbri, K.; Ghani, N.A. Palatability of black soldier fly larvae in valorizing mixed waste coconut endosperm and soybean curd residue into larval lipid and protein sources. J. Environ. Manag. 2019, 231, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Mohd-Noor, S.N.; Wong, C.Y.; Lim, J.W.; Mah-Hussin, M.I.A.; Uemura, Y.; Lam, M.K.; Ramli, A.; Bashir, M.J.K.; Tham, L. Optimization of self-fermented period of waste coconut endosperm destined to feed black soldier fly larvae in enhancing the lipid and protein yields. Renew. Energy 2017, 111, 646–654. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasnol, S.; Kiatkittipong, K.; Kiatkittipong, W.; Wong, C.Y.; Khe, C.S.; Lam, M.K.; Show, P.L.; Oh, W.D.; Chew, T.L.; Lim, J.W. RETRACTED: A Review on Insights for Green Production of Unconventional Protein and Energy Sources Derived from the Larval Biomass of Black Soldier Fly. Processes 2020, 8, 523. https://doi.org/10.3390/pr8050523
Hasnol S, Kiatkittipong K, Kiatkittipong W, Wong CY, Khe CS, Lam MK, Show PL, Oh WD, Chew TL, Lim JW. RETRACTED: A Review on Insights for Green Production of Unconventional Protein and Energy Sources Derived from the Larval Biomass of Black Soldier Fly. Processes. 2020; 8(5):523. https://doi.org/10.3390/pr8050523
Chicago/Turabian StyleHasnol, Sabrina, Kunlanan Kiatkittipong, Worapon Kiatkittipong, Chung Yiin Wong, Cheng Seong Khe, Man Kee Lam, Pau Loke Show, Wen Da Oh, Thiam Leng Chew, and Jun Wei Lim. 2020. "RETRACTED: A Review on Insights for Green Production of Unconventional Protein and Energy Sources Derived from the Larval Biomass of Black Soldier Fly" Processes 8, no. 5: 523. https://doi.org/10.3390/pr8050523
APA StyleHasnol, S., Kiatkittipong, K., Kiatkittipong, W., Wong, C. Y., Khe, C. S., Lam, M. K., Show, P. L., Oh, W. D., Chew, T. L., & Lim, J. W. (2020). RETRACTED: A Review on Insights for Green Production of Unconventional Protein and Energy Sources Derived from the Larval Biomass of Black Soldier Fly. Processes, 8(5), 523. https://doi.org/10.3390/pr8050523








