Enhancing Astaxanthin Biosynthesis by Rhodosporidium toruloides Mutants and Optimization of Medium Compositions Using Response Surface Methodology
Abstract
1. Introduction
2. Materials and Methods
2.1. Yeast Strains and Culture Conditions
2.2. Random UV Mutagenesis
2.3. Random Gamma Irradiation Mutagenesis
2.4. Optimization of Medium Compositions
2.5. Analysis
2.5.1. Determination of Survival Rate
2.5.2. Biomass Determination
2.5.3. Astaxanthin Content Determination
2.5.4. Statistical Analysis
3. Results and Discussion
3.1. Astaxanthin Production by UV-Induced Mutants
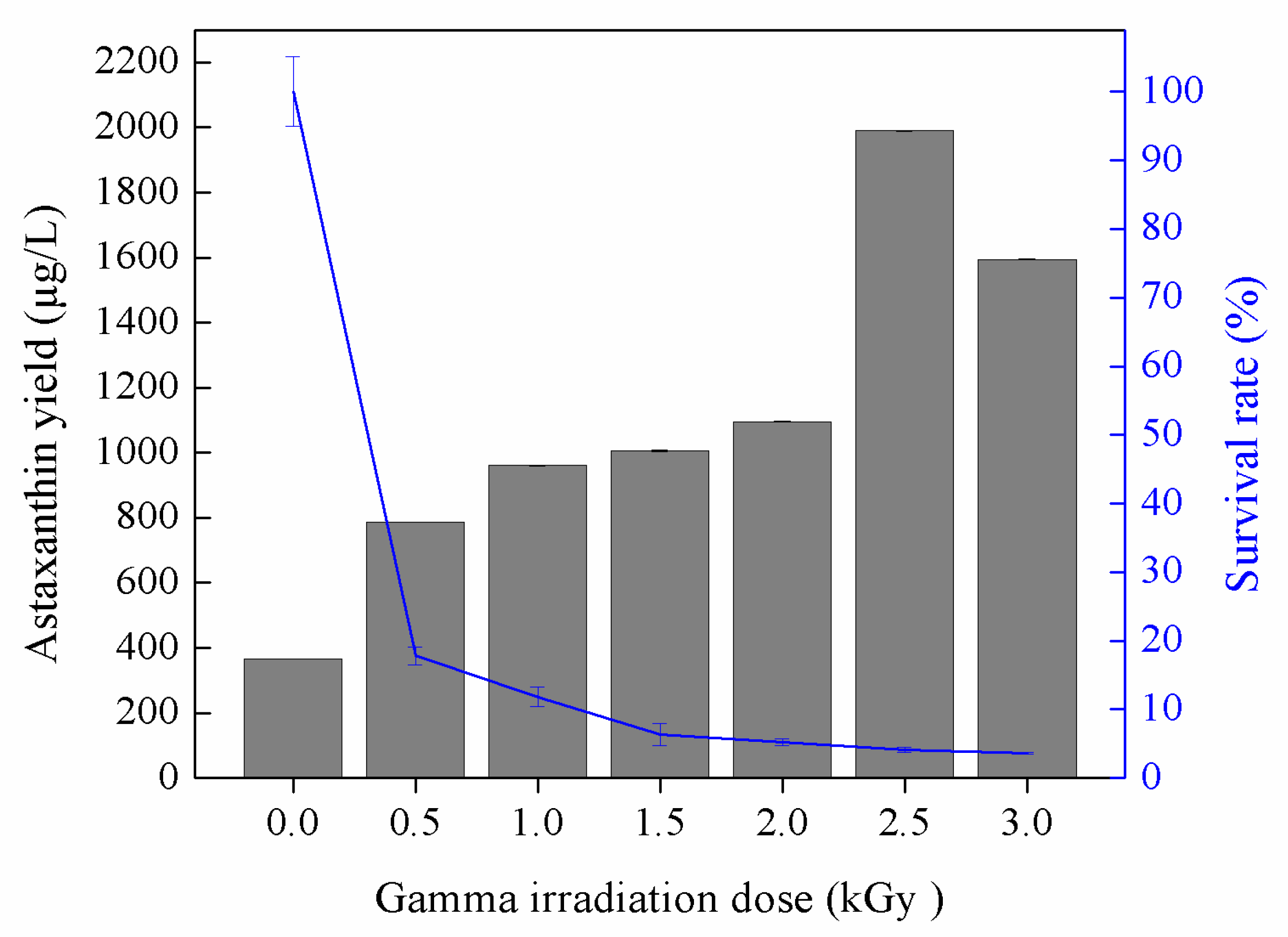
3.2. Astaxanthin Production by Gamma-Induced Mutants
3.3. Optimization of Medium Compositions Using RSM
3.3.1. Development of RSM Model
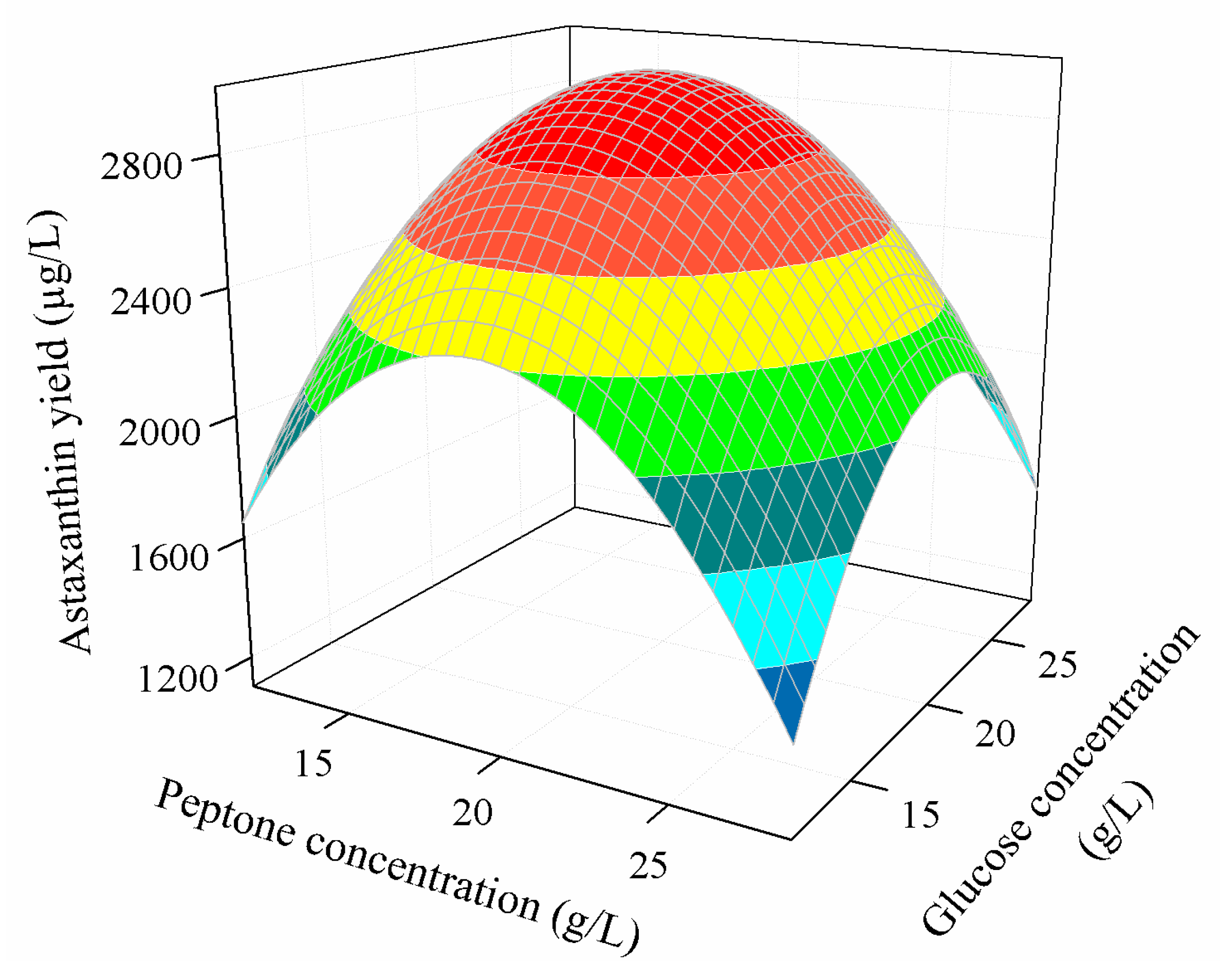
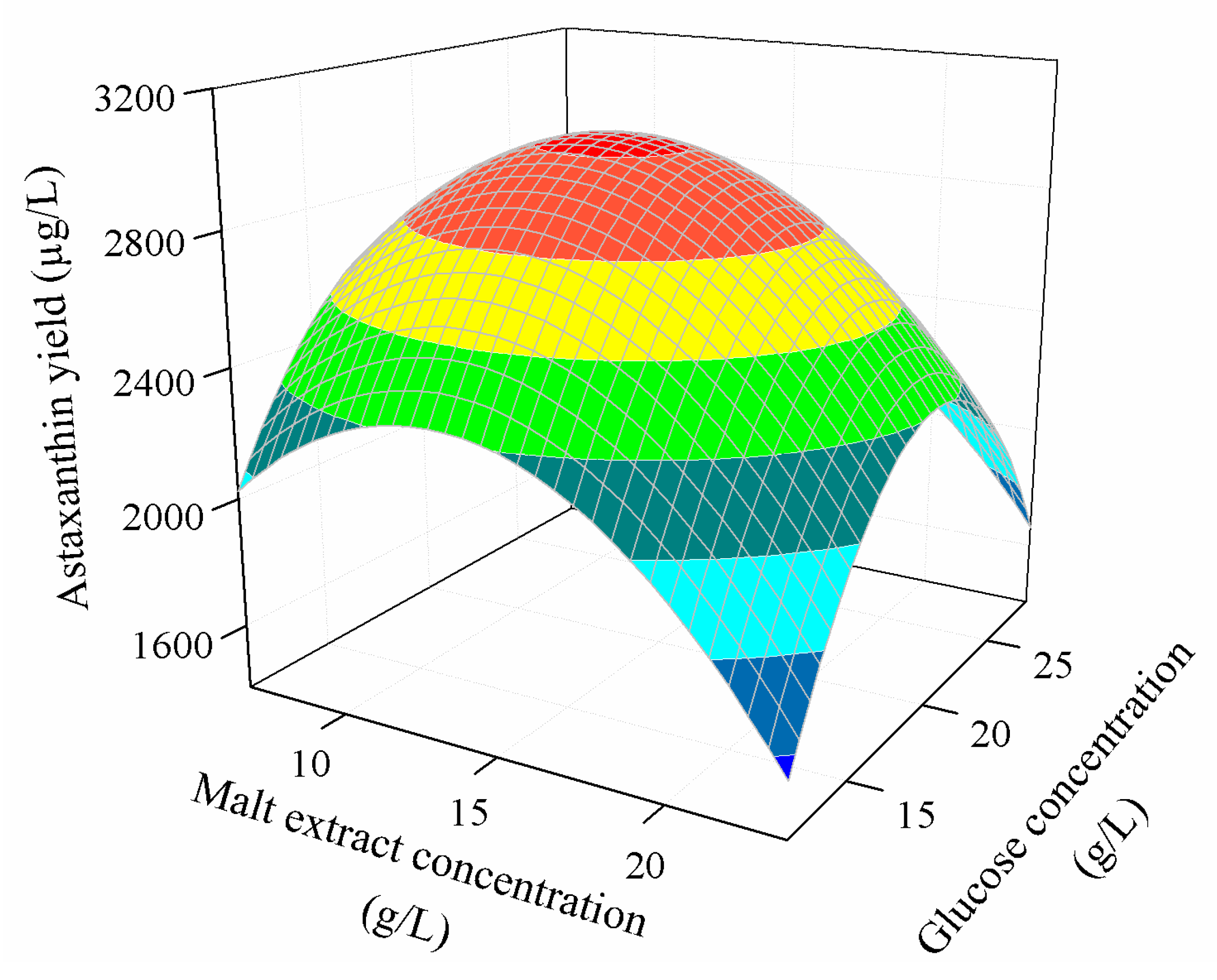
3.3.2. Combined Effect of Nutritional Parameters
3.3.3. Obtaining Optimal Medium Compositions
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Batghare, A.H.; Singh, N.; Moholkar, V.S. Investigations in ultrasound–induced enhancement of astaxanthin production by wild strain Phaffia rhodozyma MTCC 7536. Bioresour. Technol. 2018, 254, 166–173. [Google Scholar] [CrossRef]
- Tran, T.N.; Quang-Vinh, T.; Huynh, H.T.; Hoang, N.-S.; Nguyen, H.C.; Dai-Nghiep, N. Astaxanthin production by newly isolated Rhodosporidium toruloides: Optimization of medium compositions by response surface methodology. Not. Bot. Horti Agrobo. 2019, 47, 320–327. [Google Scholar] [CrossRef]
- Ambati, R.R.; Phang, S.-M.; Ravi, S.; Aswathanarayana, R.G. Astaxanthin: Sources, extraction, stability, biological activities and its commercial applications—A review. Mar. Drugs 2014, 12, 128–152. [Google Scholar] [CrossRef]
- Wu, Y.; Yan, P.; Liu, X.; Wang, Z.; Tang, Y.-J.; Chen, T.; Zhao, X. Combinatorial expression of different β-carotene hydroxylases and ketolases in Escherichia coli for increased astaxanthin production. J. Ind. Microbiol. Biotechnol. 2019, 46, 1505–1516. [Google Scholar] [CrossRef]
- Jiang, W.; Zhao, H.; Zhang, L.; Wu, B.; Zha, Z. Maintenance of mitochondrial function by astaxanthin protects against bisphenol A-induced kidney toxicity in rats. Biomed. Pharmacother. 2020, 121, 109629. [Google Scholar] [CrossRef]
- Davinelli, S.; Nielsen, M.E.; Scapagnini, G. Astaxanthin in skin health, repair, and disease: A comprehensive review. Nutrients 2018, 10, 522. [Google Scholar] [CrossRef]
- Panis, G.; Carreon, J.R. Commercial astaxanthin production derived by green alga Haematococcus pluvialis: A microalgae process model and a techno-economic assessment all through production line. Algal Res. 2016, 18, 175–190. [Google Scholar] [CrossRef]
- An, J.; Gao, F.; Ma, Q.; Xiang, Y.; Ren, D.; Lu, J. Screening for enhanced astaxanthin accumulation among Spirulina platensis mutants generated by atmospheric and room temperature plasmas. Algal Res. 2017, 25, 464–472. [Google Scholar] [CrossRef]
- Dong, H.; Li, X.; Xue, C.; Mao, X. Astaxanthin preparation by fermentation of esters from Haematococcus pluvialis algal extracts with Stenotrophomonas species. Biotechnol. Prog. 2016, 32, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cui, Y.; Chen, J.; Qin, S.; Chen, G. Metabolic engineering of Synechocystis sp. PCC6803 to produce astaxanthin. Algal Res. 2019, 44, 101679. [Google Scholar] [CrossRef]
- Choi, Y.-E.; Yun, Y.S.; Park, J. Evaluation of factors promoting astaxanthin production by a unicellular green alga, Haematococcus pluvialis, with fractional factorial design. Biotechnol. Prog. 2002, 18, 1170–1175. [Google Scholar] [CrossRef] [PubMed]
- Guyomarc’h, F.; Binet, A.; Dufossé, L. Production of carotenoids by Brevibacterium linens: Variation among strains, kinetic aspects and HPLC profiles. J. Ind. Microbiol. Biotechnol. 2000, 24, 64–70. [Google Scholar] [CrossRef]
- Zhang, C.; Seow, V.Y.; Chen, X.; Too, H.-P. Multidimensional heuristic process for high-yield production of astaxanthin and fragrance molecules in Escherichia coli. Nat. Commun. 2018, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Huang, J.J.; Sun, D.; Lee, Y.; Chen, F. Two-step cultivation for production of astaxanthin in Chlorella zofingiensis using a patented energy-free rotating floating photobioreactor (RFP). Bioresour. Technol. 2017, 224, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Azizi, M.; Hejazi, M.A.; Hashemi, M. Supplementation with polyalcohols and sequential mixotrophy dilution photoinduction strategy boost the accumulation of astaxanthin by Haematococcus pluvialis. Aquaculture 2019, 511, 734225. [Google Scholar] [CrossRef]
- Liu, Y.-H.; Alimujiang, A.; Wang, X.; Luo, S.-W.; Balamurugan, S.; Yang, W.-D.; Liu, J.-S.; Zhang, L.; Li, H.-Y. Ethanol induced jasmonate pathway promotes astaxanthin hyperaccumulation in Haematococcus pluvialis. Bioresour. Technol. 2019, 289, 121720. [Google Scholar] [CrossRef]
- Christian, D.; Zhang, J.; Sawdon, A.J.; Peng, C.-A. Enhanced astaxanthin accumulation in Haematococcus pluvialis using high carbon dioxide concentration and light illumination. Bioresour. Technol. 2018, 256, 548–551. [Google Scholar] [CrossRef]
- Kaewpintong, K.; Shotipruk, A.; Powtongsook, S.; Pavasant, P. Photoautotrophic high-density cultivation of vegetative cells of Haematococcus pluvialis in airlift bioreactor. Bioresour. Technol. 2007, 98, 288–295. [Google Scholar] [CrossRef]
- Li, J.; Zhu, D.; Niu, J.; Shen, S.; Wang, G. An economic assessment of astaxanthin production by large scale cultivation of Haematococcus pluvialis. Biotechnol. Adv. 2011, 29, 568–574. [Google Scholar] [CrossRef]
- Dias, C.; Sousa, S.; Caldeira, J.; Reis, A.; da Silva, T.L. New dual-stage pH control fed-batch cultivation strategy for the improvement of lipids and carotenoids production by the red yeast Rhodosporidium toruloides NCYC 921. Bioresour. Technol. 2015, 189, 309–318. [Google Scholar] [CrossRef]
- Wu, Y.-H.; Yang, J.; Hu, H.-Y.; Yu, Y. Lipid-rich microalgal biomass production and nutrient removal by Haematococcus pluvialis in domestic secondary effluent. Ecol. Eng. 2013, 60, 155–159. [Google Scholar] [CrossRef]
- García-Malea, M.C.; Acién, F.G.; Del Río, E.; Fernández, J.M.; Cerón, M.C.; Guerrero, M.G.; Molina-Grima, E. Production of astaxanthin by Haematococcus pluvialis: Taking the one-step system outdoors. Biotechnol. Bioeng. 2009, 102, 651–657. [Google Scholar] [CrossRef] [PubMed]
- González-García, Y.; Rábago-Panduro, L.M.; French, T.; Camacho-Córdova, D.I.; Gutiérrez-González, P.; Córdova, J. High lipids accumulation in Rhodosporidium toruloides by applying single and multiple nutrients limitation in a simple chemically defined medium. Ann. Microbiol. 2017, 67, 519–527. [Google Scholar] [CrossRef]
- Zhang, C.; Shen, H.; Zhang, X.; Yu, X.; Wang, H.; Xiao, S.; Wang, J.; Zhao, Z.K. Combined mutagenesis of Rhodosporidium toruloides for improved production of carotenoids and lipids. Biotechnol. Lett. 2016, 38, 1733–1738. [Google Scholar] [CrossRef] [PubMed]
- Yaegashi, J.; Kirby, J.; Ito, M.; Sun, J.; Dutta, T.; Mirsiaghi, M.; Sundstrom, E.R.; Rodriguez, A.; Baidoo, E.; Tanjore, D. Rhodosporidium toruloides: A new platform organism for conversion of lignocellulose into terpene biofuels and bioproducts. Biotechnol. Biofuels 2017, 10, 241. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.E.; Choi, H.I.; Kwak, H.S.; Hwang, S.-W.; Sung, Y.J.; Chang, W.S.; Sim, S.J. Rapid selection of astaxanthin-hyperproducing Haematococcus mutant via azide-based colorimetric assay combined with oil-based astaxanthin extraction. Bioresour. Technol. 2018, 267, 175–181. [Google Scholar] [CrossRef]
- Ang, F.; Khaw, S.; Few, L.; Too, W.S.; Chew, A. Isolation of a stable astaxanthin-hyperproducing mutant of Xanthophyllomyces dendrorhous through random mutagenesis. Appl. Biochem. Microbiol. 2019, 55, 255–263. [Google Scholar] [CrossRef]
- Wang, N.; Guan, B.; Kong, Q.; Sun, H.; Geng, Z.; Duan, L. Enhancement of astaxanthin production from Haematococcus pluvialis mutants by three-stage mutagenesis breeding. J. Biotechnol. 2016, 236, 71–77. [Google Scholar] [CrossRef]
- Najafi, N.; Ahmadi, A.-R.; Hosseini, R.; Golkhoo, S. Gamma irradiation as a useful tool for the isolation of astaxanthin-overproducing mutant strains of Phaffia rhodozyma. Can. J. Microbiol. 2011, 57, 730–734. [Google Scholar] [CrossRef]
- Sun, N.; Lee, S.; Song, K.B. Characterization of a carotenoid-hyperproducing yeast mutant isolated by low-dose gamma irradiation. Int. J. Food Microbiol. 2004, 94, 263–267. [Google Scholar] [CrossRef]
- Su, C.H.; Nguyen, H.C.; Nguyen, M.L.; Tran, P.T.; Wang, F.M.; Guan, Y.L. Liquid lipase-catalyzed hydrolysis of gac oil for fatty acid production: Optimization using response surface methodology. Biotechnol. Prog. 2018, 34, 1129–1136. [Google Scholar] [CrossRef] [PubMed]
- Fang, T.J.; Cheng, Y.-S. Improvement of astaxanthin production by Phaffia rhodozyma through mutation and optimization of culture conditions. J. Ferment. Bioeng. 1993, 75, 466–469. [Google Scholar] [CrossRef]
- An, G.-H.; Bielich, J.; Auerbach, R.; Johnson, E.A. Isolation and characterization of carotenoid hyperproducing mutants of yeast by flow cytometry and cell sorting. Biotechnology 1991, 9, 70–73. [Google Scholar] [CrossRef] [PubMed]
- Kelley, C.E.; Harmon, A.W. Method of determining carotenoid contents of Alaska pink shrimp and representative values for several shrimp products. Fish. Bull. 1972, 70, 111. [Google Scholar]
- Stachowiak, B. Effect of illumination intensities on astaxanthin synthesis by Xanthophyllomyces dendrorhous and its mutants. Food Sci. Biotechnol. 2013, 22, 1033–1038. [Google Scholar] [CrossRef]
- Castelblanco-Matiz, L.M.; Barbachano-Torres, A.; Ponce-Noyola, T.; Ramos-Valdivia, A.C.; García-Rojas, C.M.C.; Flores-Ortiz, C.M.; Barahona-Crisóstomo, S.K.; Baeza-Cancino, M.E.; Alcaíno-Gorman, J.; Cifuentes-Guzmán, V.H. Carotenoid production and gene expression in an astaxanthin-overproducing Xanthophyllomyces dendrorhous mutant strain. Arch. Microbiol. 2015, 197, 1129–1139. [Google Scholar] [CrossRef]
- Vázquez, M. Effect of the light on carotenoid profiles of Xanthophyllomyces dendrorhous strains (formerly Phaffia rhodozyma). Food Technol. Biotechnol. 2001, 39, 123–128. [Google Scholar]
- Stachowiak, B. Astaxanthin synthesis by Xanthophyllomyces dendrorhous DSM 5626 and its mutants on carrot extract medium under different illumination intensity. Appl. Biochem. Microbiol. 2014, 50, 471–476. [Google Scholar] [CrossRef]
- Marcoleta, A.; Niklitschek, M.; Wozniak, A.; Lozano, C.; Alcaíno, J.; Baeza, M.; Cifuentes, V. Glucose and ethanol-dependent transcriptional regulation of the astaxanthin biosynthesis pathway in Xanthophyllomyces dendrorhous. BMC Microbiol. 2011, 11, 190. [Google Scholar] [CrossRef]
- Xie, H.; Zhou, Y.; Hu, J.; Chen, Y.; Liang, J. Production of astaxanthin by a mutant strain of Phaffia rhodozyma and optimization of culture conditions using response surface methodology. Ann. Microbiol. 2014, 64, 1473–1481. [Google Scholar] [CrossRef]
- Nahidian, B.; Ghanati, F.; Shahbazi, M.; Soltani, N. Effect of nutrients on the growth and physiological features of newly isolated Haematococcus pluvialis TMU1. Bioresour. Technol. 2018, 255, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yue, C.; Geng, S.; Ning, D.; Ma, T.; Yu, X. Role of media composition in biomass and astaxanthin production of Haematococcus pluvialis under two-stage cultivation. Bioprocess Biosys. Eng. 2019, 42, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Saenge, C.; Cheirsilp, B.; Suksaroge, T.T.; Bourtoom, T. Potential use of oleaginous red yeast Rhodotorula glutinis for the bioconversion of crude glycerol from biodiesel plant to lipids and carotenoids. Process Biochem. 2011, 46, 210–218. [Google Scholar] [CrossRef]
- Ip, P.-F.; Chen, F. Production of astaxanthin by the green microalga Chlorella zofingiensis in the dark. Process Biochem. 2005, 40, 733–738. [Google Scholar] [CrossRef]
- Stoklosa, R.J.; Johnston, D.B.; Nghiem, N.P. Utilization of sweet sorghum juice for the production of astaxanthin as a biorefinery co-product by Phaffia rhodozyma. ACS Sustain. Chem. Eng. 2018, 6, 3124–3134. [Google Scholar] [CrossRef]
- Nguyen, H.C.; Su, C.-H.; Yu, Y.-K.; Huong, D.T.M. Sugarcane bagasse as a novel carbon source for heterotrophic cultivation of oleaginous microalga Schizochytrium sp. Ind. Crops Prod. 2018, 121, 99–105. [Google Scholar] [CrossRef]
- Ip, P.-F.; Wong, K.-H.; Chen, F. Enhanced production of astaxanthin by the green microalga Chlorella zofingiensis in mixotrophic culture. Process Biochem. 2004, 39, 1761–1766. [Google Scholar] [CrossRef]
- Fang, T.J.; Wang, J.-M. Extractability of astaxanthin in a mixed culture of a carotenoid over-producing mutant of Xanthophyllomyces dendrorhous and Bacillus circulans in two-stage batch fermentation. Process Biochem. 2002, 37, 1235–1245. [Google Scholar] [CrossRef]
- Hossain, T.; Miah, A.B.; Mahmud, S.A. Enhanced bioethanol production from potato peel waste via consolidated bioprocessing with statistically optimized medium. Appl. Biochem. Biotechnol. 2018, 186, 425–442. [Google Scholar] [CrossRef]
- Awad, G.E.; Amer, H.; El-Gammal, E.W.; Helmy, W.A.; Esawy, M.A.; Elnashar, M.M. Production optimization of invertase by Lactobacillus brevis Mm-6 and its immobilization on alginate beads. Carbohydr. Polym. 2013, 93, 740–746. [Google Scholar] [CrossRef]
- Tran, N.T.; Pham, D.N.; Kim, C.-J. Production of 5-aminolevulinic acid by recombinant Streptomyces coelicolor expressing hemA from Rhodobacter sphaeroides. Biotechnol. Bioprocess Eng. 2019, 24, 488–499. [Google Scholar] [CrossRef]
- Xiao, R.; Li, X.; Leonard, E.; Tharayil, N.; Zheng, Y. Investigation on the effects of cultivation conditions, fed-batch operation, and enzymatic hydrolysate of corn stover on the astaxanthin production by Thraustochytrium striatum. Algal Res. 2019, 39, 101475. [Google Scholar] [CrossRef]
- Hwang, S.-W.; Choi, H.I.; Sim, S.J. Acidic cultivation of Haematococcus pluvialis for improved astaxanthin production in the presence of a lethal fungus. Bioresour. Technol. 2019, 278, 138–144. [Google Scholar] [CrossRef] [PubMed]


| Variables | Symbols | Levels | ||||
|---|---|---|---|---|---|---|
| −1.68 (−α) | −1 | 0 | 1 | 1.68 (+α) | ||
| Peptone concentration (g/L) | X1 | 11.6 | 15 | 20 | 25 | 28.4 |
| Malt extract concentration (g/L) | X2 | 6.6 | 10 | 15 | 20 | 23.4 |
| Glucose concentration (g/L) | X3 | 11.6 | 15 | 20 | 25 | 28.4 |
| Strain | Biomass Yield (g/L) | Astaxanthin Content (µg/g) | Astaxanthin Yield (µg/L) |
|---|---|---|---|
| UV1 | 2.847 ± 0.0039 dfe | 187.641 ± 0.026 g | 534.28 ± 7.22 j |
| UV2 | 3.461 ± 0.0007 dc | 166.927 ± 0.018 l | 577.68 ± 1.14 h |
| UV3 | 2.684 ± 0.0004 fe | 253.334 ± 0.004 c | 679.95 ± 1.00 e |
| UV4 | 2.931 ± 0.0005 dfe | 194.522 ± 0.003 f | 570.21 ± 0.96 i |
| UV5 | 4.149 ± 0.0004 ab | 144.290 ± 0.002 n | 598.70 ± 0.54 g |
| UV6 | 4.629 ± 0.0004 a | 183.345 ± 0.017 h | 848.77 ± 0.77 a |
| UV7 | 2.931 ± 0.0004 dfe | 274.293 ± 0.002 b | 803.86 ± 1.21 b |
| UV8 | 2.538 ± 0.0002 dfe | 275.241 ± 0.003 a | 698.56 ± 0.55 c |
| UV9 | 3.849 ± 0.0005 bc | 172.380 ± 0.001 j | 663.55 ± 0.87 f |
| UV10 | 1.754 ± 0.0004 g | 232.629 ± 0.007 e | 407.95 ± 1.03 o |
| UV11 | 2.595 ± 0.0003 f | 182.855 ± 0.010 i | 474.51 ± 0.63 m |
| UV12 | 3.016 ± 0.0002 dfe | 171.430 ± 0.002 k | 517.09 ± 0.27 k |
| UV13 | 3.734 ± 0.0002 bc | 130.476 ± 0.007 q | 487.15 ± 0.26 l |
| UV14 | 2.753 ± 0.0003 fe | 250.245 ± 0.003 d | 688.84 ± 0.72 d |
| UV15 | 3.131 ± 0.0003 dfe | 155.485 ± 0.004 m | 486.82 ± 0.40 l |
| UV16 | 3.249 ± 0.0006dce | 142.641 ± 0.026 o | 463.49 ± 0.80 n |
| WT * | 2.777 ± 0.0003 fe | 131.664 ± 0.010 p | 365.63 ± 0.42 p |
| Strain | Biomass Yield (g/L) | Astaxanthin Content (µg/g) | Astaxanthin Yield (µg/L) |
|---|---|---|---|
| G1 | 4.737 ± 0.0007 e | 206.602 ± 0.023 o | 978.67 ± 1.0 j |
| G2 | 3.710 ± 0.0002 l | 212.161 ± 0.030 l | 787.12 ± 0.4 n |
| G3 | 4.556 ± 0.0004 f | 210.909 ± 0.019 m | 960.90 ± 0.6 k |
| G4 | 5.113 ± 0.0015 c | 196.645 ± 0.030 r | 1005.45 ± 2.0 h |
| G5 | 5.403 ± 0.0003 a | 220.475 ± 0.045 i | 1191.23 ± 0.6 c |
| G6 | 3.855 ± 0.0004 k | 229.481 ± 0.035 f | 884.65 ± 0.8 l |
| G7 | 4.236 ± 0.0006 i | 185.002 ± 0.040 s | 783.67 ± 0.9 o |
| G8 | 3.845 ± 0.0004 k | 230.779 ± 0.030 e | 887.34 ± 0.7 l |
| G9 | 4.071 ± 0.0005 j | 218.515 ± 0.025 j | 889.57 ± 0.8 l |
| G10 | 5.104 ± 0.0004 c | 214.647 ± 0.035 k | 1095.56 ± 0.7 f |
| G11 | 5.098 ± 0.0005 c | 228.490 ± 0.010 g | 1164.84 ± 0.7 d |
| G12 | 4.943 ± 0.0004 d | 200.579 ± 0.041 q | 991.46 ± 0.7 i |
| G13 | 4.474 ± 0.0004 h | 233.815 ± 0.050 d | 1046.09 ± 0.9 g |
| G14 | 4.724 ±0.0005 e | 208.408 ± 0.037 n | 984.52 ± 0.8 j |
| G15 | 4.254 ± 0.0005 i | 204.344 ± 0.077 P | 869.28 ± 1.0 m |
| G16 | 4.516 ± 0.0003 g | 253.835 ± 0.025 c | 1146.32 ± 0.5 e |
| G17 | 3.297 ± 0.0408 m | 603.659 ± 0.025 a | 1990.26 ± 1.68 a |
| G18 | 5.245 ± 0.0024 b | 222.142 ± 0.497 h | 1165.14± 6.3 d |
| G19 | 4.729 ± 0.0004 e | 337.306 ± 0.035 b | 1595.12 ± 1.0 b |
| G20 | 4.563 ± 0.0003 f | 173.364 ± 0.040 t | 791.06 ± 0.4 n |
| WT * | 2.777 ± 0.0003 l | 131.664 ± 0.010 u | 365.63 ± 0.42 p |
| Run | Variables | Response, Y (µg/L) | |||
|---|---|---|---|---|---|
| X1 | X2 | X3 | Actual Value | Predicted Value | |
| 1 | −1 | −1 | −1 | 2514.50 | 2467.02 |
| 2 | −1 | −1 | 1 | 2425.02 | 2382.75 |
| 3 | 1 | −1 | −1 | 2401.92 | 2367.29 |
| 4 | 1 | −1 | 1 | 2451.20 | 2375.71 |
| 5 | −1 | 1 | −1 | 2270.44 | 2249.67 |
| 6 | −1 | 1 | 1 | 2297.28 | 2235.65 |
| 7 | 1 | 1 | −1 | 2144.48 | 2090.50 |
| 8 | 1 | 1 | 1 | 2217.93 | 2169.16 |
| 9 | 0 | 0 | −1.68 | 2253.40 | 2300.27 |
| 10 | 0 | 0 | 1.68 | 2206.29 | 2295.55 |
| 11 | −1.68 | 0 | 0 | 2224.83 | 2280.79 |
| 12 | 1.68 | 0 | 0 | 2060.85 | 2141.02 |
| 13 | 0 | −1.68 | 0 | 2594.37 | 2666.81 |
| 14 | 0 | 1.68 | 0 | 2246.67 | 2310.36 |
| 15 | 0 | 0 | 0 | 3014.95 | 2995.97 |
| 16 | 0 | 0 | 0 | 2981.78 | 2995.97 |
| 17 | 0 | 0 | 0 | 3016.95 | 2995.97 |
| 18 | 0 | 0 | 0 | 2988.78 | 2995.97 |
| 19 | 0 | 0 | 0 | 3004.95 | 2995.97 |
| 20 | 0 | 0 | 0 | 2991.78 | 2995.97 |
| Source | DF b | SS b | MS b | F Value | Probability (P) > F |
|---|---|---|---|---|---|
| Model a | 9 | 2,251,169 | 250,130 | 49.39 | <0.0001 |
| Residual (error) | 10 | 50,642 | 5064 | ||
| Lack-of-fit | 5 | 49,582 | 9916 | 46.75 | <0.0001 |
| Total | 19 | 2,301,811 |
| Model Term | Parameter Estimate | Standard Error | t Value a | p Value |
|---|---|---|---|---|
| β0 | 2995.97 | 29.02 | 103.23 | 0.000 b |
| β1 | −41.55 | 19.26 | −2.16 | 0.056 |
| β2 | −105.97 | 19.26 | −5.503 | 0.000 b |
| β3 | −1.40 | 19.26 | −0.07 | 0.943 |
| β11 | −277.56 | 18.75 | −14.81 | 0.000 b |
| β22 | −179.39 | 18.75 | −9.57 | 0.000 b |
| β33 | −246.80 | 18.75 | −13.17 | 0.000 b |
| β12 | −14.86 | 25.16 | −0.59 | 0.568 |
| β13 | 23.17 | 25.16 | 0.92 | 0.379 |
| β23 | 17.56 | 25.16 | 0.70 | 0.501 |
| Strains | Cultivation Time (d) | Astaxanthin Yield (μg/L) | References |
|---|---|---|---|
| Spirulina platensis | 10 | 38 | [8] |
| Phaffia rhodozyma | 8 | 639.6 | [40] |
| Thraustochytrium striatum | 15 | 6200 | [52] |
| Haematococcus lacustris | 30 | 84,800 | [53] |
| R. toruloides (wild strain) | 4 | 927.1 | [2] |
| R. toruloides (mutant) | 4 | 3021.3 | This study |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tran, T.N.; Ngo, D.-H.; Tran, Q.T.; Nguyen, H.C.; Su, C.-H.; Ngo, D.-N. Enhancing Astaxanthin Biosynthesis by Rhodosporidium toruloides Mutants and Optimization of Medium Compositions Using Response Surface Methodology. Processes 2020, 8, 497. https://doi.org/10.3390/pr8040497
Tran TN, Ngo D-H, Tran QT, Nguyen HC, Su C-H, Ngo D-N. Enhancing Astaxanthin Biosynthesis by Rhodosporidium toruloides Mutants and Optimization of Medium Compositions Using Response Surface Methodology. Processes. 2020; 8(4):497. https://doi.org/10.3390/pr8040497
Chicago/Turabian StyleTran, Tuyet Nhung, Dai-Hung Ngo, Quoc Tuan Tran, Hoang Chinh Nguyen, Chia-Hung Su, and Dai-Nghiep Ngo. 2020. "Enhancing Astaxanthin Biosynthesis by Rhodosporidium toruloides Mutants and Optimization of Medium Compositions Using Response Surface Methodology" Processes 8, no. 4: 497. https://doi.org/10.3390/pr8040497
APA StyleTran, T. N., Ngo, D.-H., Tran, Q. T., Nguyen, H. C., Su, C.-H., & Ngo, D.-N. (2020). Enhancing Astaxanthin Biosynthesis by Rhodosporidium toruloides Mutants and Optimization of Medium Compositions Using Response Surface Methodology. Processes, 8(4), 497. https://doi.org/10.3390/pr8040497







