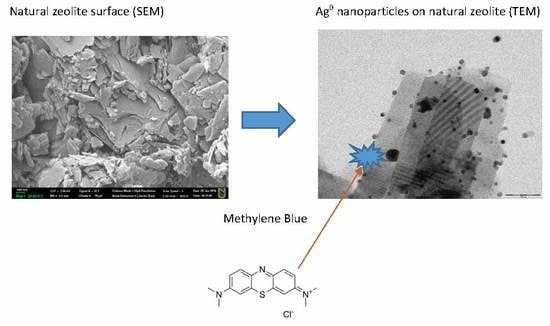
Catalytic Oxidation of Methylene Blue by Use of Natural Zeolite-Based Silver and Magnetite Nanocomposites
Abstract
1. Introduction
- The studies on zeolitic nanocomposites are few
- Magnetite zeolitic nanocomposites have not been studied
- A direct comparison of different nanocomposites in a single study is missing
- In most of studies the mineralization of MB via total carbon measurements is not studied
- The effect of the H2O2 concentration on the reaction order is rarely presented
2. Materials and Methods
2.1. Materials
2.2. Characterization Equipment
2.3. Modification of Zeolites
2.3.1. Modification with Sodium Chloride (NZU-Na)
2.3.2. Modification with Silver Oxide (Ag2O@NZU)
2.3.3. Modification with Silver Nanoparticles (Ag0@NZU)
2.3.4. Modification with Magnetite (Fe3O4@NZU)
2.4. Kinetic Measurements of Dye Discoloration
3. Results & Discussion
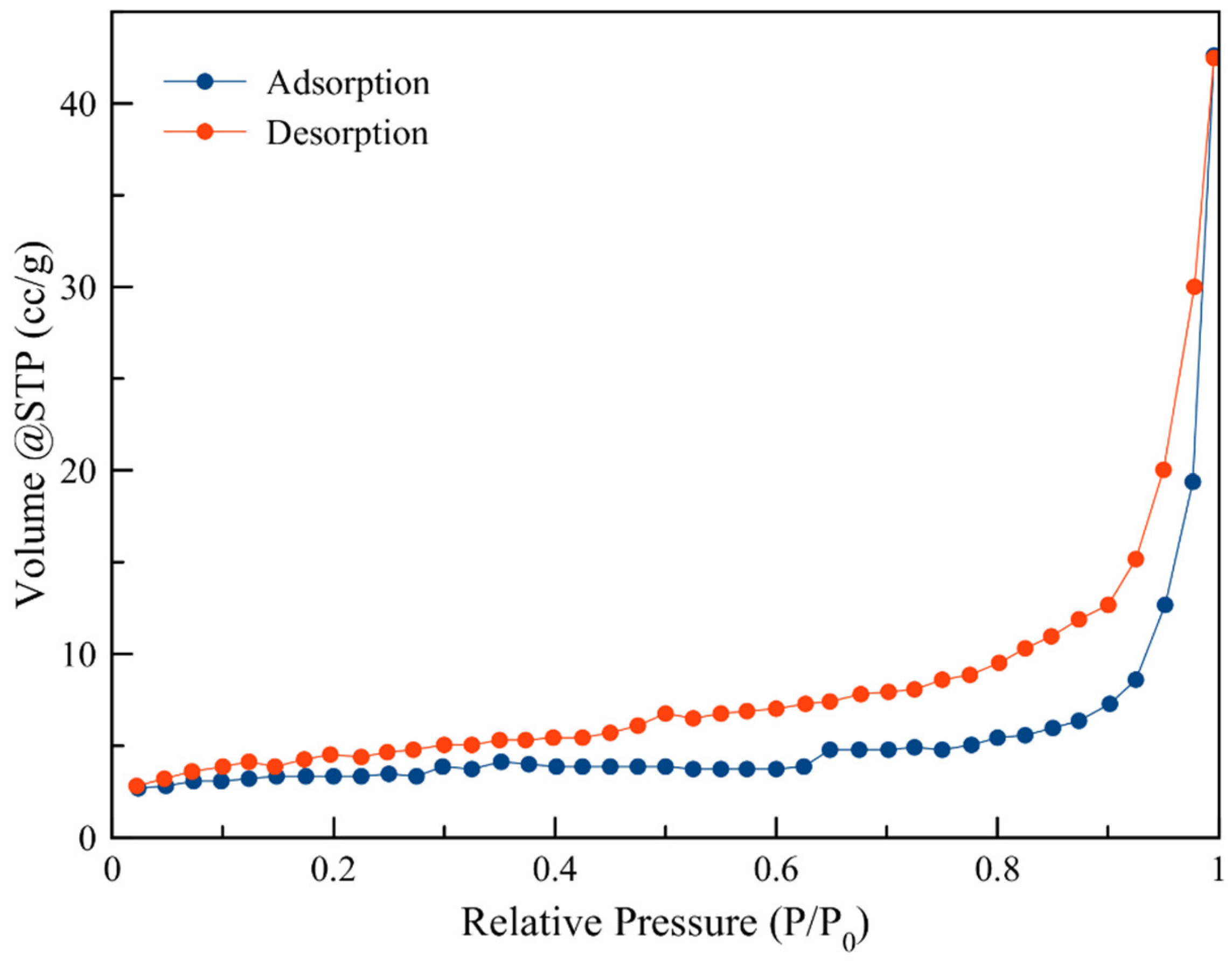
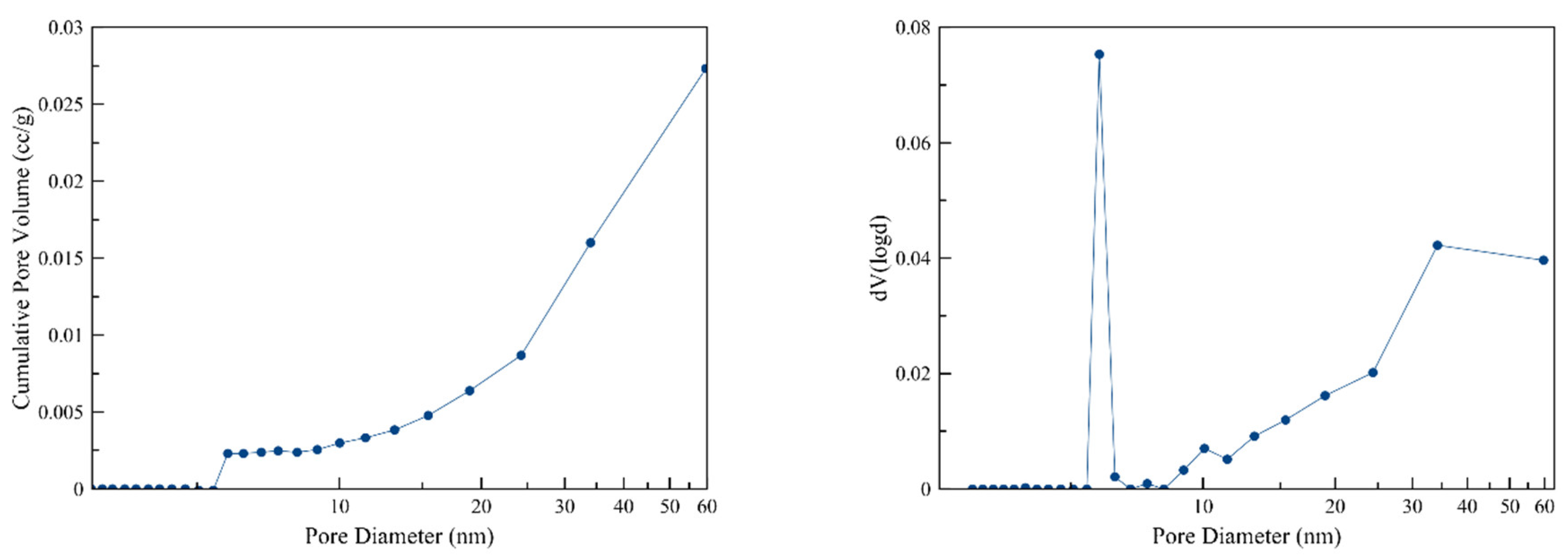
3.1. Porosimetry
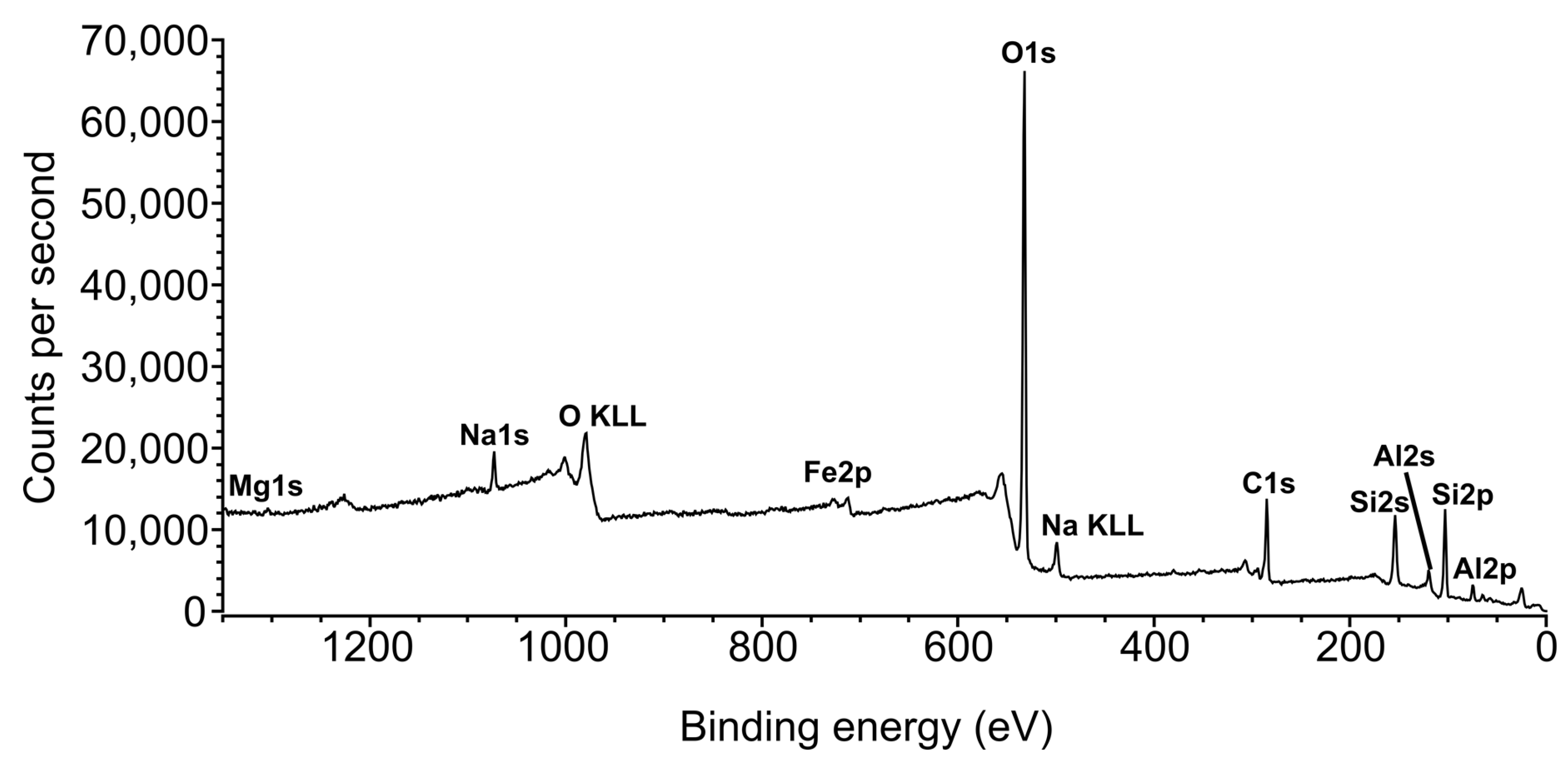
3.2. X-ray Fluorescence (XRF)
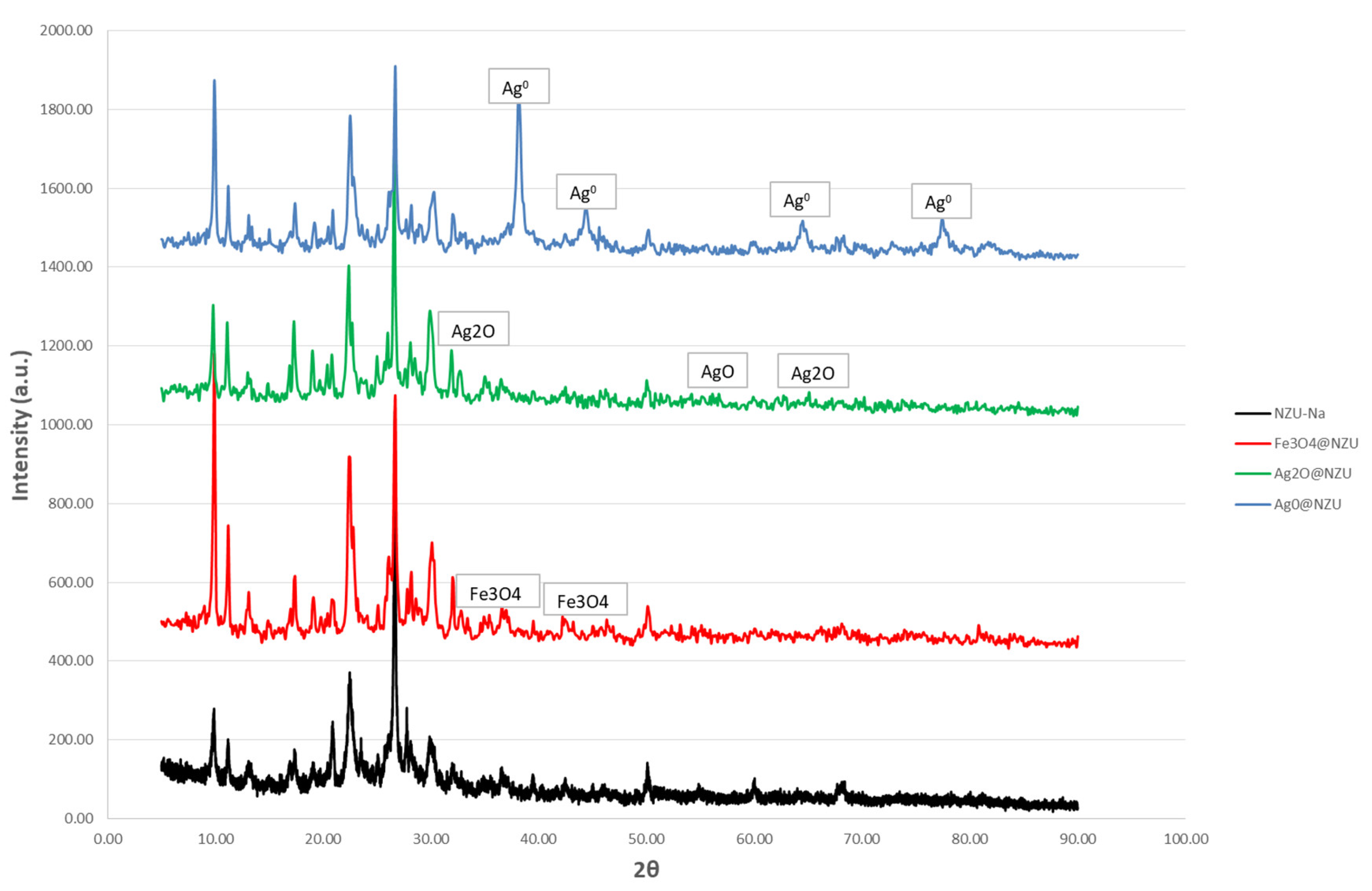
3.3. X-ray Diffraction (XRD)
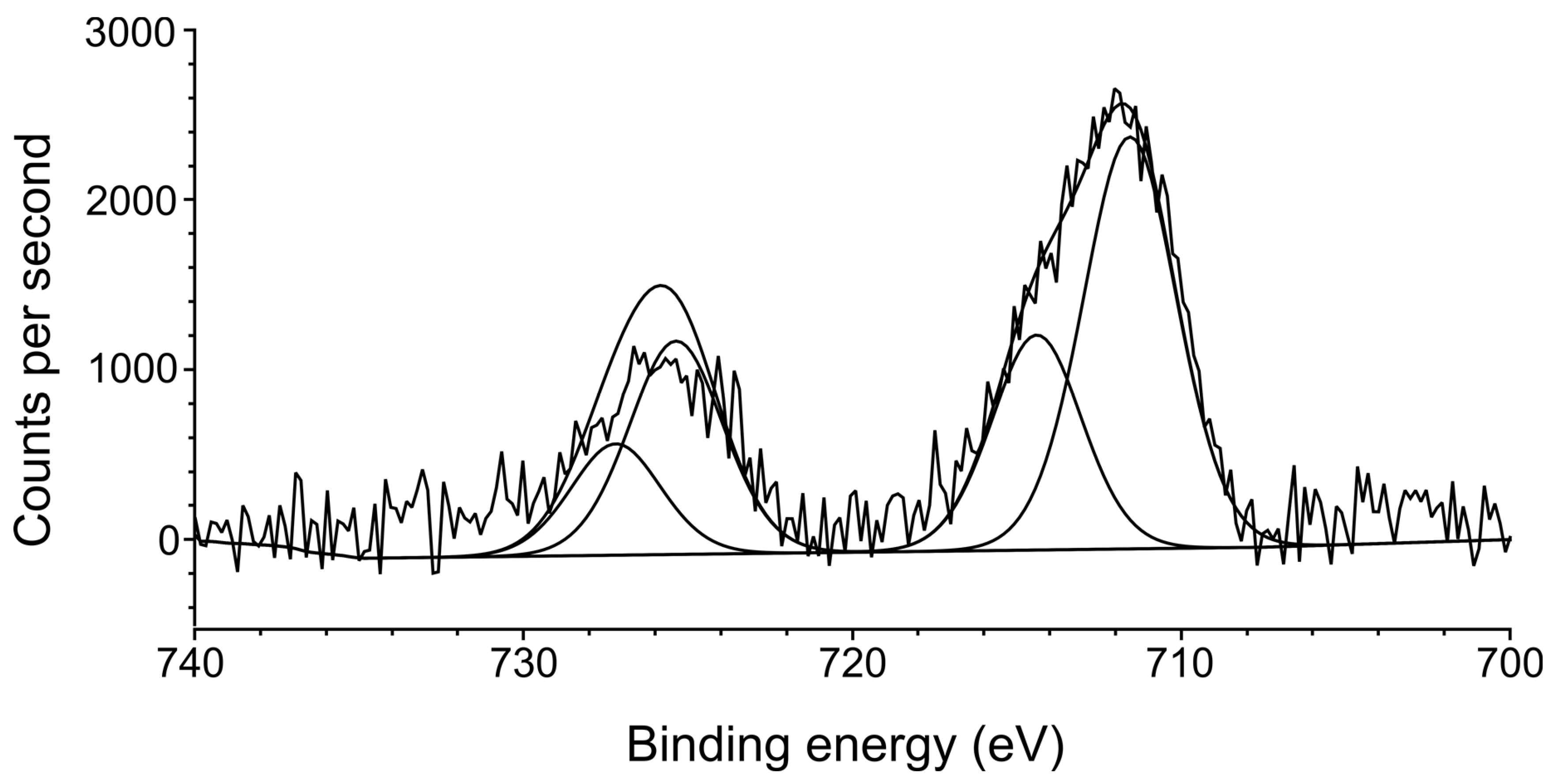
3.4. X-ray Photoelectron Spectroscopy (XPS)
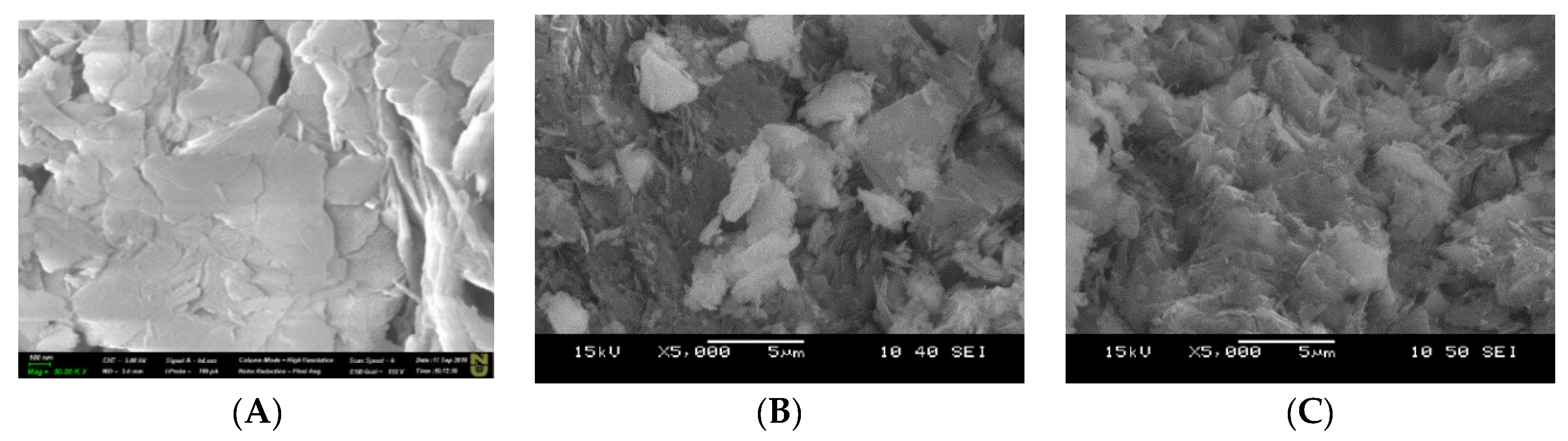
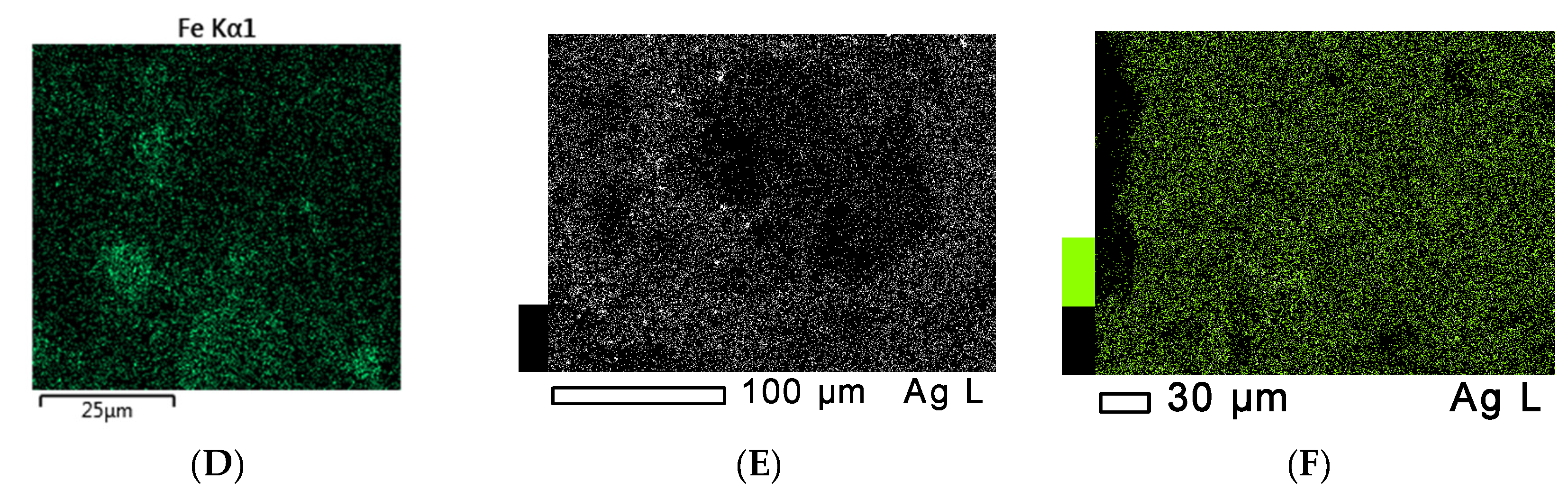
3.5. Scanning Electron Microscopy (SEM) and Energy Dispersive X-ray (EDS) Spectroscopy Measurements
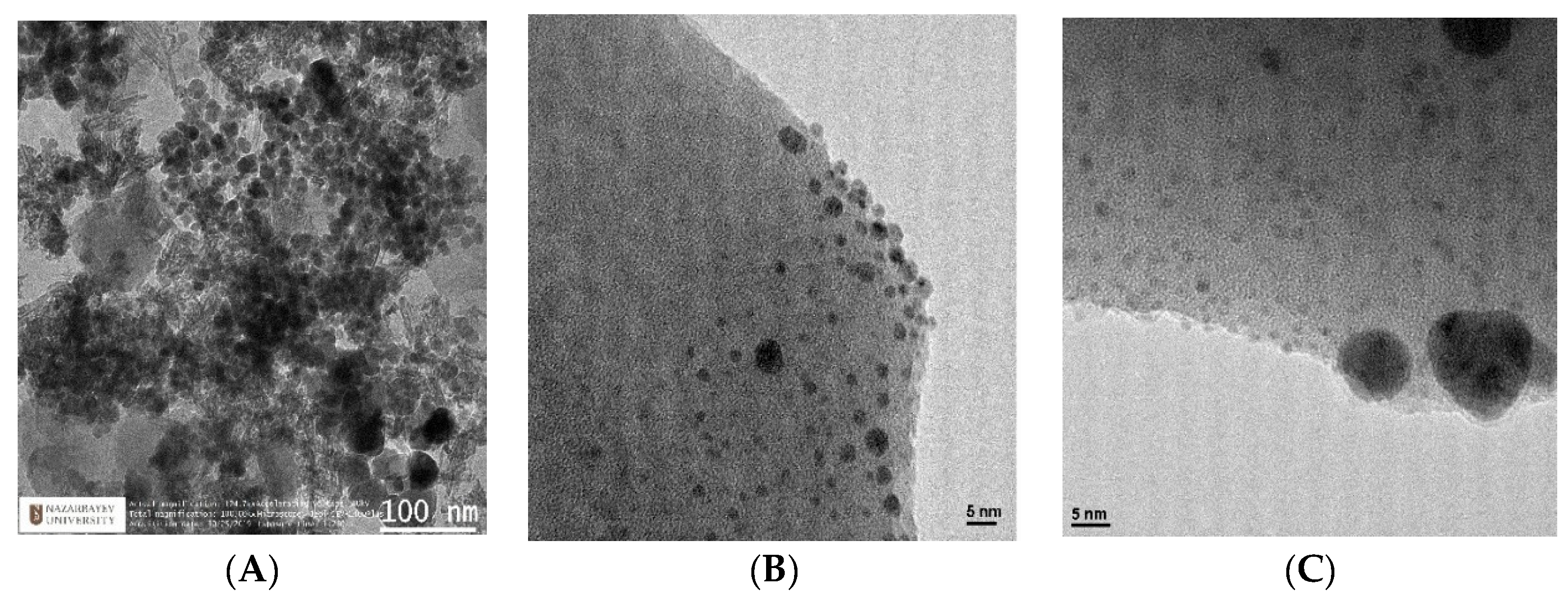
3.6. Transmission Electron Microscopy (TEM)
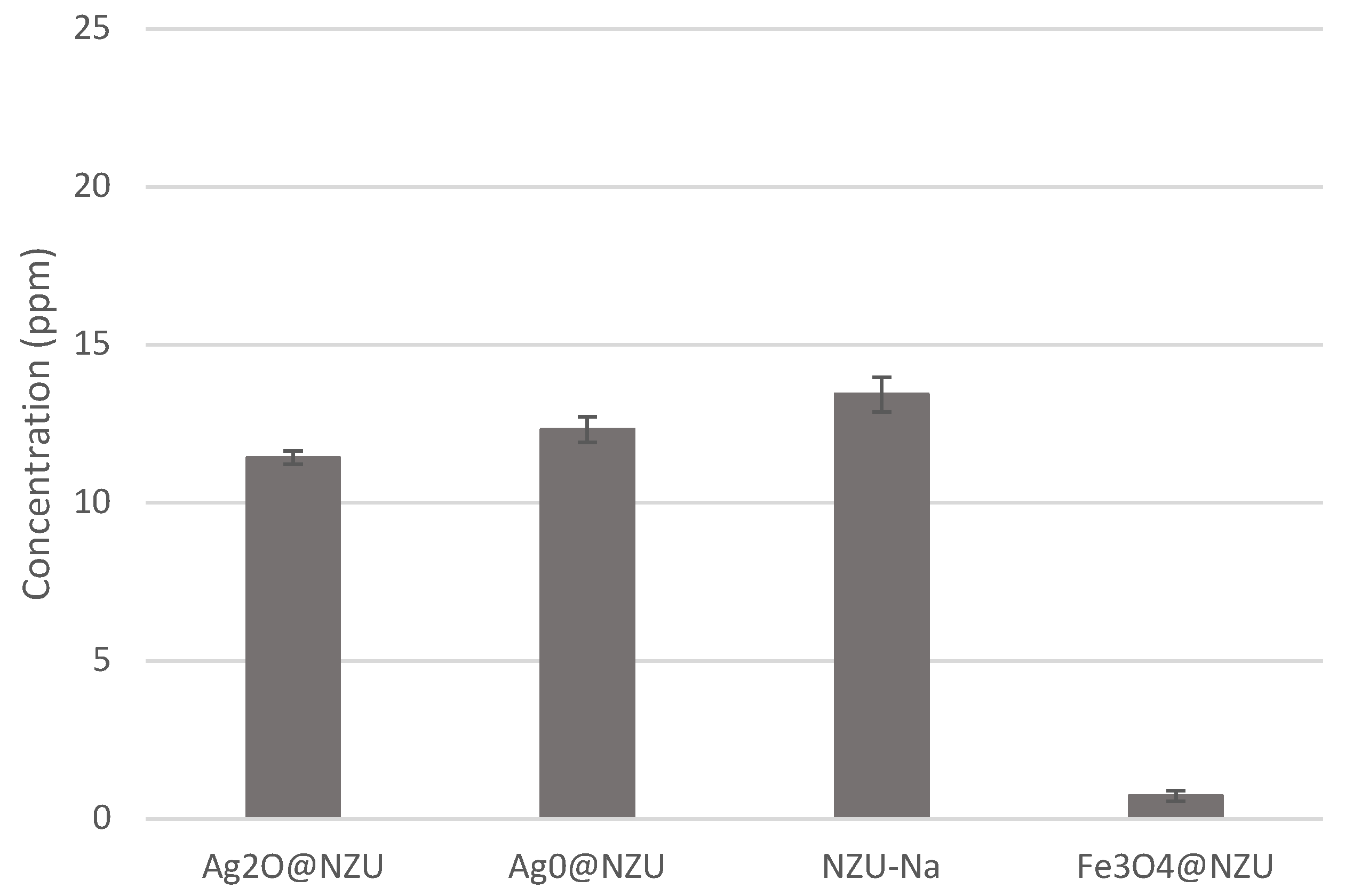
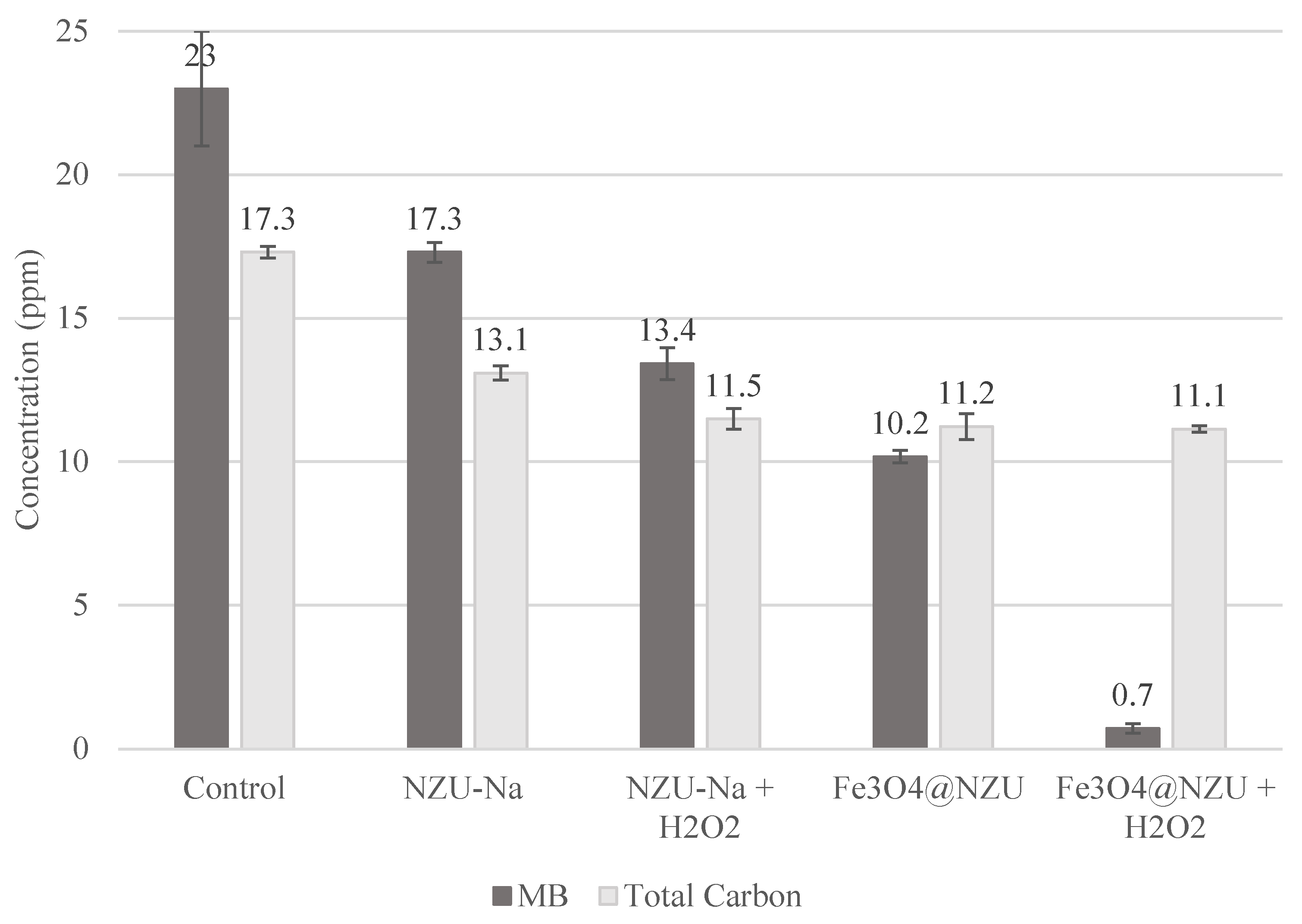
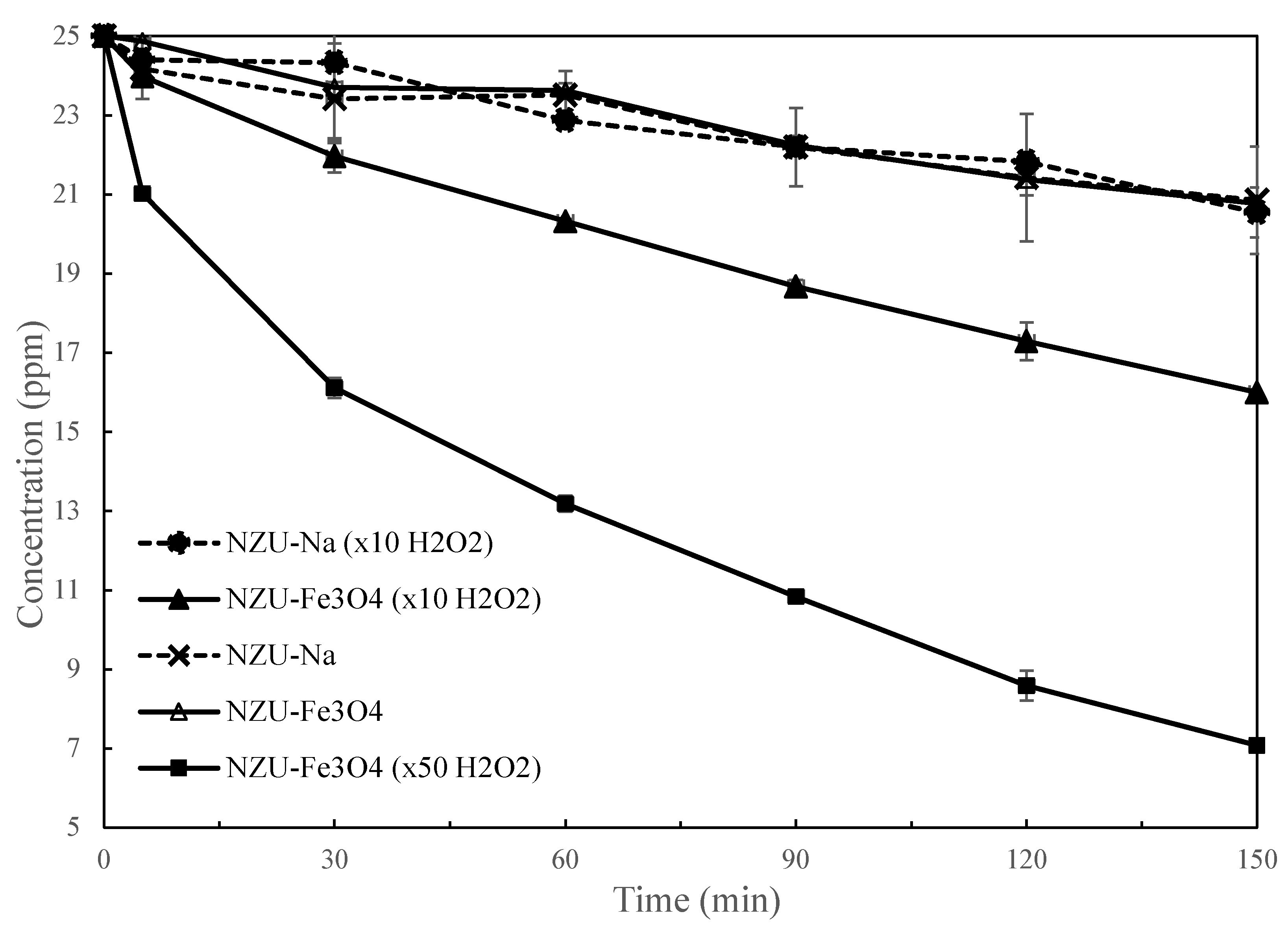
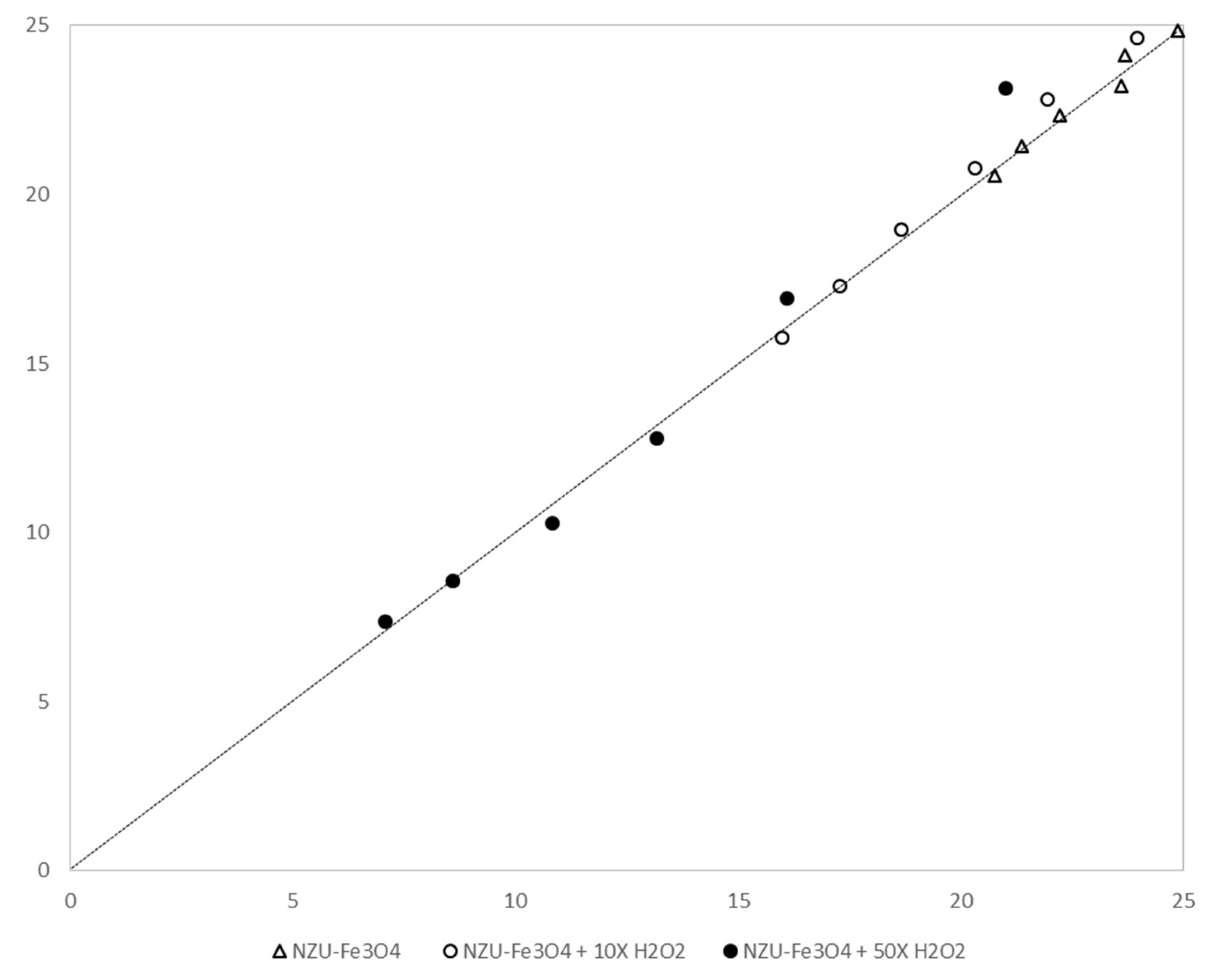
3.7. Catalytic Discoloration of MB
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Métivier-Pignon, H.; Faur-Brasquet, C.; le Cloirec, P. Adsorption of dyes onto activated carbon cloths: Approach of adsorption mechanisms and coupling of ACC with ultrafiltration to treat coloured wastewaters. Sep. Purif. Technol. 2003, 31, 3–11. [Google Scholar] [CrossRef]
- Rafatullah, M.; Sulaiman, O.; Hashim, R.; Ahmad, A. Adsorption of methylene blue on low-cost adsorbents: A review. J. Hazard. Mater. 2010, 177, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Sheng, J.; Xie, Y.; Zhou, Y. Adsorption of methylene blue from aqueous solution on pyrophyllite. Appl. Clay Sci. 2009, 46, 422–424. [Google Scholar] [CrossRef]
- Li, Y.; Du, Q.; Liu, T.; Peng, X.; Wang, J.; Sun, J.; Wang, Y.; Wu, S.; Wang, Z.; Xia, Y.; et al. Comparative study of methylene blue dye adsorption onto activated carbon, graphene oxide, and carbon nanotubes. Chem. Eng. Res. Des. 2013, 91, 361–368. [Google Scholar] [CrossRef]
- Ravikumar, K.; Deebika, B.; Balu, K. Decolourization of aqueous dye solutions by a novel adsorbent: Application of statistical designs and surface plots for the optimization and regression analysis. J. Hazard. Mater. 2005, 122, 75–83. [Google Scholar] [CrossRef]
- McMullan, G.; Meehan, C.; Conneely, A.; Kirby, N.; Robinson, T.; Nigam, P.; Banat, I.M.; Marchant, R.; Smyth, W.F. Microbial decolourisation and degradation of textile dyes. Appl. Microbiol. Biotechnol. 2001, 56, 81–87. [Google Scholar] [CrossRef]
- Pearce, C.I.; Lloyd, J.R.; Guthrie, J.T. The removal of colour from textile wastewater using whole bacterial cells: A review. Dye. Pigment. 2003, 58, 179–196. [Google Scholar] [CrossRef]
- Lee, J.W.; Choi, S.P.; Thiruvenkatachari, R.; Shim, W.G.; Moon, H. Evaluation of the performance of adsorption and coagulation processes for the maximum removal of reactive dyes. Dye. Pigment. 2006, 69, 196–203. [Google Scholar] [CrossRef]
- Robinson, T.; McMullan, G.; Marchant, R.; Nigam, P. Remediation of dyes in textile effluent: A critical review on current treatment technologies with a proposed alternative. Bioresour. Technol. 2001, 77, 247–255. [Google Scholar] [CrossRef]
- Banat, I.M.; Nigam, P.; Singh, D.; Marchant, R. Microbial decolorization of textile-dye-containing effluents: A review. Bioresour. Technol. 1996, 58, 217–227. [Google Scholar] [CrossRef]
- IARC. Chemical and physical data. Pharm. Drugs 2000, 1981, 100–104. [Google Scholar]
- Perry anc Meinhard, P.M.E. Necrotic subcutaneous abscesses following injections of methylene blue. Br. J. Clin. Pract. 1974, 28, 289–291. [Google Scholar]
- Ruhlen, J.L. Tissue necrosis. Cutaneous and subcutaneous damage following extravasation of methylene blue. J. Kans. Med. Soc. 1982, 83, 236–260. [Google Scholar] [PubMed]
- Sharr, M.M.; Weller, R.O.; Brice, J.G. Spinal cord necrosis after intrathecal injection of methylene blue. J. Neurol. Neurosurg. Psychiatry 1978, 41, 384–386. [Google Scholar] [CrossRef]
- Troche, B.I. The Methylene Blue Baby. N. Engl. J. Med. 1989, 320, 1756–1757. [Google Scholar] [CrossRef]
- Spahr, R.C.; Salsburey, D.J.; Krissberg, A.; Prin, W. Intraamniotic injection of methylene blue leading to methemoglobinemia in one of twins. Int. J. Gynecol. Obstet. 1980, 17, 477–478. [Google Scholar] [CrossRef]
- Vincer, M.J.; Allen, A.C.; Evans, J.R.; Nwaesei, C.; Stinson, D.A. Methylene-blue-induced hemolytic anemia in a neonate. Can. Med. Assoc. J. 1987, 136, 503–504. [Google Scholar]
- Serota, F.; Bernbaum, J.; Schwartz, E. The methylene-blue baby. Lancet 1979, 314, 1142–1143. [Google Scholar] [CrossRef]
- Cowett, R.M.; Hakanson, D.O.; Kocon, R.W.; Oh, W. Untoward neonatal effect of intraamniotic administration of methylene blue. Obstet. Gynecol. 1976, 48, 74S–75S. [Google Scholar]
- McEnerney, J.K.; McEnerney, L.N. Unfavorable neonatal outcome after intraamniotic injection of methylene blue. Obstet. Gynecol. 1983, 61, 35S–37S. [Google Scholar]
- Coddington, C.C.; Anderson, T.L.; Accetta, C.R.; Swanson, J.; Kruger, T.; Hodgen, G.D. Adverse effects of methylene blue on human sperm motility, components of human reproductive tract fluids, and mouse embryo cleavage. Fertil. Steril. 1989, 51, 480–485. [Google Scholar] [CrossRef]
- Turner, J. Book review. J. Transp. Geogr. 2013, 106. [Google Scholar] [CrossRef]
- Brownstein, S.; Liszauer, A.D.; Jackson, W.B. Ocular complications of a topical methylene blue-vasoconstrictor-anesthetic preparation. Can. J. Ophthalmol. 1989, 24, 317–324. [Google Scholar] [PubMed]
- Salem, I.A.; El-Maazawi, M.S. Kinetics and mechanism of color removal of methylene blue with hydrogen peroxide catalyzed by some supported alumina surfaces. Chemosphere 2000, 41, 1173–1180. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, M.; Hu, S.; Wang, L.; Yao, H. Characteristics and mechanisms of 4A zeolite supported nanoparticulate zero-valent iron as Fenton-like catalyst to degrade methylene blue. Toxicol. Environ. Chem. 2014, 96, 227–242. [Google Scholar] [CrossRef]
- Simoncic, P.; Armbruster, T. Cationic methylene blue incorporated into zeolite mordenite-Na: A single crystal X-ray study. Microporous Mesoporous Mater. 2005, 81, 87–95. [Google Scholar] [CrossRef]
- Smith, J.V. Definition of a zeolite. Zeolites 1984, 4, 309–310. [Google Scholar] [CrossRef]
- Liebau, F. Zeolites and clathrasils-Two distinct classes of framework silicates. Zeolites 1983, 3, 191–193. [Google Scholar] [CrossRef]
- Sartbaeva, A.; Wells, S.A.; Treacy, M.M.J.; Thorpe, M.F. The flexibility window in zeolites. Nat. Mater. 2006, 5, 962–965. [Google Scholar] [CrossRef]
- Groen, J.C.; Peffer, L.A.A.; Moulijn, J.A.; Pérez-Ramírez, J. On the introduction of intracrystalline mesoporosity in zeolites upon desilication in alkaline medium. Microporous Mesoporous Mater. 2004, 69, 29–34. [Google Scholar] [CrossRef]
- Roland, E. Hindustrial Production of Zeolites. Stud. Surf. Sci. Catal. 1989, 46, 645–659. [Google Scholar] [CrossRef]
- Lopes, J.H.; Nogueira, F.G.; Gonçalves, M.; Oliveira, L.C. Modified Zeolite with Transition Metals Cu and Fe for Removal of Methylene Blue from Aqueous Medium: Mass Spectrometry Study. Bull. Chem. React. Eng. Catal. 2015, 10, 237–248. [Google Scholar] [CrossRef]
- Jakab, Á.; Colar, L.A.; Pode, R.; Cocheci, L.; Manea, F. Catalytic photodegradation and mineralization of cationic dye methylene blue from aqueous solution onto copper doped zeolite. Rev. Chim. 2012, 63, 1016–1022. [Google Scholar]
- Wardhani, S.; Rahman, M.F.F.; Purwonugroho, D.; Tjahjanto, R.T.; Damayanti, C.A.; Wulandari, I.O. Photocatalytic Degradation of Methylene Blue Using TiO2-Natural Zeolite as A Photocatalyst. J. Pure Appl. Chem. Res. 2016, 5, 19–27. [Google Scholar] [CrossRef]
- Karimi-Shamsabadi, M.; Nezamzadeh-Ejhieh, A. Comparative study on the increased photoactivity of coupled and supported manganese-silver oxides onto a natural zeolite nano-particles. J. Mol. Catal. A Chem. 2016, 418–419, 103–114. [Google Scholar] [CrossRef]
- Colar, L.A.; Jakab, A.; Manea, F.; Pode, R.; Orha, C. Photocatalytic performance of Ag-modified natural zeolite catalyst for photocatalysis degradation of Methylene Blue (MB) under VIS irradiation. WIT Trans. Ecol. Environ. 2012, 164, 335–344. [Google Scholar] [CrossRef]
- SRajabi, K.; Sohrabnezhad, S. Synthesis and characterization of magnetic core with two shells: Mordenite zeolite and CuO to form Fe3O4@MOR@CuO core-shell: As a visible light driven photocatalyst. Microporous Mesoporous Mater. 2017, 242, 136–143. [Google Scholar] [CrossRef]
- Tedla, H.; Díaz, I.; Kebede, T.; Taddesse, A.M. Synthesis, characterization and photocatalytic activity of zeolite supported ZnO/Fe2O3/MnO2 nanocomposites. J. Environ. Chem. Eng. 2015, 3, 1586–1591. [Google Scholar] [CrossRef]
- Zhang, Y.; Shang, J.; Song, Y.; Rong, C.; Wang, Y.; Huang, W.; Yu, K. Selective Fenton-like oxidation of methylene blue on modified Fe-zeolites prepared via molecular imprinting technique. Water Sci. Technol. 2017, 75, 659–669. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, F.T.; Liu, Q.Q.; Zhao, X.; Liu, S.Q. Application of heterogenous catalyst of tris(1,10)-phenanthroline iron(II) loaded on zeolite for the photo-Fenton degradation of methylene blue. React. Kinet. Mech. Catal. 2011, 103, 299–310. [Google Scholar] [CrossRef]
- Subramanian, E.; Subbulekshmi, N.L. Enhanced heterogeneous wet hydrogen peroxide catalytic oxidation performance of fly ash-derived zeolite by CuO incorporation. Sci. Iran. 2017, 24, 1189–1202. [Google Scholar] [CrossRef][Green Version]
- Nassar, M.Y.; Abdelrahman, E.A. Hydrothermal tuning of the morphology and crystallite size of zeolite nanostructures for simultaneous adsorption and photocatalytic degradation of methylene blue dye. J. Mol. Liq. 2017, 242, 364–374. [Google Scholar] [CrossRef]
- Wojtaszek-Gurdak, A.; Zielinska, M.; Ziolek, M. MWW layered zeolites modified with niobium species—Surface and catalytic properties. Catal. Today 2019, 325, 89–97. [Google Scholar] [CrossRef]
- Stylianou, M.; Inglezakis, V.; Agapiou, A.; Itskos, G.; Jetybayeva, A.; Loizidou, M. A comparative study on phyllosilicate and tectosillicate mineral structural properties. Desalin. Water Treat. 2018, 112, 119–146. [Google Scholar] [CrossRef]
- Allen, G.C.; Curtis, M.T.; Hooper, A.J.; Tucker, P.M. X-ray photoelectron spectroscopy of iron–oxygen systems. J. Chem. Soc. Dalt. Trans. Dalt. Trans. 1974, 1525–1530. [Google Scholar] [CrossRef]
- Wang, S.; Zhu, Z.H. Characterisation and environmental application of an Australian natural zeolite for basic dye removal from aqueous solution. J. Hazard. Mater. 2006, 136, 946–952. [Google Scholar] [CrossRef]
- Wang, X.; Nguyen, A.V. Characterisation of electrokinetic properties of clinoptilolite before and after activation by sulphuric acid for treating CSG water. Microporous Mesoporous Mater. 2016, 220, 175–182. [Google Scholar] [CrossRef]
- Andreozzi, R.; Caprio, V.; Insola, A.; Marotta, R. Advanced oxidation processes (AOP) for water purification and recovery. Catal. Today. 1999, 53, 51–59. [Google Scholar] [CrossRef]
- Tarr, M.A. Chemical Degradation Methods for Wastes and Pollutants: Environmental and Industrial Applications; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Pignatello, J.J.; Oliveros, E.; MacKay, A. Advanced oxidation processes for organic contaminant destruction based on the fenton reaction and related chemistry. Crit. Rev. Environ. Sci. Technol. 2006, 36, 1–84. [Google Scholar] [CrossRef]
- Jain, B.; Singh, A.K.; Kim, H.; Lichtfouse, E.; Sharma, V.K. Treatment of organic pollutants by homogeneous and heterogeneous Fenton reaction processes. Environ. Chem. Lett. 2018, 16, 947–967. [Google Scholar] [CrossRef]
- Lipczynska-Kochany, E.; Bolton, J.R. Flash photolysis/HPLC applications. 2. Direct photolysis vs. hydrogen peroxide mediated photodegradation of 4-chlorophenol as studied by a flash photolysis/HPLC technique. Environ. Sci. Technol. 1992, 26, 259–262. [Google Scholar] [CrossRef]
- Poulopoulos, S.G.; Arvanitakis, F.; Philippopoulos, C.J. Photochemical treatment of phenol aqueous solutions using ultraviolet radiation and hydrogen peroxide. J. Hazard. Mater. 2006, 129, 64–68. [Google Scholar] [CrossRef] [PubMed]
| Oxidants | Zeolite | Metal 1 | Catalyst Concentration (% w/w) | Initial MB Concentration (ppm) | Zeolite Mass to Solution Volume (g/L) | Removal (Max. %) | Reaction Order 2 | Ref. |
|---|---|---|---|---|---|---|---|---|
| H2O2 (10–50 mM) and UV | Natural zeolite | Cu2+ | - | 50 | 1 | 87 | 1.3–2 * | [33] |
| H2O2 and UV | Clinoptilolite | TiO2 (anatase) | 80 | 20 | 2 | 98 | 1 | [34] |
| H2O2 and UV | Clinoptilolite | MnO (NPs) Ag2O (NPs) | 10 2.1 | 4 | 0.25 | 85 | 1 | [35] |
| Vis | Clinoptilolite | Ag0 (NPs) | - | 20–100 | 0.25–1.5 | 84 | - | [36] |
| H2O2 (0.2 mL, 3 wt%) and UV | Mordenite | CuO (NPs) Fe3O4 | 3.7 68.1 | 3.2–10 | 0.05–2 | 97 | - | [37] |
| H2O2 (10 mM) | 4A | Fe0 (NPs) | 12.7 | 30 | 0.2–5 | 100 | 1 | [25] |
| Vis | Zeolite Y | ZnO (NPs) Fe2O3 MnO2 | 3.7–13.6 (Zn) 1.9–6.9 (Fe) 0.7–0.8 (Mn) | 10–100 | 0.05–0.15 | 80 | - | [38] |
| H2O2 (2 mL, 30% v/v) | NaY Naß | Fe3O4 Cu2O | - | 100 | 1 | 100 | 0 | [39] |
| H2O2 (6% v/v) | 4A | Fe2O3 Fe2Al4Si5O18 | 17.1 | 15 | 0.3 | 88 | 1 | [39] |
| H2O2 (2.2–11 mM) and UV | NaY | Tris (1, 10) phenanthroline Fe2+ | 0.01 | 11.5 | 40 | 77 | 1 * | [40] |
| H2O2 (1 mL, 30% v/v) | Zeolite X | CuO Fe2O3 | 29.6 | 100 | 0.25 | 100 | - | [41] |
| H2O2 (2 mL, 0.5M) and UV | Analcime | C9H21O3Al | - | 10 | 2 | 100 | 1 | [42] |
| H2O2 (5 mL, 30% v/v) | Synthetic zeolite | Nb5+ | 1.9–2.2 | 80 | 1 | 100 | - | [43] |
| Sample | Al2O3 | SiO2 | Fe2O3 | Ag2O |
|---|---|---|---|---|
| NZU | 12.9 | 77.3 | 1.6 | 0 |
| NZU-Na | 12.7 | 78.5 | 1.5 | 0 |
| Fe3O4@NZU | 9.5 | 75.6 | 7.9 | 0 |
| Ag2O@NZU | 11.7 | 72.8 | 1.5 | 6.9 |
| Ag0@NZU | 11.6 | 69.9 | 1.4 | 6.8 |
| H2O2 (Mm) | k (min−1) | Reaction Order | Average Absolute Error (%) |
|---|---|---|---|
| 0 | 0.0293 | 0 | 0.8 |
| 42.4 | 0.00307 | 1 | 1.7 |
| 212 | 0.00064 | 2 | 2.8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuntubek, A.; Kinayat, N.; Meiramkulova, K.; Poulopoulos, S.G.; Bear, J.C.; Inglezakis, V.J. Catalytic Oxidation of Methylene Blue by Use of Natural Zeolite-Based Silver and Magnetite Nanocomposites. Processes 2020, 8, 471. https://doi.org/10.3390/pr8040471
Kuntubek A, Kinayat N, Meiramkulova K, Poulopoulos SG, Bear JC, Inglezakis VJ. Catalytic Oxidation of Methylene Blue by Use of Natural Zeolite-Based Silver and Magnetite Nanocomposites. Processes. 2020; 8(4):471. https://doi.org/10.3390/pr8040471
Chicago/Turabian StyleKuntubek, Aldiyar, Nurassyl Kinayat, Kulyash Meiramkulova, Stavros G. Poulopoulos, Joseph C. Bear, and Vassilis J. Inglezakis. 2020. "Catalytic Oxidation of Methylene Blue by Use of Natural Zeolite-Based Silver and Magnetite Nanocomposites" Processes 8, no. 4: 471. https://doi.org/10.3390/pr8040471
APA StyleKuntubek, A., Kinayat, N., Meiramkulova, K., Poulopoulos, S. G., Bear, J. C., & Inglezakis, V. J. (2020). Catalytic Oxidation of Methylene Blue by Use of Natural Zeolite-Based Silver and Magnetite Nanocomposites. Processes, 8(4), 471. https://doi.org/10.3390/pr8040471