Early Stage of Corrosion Formation on Pipeline Steel X70 Under Oxyfuel Atmosphere at Low Temperature
Abstract
1. Introduction
2. Materials and Methods
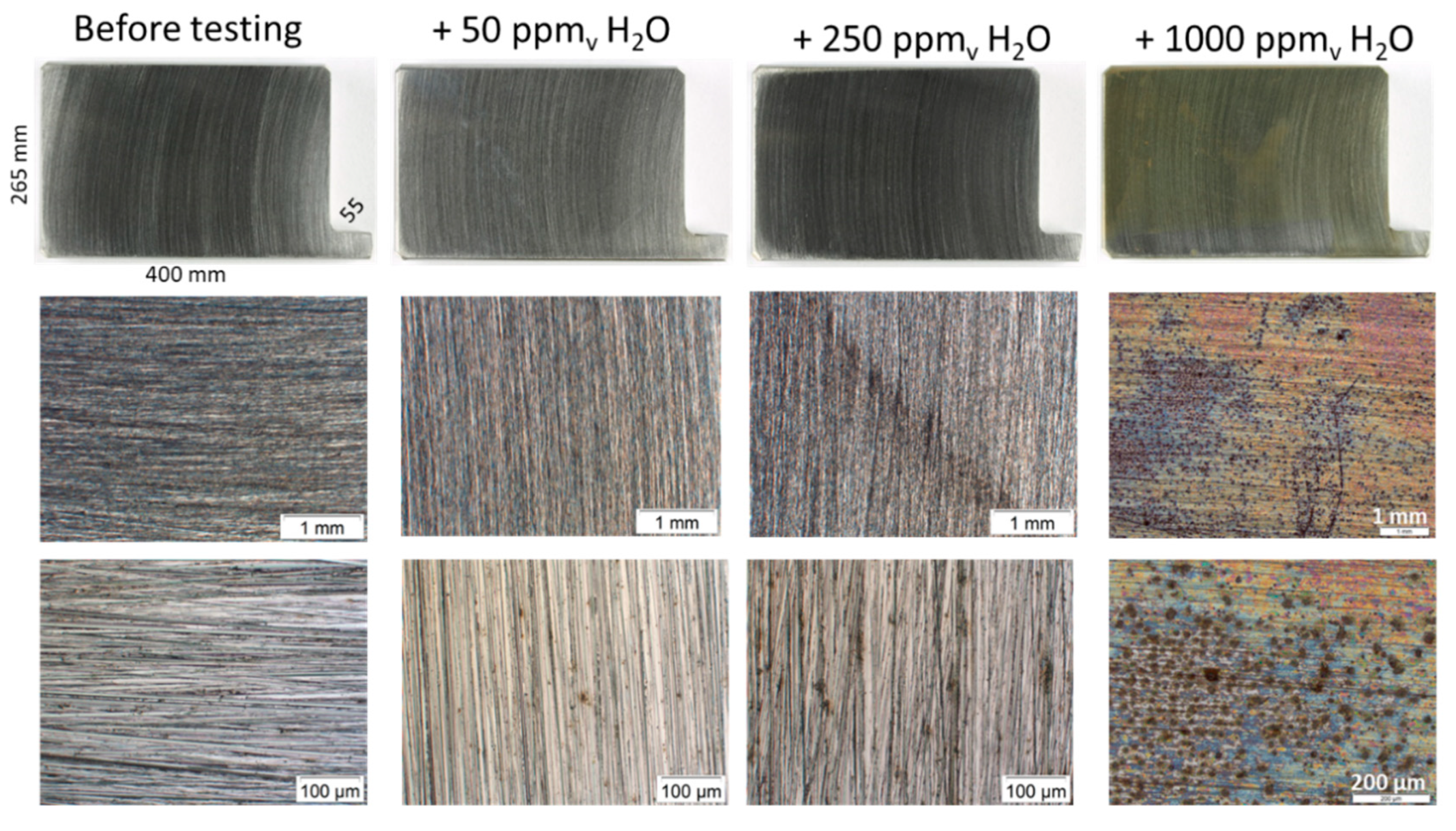
2.1. Screening Test
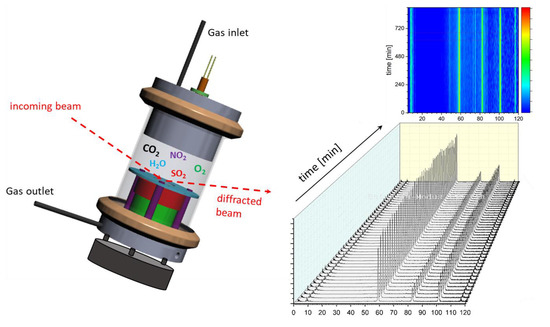
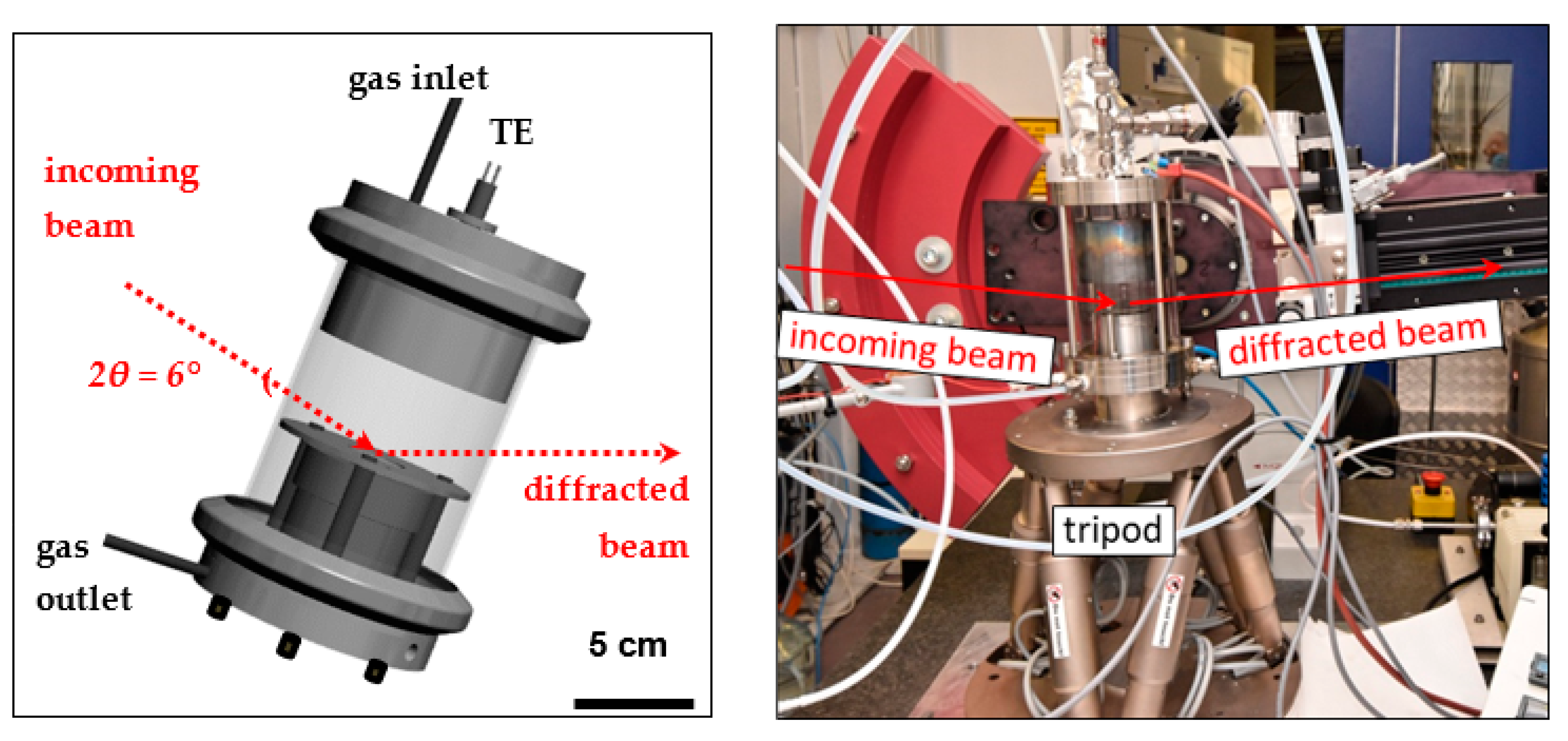
2.2. In-Situ Corrosion Experiment
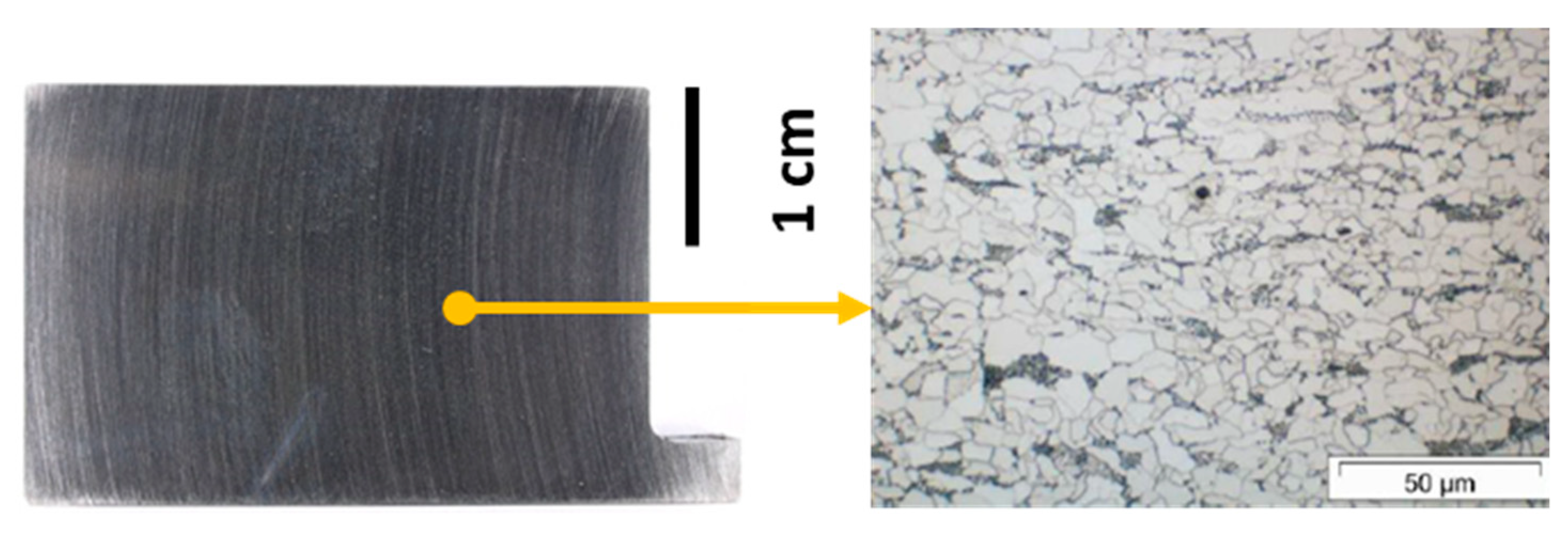
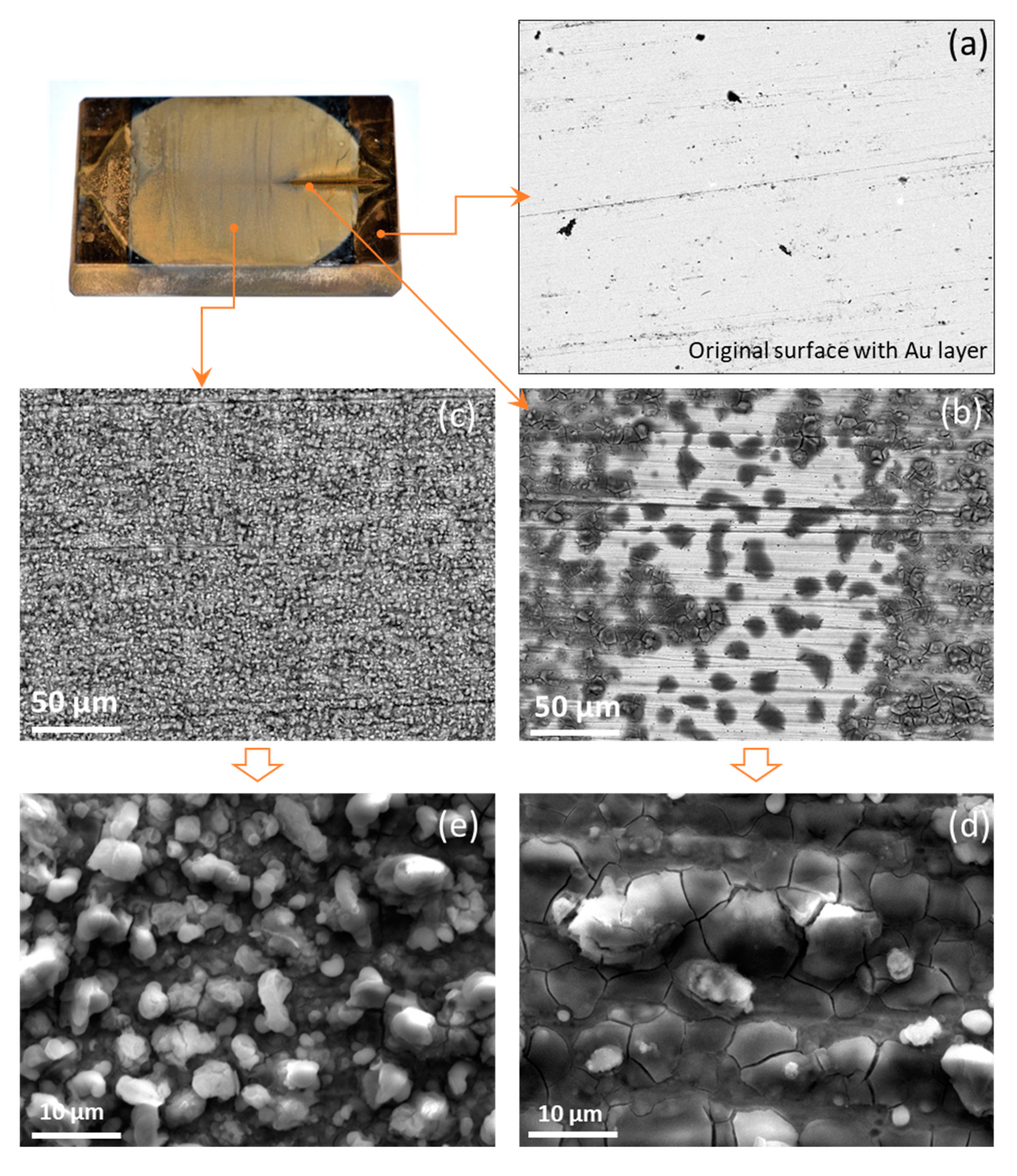
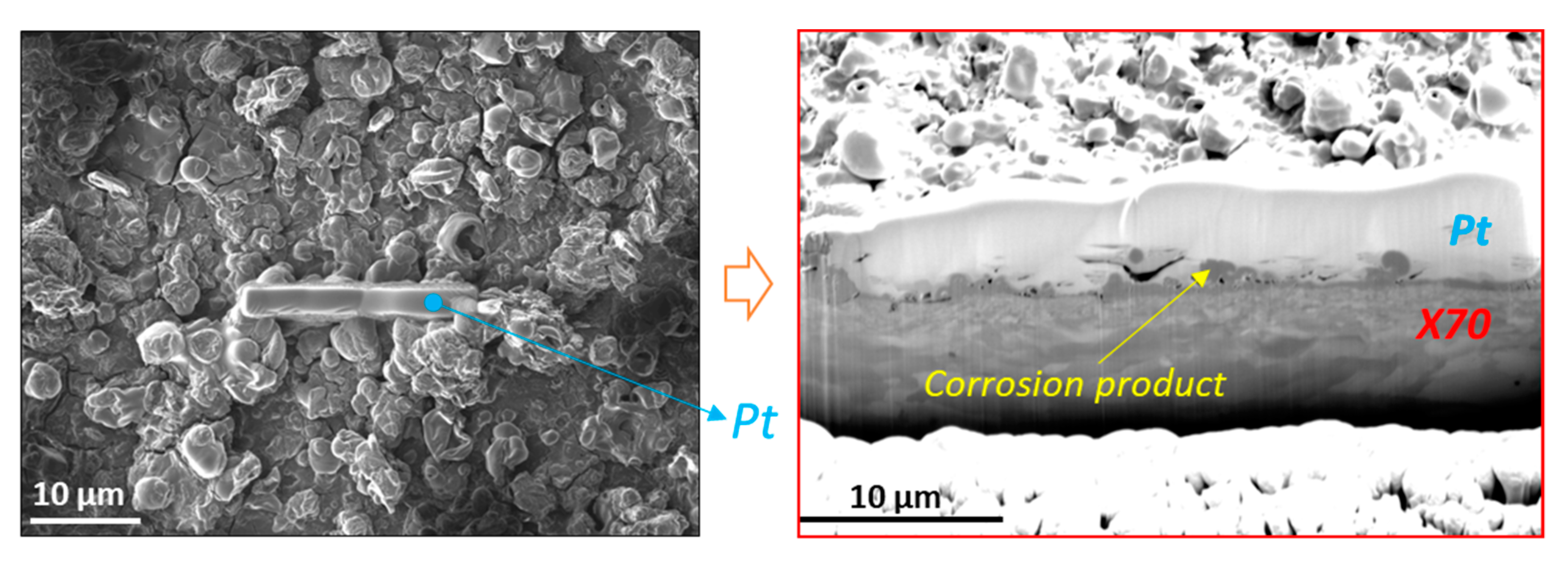
2.3. Ex-Situ Characterization Experiments
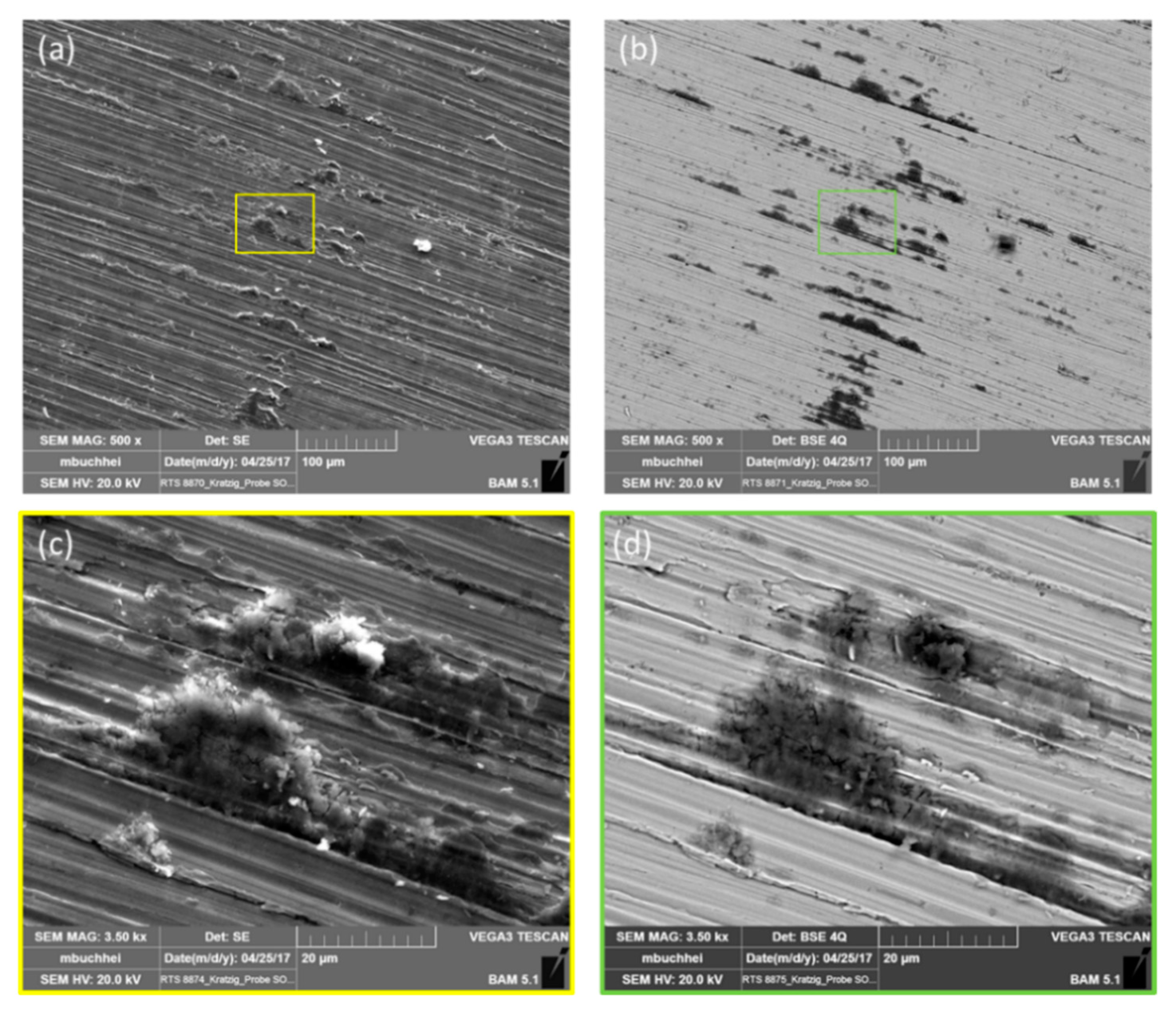
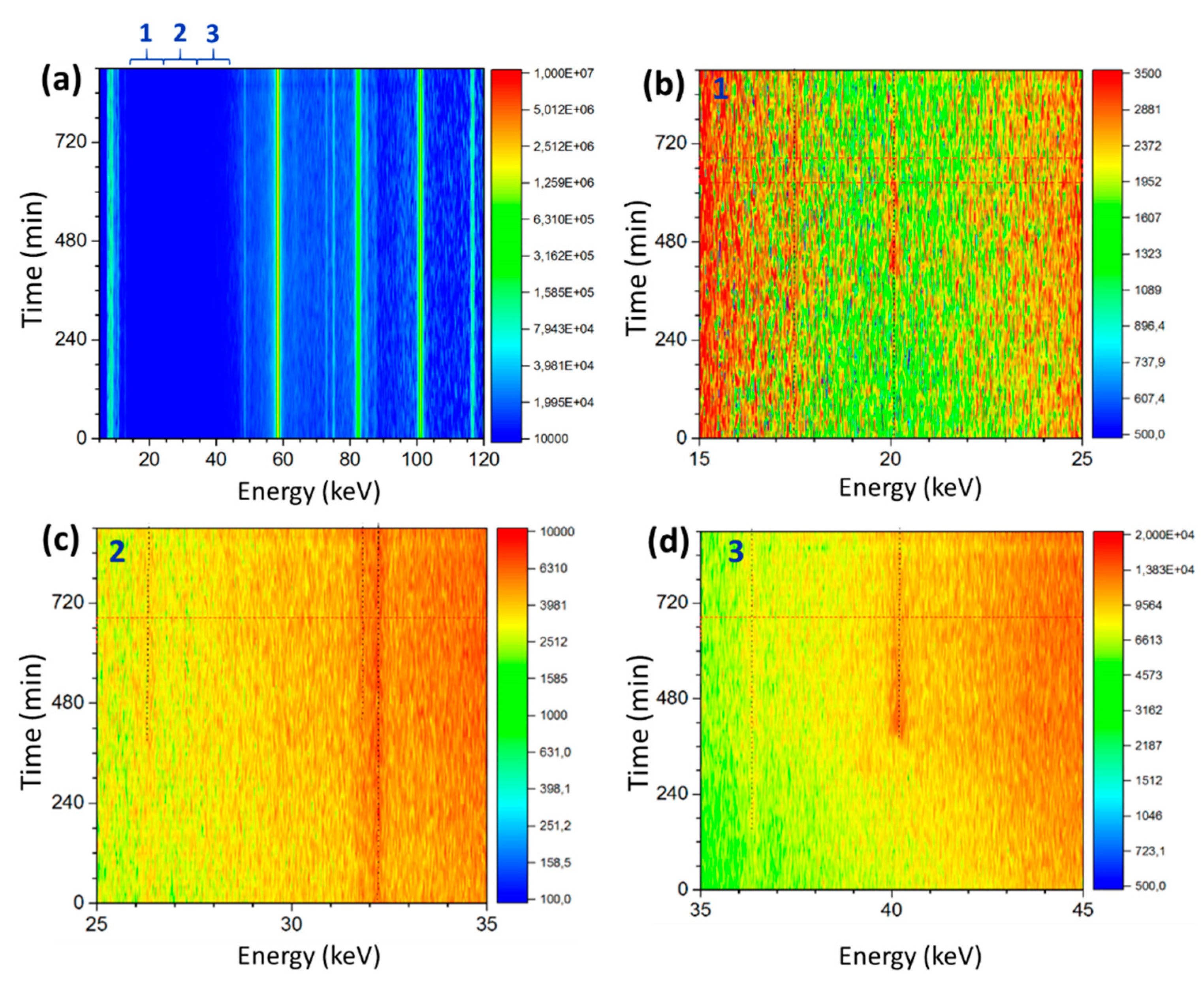
3. Results
3.1. Screening Experiments
3.2. In-Situ corrosion Experiment
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gale, J.; Davison, J. Transmission of CO2—Safety and economic considerations. Energy 2004, 29, 1319–1328. [Google Scholar] [CrossRef]
- Vandeginste, V.; Piessens, K. Pipeline design for a least-cost router application for CO2 transport in the CO2 sequestration cycle. Int. J. Greenh. Gas Control 2008, 2, 571–581. [Google Scholar] [CrossRef]
- Fleig, D.; Normann, F.; Andersson, K.; Johnsson, F.; Leckner, B. The fate of sulphur during oxyfuel combustion of lignite. Energy Procedia 2009, 9, 383–390. [Google Scholar] [CrossRef]
- Lee, J.Y.; Keener, T.C.; Yang, Y.J. Potential Flue Gas Impurities in Carbon Dioxide Streams Separated from Coal-Fired Power Plants. J. Air Waste Manag. Assoc. 2009, 59, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Rütters, H.; Stadler, S.; Bäßler, R.; Bettge, D.; Jeschke, S.; Kather, A.; Lempp, C.; Lubenau, U.; Ostertag-Henning, C.; Schmitz, S.; et al. Towards an optimization of the CO2 stream composition—A whole-chain approach. Int. J. Greenh. Gas Control 2016, 54, 682–701. [Google Scholar] [CrossRef]
- Yevtushenko, O.; Bettge, D.; Bäßler, R.; Bohraus, S. Corrosion of CO2 transport and injection pipeline steels due to the condensation effects caused by SO2 and NO2 impurities. Mater. Corros. 2015, 66, 334–341. [Google Scholar] [CrossRef]
- Quynh Hoa, L.; Baessler, R.; Bettge, D. On the Corrosion Mechanism of CO2 Transport Pipeline Steel Caused by Condensate: Synergistic Effects of NO2 and SO2. Materials 2019, 12, 364. [Google Scholar] [CrossRef]
- Choi, Y.-S.; Nesic, S.; Young, D. Effect of Impurities on the Corrosion Behavior of CO2 Transmission Pipeline Steel in Supercritical CO2-Water Environments. Environ. Sci. Technol. 2010, 44, 9233–9238. [Google Scholar] [CrossRef]
- Xiang, Y.; Wang, Z.; Xu, C.; Zhou, C.; Li, Z.; Ni, W. Impact of SO2 concentration on the corrosion rate of X70 steel and iron in water-saturated supercritical CO2 mixed with SO2. J. Supercrit. Fluids 2011, 58, 286–294. [Google Scholar] [CrossRef]
- Ruhl, A.S.; Kranzmann, A. Corrosion behavior of various steels in a continuous flow of carbon dioxide containing impurities. Int. J. Greenh. Gas Control 2012, 9, 85–90. [Google Scholar] [CrossRef]
- Morland, B.H.; Dugstad, A.; Svenningsen, G. Corrosion of Carbon Steel in Dense Phase CO2 with Water above and Below the Solubility Limit. Energy Procedia 2017, 114, 6752–6765. [Google Scholar] [CrossRef]
- Halseid, M.; Dugstad, A.; Morland, B.H. Corrosion and Bulk Phase Reactions in CO2 Transport Pipelines with Impurities: Review of Recent Published Studies. Energy Procedia 2014, 63, 2557–2569. [Google Scholar] [CrossRef]
- Lan, T.T.N.; Thoa, N.T.P.; Nishimura, R.; Tsujino, Y.; Yokoi, M.; Maeda, Y. Atmospheric corrosion of carbon steel under field exposure in the southern part of Vietnam. Corros. Sci. 2006, 48, 179–192. [Google Scholar] [CrossRef]
- Syed, S. Atmospheric corrosion of hot and cold rolled carbon steel under field exposure in Saudi Arabia. Corros. Sci. 2008, 50, 1779–1784. [Google Scholar] [CrossRef]
- Wei, L.; Pang, X.; Gao, K. Effect of flow rate on localized corrosion of X70 steel in supercritical CO2 environments. Corros. Sci. 2018, 136, 339–351. [Google Scholar] [CrossRef]
- Sun, J.; Sun, C.; Wang, Y. Effects of O2 and SO2 on Water Chemistry Characteristics and Corrosion Behavior of X70 Pipeline Steel in Supercritical CO2 Transport System. Ind. Eng. Chem. Res. 2018, 57, 2365–2375. [Google Scholar] [CrossRef]
- Qin, F.; Jiang, C.; Cui, X.; Wang, Q.; Wang, J.; Huang, R.; Yu, D.; Qu, Q.; Zhang, Y.; Peng, P.D. Effect of soil moisture content on corrosion behavior of X70 steel. Int. J. Electrochem. Sci. 2018, 13, 1603–1613. [Google Scholar] [CrossRef]
- Falk, F.; Menneken, M.; Stephan-Scherb, C. Real-Time Observation of High-Temperature Gas Corrosion in Dry and Wet SO2-Containing Atmosphere. JOM 2019, 71, 1560–1565. [Google Scholar] [CrossRef]
- Stephan-Scherb, C.; Nützmann, K.; Kranzmann, A.; Klaus, M.; Genzel, C. Real time observation of high temperature oxidation and sulfidation of Fe-Cr model alloys. Mater. Corros. 2018, 69, 678–689. [Google Scholar] [CrossRef]
- Okoro, S.C.; Niessen, F.; Villa, M.; Apel, D.; Montgomery, M.; Frandsen, F.J.; Pantleon, K. Complementary Methods for the Characterization of Corrosion Products on a Plant-Exposed Superheater Tube. Metallogr. Microstruct. Anal. 2017, 6, 22–35. [Google Scholar] [CrossRef]
- Ko, M.; Ingham, B.; Laycock, N.; Williams, D.E. In situ Synchrotron X-ray diffraction study of the effect of microstructure and boundary layer conditions on CO2 corrosion of pipeline steels. Corros. Sci. 2015, 90, 192–201. [Google Scholar] [CrossRef]
- Ko, M.; Ingham, B.; Laycock, N.; Williams, D.E. In situ Synchrotron X-ray diffraction study of the effect of chromium additions to the steel and solution on CO2 corrosion of pipeline steels. Corros. Sci. 2014, 80, 237–246. [Google Scholar] [CrossRef]
- Hassan Sk, M.; Abdullah, A.M. Corrosion of General Oil-field Grade Steel in CO2 Environment—An Update in the Light of Current Understanding. Int. J. Electrochem. Sci. 2017, 12, 4277–4290. [Google Scholar] [CrossRef]
- Ja’baz, I.; Jiao, F.; Wu, X.; Yu, D.; Ninomiya, Y.; Zhang, L. Influence of gaseous SO2 and sulphate-bearing ash deposits on the high-temperature corrosion of heat exchanger tube during oxyfuel combustion. Fuel Process. Technol. 2017, 167, 193–204. [Google Scholar] [CrossRef]
- Ghahari, M.; Krouse, D.; Laycock, N.; Rayment, T.; Padovani, C.; Stampanoni, M.; Marone, F.; Mokso, R.; Davenport, A.J. Synchrotron X-ray radiography studies of pitting corrosion of stainless steel: Extraction of pit propagation parameters. Corros. Sci. 2015, 100, 23–35. [Google Scholar] [CrossRef]
- CLUSTER—Impacts of Impurities in CO2 Streams Captured from Different Emitters in a Regional Cluster on Transport, Injection and Storage. Available online: www.bgr.bund.de/CLUSTER-EN (accessed on 1 May 2019).
- Schutz, R.W.; Watkins, H.B. Recent developments in titanium alloy application in the energy industry. Mater. Sci. Eng. A 1998, 243, 305–315. [Google Scholar] [CrossRef]
- Ruhl, A.S.; Kranzmann, A. Investigation of corrosive effects of sulphur dioxide, oxygen and water vapour on pipeline steels. Int. J. Greenh. Gas Control 2013, 13, 9–16. [Google Scholar] [CrossRef]
- Engel, F.; Kather, A. Conditioning of a Pipeline CO2 Stream for Ship Transport from Various CO2 Sources. Energy Procedia 2017, 114, 6741–6751. [Google Scholar] [CrossRef]
- Bodentemperatur. Available online: https://www.pik-potsdam.de/services/klima-wetter-potsdam/klimazeitreihen/bodentemperatur (accessed on 1 May 2019).
- Genzel, C.; Denks, I.A.; Gibmeier, J.; Klaus, M.; Wagener, G. The materials science Synchrotron beamline EDDI for energy-dispersive diffraction analysis. Nucl. Instrum. Meth. Phys. Res. A 2007, 578, 23–33. [Google Scholar] [CrossRef]
- ICSD-Fiz-Karlsruhe Inorganic Crystal Structure Database. Available online: https://www.fiz-karlsruhe.de/en/produkte-und-dienstleistungen/inorganic-crystal-structure-database-icsd (accessed on 1 May 2019).
- Huijbregts, W.M.M.; Leferink, R.G.I. Latest advances in the understanding of acid dewpoint corrosion: Corrosion and stress corrosion cracking in combustion gas condensates. Anti-Corros. Methods Mater. 2004, 51, 173–188. [Google Scholar] [CrossRef]
- Atkins, P.; Paula, J.; Keeler, J. Physical Chemistry, 11th ed.; Oxford University Press: Oxford, UK, 2018. [Google Scholar]
- Morland, B.H.; Norby, T.; Tjelta, M.; Svenningsen, G. Effect of SO2, O2, NO2, and H2O concentrations on chemical reactions and corrosion of carbon steel in dense phase CO2. Corrosion 2019, 75, 1327–1338. [Google Scholar] [CrossRef]
- Morland, B.H.; Tjelta, M.; Norby, T.; Svenningsen, G. Acid reactions in hub systems consisting of separate non-reactive CO2 transport lines. Int. J. Greenh. Gas Control 2019, 87, 246–255. [Google Scholar] [CrossRef]
- Morland, B.H.; Tadesse, A.; Svenningsen, G.; Springer, R.D.; Anderko, A. Nitric and Sulfuric Acid Solubility in Dense Phase CO2. Ind. Eng. Chem. Res. 2019, 58, 22924–22933. [Google Scholar] [CrossRef]
- Majzlan, J.; Botez, C.; Stephens, P.W. The crystal structures of synthetics Fe2(SO4)3(H2O)5 and the type specimen of lausenite. Am. Mineral. 2005, 90, 411–416. [Google Scholar] [CrossRef]
- Ackermann, A.; Lazic, B.; Armbruster, T.; Doyle, S.; Grevel, K.-S.; Majzlan, J. Thermodynamic and crystallographic properties of kornelite [Fe2(SO4)3-7.75H2O] and paracoquimbite [Fe2(SO4)3·9H2O]. Am. Mineral. 2009, 94, 1620–1628. [Google Scholar] [CrossRef]
- Scordari, F.; Ventruti, G.; Gualtieri, A.F. The structure of metahohmannite, Fe23 + [O(SO4)2]·4H2O, by in situ Synchrotron powder diffraction. Am. Mineral. 2004, 89, 365–370. [Google Scholar] [CrossRef]
- Kammeyer, C.W.; Whitman, D.R. Quantum Mechanical Calculation of Molecular Radii. I. Hydrides of Elements of Periodic Groups IV through VII. J. Chem. Phys. 1972, 56, 4419–4421. [Google Scholar] [CrossRef]
- Matito-Martos, I.; Martin-Calvo, A.; Gutierrez-Sevillano, J.J.; Haranczyk, M.; Doblare, M.; Parra, J.B.; Ania, C.O.; Calero, S. Zeolite screening for the separation of gas mixtures containing SO2, CO2 and CO. Phys. Chem. Chem. Phys. 2014, 16, 19884–19893. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised Effective Ionic Radii and Systematic Studies of Interatomie Distances in Halides and Chaleogenides. Acta Cryst. 1976, A32, 751–767. [Google Scholar] [CrossRef]
- Ribbe, P.H.; Gibbs, G.H. Crystal Structures of the Humite Minerals: III. Mg/Fe Ordering in Humite and its Relation to Other Ferromagnesian Silicates. Am. Mineral. 1971, 56, 1155–1173. [Google Scholar]
- Jenkins, H.D.B.; Thakur, K.P. Reappraisal of thermochemical radii for complex ions. J. Chem. Educ. 1979, 56, 576–577. [Google Scholar] [CrossRef]
- Sun, Q.; Zheng, H. Raman OH stretching vibration of ice Ih. Prog. Nat. Sci. 2009, 19, 1651–1654. [Google Scholar] [CrossRef]
- Sharma, S.K. Raman study of ferric nitrate crystalline hydrate and variably hydrated liquids. J. Chem. Phys. 1974, 61, 1748–1754. [Google Scholar] [CrossRef]
- Hua, Y.; Barker, R.; Neville, A. Corrosion behaviour of X65 steels in water-containing supercritical CO2 environments with NO2/O2. In Proceedings of the CORROSION 2018, NACE International, Phoenix, AZ, USA, 15–19 April 2018; p. 11085. [Google Scholar]
- Chio, C.H.; Sharma, S.K.; Muenow, D.W. The hydrates and deuterates of ferrous sulfate (FeSO4): A Raman spectroscopic study. J. Raman Spectrosc. 2007, 38, 87–99. [Google Scholar] [CrossRef]
- Chio, C.H.; Sharma, S.K.; Muenow, D.W. Micro-Raman studies of hydrous ferrous sulfates and jarosites. Spectrochim. Acta A 2005, 61, 2428–2433. [Google Scholar] [CrossRef]
- Chourpa, I.; Douziech-Eyrolles, L.; Ngaboni-Okassa, L.; Fouquenet, J.F.; Cohen-Jonathan, S.; Souce, M.; Marchais, H.; Dubois, P. Molecular composition of iron oxide nanoparticles, precursors for magnetic drug targeting, as characterized by confocal Raman microspectroscopy. Analyst 2005, 130, 1395–1403. [Google Scholar] [CrossRef]
- Hua, Y.; Barker, R.; Neville, A. Understanding the Influence of SO2 and O2 on the Corrosion of Carbon Steel in Water-Saturated Supercritical CO2. Corrosion 2014, 71, 667–683. [Google Scholar] [CrossRef]
- Ruhl, A.S.; Goebel, A.; Kranzmann, A. Corrosion Behavior of Various Steels for Compression, Transport and Injection for Carbon Capture and Storage. Energy Procedia 2012, 23, 216–225. [Google Scholar] [CrossRef]
- Le, Q.H.; Baessler, R.; Knauer, S.; Jaeger, P.; Kratzig, A.; Bettge, D.; Kranzmann, A. Droplet Corrosion of CO2 Transport Pipeline Steels in Simulated Oxyfuel Flue Gas. Corrosion 2018, 74, 1406–1420. [Google Scholar] [CrossRef]




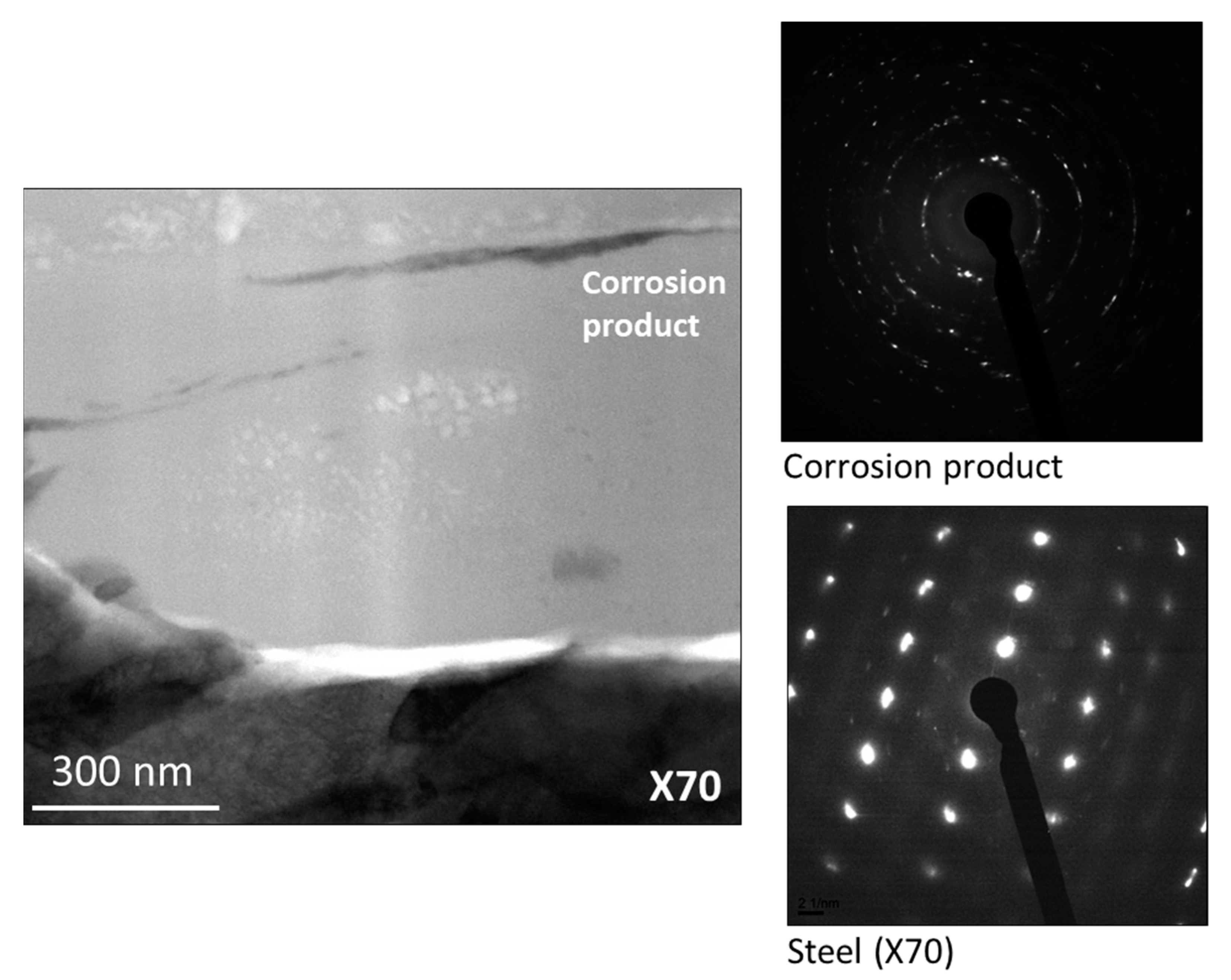


| Element | C | Mn | Si | P | S | Cr | Ni |
|---|---|---|---|---|---|---|---|
| % by mass | 0.16 | 1.70 | 0.45 | 0.02 | 0.02 | 0.03 | 0.05 |
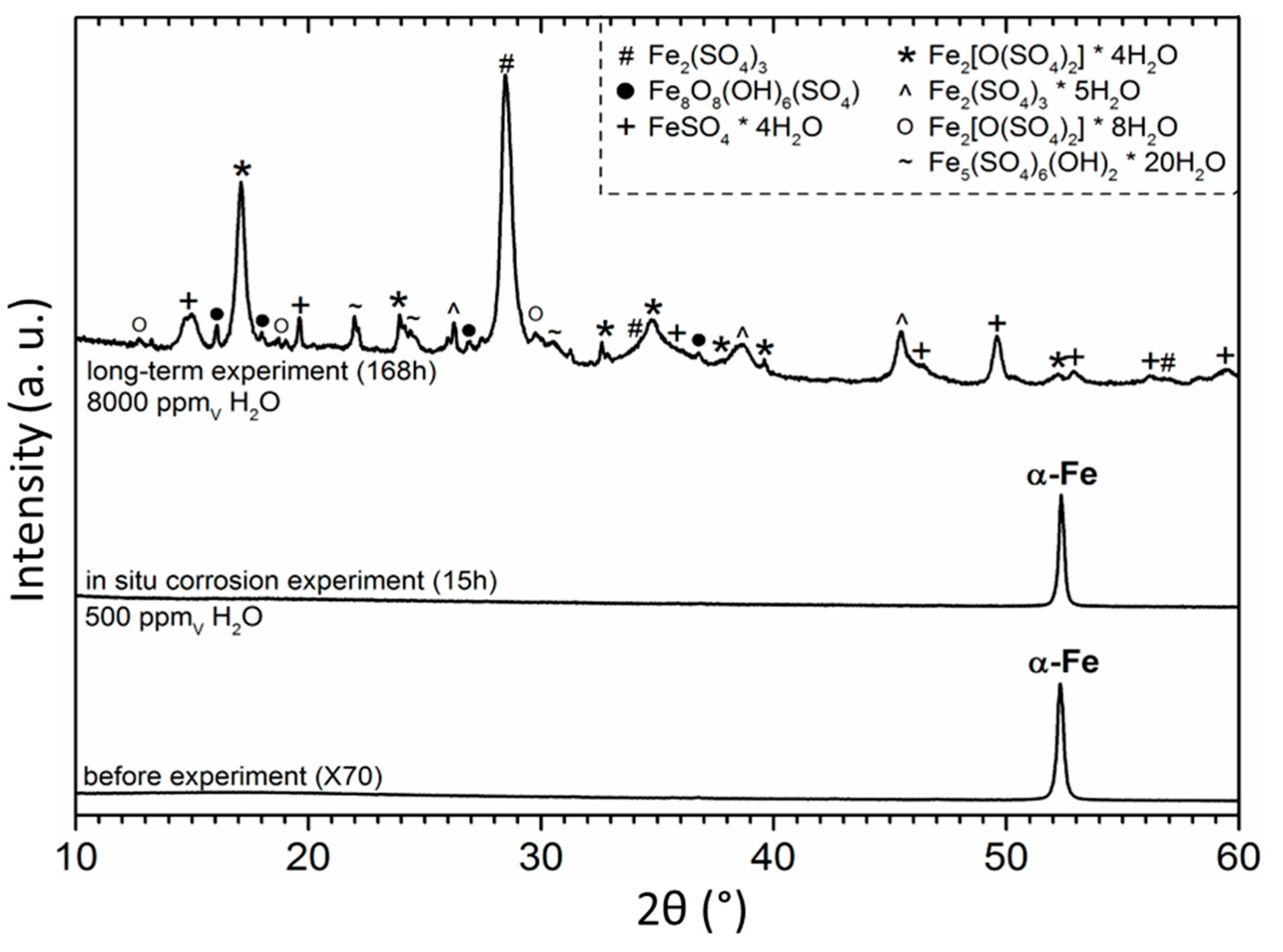
| Emission Line (keV) | Lattice Parameter (Å) | Onset (min) | Phase Formula (hkl) | ||
|---|---|---|---|---|---|
| 20.10 | 5.993 | ~360 | Metahohmannite | Fe2(SO4)2O·4 H2O | (110) |
| 26.35 | 4.500 | ~240 | Rozenite | FeSO4·4 H2O | (111) |
| 31.81 | 3.757 | ~360 | Metahohmannite | Fe2(SO4)2O·4 H2O | (12–1) |
| 36.35 | 3.204 | ~150 | Lausenite | Fe2(SO4)3·5 H2O | (30–1) |
| 40.18 | 2.946 | ~360 | Metahohmannite | Fe2(SO4)2O·4 H2O | (211) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kratzig, A.; Quynh Hoa, L.; Bettge, D.; Menneken, M.; Bäßler, R. Early Stage of Corrosion Formation on Pipeline Steel X70 Under Oxyfuel Atmosphere at Low Temperature. Processes 2020, 8, 421. https://doi.org/10.3390/pr8040421
Kratzig A, Quynh Hoa L, Bettge D, Menneken M, Bäßler R. Early Stage of Corrosion Formation on Pipeline Steel X70 Under Oxyfuel Atmosphere at Low Temperature. Processes. 2020; 8(4):421. https://doi.org/10.3390/pr8040421
Chicago/Turabian StyleKratzig, Andreas, Le Quynh Hoa, Dirk Bettge, Martina Menneken, and Ralph Bäßler. 2020. "Early Stage of Corrosion Formation on Pipeline Steel X70 Under Oxyfuel Atmosphere at Low Temperature" Processes 8, no. 4: 421. https://doi.org/10.3390/pr8040421
APA StyleKratzig, A., Quynh Hoa, L., Bettge, D., Menneken, M., & Bäßler, R. (2020). Early Stage of Corrosion Formation on Pipeline Steel X70 Under Oxyfuel Atmosphere at Low Temperature. Processes, 8(4), 421. https://doi.org/10.3390/pr8040421