1. Introduction
Globally, energy demand is escalating with the production of petrochemical products, which ends up generating high amounts of wastewater due to its complex process. This creates a global problem of contaminated oily wastewater which needs to be decontaminated [
1]. Meanwhile, coagulation and dissolved air flotation (CDAF) systems have been the most widely used physio-chemical processes in the water and wastewater treatment settings [
2,
3]. Of these processes, the DAF can be operated alone or combined with other processes at different stages of the WWTPs for primary, secondary or tertiary treatment purposes [
4]. In this context, the DAF was employed for primary purposes to improve separation of industrial oil and suspended materials from oily wastewater.
In South Africa, the concern about the environmental threats of oily waste pollution produced during oil exploration and production activities impedes the united nations (UN’s) sustainable goal for 2050, with a focus on clean water and sanitation [
5,
6]. It is therefore important to improve the treatment process to meet the stringent discharge limits of soap oil and grease (SOG) below discharge limits of 50 mg/L [
7,
8]. Some of the waste streams, including petrochemical industries, contain high amounts of contaminants viz. turbidity, total suspended solids (TSS), chemical oxygen demand (COD), SOG and other organic compounds’ derivatives [
7,
9,
10]. These contaminants are not just detrimental to the environment, but also to aquatic life and human health [
8].
Conventionally, treatment technologies including gravity separation, electrocoagulation, coagulation, flotation, advanced oxidation processes, membrane filtration, and biodegradation are used [
11,
12,
13,
14,
15,
16]. However, some of the aforementioned technologies are unsatisfactory relating to the separation of oil from water, whereby flotation remains remarkable [
17]. Flotation involves the separation of bulk solids from the liquid medium using microbubbles [
2,
18]. Among the flotation processes, such as electrolytic flotation, dissolved-air flotation (DAF) and dispersed-air flotation, DAF has been the most commonly used flotation technology in mineral processing, potable water, and wastewater treatment industries [
2,
19].
The DAF system was firstly used in the early 1960s for the treatment of drinking water in Scandinavia, the UK and South Africa [
2,
18,
20]. In this process, dissolved air is saturated at high pressure (300–600 kPa), which forms microbubbles when released into the flotation cell, serving as a driving force to move the aggregated flocs to the surface [
18,
19]. This phenomenon includes: (a) air bubble generation, (b) contact between air bubbles and oil droplets, (c) attachment of gas bubbles to oil droplets, and (d) the rising up of the air–oil combination [
2,
18,
19]. Edzwald [
2] reported some of the technical advantages of DAF as compared to conventional sedimentation process, including rapid output, a high loading rate, and low hydraulic retention time and the low cost of construction. Based on experience, DAF is easy to operate with a higher capacity and a more acceptable footprint than sedimentation processes [
19,
20]. A study by Adlan et al., [
21] shows a 75% reduction in COD from synthetic landfill leachate with an initial COD of 2010 mg/L by using ferric chloride coagulation in a DAF treatment process [
1,
8]. In South Africa, to ascertain the variation in oil refinery wastewater (ORW) composition, combining chemical and physical treatment processes is implemented within an oil refinery plant for the recovery of valuable industrial oil potential from the oily wastewater [
10].
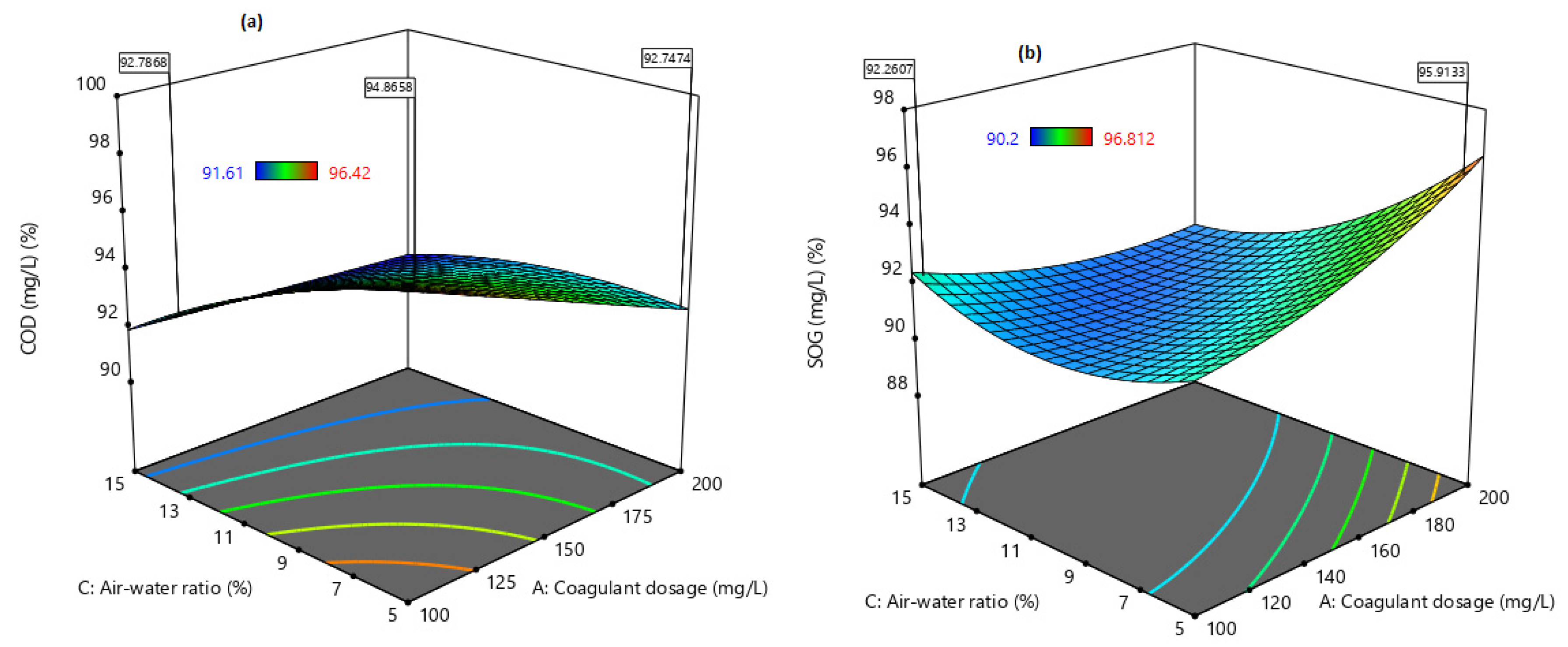
Consequently, the optimisation of DAF in most industries is very complex due to the multivariable delays in responses and resources associated with the one-factor-at time (OFAT) approach. Optimising DAF by OFAT and keeping the rest of the factors constant does not provide much information on the interactive effects of the system operating conditions [
21]. Some of these setbacks include the mixing mechanism, charge neutralisation, interfacial bridging and entrapment of air bubble–colloidal particles [
2]. Meanwhile, response surface methodology (RSM), is used empirically to study the relationship between one or more responses as a function of specified input factors in the chemical, biological and wastewater facilities [
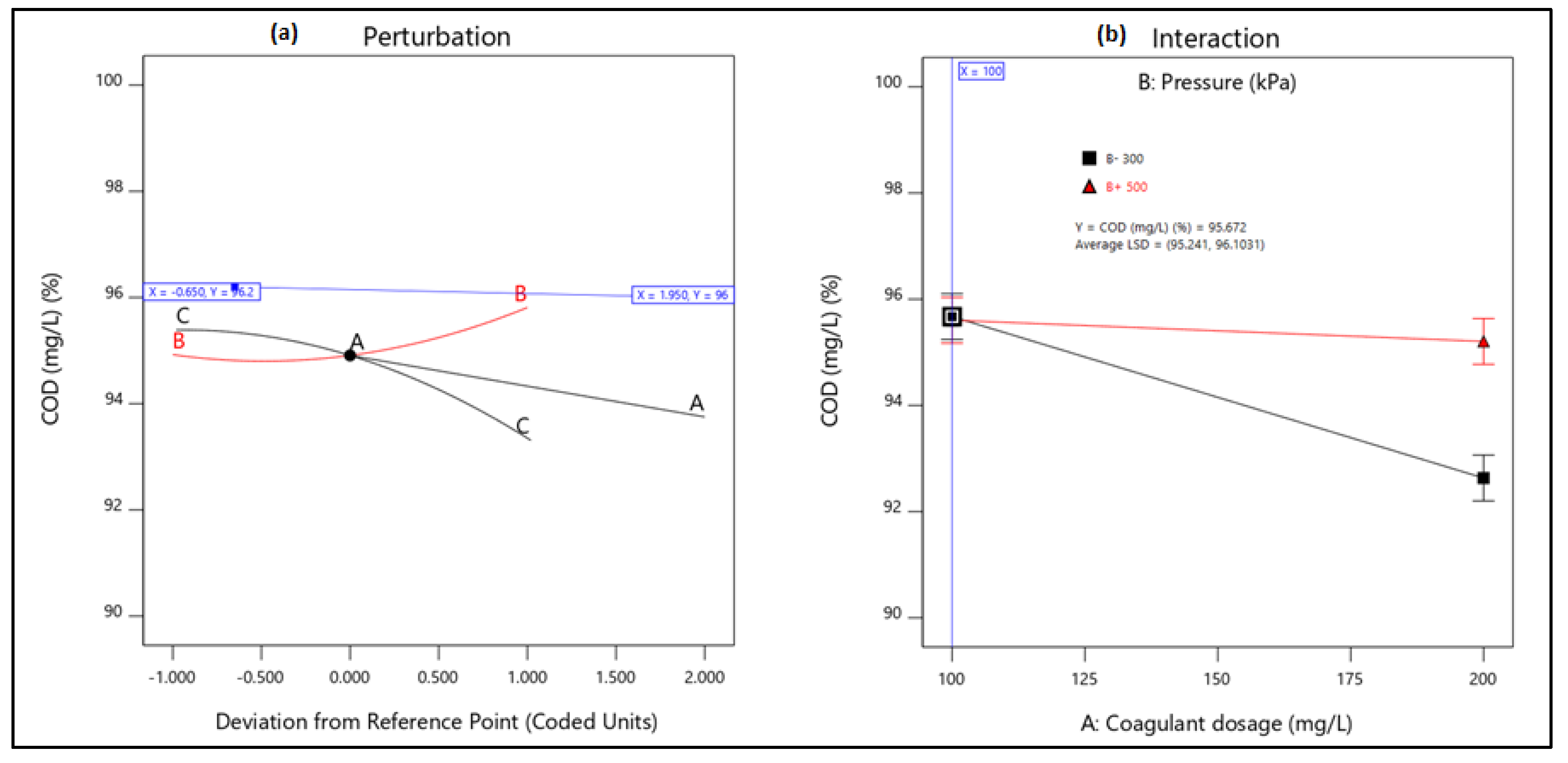
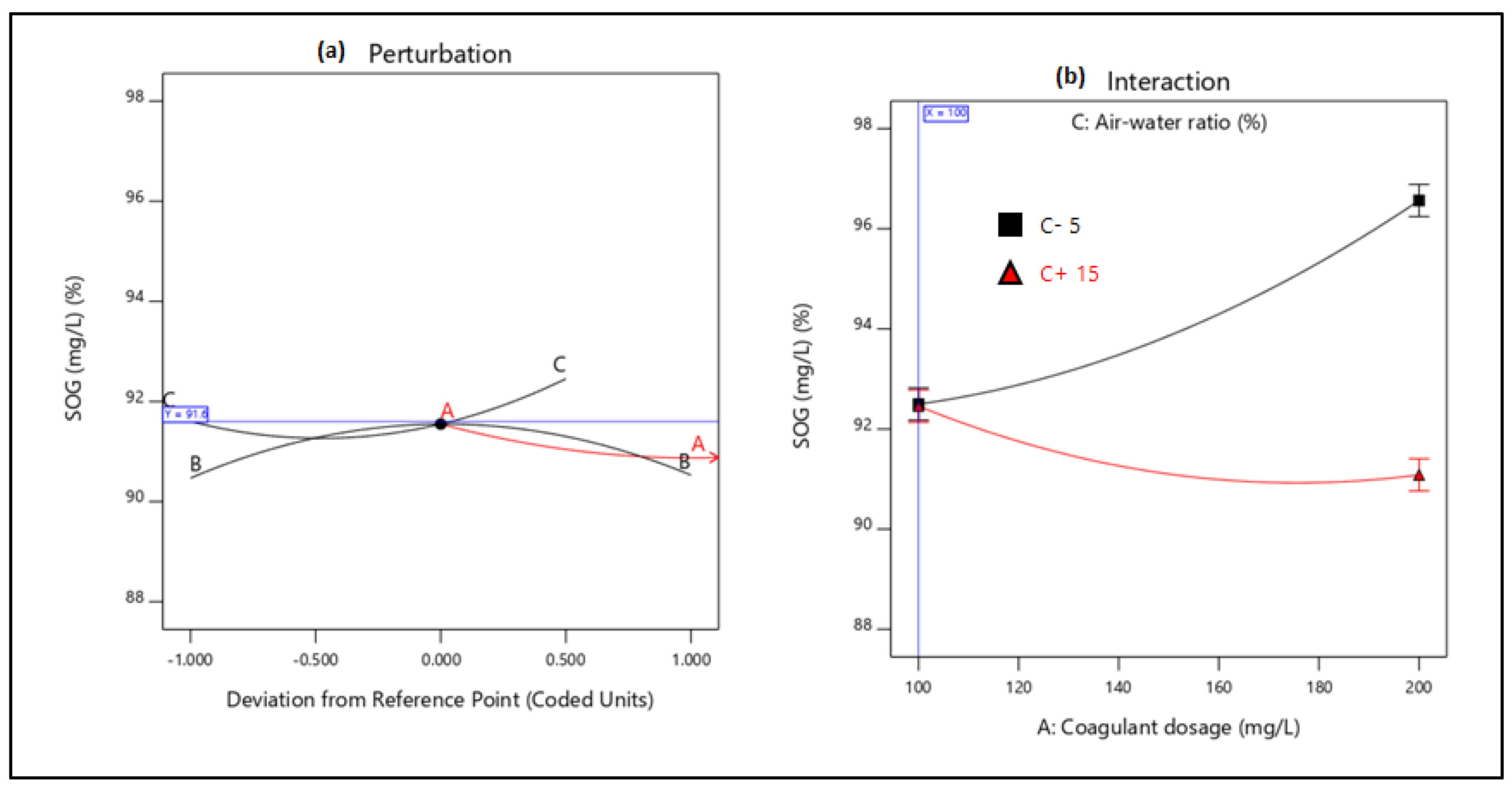
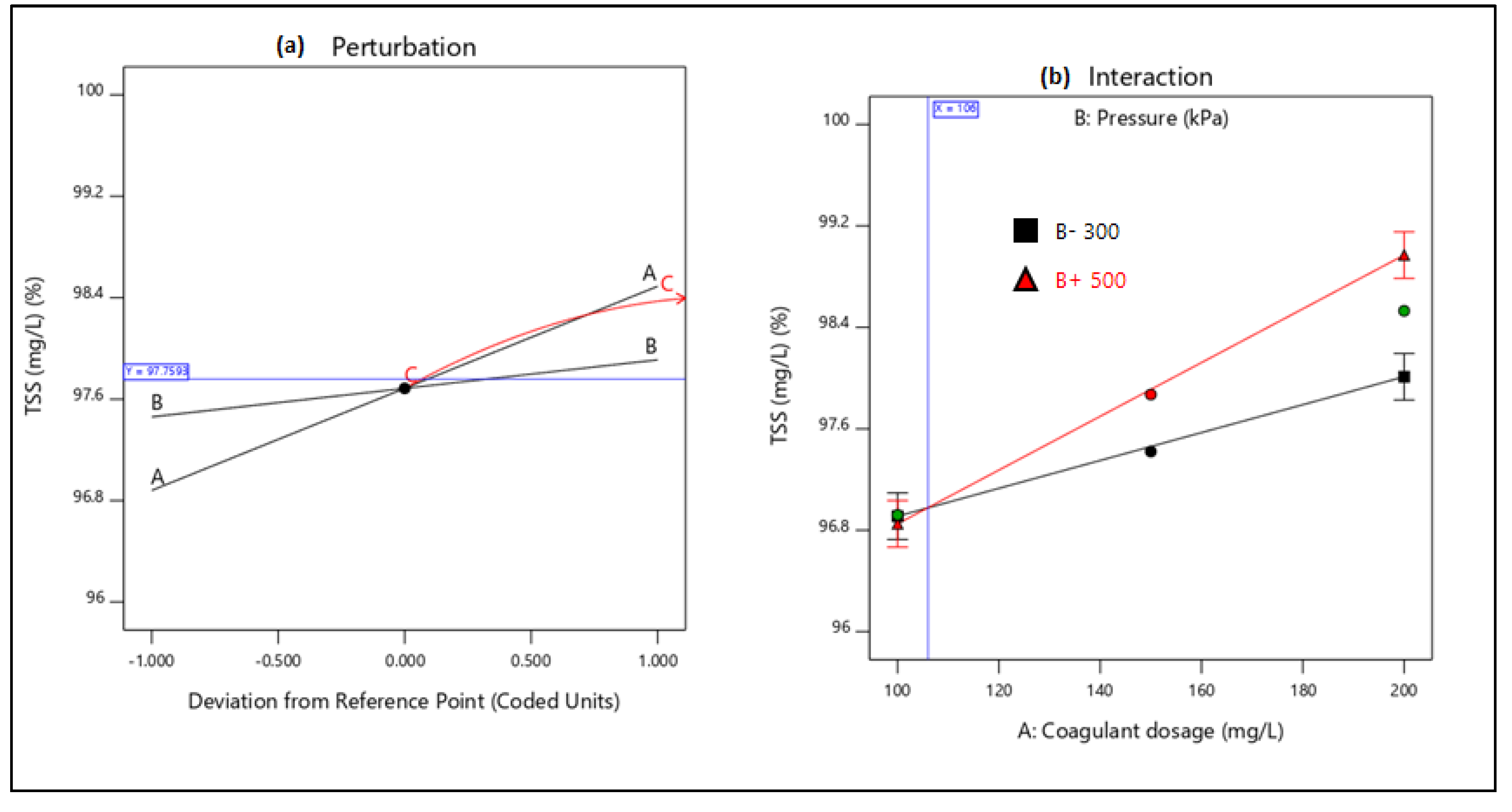
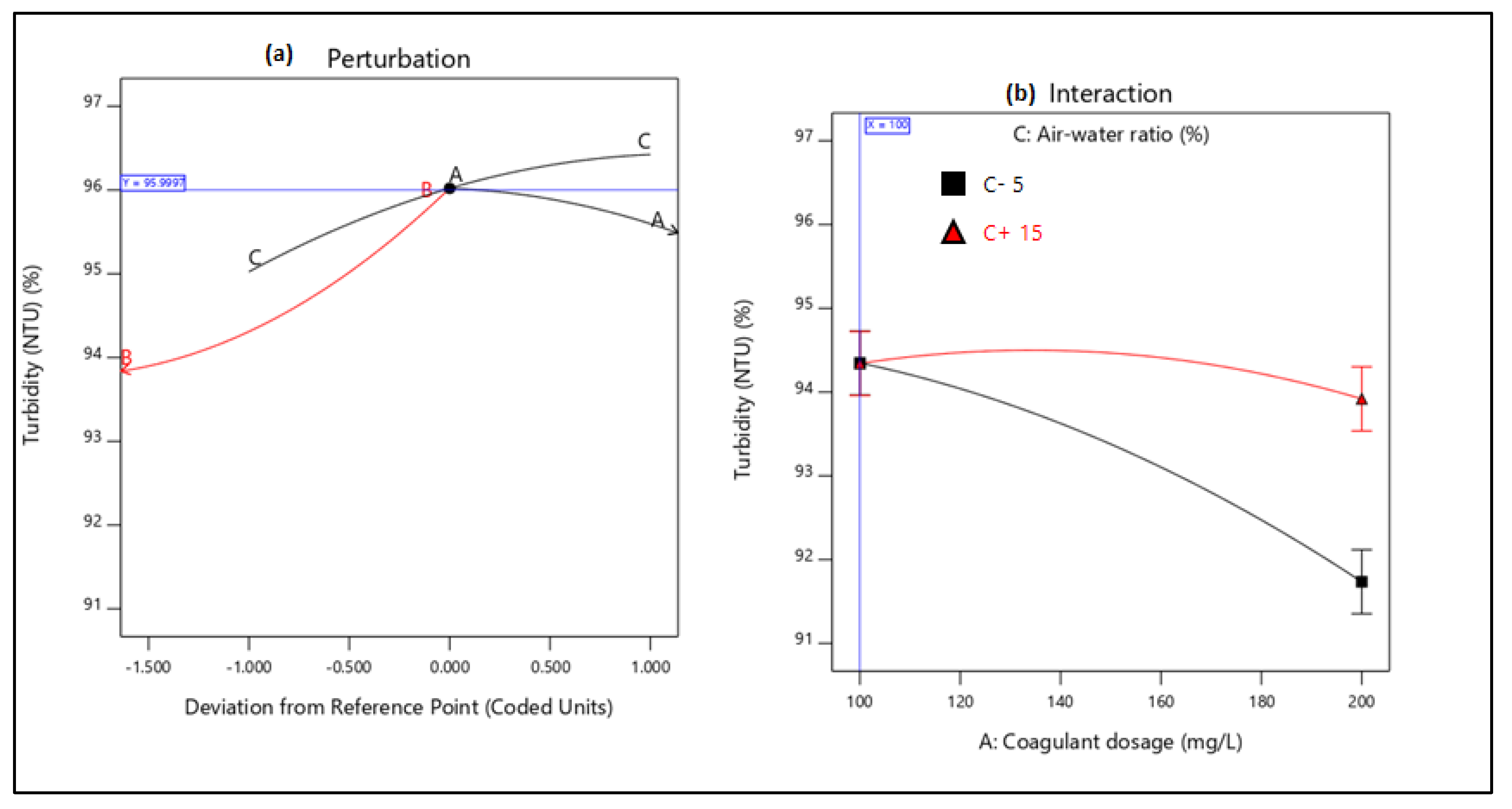
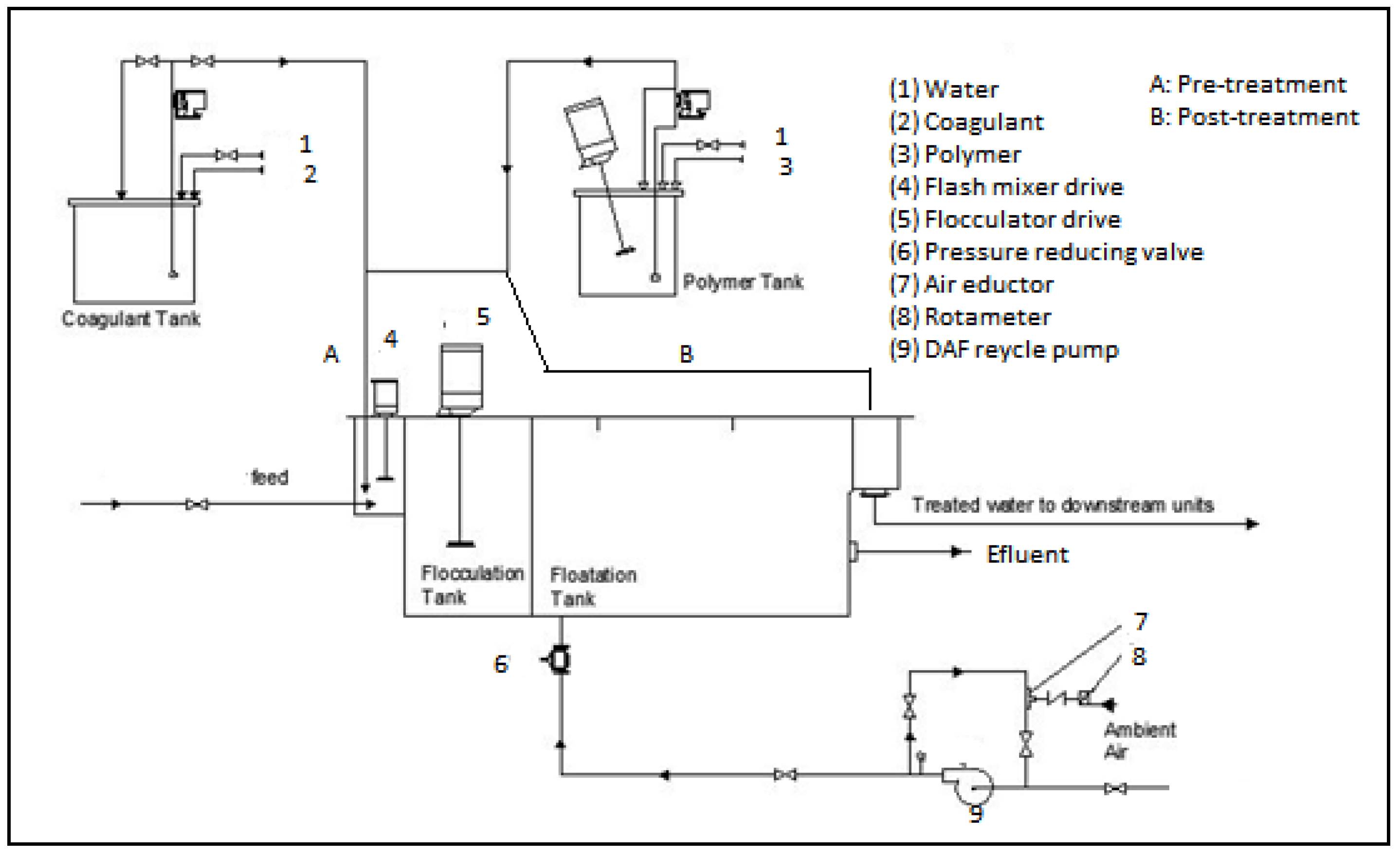
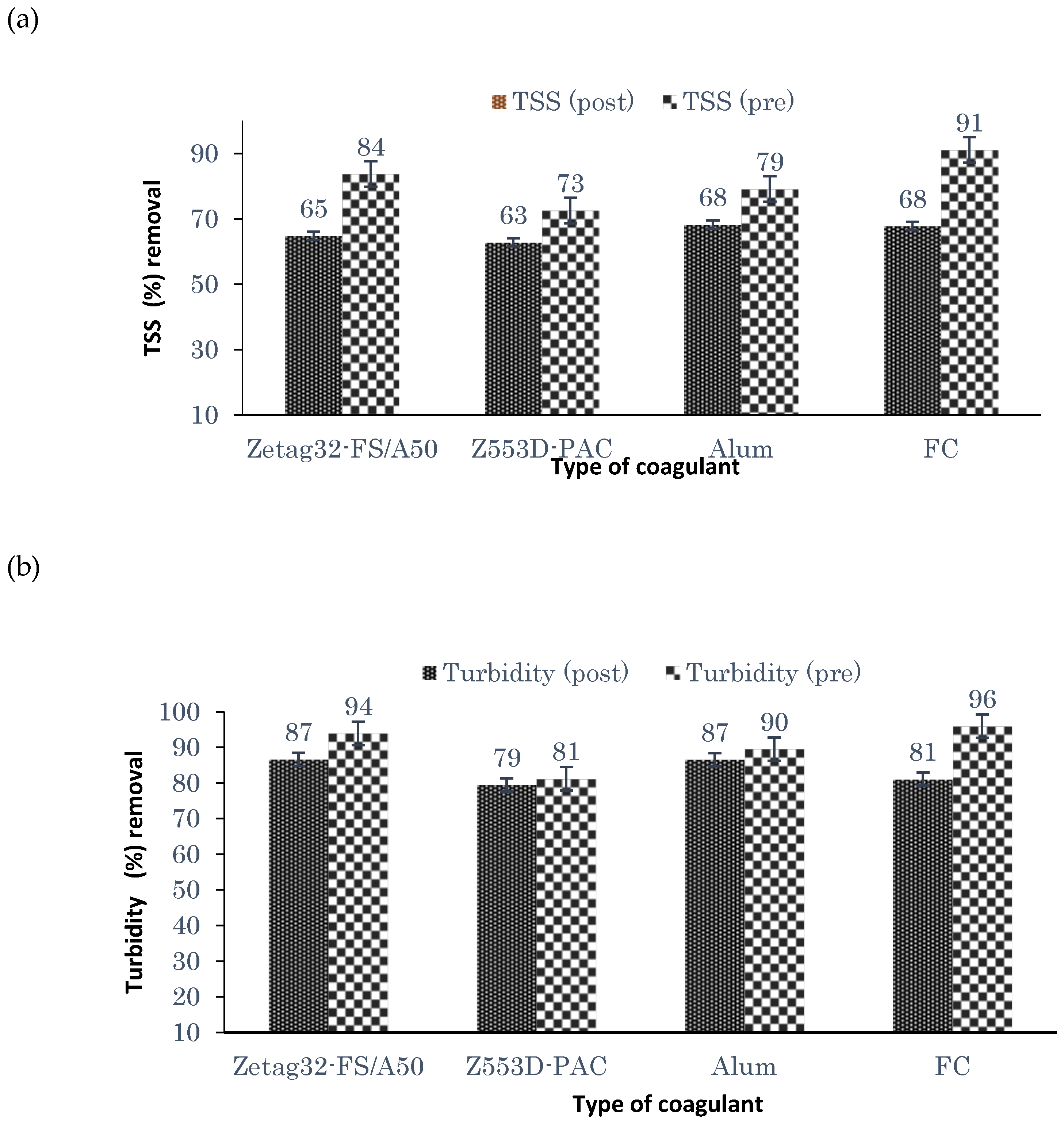
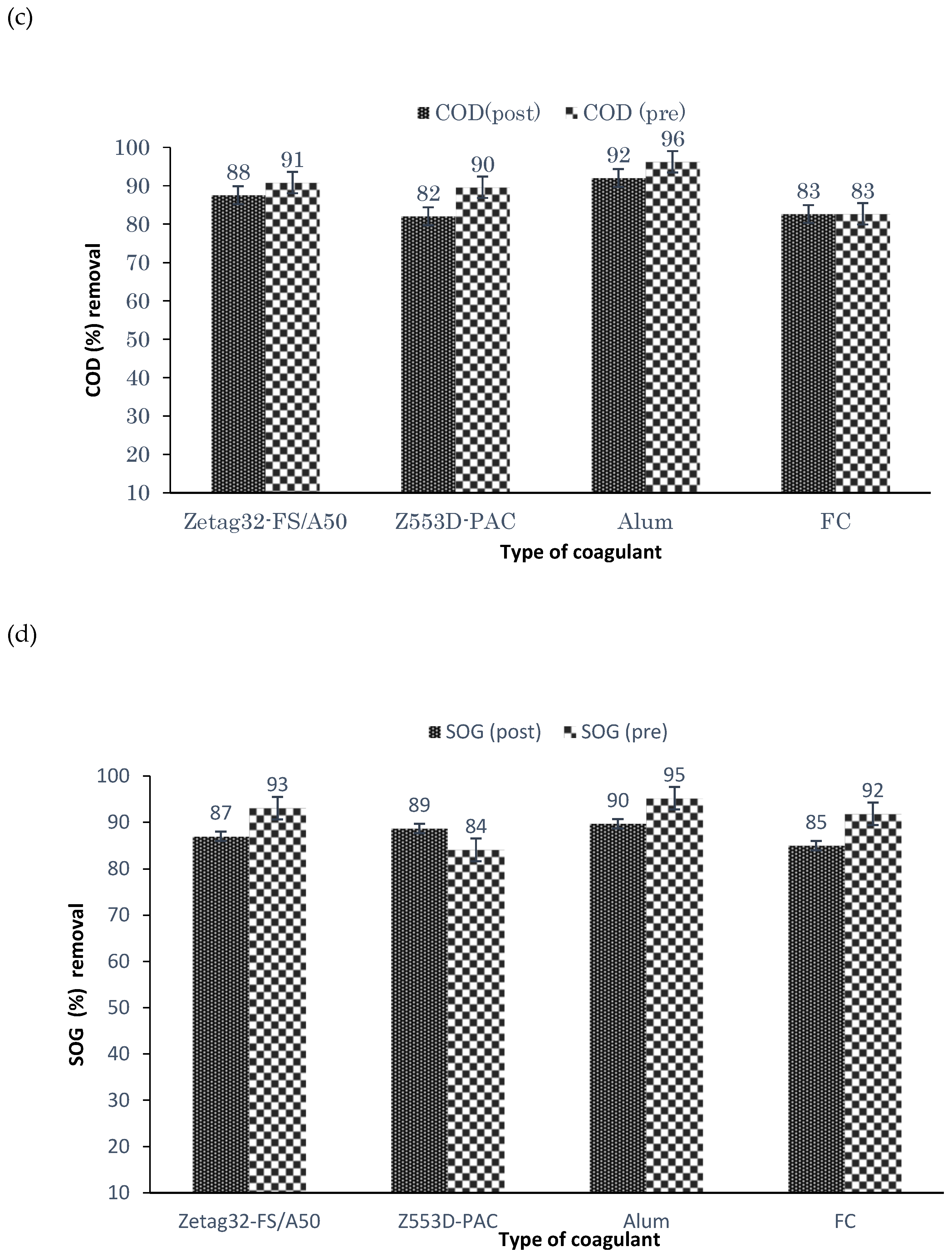
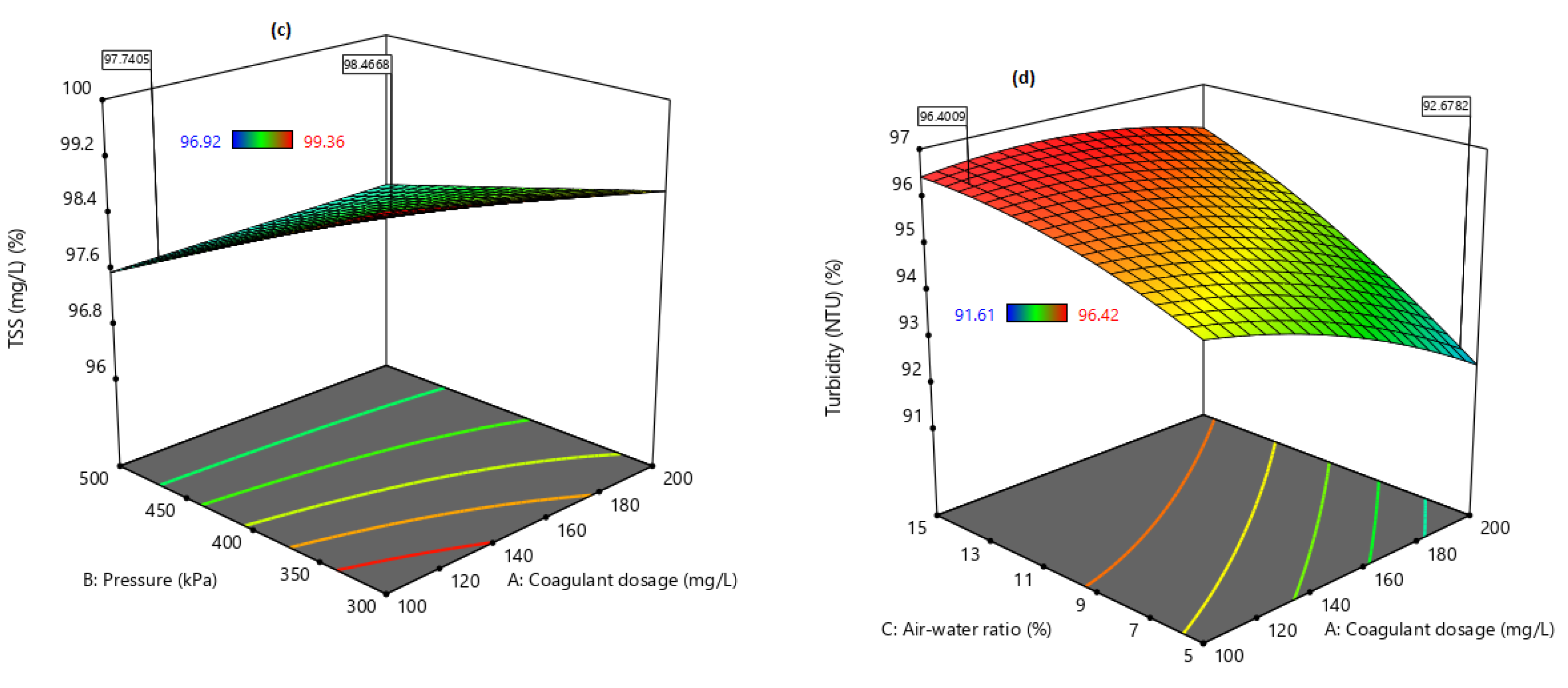
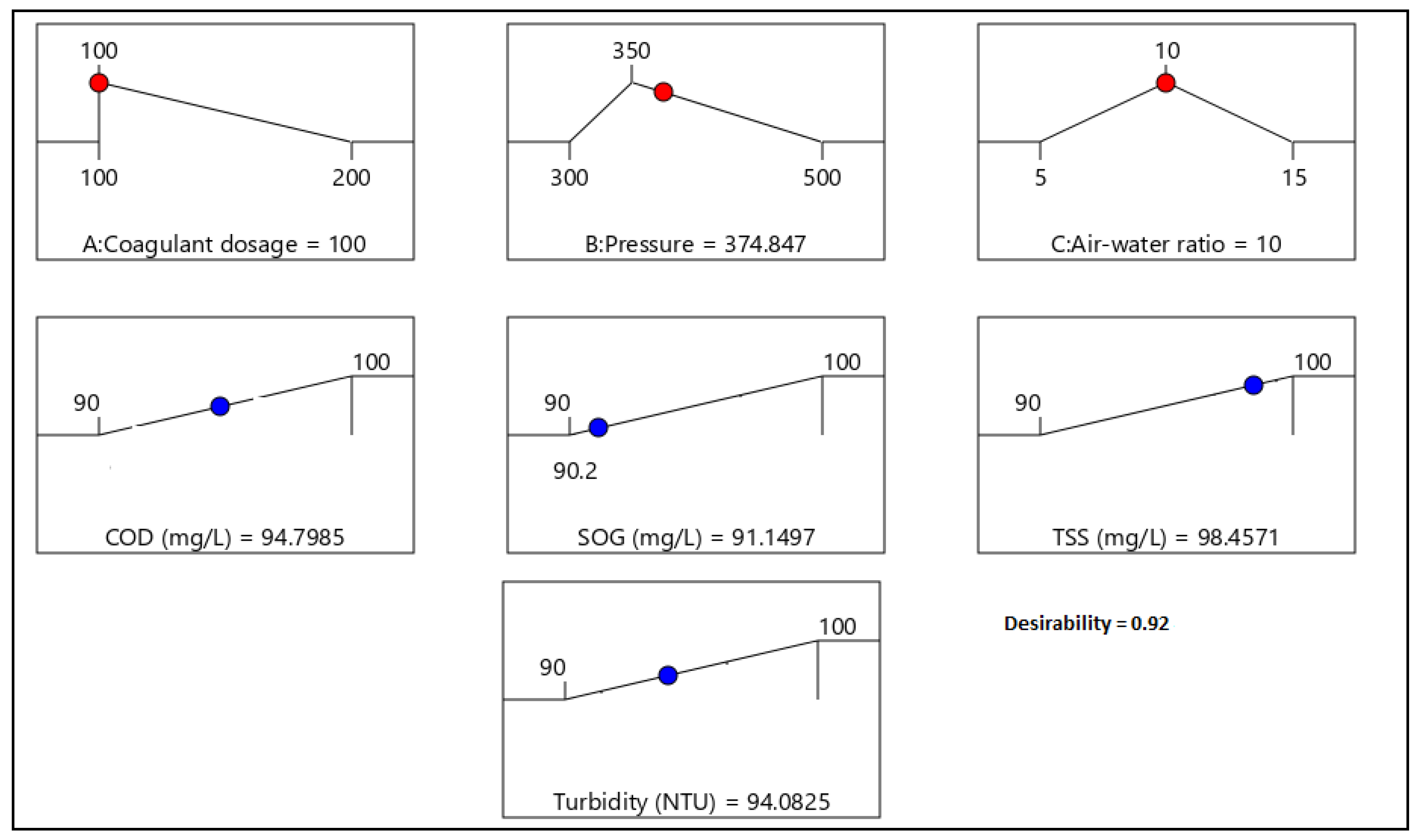
21]. Therefore, this study aimed to evaluate the performance of combining coagulation and DAF in two configuration streams, namely (a) acid-coagulation-DAF (pre-treatment) and (b) acid-DAF-coagulation (post-treatment) using two polymeric organic coagulants (Z553D and Zetag-FS/A50) and two inorganic coagulants (ferric sulphate (FS) and alum). The study also examined the individual and interactive influence of three independent factors (coagulant dosage, air saturator pressure and air–water ratio) on the removal of COD, SOG, TSS and turbidity using RSM.
5. Conclusions
This study presents coagulation before DAF (pre-treatment) as the better configuration option compared to coagulation after DAF (post-treatment). Among the four coagulants used to evaluate the DAF configuration’s treatability performance, Alum was found to be superior to the other types of coagulants. Alum, due to its trivalent ions, was able to neutralise the negatively charged oil droplets. The use of RSM was seen as far better than the OFAT approach, in terms of cost-effectiveness and optimising the complex factors of the coagulation–DAF process. The Box-Behnken design for the RSM shows valuable information on the interactions between the factors and the possibility of obtaining optimum conditions with a smaller number of experiments, time and resources. The impact of the three factors (coagulant dosage, pressure and air–water ratio) and their interactions were modelled and optimised simultaneously to maximise the response variables (%removal of COD, SOG, TSS, and Turbidity). The ANOVA indicated that the quadratic models developed were highly significant at a 95% confidence level, and the predicted results at the optimum conditions were in good agreement with the experimental data. Consequently, coagulant dosage was the most influential factor for contaminant removal.