Effect of Nitrate and Perchlorate on Selenate Reduction in a Sequencing Batch Reactor
Abstract
1. Introduction
2. Materials and Methods
2.1. Selective Enrichment of Selenate-Reducing Bacteria
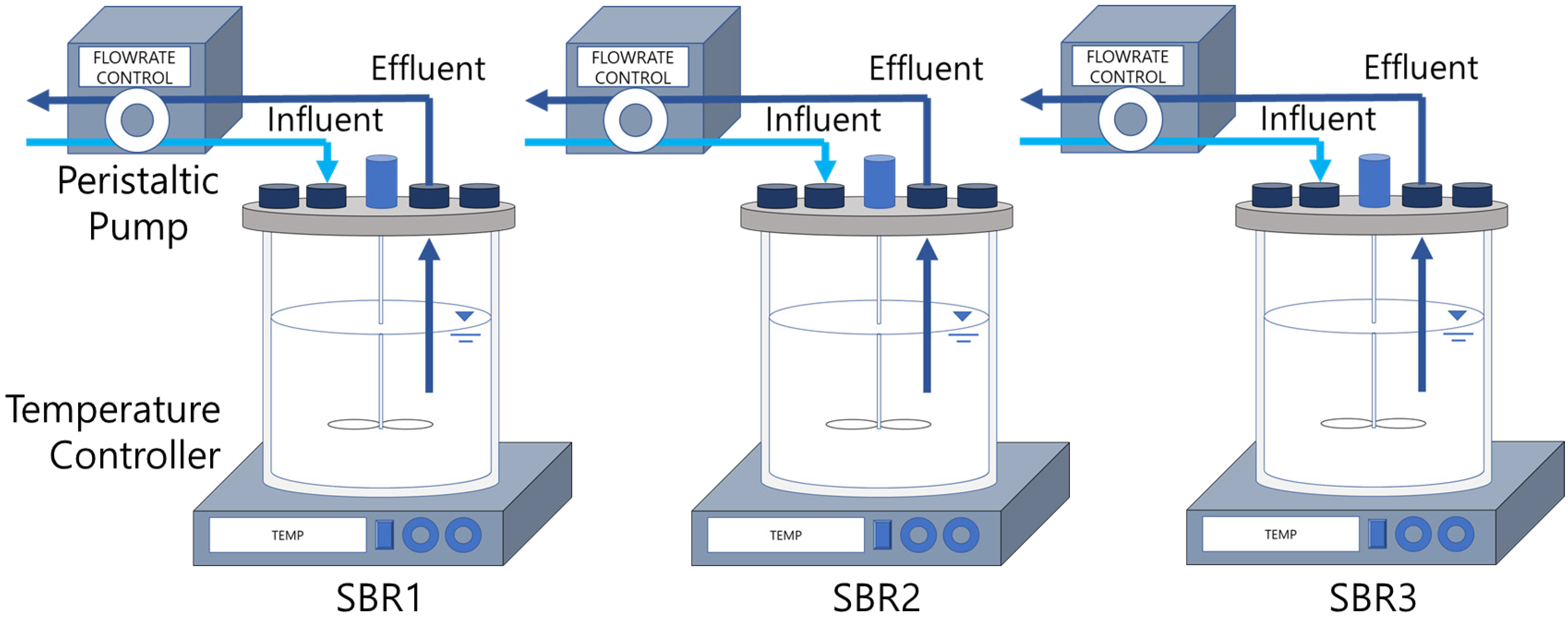
2.2. Operating Condition of SBRs
2.3. Analytical Methods
3. Results and Discussion
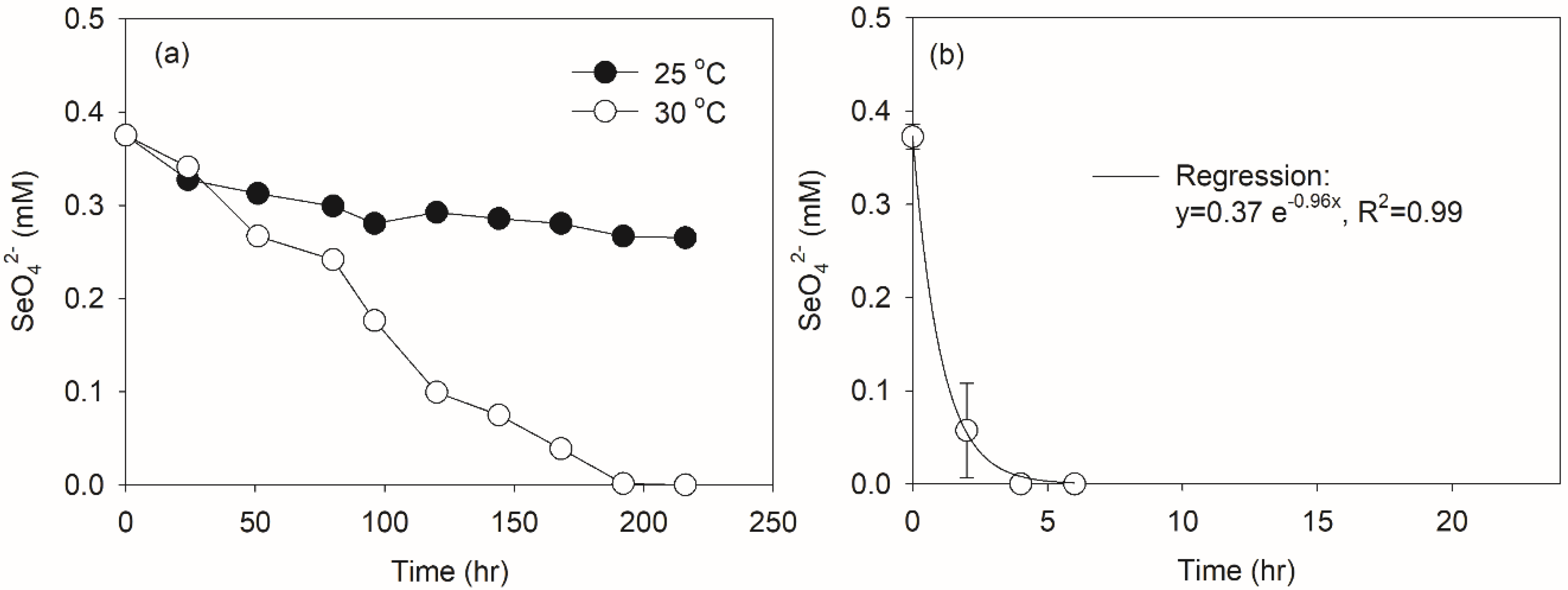
3.1. Appropriate Temperature for Selenate-Reducing Bacteria Acclimation in SBRs
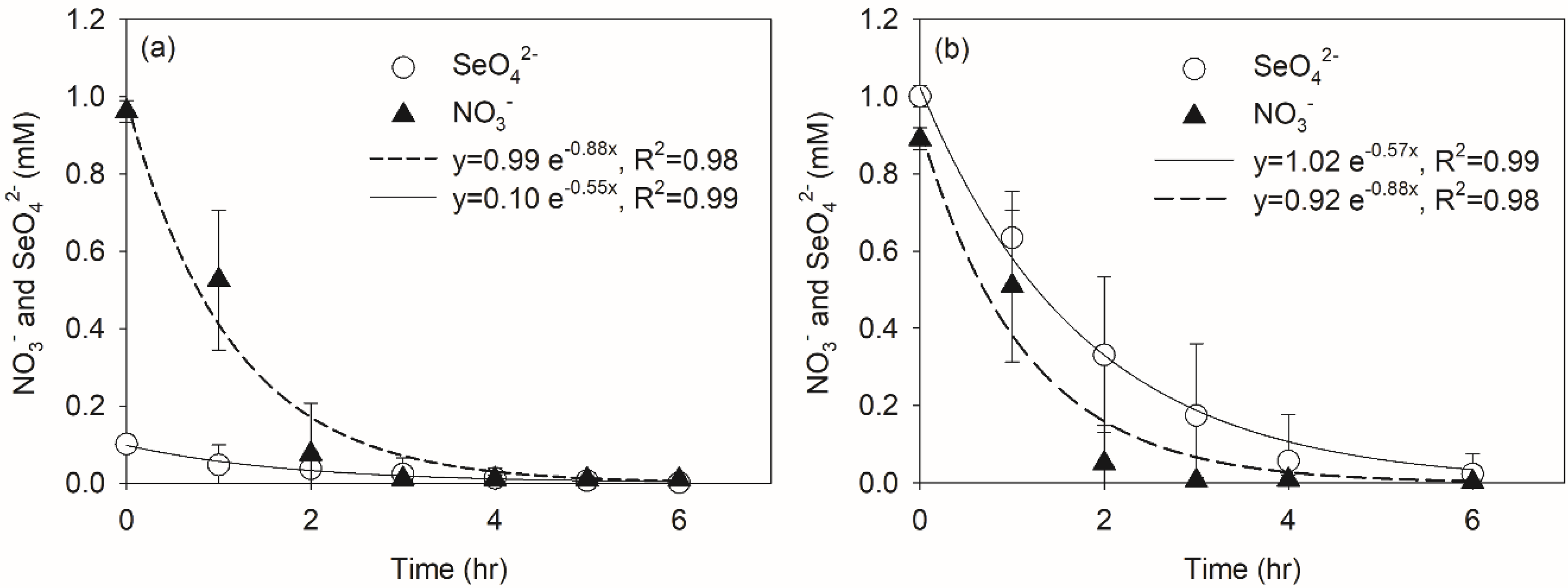
3.2. Effect of Nitrate on Selenate Reduction
3.3. Effect of External Carbon Limitation on Selenate Reduction
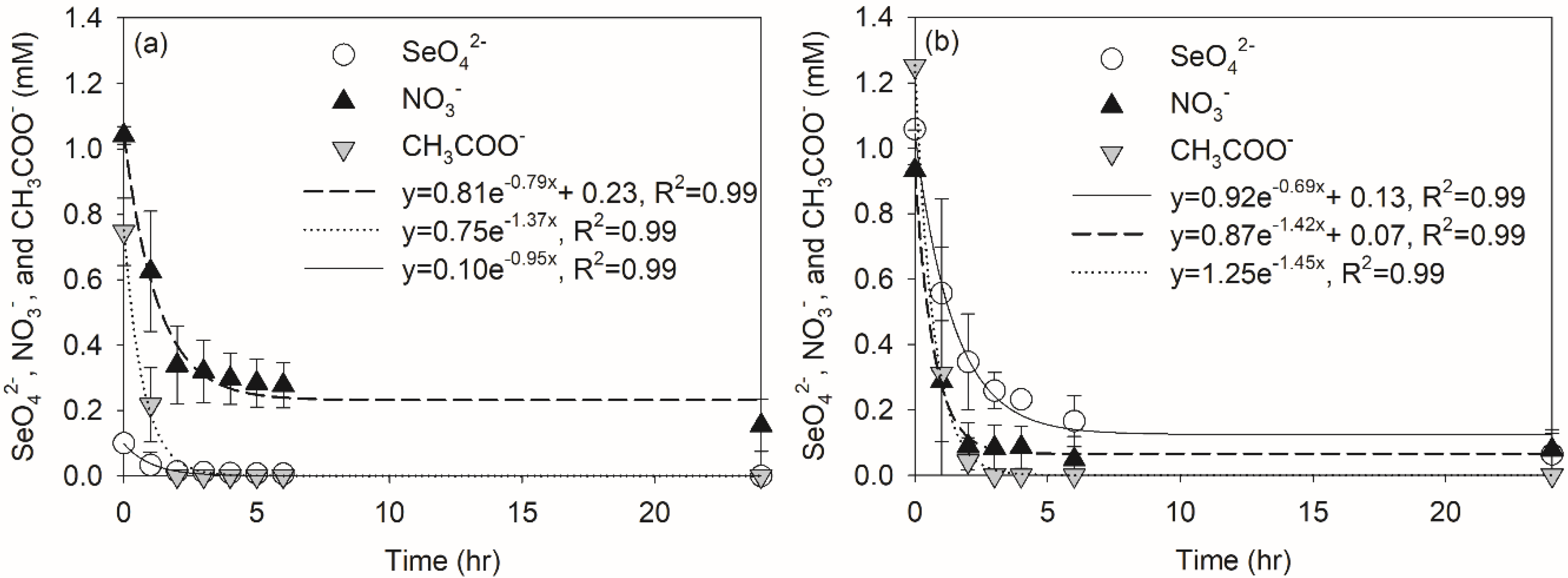
3.4. Nitrate and Perchlorate Effect on Selenate Reduction in SBRs
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Stranges, S.; Navas-Acien, A.; Rayman, M.P.; Guallar, E. Selenium status and cardiometabolic health: state of the evidence. Nutr. Metab. Cardiovasc. Dis. 2010, 20, 754–760. [Google Scholar] [CrossRef] [PubMed]
- USEPA. National Primary Drinking Water Regulations. Available online: https://www.epa.gov/ground-water-and-drinking-water/national-primary-drinking-water-regulations (accessed on 9 September 2019).
- Lemly, A.D. Aquatic selenium pollution is a global environmental safety issue. Ecotox. Environ. Safe 2004, 59, 44–56. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for Drinking-Water Quality: Fourth Edition Incorporating the First Addendum; World Health Organization Press: Geneva, Switzerland, 2017. [Google Scholar]
- MOE. Drinking Water Regulation; Ministry of Environment: Seoul, Korea, 2019.
- Nancharaiah, Y.V.; Lens, P.N. Selenium biomineralization for biotechnological applications. Trends Biotechnol. 2015, 33, 323–330. [Google Scholar] [CrossRef] [PubMed]
- CH2MHill, Inc. Review of Available Technologies for the Removal of Selenium from Water; Selenium Working Group, North American Metals Council: Washington, WA, USA, 2010. [Google Scholar]
- Snyder, M.M.; Um, W. Adsorption mechanisms and transport behavior between selenate and selenite on different sorbents. Int. J. Waste Resources 2014, 4, 1–8. [Google Scholar] [CrossRef]
- Buchs, B.; Evangelou, M.W.; Winkel, L.H.; Lenz, M. Colloidal properties of nanoparticular biogenic selenium govern environmental fate and bioremediation effectiveness. Environ. Sci. Technol. 2013, 47, 2401–2407. [Google Scholar] [CrossRef]
- Siddique, T.; Okeke, B.C.; Zhang, Y.; Arshad, M.; Han, S.K.; Frankenberger, W.T. Bacterial Diversity in Selenium Reduction of Agricultural Drainage Water Amended with Rice Straw. J. Environ. Qual. 2005, 34, 217–226. [Google Scholar] [CrossRef]
- Zhang, Y.; Okeke, B.C.; Frankenberger, W.T. Bacterial reduction of selenate to elemental selenium utilizing molasses as a carbon source. Bioresour. Technol. 2008, 99, 1267–1273. [Google Scholar] [CrossRef]
- Khamkhash, A.; Srivastava, V.; Ghosh, T.; Akdogan, G.; Ganguli, R.; Aggarwal, S. Mining-Related Selenium Contamination in Alaska, and the State of Current Knowledge. Minerals 2017, 7, 46. [Google Scholar] [CrossRef]
- Tang, Q.; Liu, G.; Yan, Z.; Sun, R. Distribution and fate of environmentally sensitive elements (arsenic, mercury, stibium and selenium) in coal-fired power plants at Huainan, Anhui, China. Fuel 2012, 95, 334–339. [Google Scholar] [CrossRef]
- Lenz, M.; Enright, A.M.; O’Flaherty, V.; van Aelst, A.C.; Lens, P.N. Bioaugmentation of UASB reactors with immobilized Sulfurospirillum barnesii for simultaneous selenate and nitrate removal. Appl. Microbiol. Biotechnol. 2009, 83, 377–388. [Google Scholar] [CrossRef]
- Lai, C.Y.; Yang, X.; Tang, Y.; Rittmann, B.E.; Zhao, H.P. Nitrate shaped the selenate-reducing microbial community in a hydrogen-based biofilm reactor. Environ. Sci. Technol. 2014, 48, 3395–3402. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.Y.; Wen, L.L.; Shi, L.D.; Zhao, K.K.; Wang, Y.Q.; Yang, X.; Rittmann, B.E.; Zhou, C.; Tang, Y.; Zheng, P.; et al. Selenate and Nitrate Bioreductions Using Methane as the Electron Donor in a Membrane Biofilm Reactor. Environ. Sci. Technol. 2016, 50, 10179–10186. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.; Rittmann, B.E.; Wright, W.F.; Bowman, R.H. Simultaneous bio-reduction of nitrate, perchlorate, selenate, chromate, arsenate, and dibromochloropropane using a hydrogen-based membrane biofilm reactor. Biodegradation 2007, 18, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Hageman, S.P.; van der Weijden, R.D.; Weijma, J.; Buisman, C.J. Microbiological selenate to selenite conversion for selenium removal. Water Res. 2013, 47, 2118–2128. [Google Scholar] [CrossRef]
- Fujita, M.; Ike, M.; Kashiwa, M.; Hashimoto, R.; Soda, S. Laboratory-scale continuous reactor for soluble selenium removal using selenate-reducing bacterium, Bacillus sp. SF-1. Biotechnol. Bioeng. 2002, 80, 755–761. [Google Scholar] [CrossRef]
- Wan, H.S.; Hao, O.J.; Kim, H. Environmental factors affecting selenite reduction by a mixed culture. J. Environ. Eng.-Asce 2001, 127, 175–178. [Google Scholar] [CrossRef]
- Fujita, M.; Ike, M.; Nishimoto, S.; Takahashi, K.; Kashiwa, M. Isolation and characterization of a novel selenate-reducing bacterium, Bacillus sp. SF-1. J. Ferment. Bioeng. 1997, 83, 517–522. [Google Scholar] [CrossRef]
- Astratinei, V.; van Hullebusch, E.; Lens, P. Bioconversion of Selenate in Methanogenic Anaerobic Granular Sludge. J. Environ. Qual. 2006, 35, 1873–1883. [Google Scholar] [CrossRef]
- Lenz, M.; Smit, M.; Binder, P.; van Aelst, A.C.; Lens, P.N. Biological alkylation and colloid formation of selenium in methanogenic UASB reactors. J. Environ. Qual. 2008, 37, 1691–1700. [Google Scholar] [CrossRef]
- Kashiwa, M.; Nishimoto, S.; Takahashi, K.; Ike, M.; Fujita, M. Factors affecting soluble selenium removal by a selenate-reducing bacterium Bacillus sp. SF-1. J. Biosci. Bioeng. 2000, 89, 528–533. [Google Scholar] [CrossRef]
- Martín-Hernández, M.; Carrera, J.; Pérez, J.; Suárez-Ojeda, M.E. Enrichment of a K-strategist microbial population able to biodegrade p-nitrophenol in a sequencing batch reactor. Water Res. 2009, 43, 3871–3883. [Google Scholar] [CrossRef] [PubMed]
- Dytczak, M.A.; Londry, K.L.; Oleszkiewicz, J.A. Activated sludge operational regime has significant impact on the type of nitrifying community and its nitrification rates. Water Res. 2008, 42, 2320–2328. [Google Scholar] [CrossRef] [PubMed]
- Nozawa-Inoue, M.; Scow, K.M.; Rolston, D.E. Reduction of perchlorate and nitrate by microbial communities in vadose soil. Appl. Environ. Microbiol. 2005, 71, 3928–3934. [Google Scholar] [CrossRef] [PubMed]

| Division | Initial Concentration of Target Contaminants | C: N (CH3COO−-C: NO3-N) | |||
|---|---|---|---|---|---|
| Selenate (mM SeO42−) | Nitrate (mM NO3−) | Perchlorate (mM ClO4−) | Acetate (mM CH3COO−) | ||
| Phase 0 a | 0.35 | 0.00 | 0.0 | 3.4 | N.A. |
| Phase 1 | 0.1 | 1.0 | 0.0 | 3.4 | 6.7: 1 |
| Phase 2 | 1.0 | 1.0 | 0.0 | 5.1 | 11.1: 1 |
| Phase 3 | 0.1 | 1.0 | 0.0 | 0.6 | 1.2: 1 |
| Phase 4 | 1.0 | 1.0 | 0.0 | 0.9 | 2.3: 1 |
| Phase 5 | 0.1 | 1.0 | 0.2 | 3.4 | 5.8: 1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.-W.; Hong, S.H.; Choi, H. Effect of Nitrate and Perchlorate on Selenate Reduction in a Sequencing Batch Reactor. Processes 2020, 8, 344. https://doi.org/10.3390/pr8030344
Kim H-W, Hong SH, Choi H. Effect of Nitrate and Perchlorate on Selenate Reduction in a Sequencing Batch Reactor. Processes. 2020; 8(3):344. https://doi.org/10.3390/pr8030344
Chicago/Turabian StyleKim, Hyun-Woo, Seong Hwan Hong, and Hyeoksun Choi. 2020. "Effect of Nitrate and Perchlorate on Selenate Reduction in a Sequencing Batch Reactor" Processes 8, no. 3: 344. https://doi.org/10.3390/pr8030344
APA StyleKim, H.-W., Hong, S. H., & Choi, H. (2020). Effect of Nitrate and Perchlorate on Selenate Reduction in a Sequencing Batch Reactor. Processes, 8(3), 344. https://doi.org/10.3390/pr8030344





