Numerical Investigation of Centrifugal Blood Pump Cavitation Characteristics with Variable Speed
Abstract
1. Introduction
2. Methods
2.1. Pump Geometry
2.2. Mesh
2.3. Numerical Simulation Methods
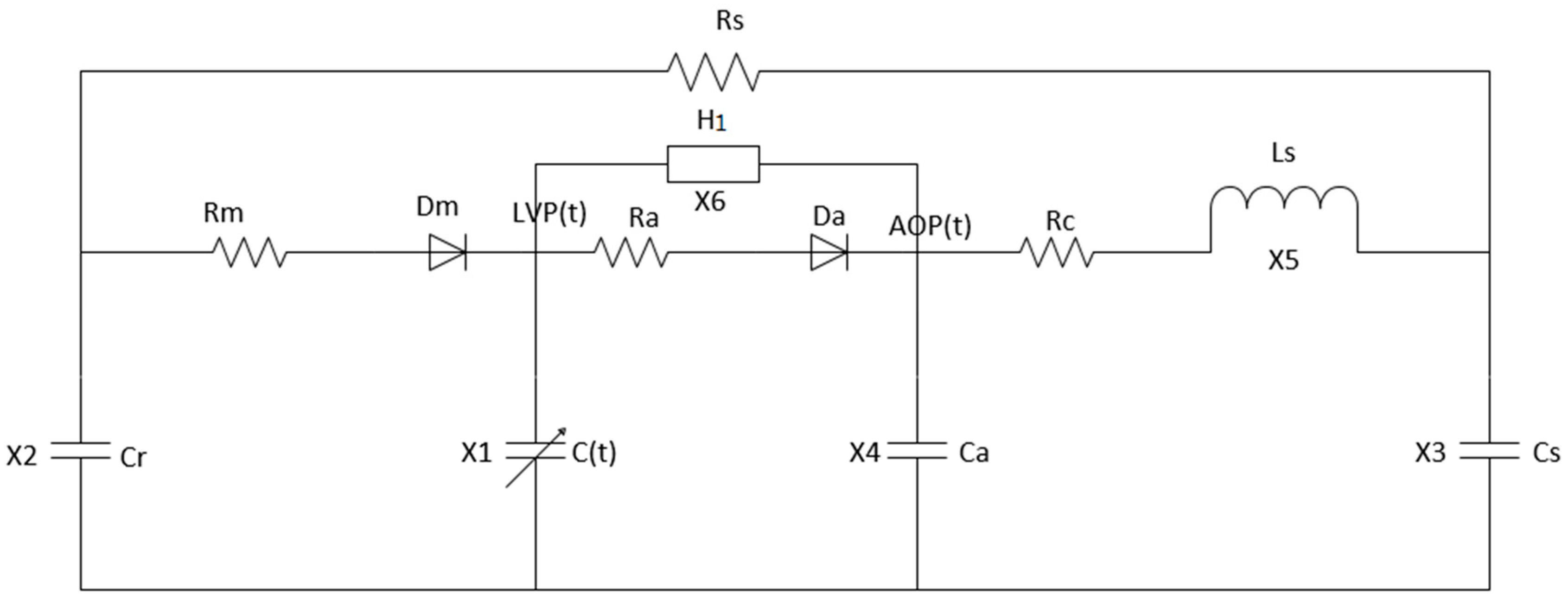
3. Centrifugal Blood Pump—Left Heart Circulation System Coupling Model
3.1. Centrifugal Blood Pump Model
3.2. Centrifugal Blood Pump–Left Heart Blood Circulation System
4. Numerical Simulation Results
4.1. Numerical Simulation of a Centrifugal Blood Pump without Cavitation
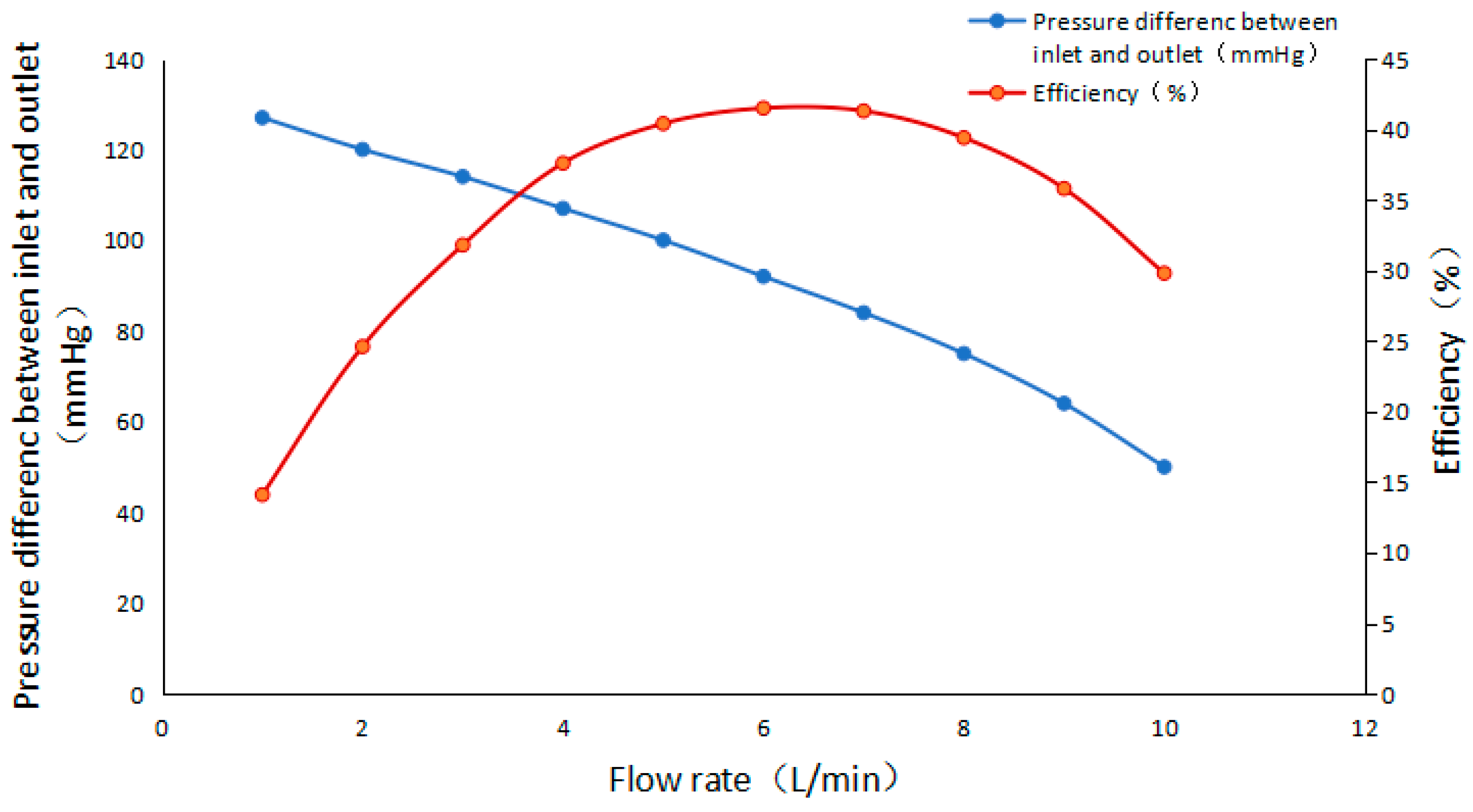
4.1.1. Performance Characteristics
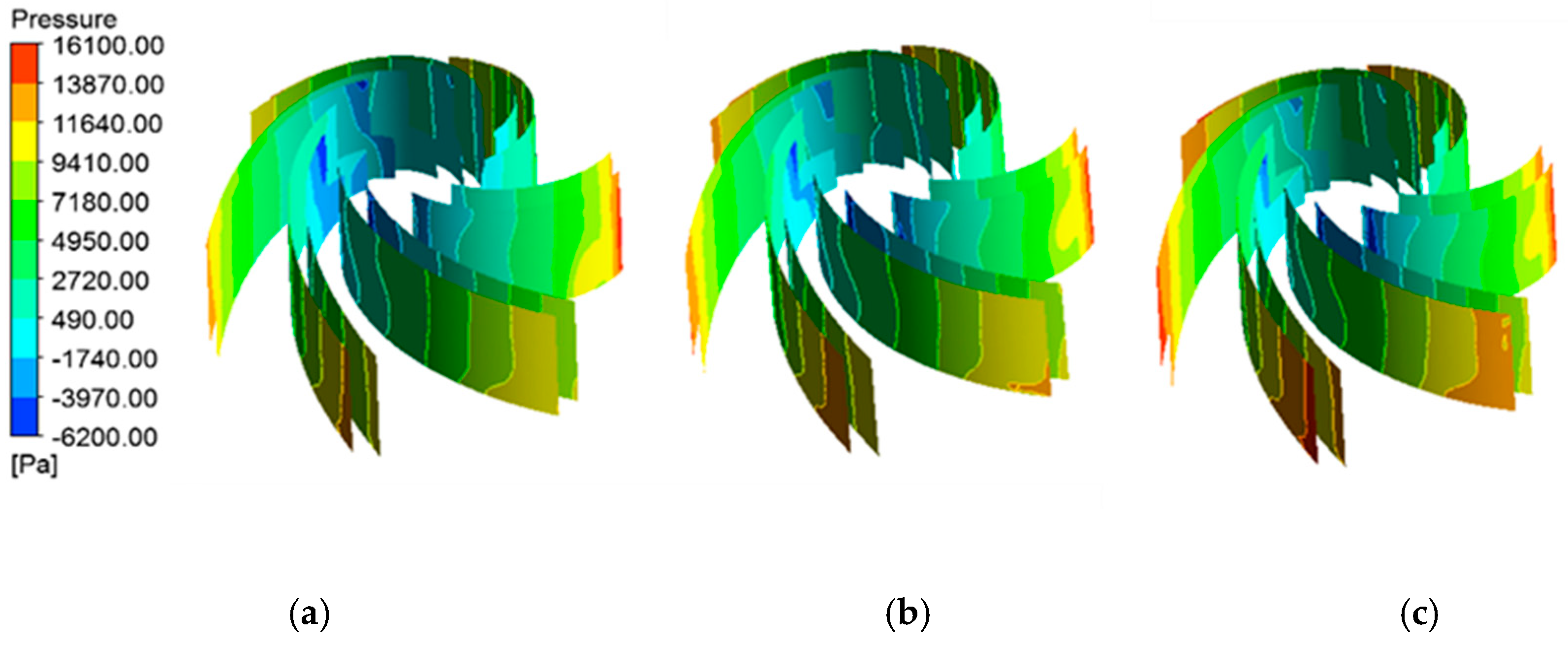
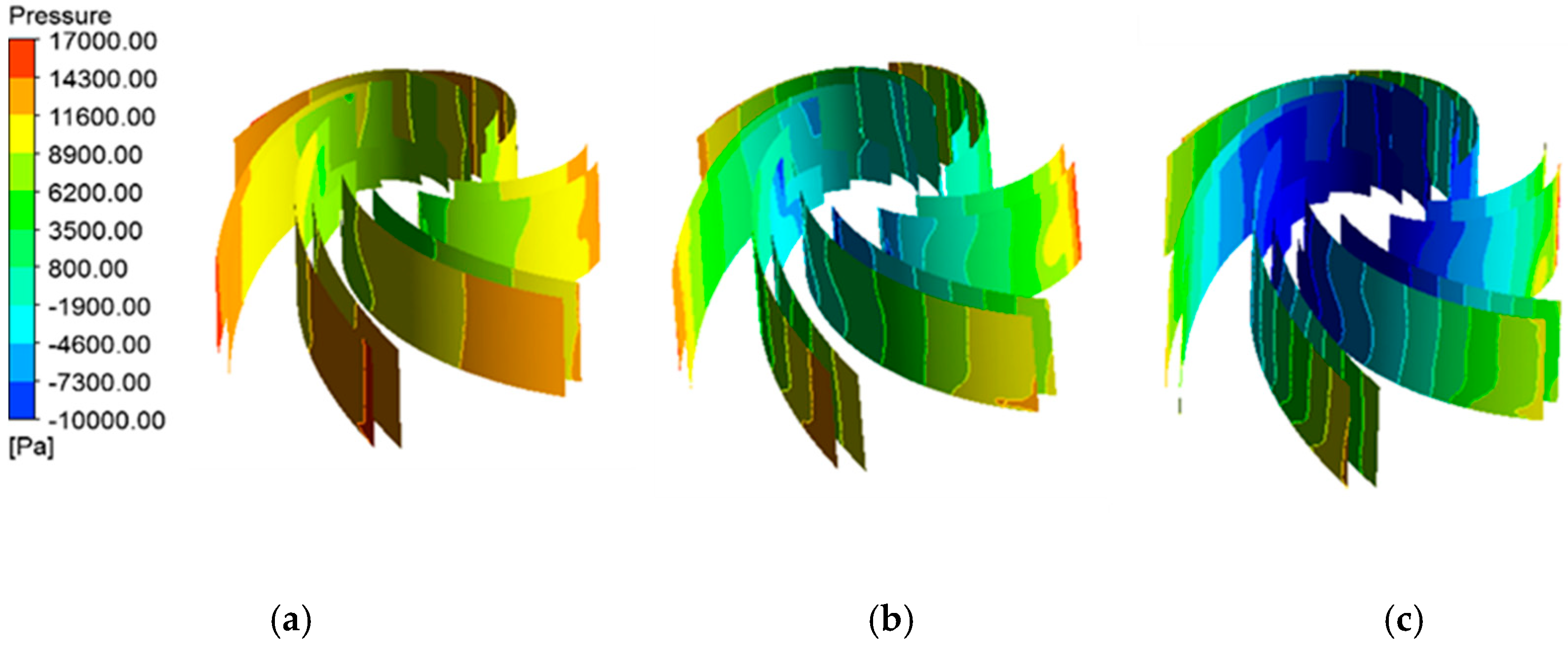
4.1.2. Pressure Distribution of Blade Surfaces
4.2. Numerical Simulation of Centrifugal Blood Pump with Cavitation
4.2.1. Prediction of Cavitation Performance
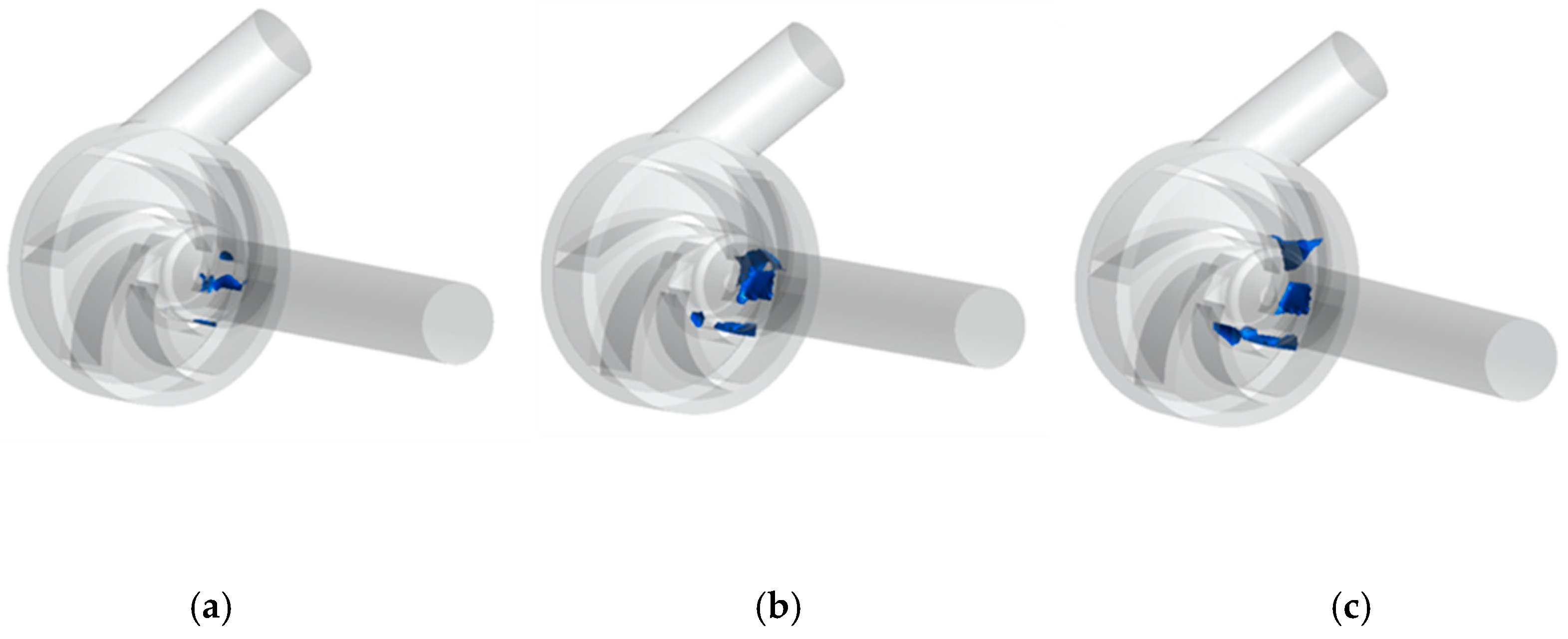
4.2.2. Cavitation Distribution
5. Cavitation Characteristics Analysis Under Variable Speed
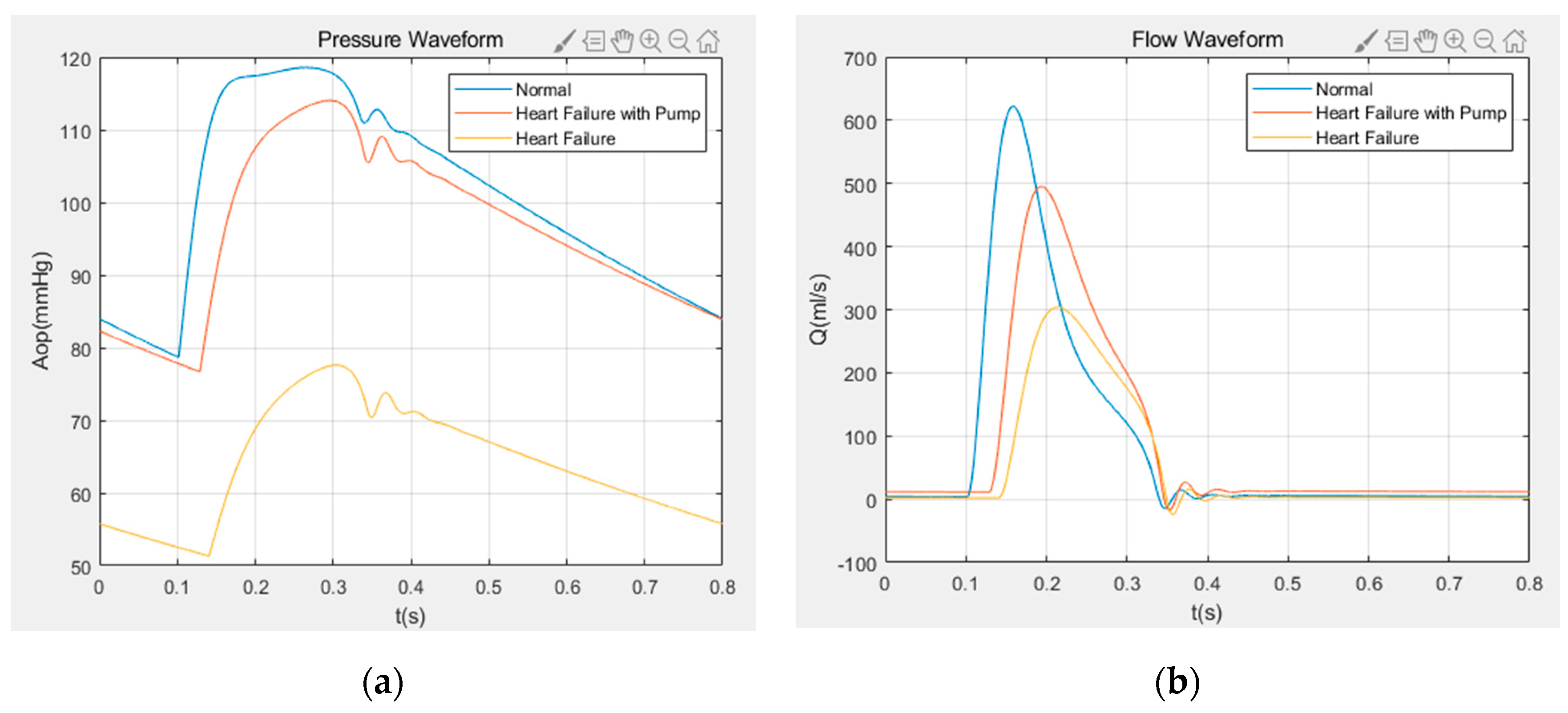
5.1. Numerical Results of Variable Speed Assist
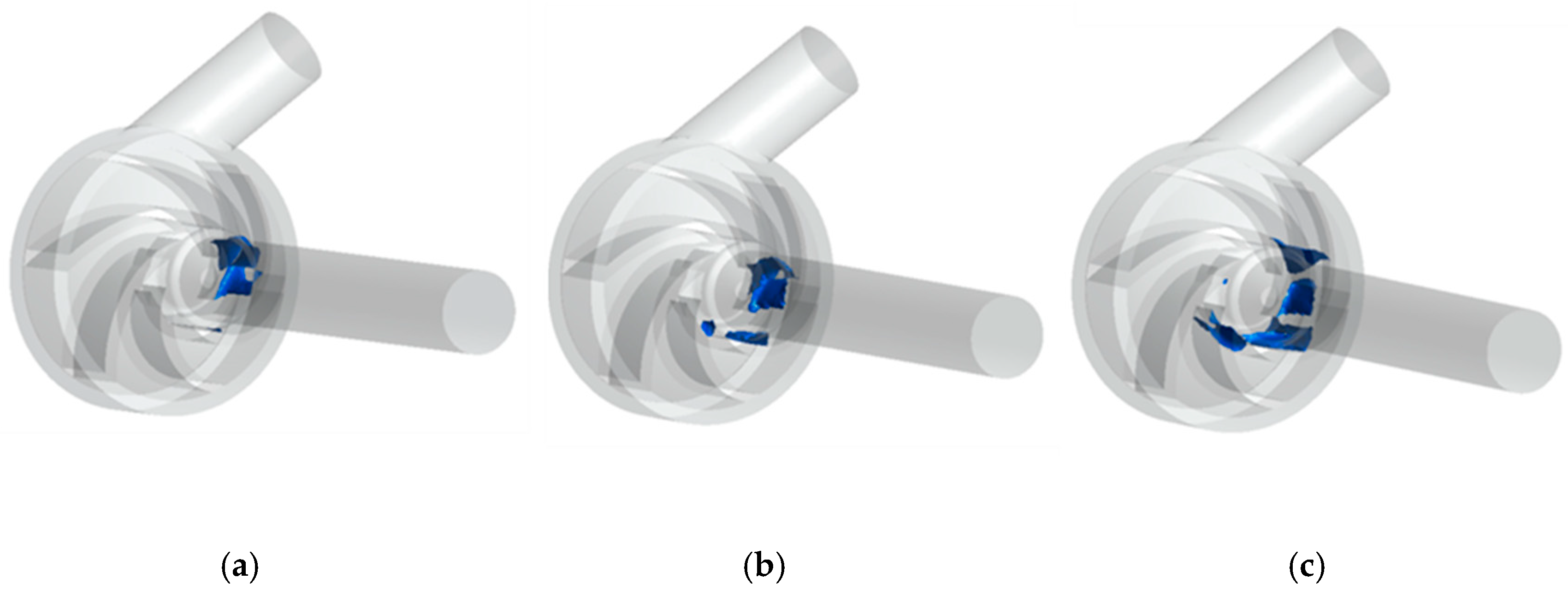
5.2. Cavitation Characteristics under Variable Speed
6. Conclusions
- (1).
- As the inlet pressure decreases, the cavitation in the impeller flow channel of the centrifugal blood pump appears near the hub of the impeller flow channel. Then, as the inlet pressure decreases further, the degree of cavitation increases gradually and the bubbles develop towards the impeller outlet.
- (2).
- With the increase of impeller speed, the cavitation degree in the passage of the centrifugal blood pump increases accordingly, and the bubbles produced by cavitation distribute asymmetrically in the impeller.
- (3).
- The obtained hemodynamic results show that the aortic flow rate and the aortic pressure pulsatility, obtained by variable speed assist in the case of heart failure, are significantly improved.
- (4).
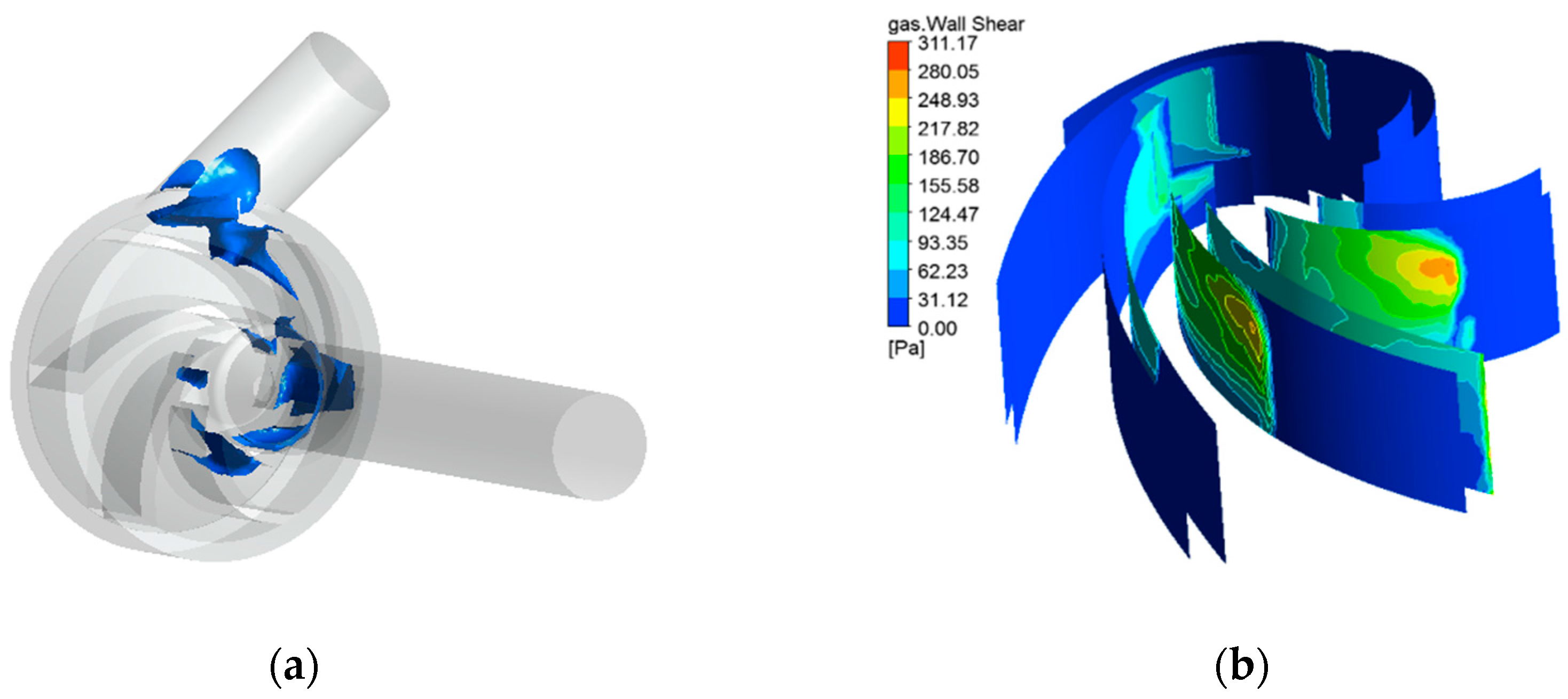
- In a cardiac cycle assisted by the variable speed of the centrifugal blood pump, cavitation is most likely to occur during ejection and most unlikely to occur during isovolumetric contraction. Cavitation not only affects the working performance of the pump but also generates large shear stress, which increases the chances of hemolysis.
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| Qx | flow rate of the blood pump (L/min) |
| constant | |
| mitral valve resistance | |
| aortic resistance | |
| left atrial compliance | |
| aortic compliance | |
| aortic blood inertia | |
| aortic valve | |
| left atrial pressure (mmHg) | |
| aortic pressure (mmHg) | |
| pump flow (mL/s) | |
| torque (N·m) | |
| inlet pressure (Pa) | |
| inlet flow velocity (m/s) | |
| inlet height of the pump (m) | |
| liquid density (kg/) | |
| available net positive suction head (m) | |
| critical net positive suction head (m) | |
| saturated vapor pressure of the liquid (mmHg) | |
| Hb | hemoglobin concentration (g/L) |
| τ | shear stress on the red blood cells (Pa) |
| pressure difference between the inlet and outlet of the blood pump (Pa) | |
| ω | speed (r/min) |
| Ra | aortic valve resistance |
| systemic vascular resistance | |
| left ventricular compliance | |
| peripheral vascular compliance | |
| mitral valve | |
| left ventricular pressure (mmHg) | |
| arterial pressure (mmHg) | |
| aortic flow (mL/s) | |
| efficiency (%) | |
| angular velocity (rad/s) | |
| outlet pressure (Pa) | |
| outlet flow velocity (m/s) | |
| outlet height of the pump (m) | |
| g | acceleration of gravity () |
| required net positive suction head(m) | |
| constant | |
| centrifugal blood pump | |
| ∆Hb | increased plasma free hemoglobin concentration (g/L) |
| exposure time (s) |
References
- Simaan, M.A.; Ferreira, A.; Chen, S.; Antaki, J.F.; Galati, D.G. A Dynamical State Space Representation and Performance Analysis of a Feedback-Controlled Rotary Left Ventricular Assist Device. IEEE Trans. Control Syst. Technol. 2009, 17, 15–28. [Google Scholar] [CrossRef]
- Bai, L.; Zhou, L.; Jiang, X.; Pang, Q.; Ye, D. Vibration in a multistage centrifugal pump under varied conditions. Shock Vib. 2019, 2057031. [Google Scholar] [CrossRef]
- Wu, Y.; Allaire, P.; Tao, G.; Wood, H.; Olsen, D.; Tribble, C. An Advanced Physiological Controller Design for a Left Ventricular Assist Device to Prevent Left Ventricular Collapse. Artif. Organs 2003, 27, 926–930. [Google Scholar] [CrossRef]
- Konishi, H.; Antaki, J.F.; Amin, D.V.; Boston, J.R.; Kerrigan, J.P.; Mandarino, W.A.; Litwak, P.; Yamazaki, K.; Macha, M.; Butler, K.C.; et al. Controller for an Axial Flow Blood Pump. Artif. Organs 1996, 20, 618–620. [Google Scholar] [CrossRef]
- Brennen, C.E. Hydrodynamics of Pumps; Cambridge University Press: Cambridge, UK, 2011. [Google Scholar]
- Lokhandwalla, M.; McAteer, J.A.; Williams Jr, J.C.; Sturtevant, B. Mechanical haemolysis in shock wave lithotripsy (SWL): II. In vitro cell lysis due to shear. Phys. Med. Biol. 2001, 46, 1245–1264. [Google Scholar] [CrossRef]
- Walker, W. Cavitation in pulsatile blood pumps. Adv. Bioeng. 1974, 1, 148–150. [Google Scholar]
- Walker, W.F. Pulsatile blood pump induced cavitation. In Proceedings of the 27th Annual Conference on Engineering in Medicine and Biology, San Francisco, CA, USA, 23–27 July 1974; Volume 16, p. 53. [Google Scholar]
- Freed, D.; Walker, W.F.; Dube, C.M.; Tokuno, T. Effects of vaporous cavitation near prosthetic surfaces. Trans. Am. Soc. Artif. Intern. Organs 1981, 27, 105–109. [Google Scholar]
- Lin, Z.; Xiaodong, R.; Zou, J.; Fu, X. Experimental Study of Cavitation Phenomenon in a Centrifugal Blood Pump Induced by the Failure of Inlet Cannula. Chin. J. Mech. Eng. 2014, 27, 165–170. [Google Scholar] [CrossRef]
- Kijima, T.; Oshiyama, H.; Horiuchi, K.; Nogawa, A.; Hamasaki, H.; Amano, N.; Nojiri, C.; Fukasawa, H.; Akutsu, T. A Straight Path Centrifugal Blood Pump Concept in the Capiox Centrifugal Pump. Artif. Organs 1993, 17, 593–598. [Google Scholar] [CrossRef]
- Medvitz, R.B.; Kunz, R.F.; Boger, D.A.; Lindau, J.W.; Yocum, A.M.; Pauley, L.L. Performance Analysis of Cavitating Flow in Centrifugal Pumps Using Multiphase CFD. J. Fluids Eng. 2002, 124, 377–383. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, M.; Xu, Z.; Zhuang, X.; Li, Q.; Xu, L. Hemodynamic analysis of a centrifugal blood pump. Chin. J. Med Instrum. 2015, 39, 16–20. [Google Scholar]
- Procedure for Estimation and Reporting of Uncertainty Due to Discretization in CFD Applications. J. Fluids Eng. 2008, 130, 078001. [CrossRef]
- Barrio, R.; Blanco, E.; Parrondo, J.; González, J.; Fernández, J. The effect of impeller cutback on the fluid-dynamic pulsations and load at the blade-passing frequency in a centrifugal pump. Trans. ASME J. Fluids Eng. 2008, 130, 1–11. [Google Scholar] [CrossRef]
- Zhou, L.; Deshpande, K.; Zhang, X.; Agarwal, R. Process Simulation of Chemical Looping Combustion using ASPEN Plus for a Mixture of Biomass and Coal with Various Oxygen Carriers. Energy 2020, 195, 116955. [Google Scholar] [CrossRef]
- Untaroiu, A.; Throckmorton, A.L.; Patel, S.M.; Wood, H.G.; Allaire, P.E.; Olsen, D.B. Numerical and Experimental Analysis of an Axial Flow Left Ventricular Assist Device: The Influence of the Diffuser on Overall Pump Performance. Artif. Organs 2005, 29, 581–591. [Google Scholar] [CrossRef]
- Lee, H.; Taenaka, Y. Characteristics of Mechanical Heart Valve Cavitation in a Pneumatic Ventricular Assist Device. Artif. Organs 2008, 32, 453–460. [Google Scholar] [CrossRef]
- Graf, T.; Reul, H.; Detlefs, C.; Wilmes, R.; Rau, G. Causes and formation of cavitation in mechanical heart valves. J. Heart Valve Dis. 1994, 3, S49–S64. [Google Scholar]
- Choi, S.; Boston, J.R.; Thomas, D.; Antaki, J.F. Modeling and identification of an axial flow blood pump. In Proceedings of the 1997 American Control Conference, Albuquerque, NM, USA, 6 June 1997; pp. 3714–3715. [Google Scholar]
- Gu, K.; Chang, Y.; Gao, B.; Liu, Y.; Zhang, Z.; Wan, F. Lumped Parameter Model for Heart Failure with Novel Regulating Mechanisms of Peripheral Resistance and Vascular Compliance. ASAIO J. 2012, 58, 223–231. [Google Scholar] [CrossRef]
- Deswysen, B.; Charlier, A.A.; Gevers, M. Quantitative evaluation of the systemic arterial bed by parameter estimation of a simple model. Med Biol. Eng. Comput. 1980, 18, 153–166. [Google Scholar] [CrossRef]
- He, Z.; Liu, Y.; Jing, T.; Zhang, G.; Liu, H.; Wang, H. Heart valve model with controllable closing volume. J. Drain. Irrig. Mach. Eng. 2019, 37, 1–6. [Google Scholar]
- Suga, H. Cardiac energetics: From Emax to pressure-volume area. Clin. Exp. Pharmacol. Physiol. 2003, 30, 580–585. [Google Scholar] [CrossRef]
- Yu, Y.C.; Boston, J.R.; Simaan, M.A.; Antaki, J.F. Estimation of systemic vascular bed parameters for artificial heart control. IEEE Trans. Autom. Control 1998, 43, 765–778. [Google Scholar]
- Zhou, B.; Zhang, G.; Jing, T.; Wang, H.; He, Z. Numerical simulation on hemolysis induced by two-stage axial-flow blood pump during pulsating heart failure. J. Drain. Irrig. Mach. Eng. 2018, 36, 28–34. [Google Scholar]
- Zhu, Y.; Tang, S.; Quan, L.; Jiang, W.; Zhou, L. Extraction method for signal effective component based on extreme-point symmetric mode decomposition and Kullback-Leibler divergence. J. Brazil. Soc. Mech. Sci. Eng. 2019, 41, 100. [Google Scholar] [CrossRef]
- Bachert, R.; Stoffel, B.; Dular, M. Unsteady cavitation at the tongue of the volute of a centrifugal pump. Trans. ASME J. Fluids Eng. 2010, 132, 1–6. [Google Scholar] [CrossRef]
- Hong, F.; Yuan, J.; Zhang, J.; Lu, J.; Zhang, Y. Numerical analysis of cavitating flow characteristics in residual heat removal pumps during the SBLOCA. J. Harbin Eng. Univ. 2015, 36, 297–301. [Google Scholar]
- Bai, L.; Zhou, L.; Han, C.; Zhu, Y.; Shi, W. Numerical study of pressure fluctuation and unsteady flow in a centrifugal pump. Processes 2019, 7, 354. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, L.; Zhou, L.; Li, W.; Wang, C. Transient response analysis of cantilever multistage centrifugal pump based on multi-source excitation. J. Low Freq. Noise Vib. Act. Control 2020, 1–17. [Google Scholar] [CrossRef]
- Li, W.; Li, E.; Ji, L.; Zhou, L.; Shi, W.; Zhu, Y. Mechanism and propagation characteristics of rotating stall in a mixed-flow pump. Renew. Energy 2020. [Google Scholar] [CrossRef]
- Giersiepen, M.; Wurzinger, L.J.; Opitz, R.; Reul, H. Estimation of shear stress-related blood damage in heart valve prostheses—In vitro comparison of 25 aortic valves. Int. J. Artif. Organs 1990, 13, 300. [Google Scholar] [CrossRef]
- Wang, F.; Lan, L.; Feng, Z.; Qian, K. Prediction of shear stress-related hemolysis in centrifugal blood pumps by computational fluid dynamics. Prog. Nat. Sci. 2005, 15, 951–955. [Google Scholar]

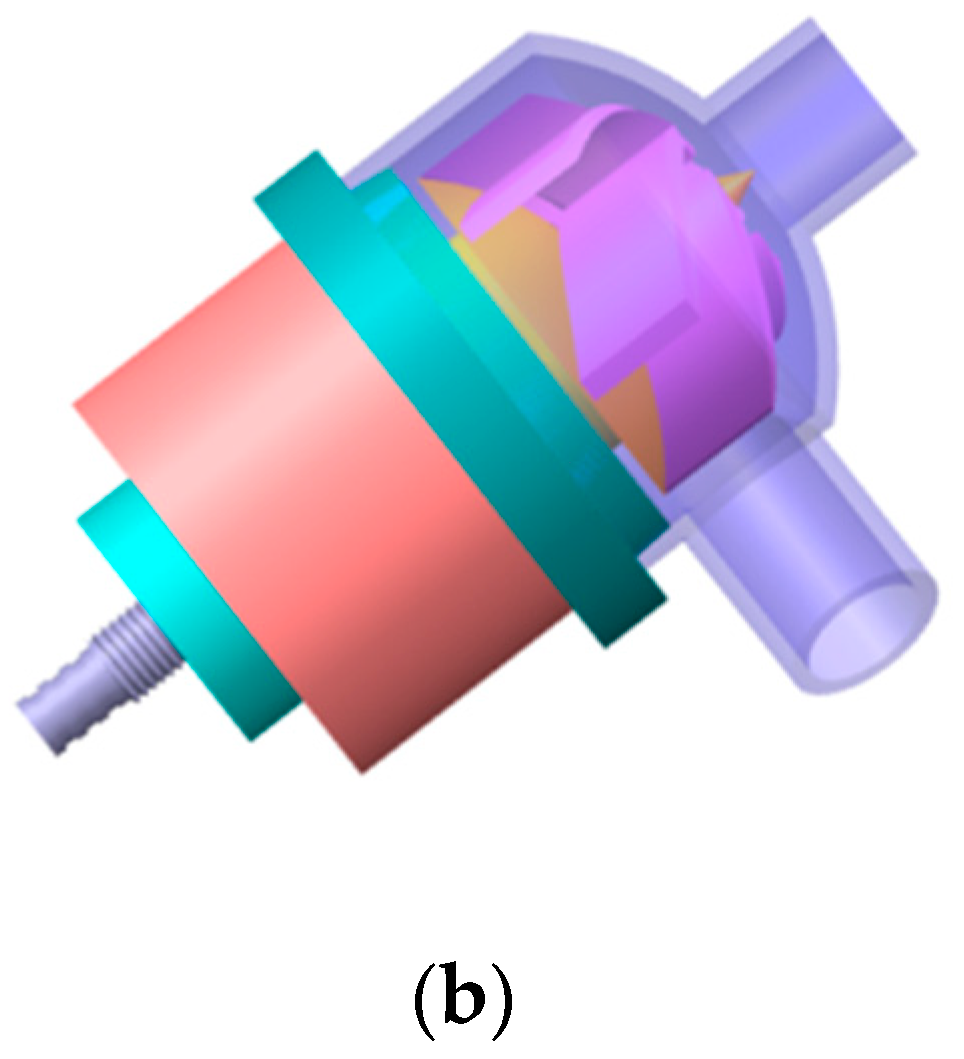
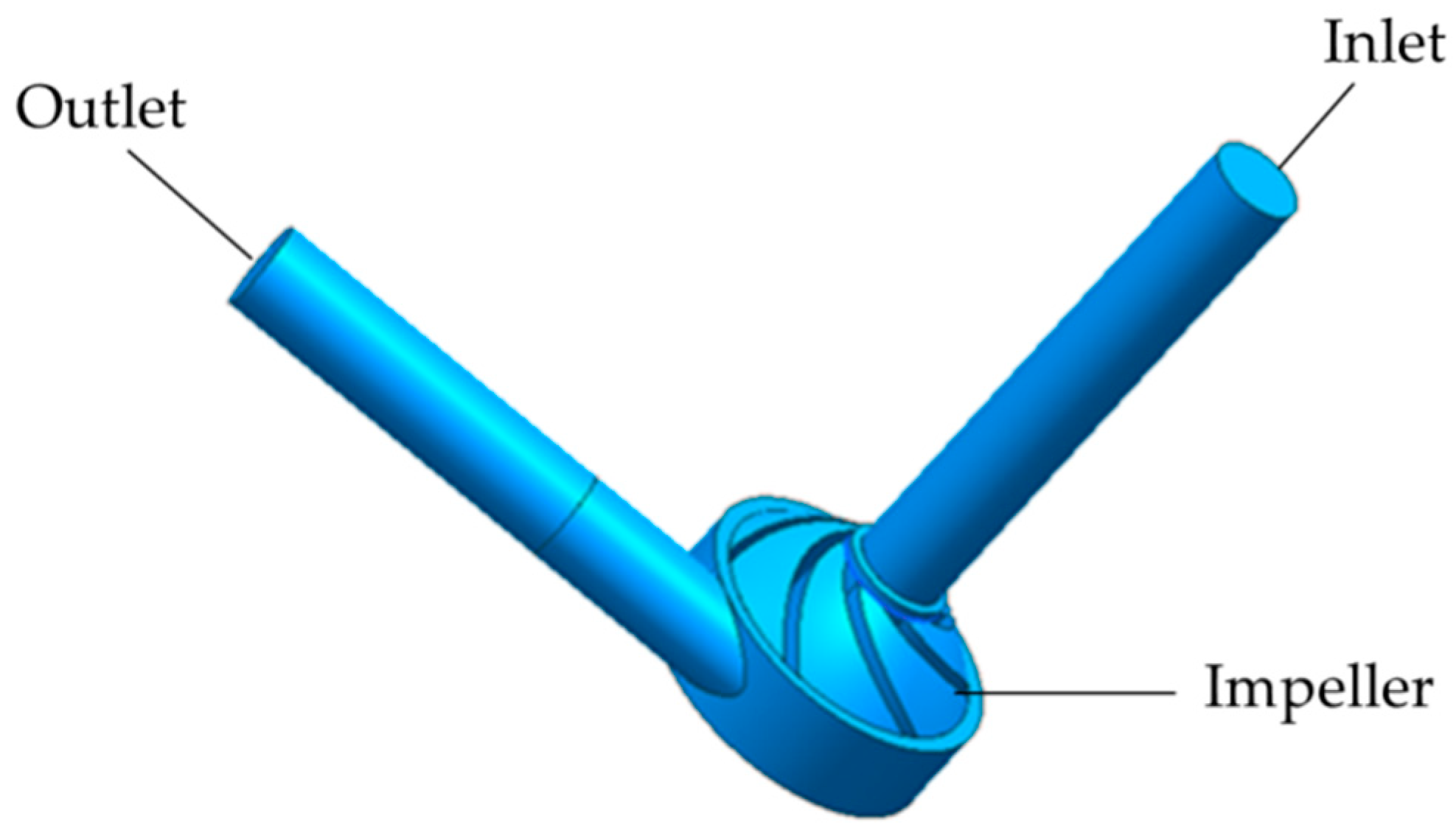
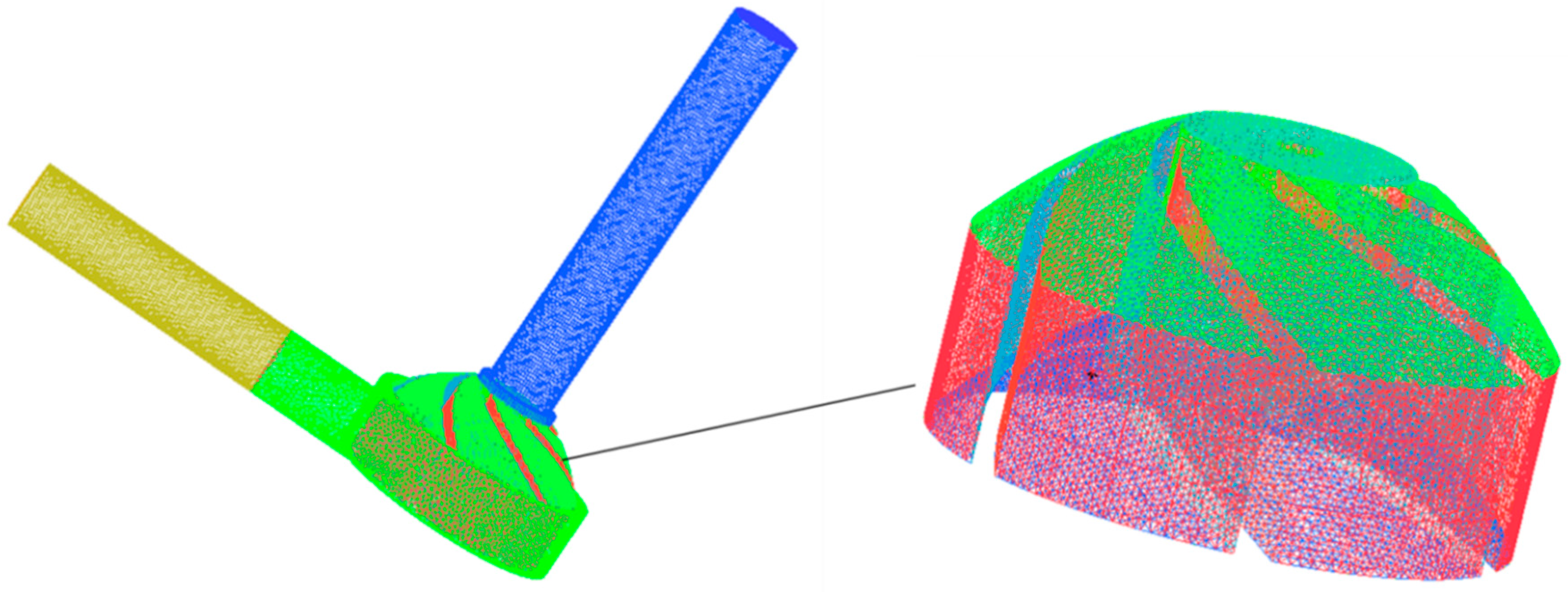
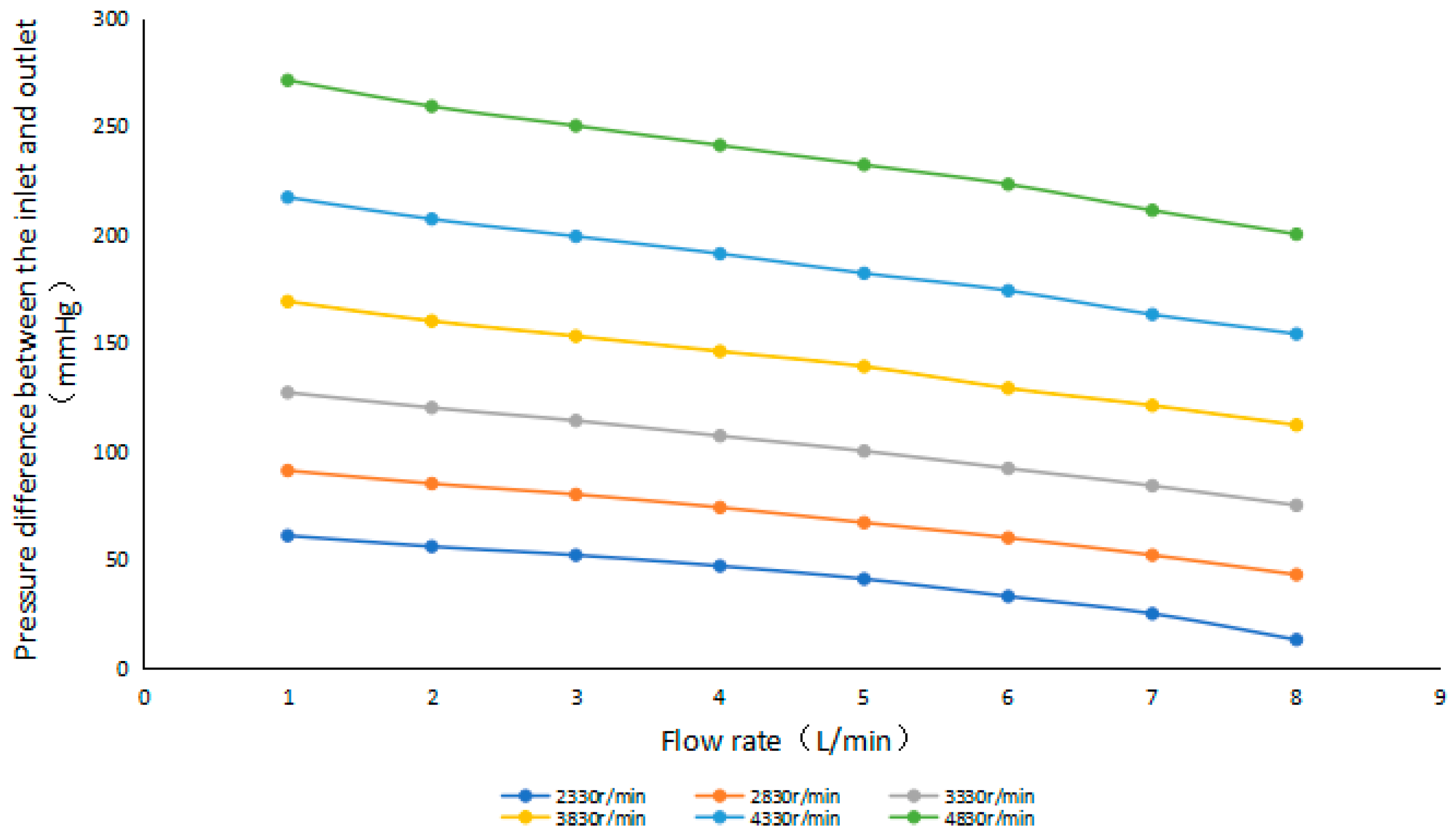



| Design Parameter | Value | Unit |
|---|---|---|
| Rated point head | 100 | mmHg |
| Rated point flow | 5 | L/min |
| Impeller speed | 3330 | r/min |
| Pump inlet diameter | 10 | mm |
| Impeller diameter | 30 | mm |
| Pump outlet diameter | 10 | mm |
| Blade number | 6 | mm |
| Blade spiral angle | 30 | ° |
| Project Name | CFX Settings |
|---|---|
| Inlet | Mass Flow |
| Outlet | Pressure |
| Reference pressure | 0 atm |
| Turbulence model | Standard k-ε |
| Wall | No-slip |
| Cavitation model | Rayleigh–Plesset |
| Near wall area | Standard Wall Function |
| Control equation | RANS |
| Parameter | Value | Physiological Meaning |
|---|---|---|
| Rm | 0.0050 mmHg·s/mL | Mitral valve resistance |
| Ra | 0.0010 mmHg·s/mL | Aortic valve resistance |
| Rc | 0.0398 mmHg·s/mL | Aortic resistance |
| Rs | 1.0000 mmHgs/mL | Systemic vascular resistance |
| Cr | 4.4000 mL/mmHg | Left atrial compliance |
| C (t) | Time-varying | Left ventricular compliance |
| Ca | 0.0800 mL/mmHg | Aortic compliance |
| Cs | 1.3300 mL/mmHg | Peripheral vascular compliance |
| Ls | 0.0005 mmHg·/mL | Aortic blood inertia |
| Dm | Mitral Valve | |
| Da | Aortic Valve | |
| Centrifugal blood pump |
| Variable | Name | Physiological Meaning | Unit |
|---|---|---|---|
| x1(t) | LVP (t) | Left ventricular pressure | mmHg |
| x2(t) | LAP (t) | Left atrial pressure | mmHg |
| x3(t) | AP (t) | Arterial pressure | mmHg |
| x4(t) | AoP (t) | Aortic pressure | mmHg |
| x5(t) | Qt (t) | Aortic flow | mL/s |
| x6(t) | Q (t) | Pump flow | mL/s |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jing, T.; Cheng, Y.; Wang, F.; Bao, W.; Zhou, L. Numerical Investigation of Centrifugal Blood Pump Cavitation Characteristics with Variable Speed. Processes 2020, 8, 293. https://doi.org/10.3390/pr8030293
Jing T, Cheng Y, Wang F, Bao W, Zhou L. Numerical Investigation of Centrifugal Blood Pump Cavitation Characteristics with Variable Speed. Processes. 2020; 8(3):293. https://doi.org/10.3390/pr8030293
Chicago/Turabian StyleJing, Teng, Yujiao Cheng, Fangqun Wang, Wei Bao, and Ling Zhou. 2020. "Numerical Investigation of Centrifugal Blood Pump Cavitation Characteristics with Variable Speed" Processes 8, no. 3: 293. https://doi.org/10.3390/pr8030293
APA StyleJing, T., Cheng, Y., Wang, F., Bao, W., & Zhou, L. (2020). Numerical Investigation of Centrifugal Blood Pump Cavitation Characteristics with Variable Speed. Processes, 8(3), 293. https://doi.org/10.3390/pr8030293






