Abstract
Pinene is a secondary plant metabolite that has functional properties as a flavor additive as well as potential cognitive health benefits. Although pinene is present in low concentrations in several plants, it is possible to engineer microorganisms to produce pinene. However, feedstock cost is currently limiting the industrial scale-up of microbial pinene production. One potential solution is to leverage waste streams such as whey permeate as an alternative to expensive feedstocks. Whey permeate is a sterile-filtered dairy effluent that contains 4.5% weight/weight lactose, and it must be processed or disposed of due its high biochemical oxygen demand, often at significant cost to the producer. Approximately 180 million m3 of whey is produced annually in the U.S., and only half of this quantity receives additional processing for the recovery of lactose. Given that organisms such as recombinant Escherichia coli grow on untreated whey permeate, there is an opportunity for dairy producers to microbially produce pinene and reduce the biological oxygen demand of whey permeate via microbial lactose consumption. The process would convert a waste stream into a valuable coproduct. This review examines the current approaches for microbial pinene production, and the suitability of whey permeate as a medium for microbial pinene production.
1. Introduction
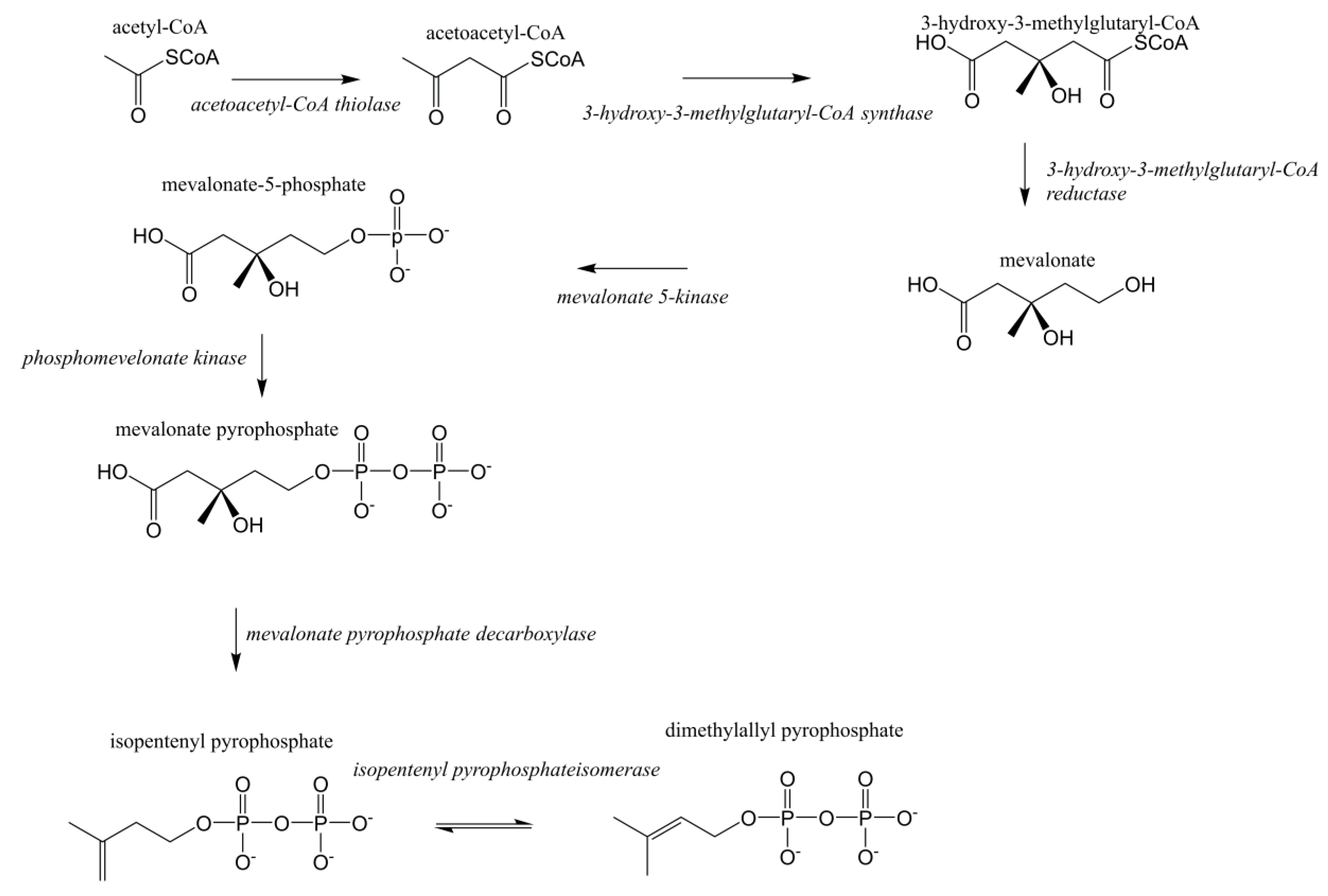
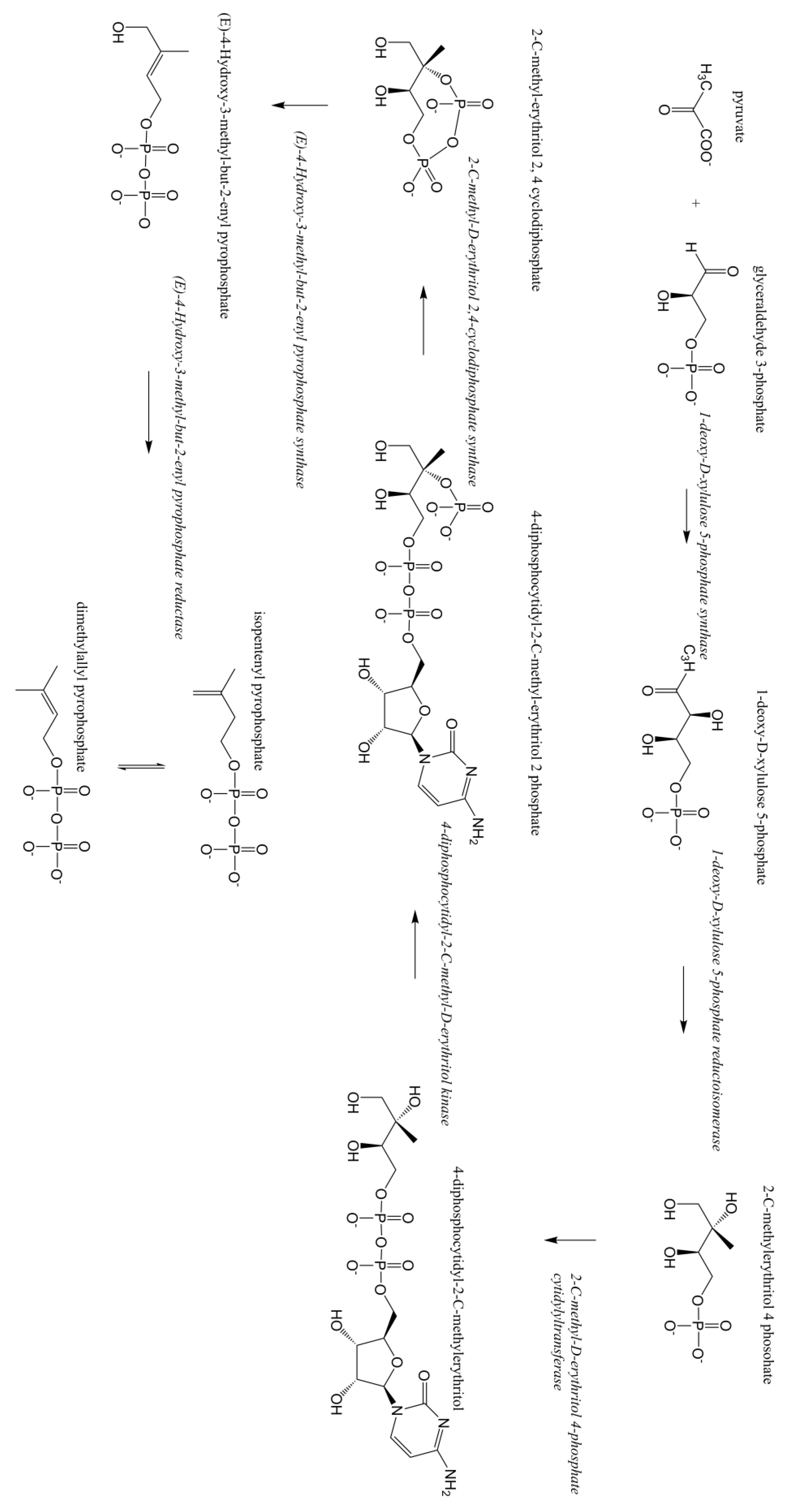
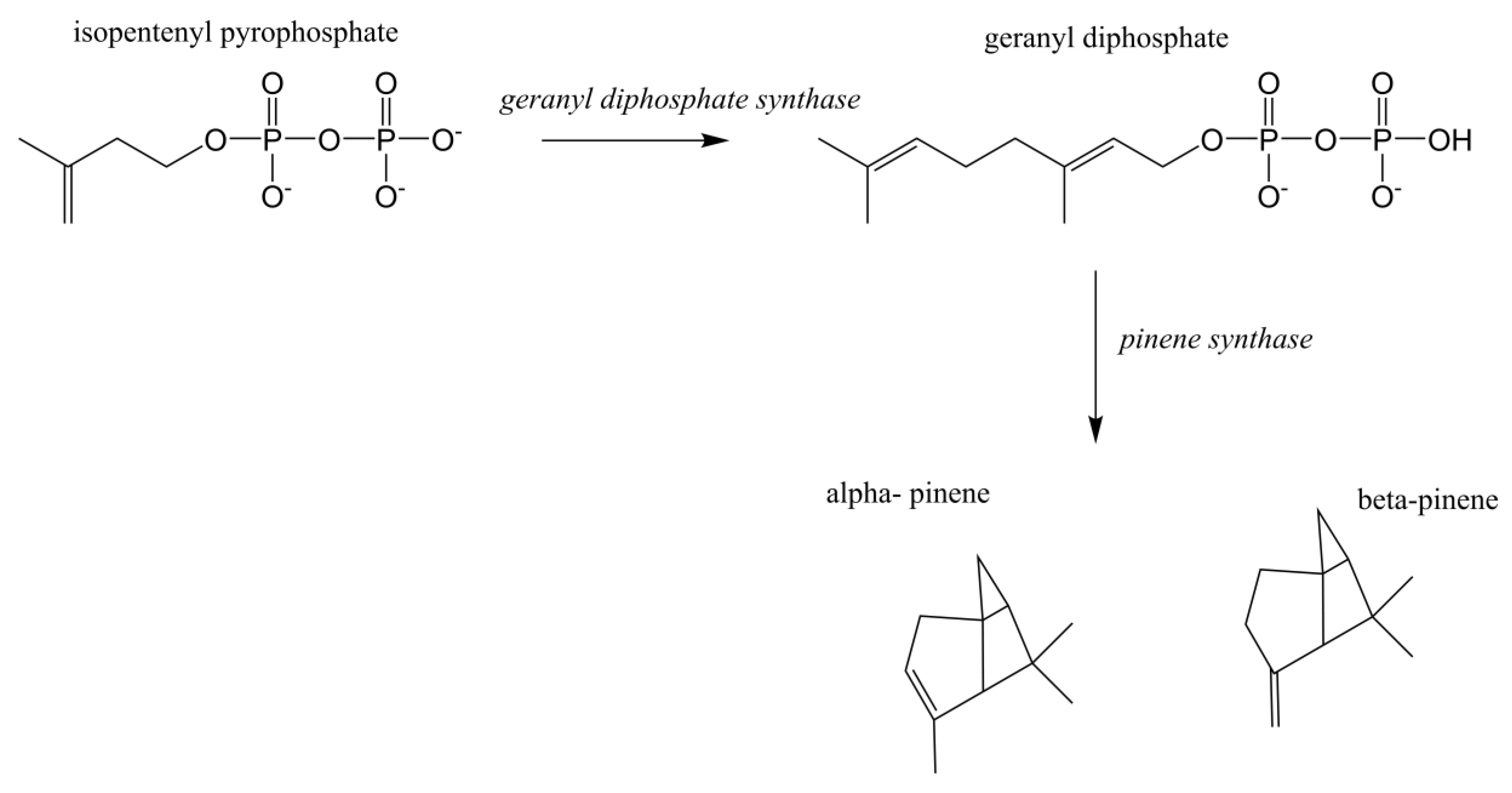
Terpenoids/isoprenoids are multifunctional organic compounds that contribute to an array of applications and are currently used as solvents, fragrances, natural pesticides, lubricants, flavoring agents, and in nutraceutical/medical applications [1,2,3,4]. Terpenoids/isoprenoids are hydrocarbons comprised of five carbon isoprene units and are classified by their number of carbon atoms, namely hemiterpenoids (C5), monoterpenoids (C10), sesquiterpenoids (C15), diterpenoids (C20), triterpenoids (C30) tetraterpenoids (C40) and polyterpenoids (C45+). Terpenoid precursors are produced in most organisms via the mevalonate (MVA) (Figure 1) and/or the 2-C-methyl-D-erythrithol 4-phosphate/1-deoxy-D-xylose 5 phosphate (MEP/DOXP) pathway (Figure 2) and are synthesized via terpenoid synthases [5]. Pinene is synthesized from isopentenyl pyrophosphate produced from the MVA or MEP/DOXP pathway via geranyl diphosphate synthase and pinene synthase (Figure 3).
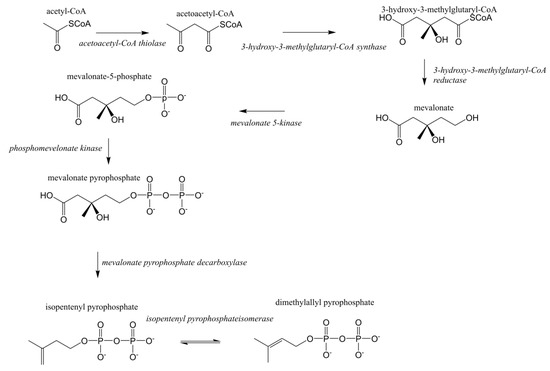
Figure 1.
Mevalonate (MVA) pathway for eukaryotes.
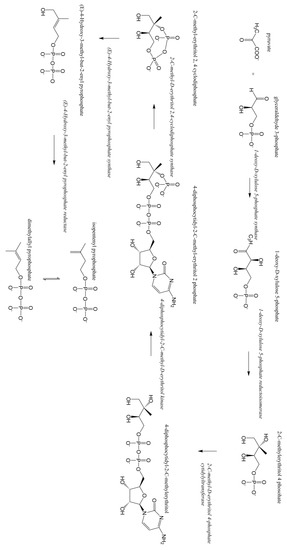
Figure 2.
Methylerythritol phosphate (MEP/DOX) pathway.
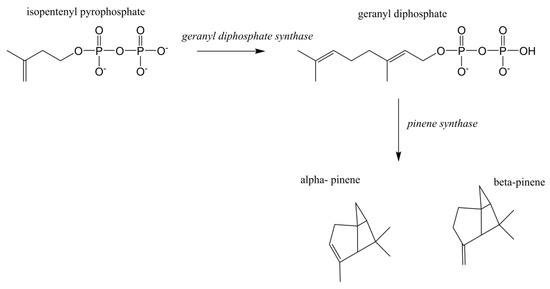
Figure 3.
Pinene biosynthesis from isopentenyl pyrophosphate.
Pinene is a monoterpene with two structural isomers, α-pinene and β-pinene (IUPAC names: (2,6,6-trimethylbicyclo[3.1.1]hept-2-ene and 6,6-dimethyl-2-methylenebicyclo[3.1.1]heptane, respectively). Both α-pinene and β-pinene have multiple commercial applications. They are commonly used as flavor compounds to produce herbal or earthy flavors in food [6]. Additional potential commercial applications include uses as a antimicrobial agent, potential use as a jet fuel alternative and functionality as a starting compound for the synthesis of other terpenoids [3,7,8]. This review will examine the potential industrial applications of pinene, the current approaches for microbial pinene production, and the suitability of whey permeate as a medium for microbial pinene production.
2. Applications of Pinene
Pinene is a compound with several current and potential applications in medicine, as a flavor/aroma agent, and as a precursor for fuel production. α- and β-Pinene have several potential applications in medicine. Firstly, it exhibited antimicrobial properties and increased the effectiveness of commercial antibiotics against methicillin resistant Staphylococcus aureus [7]. α-pinene also reversibly inhibited acetylcholinesterase, and therefore has potential to treat neurodegenerative diseases such as Alzheimer’s disease and Parkinson’s disease [1,9]. Additionally, α-Pinene reduced the impact of scopolamine-induced cognitive impairment in mice and 6-hydroxydopamine-induced Parkinson’s disease in rats [9,10]. Pinene itself is used as flavoring agent and in cosmetics, but is also used to derive the additional aromatic compounds, verbenone and carvone, which are used in perfumery and as insect repellents [8,11,12]. Collectively, this evidence indicates that pinene has flavoring applications, potential medical value, or could be used as an ingredient in nutraceutical products.
Dimerized pinene also has a volumetric energy similar to that of commercial jet fuel (JP-10) [3]. Although it is not applied commercially as a fuel at this time, increased availability of dimerized pinene could assist airlines in shifting from reliance on fossil fuels towards biofuels. For dimerized pinene to be an economically viable fuel, a sustainable production method must be developed. The combined annual production volume of α and β-pinene is approximately 49 million liters [13]. This is several orders of magnitude less than what would be needed to make a tangible contribution to the jet fuel market, considering that U.S. airline carriers consumed 67 billion liters of jet fuel in 2016 [14].
3. Plant Pinene Biosynthesis and Purification
Pinene is a secondary plant metabolite produced in a wide variety of aromatic plants in small concentrations, however it is produced industrially during the paper making process [15]. Plant-derived isopentenyl diphosphate (IPP) and dimethylallyl diphosphate (DMAPP) are the precursors of pinene and other terpenoids [16]. Geranyl diphosphate (GPP), a monoterpenoid precursor, is formed through the condensation of IPP and DMAPP, which is catalyzed by a terpene synthase (GPPS). GPP is cyclized by pinene synthase (PS) to form α- or β-pinene (Figure 3) [16]. IPP and DMAPP are biosynthesized in plants via the MVA pathway or the MEP pathway [5]. The MVA pathway is initiated by the condensation of two acetyl coenzyme A (acetyl CoA) molecules (Figure 1). The MEP pathway is initiated by the condensation of glyceraldehyde 3-phosohate and pyruvate (Figure 2). Glyceraldehyde 3-phosohate, pyruvate and acetyl CoA are inputs into several metabolic pathways (glycolysis, tricarboxylic acid cycle, gluconeogenesis and the pentose phosphate pathway), thus the MVA and MEP pathways are heavily regulated [5]. Plants can utilize both pathways for terpenoid production; however, pinene and other terpenoids are produced in relatively small quantities in plants from these pathways due to metabolic regulation [3,5].
Currently, in the United States, pinene is produced as a byproduct from the wood pulp production process known as kraft pulping [6,17,18]. Turpentine is collected during the heated digestion process for wood pulp production and pinene can be extracted from turpentine via fractional vacuum distillation [19]. One ton of fresh pine wood yields between 6–18 L of turpentine [20]. Turpentine is composed of a variety of terpenoids and its concentration is variable. α-Pinene is the primary constituent (75%–85%) with variable amounts of β-pinene (up to 3%), camphene (4%–15%), and limonene (5%–15%) [21]. Pinene can also be found in low concentrations in variety of other plants including rosemary (Rosmarinus officinalis), sage (Salvia officinalis) [22], wild thyme (Thymus serpyllum) and a variety of conifers (plants from the class Pinosida) [23]. Additional refining and processing are required for pinene to be used as a flavor ingredient, nutraceutical, or fuel additive.
4. Microbial Pinene Biosynthesis
With only small quantities produced in plants, there is an opportunity to produce pinene and other terpenoids microbially. A common strategy for the microbial production of pinene is the transformation of plasmids containing genes isolated from pinene producing plants [6,8,16,24].
These plasmids encode for the production of the enzymes within the pathways illustrated in Figure 1, Figure 2 and Figure 3, which enables for the production of the intermediate compounds and subsequently, pinene. The precursors to these pathways are pyruvate and acetyl-CoA, products of the glycolysis and citric acid cycle, which are commonly utilized for cellular energy production. A portion of pyruvate and acetyl-CoA produced during cellular metabolism can be potentially utilized for microbial pinene production. For example, pinene could be produced in a similar manner to the microbial production of β-farnesene, another terpenoid [25]. Currently, recombinant Saccharomyces cerevisiae is being used to produce β-farnesene in 200,000 L bioreactors for industrial production; β- farnesene is then used in variety of commercial applications [25]. The following sections discuss the pros and cons of utilizing some promising organisms for the microbial production of pinene and highlights the strategies used for microbial engineering (Table 1).

Table 1.
Pros and cons of potential recombinant microorganisms that produce pinene and their use of whey permeate as medium *.
4.1. E. coli
The MVA pathway was expressed in E. coli using genes from Enterococcus faecalis (mvaE, mvaS, mvaE) and S. cerevisiae (ERG12, ERG8, ERG19, IDI1) ([6,8,16,26]. Geranyl diphosphate synthase (GPPS) and pinene synthase (PS) were expressed using GPPS2 and Pt30 genes derived from Abies grandis and Pinus taeda, respectively [8]. GPPS catalyzes the conversion of isopentenyl pyrophosphate, also known as isopentenyl diphosphate (IPP) into geranyl diphosphate (GPP), which is the substrate for PS whose final product is pinene [16]. Using the expressed pathways described above, an accumulation of 5.44 mg/L of E. coli produced α-pinene was reported [8].
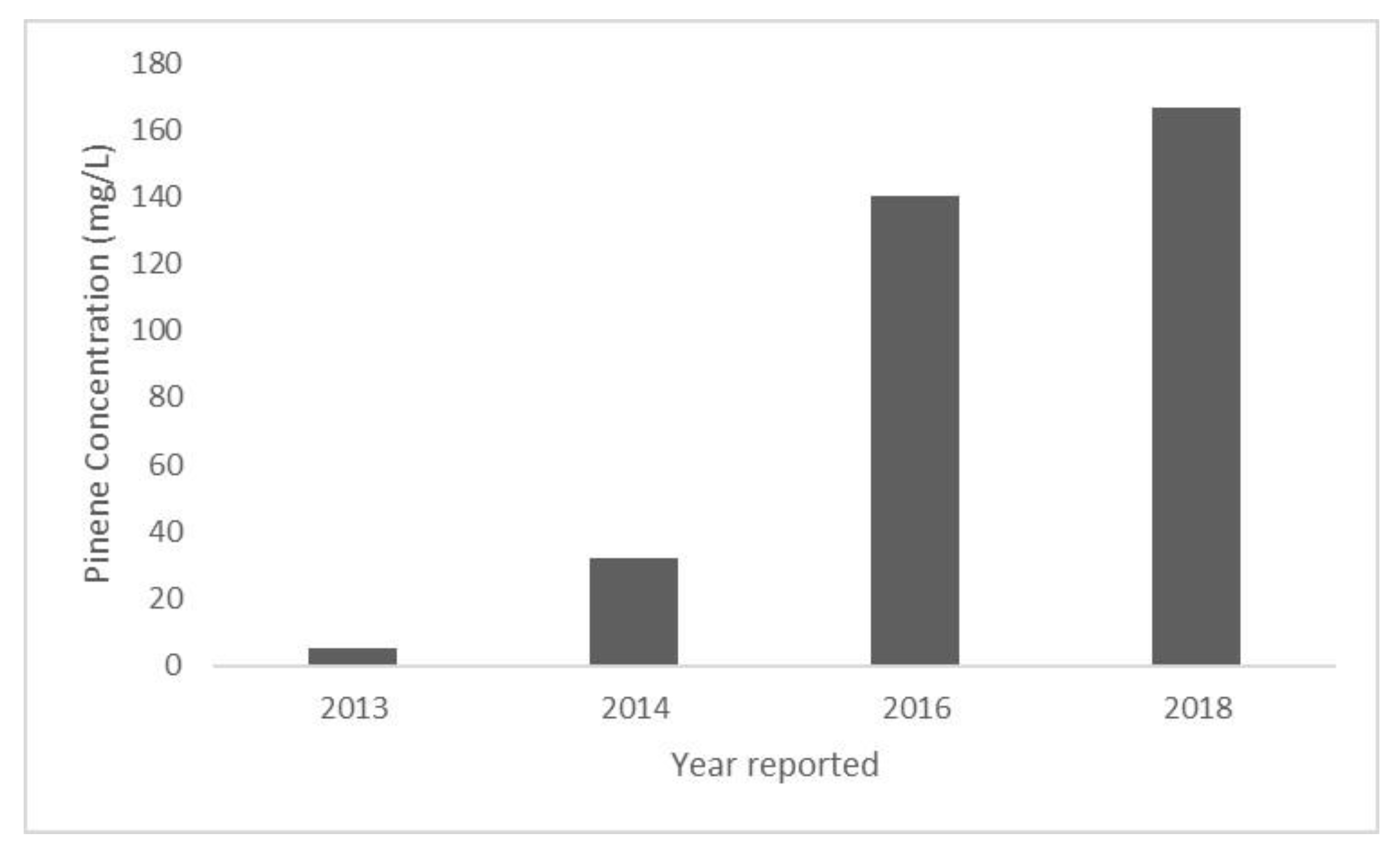
A 30-fold increase in E. coli pinene production was reported from 2013 to 2018 (Figure 4). These advancements were initiated with screening PS and GPPS from three different species of plants in a strain of E. coli harboring the MVA pathway [6]. E. coli expression of a combination of PS and GPPS from Abies grandis resulted in the production of 28 mg/L of pinene [6]. It was hypothesized that pinene production was limited by the toxicity of GPP, an intermediate in the pinene production pathway. To overcome GPP toxicity, a GPPS-PS protein fusion was produced in order to reduce GPPS inhibition [6]. That step resulted in an increased pinene yield of 32 mg/L [6].
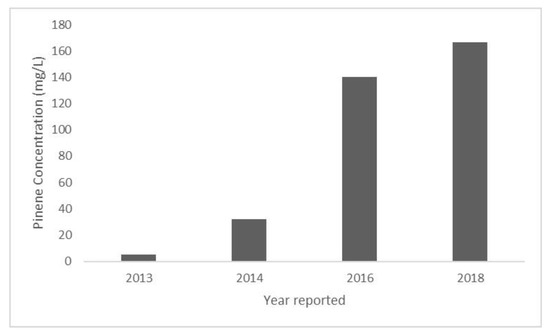
Figure 4.
Reported pinene concentration produced by genetically engineered E. coli in a single batch laboratory production system. Values obtained from the following reports: 2013—Reported in Biotechnology for Biofuels [8], 2014—Reported in ACS Synthetic Biology [6], 2016—Reported in ACS Synthetic Biology [26], 2018—Reported in Frontiers in Microbiology [16], reported standard deviation ± 0.3.
An even higher level of pinene synthesis by E. coli (140 mg/L) was subsequently reported, due to the introduction of a laboratory-evolved pinene synthase variant with a higher activity than the parent enzyme native to Pinus taeda [26]. Most recently, E. coli production of pinene at levels of 166 mg/L was shown using two recombinant E. coli strains. One strain expressed the genes for the MVA pathway and the other expressed the genes for GPPS and PS [16]. Each strain was propagated separately and then were combined into a whole cell biocatalyst that was a phosphate buffer overlaid with dodecane. The dodecane accumulated 166 mg/L of pinene after 28 h [16]. This finding indicates that the scale-up of the whole cell biocatalyst system may warrant further research for pinene production.
There are several challenges related to increasing E. coli-produced pinene titers. The heterologous enzymes introduced to E. coli for pinene production produce intermediates that cannot be utilized by the E. coli cell and this metabolic burden can cause plasmid instability [36]. Plasmid recombination can be reduced and pinene titer increased by altering the transcriptional rrnB-T1 terminators on the plasmid to strong terminators that are not homologous [36]. An E. coli strain with the genes recA, recE, recF, recI and endA removed was transformed with a plasmid encoded for pinene production with strong terminators. This E. coli strain increased pinene titer by 6.9 fold [36].
The catalytic process of mevalonic kinase and mevalonate diphosphate decarboxylase were identified as the bottlenecks of the MVA pathway in recombinant E. coli [36]. A strain of laboratory involved pinene-producing E. coli strain was whole genome sequenced, then aligned with the E. coli reference strain MG1655 genome sequence, then non-synonymous mutant genes were up and downregulated [37]. Using comparative genomics and transcriptional level analysis, it was shown that mutations and up-regulation of transcription levels of the MEP pathway genes (dxr, dxs, ispH, and ispU) and some protein membrane genes (gsiA, nlpA, sufBCDS, opgB, setC, oppF, ypjK, btuB, pitA and cusA) are related to increased pinene tolerance and production in E. coli [37]. Overexpression of genetic information processing genes (cbpA, rpoA, mutS and dusB) and cellular process genes (tabA and flgFGH) also improved pinene titer [37]. The mutation and transcriptional downregulation of cellular division modulation genes (ydiJ, yjbQ, prpR, marR, fabR and cedA) was also associated with increased pinene tolerance and production [37].
Although the use of E. coli for pinene production has many advantages, including but not limited to its genetic tractability and its current use for the production of other isoprenoids [24], a limitation with this organism is that E. coli’s outer membrane is comprised of LPS, a potential food safety concern if pinene is destined for human consumption.
4.2. S. cereviseae and K. marxianus
S. cerevisiae is another commonly used microorganism for genetic engineering, and has been successfully used to commercially produce β-farnesene, a sesquiterpene [25]. Monoterpenoid production has also been reported for genetically engineered S. cerevisiae in conjunction with other isoprenoids [29,30,38]. S. cerevisiae and Kluyveromyces marxianus that are generally recognized as safe may be better candidates for pinene production if the genetic engineering challenges can be overcome. K. marxianus can metabolize lactose; however, it has the same genetic engineering challenge as S. cerevisiae; the presence of a native competing MVA pathway.
S. cerevisiae is an attractive organism to use for monoterpenoid production due it’s genetic traceability and being GRAS. The MVA pathway is native to S. cerevisiae, which initially seems promising; however, it is highly regulated and critical for cell survival. Monoterpene synthesis is more difficult to engineer into recombinant S. cerevisiae due to the presence of the farnesyl pyrophosphate synthase (Erg20p), an enzyme which competes for GPP, the precursor to pinene and other monoterpenoids [30,39]. Farnesyl pyrophosphate is an intermediate in the ergosterol production pathway and ergosterol plays a critical role in S. cerevisiae cell membrane fluidity, permeability, and structure [40]. Deletion of ERG20, the gene responsible for Erg20p production, is lethal [30]. It has been reported that direct downregulation of ERG20 did not increase monoterpene production, and instead may have decreased production [40]. Evidence also indicates that Erg20p has greater activity for GPP than PS, another factor which could limit pinene production [41,42].
Several strategies have been employed to overcome the necessity of ergosterol production and the enzymatic competition of the synthases for S. cerevisiae monoterpenoid production. N-degron protein degradation was employed to regulate Erg20p/ERG20; this yielded reported titers of 76 mg/L of limonene [39]. It was also shown that fusion of a terpene synthase with Erg20p increased S. cerevisiae monoterpene titers by 69% [41]. Another strategy is to replace the native ERG20 promoter with a heterologous promoter [42]. The native ERG20 promoter was replaced with a glucose sensing promoter (HXT1) in a geraniol producing strain of S. cerevisiae. This resulted in 897 mg/L of geraniol being produced when the organisms were fed pure ethanol. It was further reported that a titer of 1.69 g/L was achieved via gene deletion (OYE2) and engineering for complemented LEU2 auxotrophy [42]. These strategies could be employed to produce pinene using S. cerevisiae as the host organism. Further, it may be advantageous to use a microorganism for which the MVA pathway is not critical for cell survival, or a strain of S. cerevisiae with lower Erg20p activity.
4.3. Corynebacterium glutamicum and Rhodosporidium torulides
C. glutamicum is used for microbial amino acid production on a commercial scale GRAS status and, in one study, was used to produce pinene [31]. Pinene synthesis was shown using plasmid-encoded genes for deoxy-D-xylose-5-phosphate synthase, isopentenyl diphosphate isomerase, two geranyl diphosphate synthases, and pinene synthase. The native MEP pathway genes responsible for deoxy-D-xylose-5-phosphate synthase and isopentenyl diphosphate isomerase production (dxs and idi genes) were also overexpressed. The maximum pinene titer reported was 176 µg/L, a quantity that is several orders of magnitude lower than the pinene production reported for E. coli [31]. A potential solution to the lower pinene yields is the introduction of a heterologous MVA pathway into C. glutamicum. The use of a heterologous MVA pathway has led to increased pinene titers in E. coli [8,16,37]. Introduction of the MVA pathway could increase the ability of a strain of C. glutamicum to produce pinene.
Monoterpene production using a strain of carotenogenic yeast, Rhodosporidium torulides, was also reported [33]. Recombinant R. torulides harboring genes to produce nine different monoterpenes produced very little pinene according to a dodecane overlay analyzed using GC-MS; however, it was detected using solid-phase microextraction. It was determined that the synthesis of GPP, which is an intermediate in ergosterol production, was found to be a limiting factor [33]. Strategies used for S. cerevisiae to increase monoterpene production, such as using N-degron protein degradation to regulate Erg20p/ERG20 or replacement of the ERG20 promoter with a heterologous promoter, could be employed.
5. Possible Challenges for Large-Scale Synthesis of Pinene by Microorganisms
Besides increasing the quantities of pinene produced by microorganisms, several other genetic and physiological challenges must be overcome for the microbial production of pinene or other monoterpenoids to be economically feasible. Cell toxicity to pinene must be overcome for pinene production to achieve commercial viability. This might be achieved by strategies such as the serial culturing of cells with increasing pinene concentrations. Serial culturing of E. coli improved pinene tolerance and increased pinene titer 31% compared with the starting strain and this may be an applicable strategy for other microorganisms [16]. Overexpression of efflux pumps has been shown to increase tolerance to harmful secondary metabolites. The over expression of efflux pumps which remove pinene from the cell such as E. coli acrAB and Pseudomonas putida KT2440 TtgB could be employed in E. coli or other microorganisms [16]. The over expression of E. coli acrAB and Pseudomonas putida KT2440 TtgB efflux pumps increased the pinene titer from 7.3 ± 0.2 mg/L to 9.1 mg/L, a 25% increase in E. coli [16].
It will be important for the pinene-producing microorganisms to withstand the fluctuating hydrostatic pressures of industrial bioreactors [25]. Standard industrial bioreactors are reported to contain up to 200,000 L [25,43]. Thermotolerance is also important because removal of heat produced from cellular metabolism is a challenge as bioreactor size is increased [43]. Heat accumulates due to the volume of fluid being much greater than the heat transfer surface area [43]. The addition of oxygen to medium could potentially cause facultative anaerobes to respire which releases more energy (74.4 vs. 2808 kJ per C6H12O6 molecule metabolized) [44] into the system, which must subsequently be removed. The microorganisms would also ideally grow on an inexpensive and readily available medium.
Recent studies focused on the production of pinene by E. coli in a single-batch system [6,8,16,37]. Alternatively, a fed-batch system can be used. A titer of 970 mg/L of E. coli-produced pinene was reported in fed-batch fermentation conditions, whereas the same strain in the single-batch system produced 5.44 mg/L [8]. This indicates that a fed-batch system may be more commercially viable on an industrial scale. This yielded a conversion efficiency of 2.61% by mass of pinene to mass of glucose for the fed batch system [8]. The production of harmful metabolites may reduce microbial pinene production efficiency. An example of this was the production acetic acid, which is a harmful metabolic by-product of the E. coli in a single-batch system [8]. This was found to be a non-limiting factor in a fed-batch system using the same strain of E. coli in a fed-batch system maintained at 0.5 g/L of glucose [8]. These highly controlled conditions are the likely reason for the increase in pinene titer when compared with the single-batch system. These findings indicate that more research is required to understand if increases in pinene concentration in a single-batch bioreactor will translate to increased pinene production in a fed-batch bioreactor or chemostat.
Lastly, increased titers of E. coli produced pinene was reported using a laboratory-scale whole cell biocatalyst system [16,37]. This system involved growing strains independently, then combining them into an agitated bioreactor. The biocatalyst system has produced a titer 2.6-fold higher than a fermentation system (166 vs. 64 mg/L) [16]. This indicates that the scale-up of a whole cell biocatalyst system for pinene production should be explored.
The differences in titer in the fed-batch bioreactor and the single-batch system indicate that environmental factors other than genetics influence microbial pinene production yields such as pH, temperature, solute or ion concentration, etc. Pinene producing microorganisms would ideally grow on an inexpensive and readily available medium. Given that feedstock is a major expense for biomanufacturers, the use of an inexpensive feedstock could potentially impact the economic feasibility of microbial pinene production.
6. Whey as a Feedstock for Microbial Pinene Production
A recent techno-economic assessment of microbial terpenoid production indicated that 80%–95% of the costs can be attributed to the cost of the feedstock [13]. Cane syrup is often utilized as the industrial feedstock for S. cerevisiae β-farnesene synthesis [25,45], but its cost is a current limitation to commercializing the microbial production of pinene due to lower titers [13]. Thus, the identification of an alternative low-cost feedstock for microbial pinene production could significantly improve the cost-effectiveness.
Whey permeate may be an economically viable feedstock for microbial pinene production. Whey and whey permeate have been used as a microbial medium to industrially produce ethanol since 1978, indicating that it may be an economically viable microbial medium [46]. It is produced in large quantities during cheese production, undergoes a filtration process for protein recovery, and is considered a waste stream in many production facilities. The ultrafiltration and diafiltration of whey produce a sterile permeate composed of water, lactose, non-protein nitrogen and minerals. The sterile filtration of whey and the composition of the permeate indicate that it may be a viable microbial medium. Table 2 illustrates the approximate composition of whey and whey permeate [47]. For most microbes that can utilize lactose, it is hydrolyzed intracellularly into its constituents, glucose and galactose. Glucose can be utilized for cellular energy production via glycolysis and galactose can be converted to glucose-6-phosphate, an intermediate in glycolysis. The product of glycolysis is pyruvate, which can be converted to acetyl-CoA. Both of these compounds serve as the precursors to the MEP and MVA pathways, respectively. Trace amounts of oligosaccharides, peptides and (β-casein, αs1-casein, GLCM1, PIGR, SAA, κ-casein and αs2-casein) and enzymes (plasmin, cathepsin and elastase) have been identified with whey permeate [48,49].

Table 2.
Approximate composition of whey and whey permeate.
The majority of value-added processing of whey permeate currently focuses on recovering lactose. However, lactose recovery is an energetically expensive process and requires significant capital investment. Lactose recovery from whey permeate is a multi-stage process that requires an evaporator, crystallization tanks, decanter centrifuges, fluid-bed drier and hammer mill [47]. As such, lactose recovery is not a viable economic option for many creameries (especially small to medium-size creameries).
If not used for other purposes, whey permeate is a costly waste stream in the dairy industry, and in some situations can represent an economic limitation to creamery expansion. The biological oxygen demand (BOD) (approximately 30–50 g/L) of whey permeate is the main driver of the high cost of disposal. Importantly, microbial lactose consumption can lower the BOD up to 75% [50], and thus, microbial treatment of whey permeate represents the potential combined benefit of delivering value-added product while simultaneously reducing treatment and disposal costs of whey permeate.
Whey permeate is currently used as a fermentation medium for the production of single cell protein, lactic acid, vitamin B12 and ethanol. More specifically, recombinant E. coli strains have produced ethanol using untreated whey permeate as a fermentation medium (Table 1) [51]. This indicates that whey permeate may be a suitable medium for other recombinant E. coli strains or other recombinant microorganisms. In fact, multiple studies have demonstrated the ability of E. coli to metabolize the lactose within whey permeate [6,8,16,26,52]. It was reported in 2017 that an E. coli strain efficiently grew and fermented untreated whey permeate without nutritional supplementation in a pH-controlled bioreactor [51]. Recent efforts also indicate that recombinant homofermentative E. coli strains expressing the Vitreoscilla hemoglobin successfully produce ethanol using rehydrated whey powder and autoclaved cheese whey as a medium [53,54]. This indicates that recombinant E. coli used to produce pinene may be able grow on whey permeate.
S. cerevisiae, the organism traditionally used for ethanol production, cannot metabolize lactose. Native S. cerevisiae does not produce lactose permease to facilitate the transmembrane transport of lactose, nor does it produce β-galactosidase to hydrolyze lactose to D-glucose and D-galactose [55,56]. The addition of β-galactosidase to whey permeate can make whey permeate a suitable medium for S. cerevisiae [57]. Alternatively, engineered S. cerevisiae strains can metabolize lactose directly [55,56,58]. This indicates that a recombinant strain of S. cerevisiae may be utilized to produce pinene from whey permeate.
C. glutamicum lacks the necessary genes to metabolize lactose or galactose, but similar to the case with S. cerevisiae, recombinant strains of C. glutamicum have been produced that can metabolize lactose and galactose directly [59]. The inability of C. glutamicum to metabolize galactose indicates that the addition of exogenous β-galactosidase to whey permeate would not be an effective treatment for the medium. The additional metabolic engineering requirement for C. glutamicum may make it an inappropriate candidate for microbial pinene production using whey permeate.
R. torulides can metabolize glucose and galactose; however, it grows poorly using whey permeate as a medium [60]. Treatment of whey permeate with exogenous β-galactosidase would likely make the whey permeate a more suitable medium for R. torulides as it can metabolize galactose.
The production of ethanol using whey permeate as a feedstock has been accomplished on a commercial scale using K. marxianus as the fermentation organism. K. marxianus is GRAS and can metabolize lactose [46]. K. marxianus could be a suitable recombinant microorganism for the microbial production of pinene using whey permeate as a medium (Table 1). K. marxianus is a fast growing microorganism and has been observed to grow at temperatures greater than 50 °C [61,62,63]. K. marxianus has not traditionally been used in genetic engineering; however, K. marxianus’ genome has been sequenced and tools have been developed for the genetic engineering of K. marxianus NBRC1777 [35]. A plasmid-based CRISPR-Cas9 was adapted for the genome editing of K. marxianus Km17 [63]. This enabled the engineering of stable heterothallic haploid strains of K. marxianus [63]. Recombinant K. marxianus KM-L9-20 has been cultured in an optimized whey medium to overproduce lactase [34]. This indicates that whey permeate may be a suitable medium for K. marxianus genetically engineered to produce pinene.
7. Whey Standardization and Pinene Recovery
To scale-up pinene production utilizing dairy waste streams, the composition of whey and whey permeate feedstocks would need to be standardized. Evaporators, already commonly used for lactose recovery, could be used to standardize the lactose concentration of the permeate and/or increase the lactose concentration to a desired level. The evaporators could also be used to make a concentrate, which could then be used in a fed-batch bioreactor system. This could make whey permeate a more consistent and desirable microbial medium for pinene production.
Due to the fact that the commercial production of pinene utilizes condensers to collect the volatile compound from heated wood pulp digestors [20,64], the recovery method for pinene from whey would need to employ a different technology. Liquid-liquid extraction is the common method for recovery of microbially produced non-polar compounds [65]. Disruption of the cell wall may increase pinene yields, and due to pinene being a small non-polar compound, counter-current supercritical CO2 extraction could be used as an alternative recovery process [65,66].
Currently, the highest reported yield for E. coli pinene production is 2.61% g pinene/g glucose [8]. If the 10% of annual lactose produced from cheese production (860,000 metric tons) can be utilized in a similar manner as glucose, this would equate to 22,446 metric tons of pinene that could be produced a year. This represents approximately half of the current market for pinene [13]. The 2018 market size for α- and β -pinene was approximately $56 and $102 million/year, respectively [13]. If the challenges of monoterpenoid production can be overcome and microbial production yields are similar to those of the microbially produced sesquiterpene, β-farnesene (17.3% g β-farnesene/g glucose) then this could equate to the production of 148,780 metric tons of pinene [25]. This level of production could lend itself to production as a fuel additive precursor like β-farnesene. This highlights the potential of using whey permeate, a sterile-filtered cheese production by-product, as a microbial medium for recombinant microorganisms to produce pinene. Whey and whey permeate are considered waste streams at many cheese production facilities. The production of pinene using a whey permeate as a medium potentially decreases the BOD of whey, due to the consumption of lactose, and produces a valuable compound that has several different commercial applications. This process could potentially be applied to other microbially produced terpenoids which have health, perfumery, and flavoring applications.
8. Conclusions
Whey permeate, a dairy processing effluent, has the potential to become a feedstock to produce high-value bioactive compounds such as pinene. As the capacity for microbial synthesis of high-value bioactive compounds progresses, the need for a sterile growth medium will become increasingly relevant to biotechnology companies. This opportunity coincides with the need of cheese production facilities to process whey permeate, to reduce its BOD. Such synergy could lead to an opportunity to create a value-added product—pinene—from what is currently a waste stream in many cheese production facilities. Although several challenges must be overcome for the microbial production of pinene or other terpenoids to be economically feasible, they could be overcome by improving genetic methods for non-model organisms that also have GRAS status (e.g., K. marxianus). Research related to the scale-up of a co-modular biocatalyst system and a pinene separation system is also needed. An economic evaluation, such as a techno-economic assessment, should be conducted to understand the economic feasibility of this proposed process. An environmental evaluation, such as a life cycle assessment comparing the current processing methods of whey permeate with the proposed process, should be conducted to understand the environmental implications of embracing this emerging technology. This review calls for further research into the use of whey permeate as a microbial medium for recombinant microorganisms that produce plant secondary metabolites.
Author Contributions
Authors contributed in the following roles: conceptualization, D.R., M.L.M., S.A.P. investigation, D.R. writing, original draft preparation, D.R. writing, review and editing, D.R., M.L.M., S.A.P. and E.S.S. supervision, E.S.S. and M.L.M. project administration, E.S.S. funding acquisition, E.S.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Miyazawa, M.; Yamafuji, C. Inhibition of Acetylcholinesterase Activity by Bicyclic Monoterpenoids. J. Agric. Food Chem. 2005, 53, 1765–1768. [Google Scholar] [CrossRef]
- Miller, J.A.; Thompson, P.A.; Hakim, I.A.; Chow, H.-H.S.; Thomson, C.A. d-Limonene: A Bioactive Food Component from Citrus and Evidence for a Potential Role in Breast Cancer Prevention and Treatment. Oncol. Rev. 2011, 5, 31–42. [Google Scholar] [CrossRef]
- George, K.W.; Alonso-Gutierrez, J.; Keasling, J.D.; Lee, T.S. Isoprenoid Drugs, Biofuels, and Chemicals—Artemisinin, Farnesene, and Beyond; Springer: Cham, Swizerland, 2015; pp. 355–389. [Google Scholar]
- Clomburg, J.M.; Crumbley, A.M.; Gonzalez, R. Industrial Biomanufacturing: The Future of Chemical production. Science (80-. ) 2017, 355, aag0804. [Google Scholar] [CrossRef]
- Chang, M.C.Y.; Keasling, J.D. Production of Isoprenoid Pharmaceuticals by Engineered Microbes. Nat. Chem. Biol. 2006, 2, 674–681. [Google Scholar] [CrossRef]
- Sarria, S.; Wong, B.; Martín, H.G.; Keasling, J.D.; Peralta-Yahya, P. Microbial Synthesis of Pinene. ACS Synth. Biol. 2014, 3, 466–475. [Google Scholar] [CrossRef]
- Da Silva, A.C.R.; Lopes, P.M.; de Azevedo, M.M.B.; Costa, D.C.M.; Alviano, C.S.; Alviano, D.S. Biological Activities of a-Pinene and β-Pinene Enantiomers. Molecules 2012, 17, 6305–6316. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Nie, Q.; Ren, M.; Feng, H.; Jiang, X.; Zheng, Y.; Liu, M.; Zhang, H.; Xian, M. Metabolic Engineering of Escherichia coli for the Biosynthesis of Alpha-Pinene. Biotechnol. Biofuels 2013, 6, 60. [Google Scholar] [CrossRef] [PubMed]
- Goudarzi, S.; Rafieirad, M. Evaluating the Effect of α-Pinene on Motor Activity, Avoidance Memory and Lipid Peroxidation in Animal Model of Parkinson Disease in Adult Male Rats. Res. J. Pharmacogn. 2017, 4, 53–63. [Google Scholar]
- Lee, G.-Y.; Lee, C.; Park, G.H.; Jang, J.-H. Amelioration of Scopolamine-Induced Learning and Memory Impairment by α -Pinene in C57BL/6 Mice. Evidence-Based Complement. Altern. Med. 2017, 2017, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Vermaak, I.; Viljoen, A. Camphor—A Fumigant during the Black Death and a Coveted Fragrant Wood in Ancient Egypt and Babylon—A Review. Molecules 2013, 18, 5434–5454. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Evaluations of the Joint FAO/WHO Expert Committee on Food Additives-Verbenone; World Health Organization: Geneva, Switzerland, 2019; p. 1. [Google Scholar]
- Wu, W.; Maravelias, C.T. Synthesis and Techno-Economic Assessment of Microbial-Based Processes for Terpenes Production. Biotechnol. Biofuels 2018, 11, 294. [Google Scholar] [CrossRef] [PubMed]
- Schipper, M. As U.S. Airlines Carry More Passengers, Jet Fuel Use Remains Well Below its Previous Peak—Today in Energy; U.S. Energy Information Administration (EIA) United States Department of Energy: Washington, DC, USA, 2017.
- Al-Hamimi, S.; Abellan Mayoral, A.; Cunico, L.P.; Turner, C. Carbon Dioxide Expanded Ethanol Extraction: Solubility and Extraction Kinetics of α-Pinene and cis-Verbenol. Anal. Chem. 2016, 88, 4336–4345. [Google Scholar] [CrossRef] [PubMed]
- Niu, F.-X.; He, X.; Wu, Y.-Q.; Liu, J.-Z. Enhancing Production of Pinene in Escherichia coli by Using a Combination of Tolerance, Evolution, and Modular Co-culture Engineering. Front. Microbiol. 2018, 9, 1623. [Google Scholar] [CrossRef] [PubMed]
- Behr, A.; Johnen, L. Myrcene as a Natural Base Chemical in Sustainable Chemistry: A Critical Review. ChemSusChem 2009, 2, 1072–1095. [Google Scholar] [CrossRef]
- Jawjit, W.; Kroeze, C.; Soontaranun, W.; Hordijk, L. Options to Reduce the Environmental Impact by Eucalyptus-based Kraft Pulp Industry in Thailand: Model Description. J. Clean. Prod. 2007, 15, 1827–1839. [Google Scholar] [CrossRef]
- Bajpai, P. Pulping Fundamentals. In Biermann’s Handbook of Pulp and Paper; Elsevier: Amsterdam, The Netherlands, 2018; pp. 295–351. ISBN 978-0-12-814240-0. [Google Scholar]
- Bajpai, P. Kraft Spent Liquor Recovery. In Biermann’s Handbook of Pulp and Paper; Elsevier: Amsterdam, The Netherlands, 2018; pp. 425–451. ISBN 978-0-12-814240-0. [Google Scholar]
- Haneke, K. Turpentine (Turpentine Oil, Wood Turpentine, Sulfate Turpentine, Sulfite Turpentine) [8006-64-2] Review of Toxicological Literature; Integrated Laboratory Systems: Morrisville, NC, USA, 2002. [Google Scholar]
- Al-Asheh, S.; Allawzi, M.; Al-Otoom, A.; Allaboun, H.; Al-Zoubi, A. Supercritical Fluid Extraction of Useful Compounds from Sage. Nat. Sci. 2012, 04, 544–551. [Google Scholar] [CrossRef]
- Burdock, G. Fenaroli’s handbook of flavor ingredients, 6th ed.; CRC Press: Boca Raton, FL, USA, 2010; Volume 14, ISBN 9781420090772. [Google Scholar]
- Vickers, C.E.; Behrendorff, J.B.Y.H.; Bongers, M.; Brennan, T.C.R.; Bruschi, M.; Nielsen, L.K. Production of Industrially Relevant Isoprenoid Compounds in Engineered Microbes. In Microorganisms in Biorefineries; Springer: Berlin/Heidelberg, Germany, 2015; pp. 303–334. ISBN 978-3-662-45209-7. [Google Scholar]
- Meadows, A.L.; Hawkins, K.M.; Tsegaye, Y.; Antipov, E.; Kim, Y.; Raetz, L.; Dahl, R.H.; Tai, A.; Mahatdejkul-Meadows, T.; Xu, L.; et al. Rewriting Yeast Central Carbon Metabolism for Industrial Isoprenoid Production. Nature 2016, 537, 694–697. [Google Scholar] [CrossRef]
- Tashiro, M.; Kiyota, H.; Kawai-Noma, S.; Saito, K.; Ikeuchi, M.; Iijima, Y.; Umeno, D. Bacterial Production of Pinene by a Laboratory-Evolved Pinene-Synthase. ACS Synth. Biol. 2016, 5, 1011–1020. [Google Scholar] [CrossRef]
- Amiri, P.; Shahpiri, A.; Asadollahi, M.A.; Momenbeik, F.; Partow, S. Metabolic Engineering of Saccharomyces cerevisiae for Linalool Production. Biotechnol. Lett. 2016, 38, 503–508. [Google Scholar] [CrossRef]
- Bröker, J.N.; Müller, B.; van Deenen, N.; Prüfer, D.; Schulze Gronover, C. Upregulating the Mevalonate Pathway and Repressing Sterol Synthesis in Saccharomyces cerevisiae Enhances the Production of Triterpenes. Appl. Microbiol. Biotechnol. 2018, 102, 6923–6934. [Google Scholar] [CrossRef]
- Ignea, C.; Cvetkovic, I.; Loupassaki, S.; Kefalas, P.; Johnson, C.B.; Kampranis, S.C.; Makris, A.M. Improving Yeast Strains using Recyclable Integration Cassettes, for the Production of Plant Terpenoids. Microb. Cell Fact. 2011, 10, 4. [Google Scholar] [CrossRef] [PubMed]
- Ignea, C.; Pontini, M.; Maffei, M.E.; Makris, A.M.; Kampranis, S.C. Engineering Monoterpene Production in Yeast Using a Synthetic Dominant Negative Geranyl Diphosphate Synthase. ACS Synth. Biol. 2014, 3, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.-K.; Eom, J.-H.; Kim, Y.; Um, Y.; Woo, H.M. Biosynthesis of Pinene from Glucose using Metabolically-Engineered Corynebacterium glutamicum. Biotechnol. Lett. 2014, 36, 2069–2077. [Google Scholar] [CrossRef] [PubMed]
- International Mycological Association Rhodosporidium toruloide 2016, Online Database. Available online: http://www.mycobank.org/BioloMICS.aspx?TableKey=14682616000000089&Rec=1019&Fields=All (accessed on 16 December 2019).
- Zhuang, X.; Kilian, O.; Monroe, E.; Ito, M.; Tran-Gymfi, M.B.; Liu, F.; Davis, R.W.; Mirsiaghi, M.; Sundstrom, E.; Pray, T.; et al. Monoterpene Production by the Carotenogenic Yeast Rhodosporidium toruloides. Microb. Cell Fact. 2019, 18, 54. [Google Scholar] [CrossRef]
- Ren, Z.-Y.; Liu, G.-L.; Chi, Z.; Han, Y.-Z.; Hu, Z.; Chi, Z.-M. Overexpression of Both the Lactase Gene and its Transcriptional Activator Gene Greatly Enhances Lactase Production by Kluyveromyces marxianus. Process Biochem. 2017, 61, 38–46. [Google Scholar] [CrossRef]
- Nambu-Nishida, Y.; Nishida, K.; Hasunuma, T.; Kondo, A. Development of a Comprehensive Set of Tools for Genome Engineering in a Cold- and Thermo-Tolerant Kluyveromyces marxianus Yeast Strain. Sci. Rep. 2017, 7, 8993. [Google Scholar] [CrossRef]
- Bao, S.-H.; Zhang, D.-Y.; Meng, E. Improving Biosynthetic Production of Pinene through Plasmid Recombination Elimination and Pathway Optimization. Plasmid 2019, 105, 102431. [Google Scholar] [CrossRef]
- Niu, F.-X.; Huang, Y.-B.; Ji, L.-N.; Liu, J.-Z. Genomic and Transcriptional Changes in Response to Pinene Tolerance and Overproduction in Evolved Escherichia coli. Synth. Syst. Biotechnol. 2019, 4, 113–119. [Google Scholar] [CrossRef]
- Fischer, M.J.C.; Meyer, S.; Claudel, P.; Bergdoll, M.; Karst, F. Metabolic Engineering of Monoterpene Synthesis in Yeast. Biotechnol. Bioeng. 2011, 108, 1883–1892. [Google Scholar] [CrossRef]
- Peng, B.; Nielsen, L.K.; Kampranis, S.C.; Vickers, C.E. Engineered Protein Degradation of Farnesyl Pyrophosphate Synthase is an Effective Regulatory Mechanism to Increase Monoterpene Production in Saccharomyces cerevisiae. Metab. Eng. 2018, 47, 83–93. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Esquivel, B.D.; White, T.C. Overexpression or Deletion of Ergosterol Biosynthesis Genes Alters Doubling Time, Response to Stress Agents, and Drug Susceptibility in Saccharomyces cerevisiae. MBio 2018, 9, e01291-18. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Sun, M.; Xu, S.; Zhou, J. Enhanced (S)-Linalool Production by Fusion Expression of Farnesyl Diphosphate Synthase and Linalool Synthase in Saccharomyces cerevisiae. J. Appl. Microbiol. 2016, 121, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Li, C.; Zhang, Y.; Shen, Y.; Hou, J.; Bao, X. Dynamic control of ERG20 Expression Combined with Minimized Endogenous Downstream Metabolism Contributes to the Improvement of Geraniol Production in Saccharomyces cerevisiae. Microb. Cell Fact. 2017, 16, 17. [Google Scholar] [CrossRef] [PubMed]
- Parulekar, S.; Birol, G.; Cinar, A.; Undey, C. Introduction. In Batch Fermentation Modeling: Monitoring and Control; Chemical Industries; CRC Press: Boca Raton, FL, USA, 2003; Volume 93, pp. 1–19. ISBN 978-0-8247-4034-4. [Google Scholar]
- Campbel, J.A. Energy from Aerobic vs. Anaerobic Fermentation. J. Chem. Educ. 1973, 50, 535. [Google Scholar]
- Abram, T. Sugars Defined; Michigan State University Extension: Lansing, MI, USA, 2014. [Google Scholar]
- Hughes, P.; Risner, D.; Goddik, L.M. Whey to Vodka. In Whey-Biological Properties and Alternatives; IntechOpen: London, UK, 2018; Available online: https://www.intechopen.com/books/whey-biological-properties-and-alternative-uses/whey-to-vodka (accessed on 16 December 2019).
- Tetra Pak Whey Processing. In Dairy Processing Handbook; Tetrapak, 2019; Available online: https://dairyprocessinghandbook.tetrapak.com/chapter/whey-processing (accessed on 16 December 2019).
- Lee, H.; Cuthbertson, D.J.; Otter, D.E.; Barile, D. Rapid Screening of Bovine Milk Oligosaccharides in a Whey Permeate Product and Domestic Animal Milks by Accurate Mass Database and Tandem Mass Spectral Library. J. Agric. Food Chem. 2016, 64, 6364–6374. [Google Scholar] [CrossRef] [PubMed]
- Dallas, D.C.; Weinborn, V.; de Moura Bell, J.M.L.N.; Wang, M.; Parker, E.A.; Guerrero, A.; Hettinga, K.A.; Lebrilla, C.B.; German, J.B.; Barile, D. Comprehensive Peptidomic and Glycomic Evaluation Reveals that Sweet Whey Permeate from Colostrum is a Source of Milk Protein-Derived Peptides and Oligosaccharides. Food Res. Int. 2014, 63, 203–209. [Google Scholar] [CrossRef]
- Siso, M.I.G. The biotechnological utilization of cheese whey: A review. Bioresour. Technol. 1996, 57, 1–11. [Google Scholar] [CrossRef]
- Pasotti, L.; Zucca, S.; Casanova, M.; Micoli, G.; Cusella De Angelis, M.G.; Magni, P. Fermentation of Lactose to Ethanol in Cheese Whey Permeate and Concentrated Permeate by Engineered Escherichia coli. BMC Biotechnol. 2017, 17, 48. [Google Scholar] [CrossRef]
- Risner, D.; Tomasino, E.; Hughes, P.; Meunier-Goddik, L. Volatile Aroma Composition of Distillates Produced from Fermented Sweet and Acid Whey. J. Dairy Sci. 2019, 102, 202–210. [Google Scholar] [CrossRef]
- Akbas, M.Y.; Sar, T.; Ozcelik, B. Improved Ethanol Production from Cheese Whey, Whey Powder, and Sugar Beet Molasses by “ Vitreoscilla hemoglobin expressing” Escherichia coli. Biosci. Biotechnol. Biochem. 2014, 78, 687–694. [Google Scholar] [CrossRef]
- Sar, T.; Stark, B.C.; Yesilcimen Akbas, M. Effective Ethanol Production from Whey Powder through Immobilized E. coli Expressing Vitreoscilla Hemoglobin. Bioengineered 2017, 8, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, P.M.R.; François, J.; Parrou, J.L.; Teixeira, J.A.; Domingues, L. Adaptive Evolution of a Lactose-consuming Saccharomyces cerevisiae recombinant. Appl. Environ. Microbiol. 2008, 74, 1748–1756. [Google Scholar] [CrossRef] [PubMed]
- Sreekrishna, K.; Dickson, R.C. Construction of Strains of Saccharomyces cerevisiae that Grow on Lactose. Proc. Natl. Acad. Sci. USA 1985, 82, 7909–7913. [Google Scholar] [CrossRef] [PubMed]
- Lawton, M.R.; Alcaine, S.D. Leveraging Endogenous Barley Enzymes to Turn Lactose-Containing Dairy By-products into Fermentable Adjuncts for Saccharomyces cerevisiae-based Ethanol Fermentations. J. Dairy Sci. 2019, 102, 2044–2050. [Google Scholar] [CrossRef]
- Liu, J.-J.; Zhang, G.-C.; Oh, E.J.; Pathanibul, P.; Turner, T.L.; Jin, Y.-S. Lactose Fermentation by Engineered Saccharomyces cerevisiae Capable of Fermenting Cellobiose. J. Biotechnol. 2016, 234, 99–104. [Google Scholar] [CrossRef]
- Barrett, E.; Stanton, C.; Zelder, O.; Fitzgerald, G.; Ross, R.P. Heterologous Expression of Lactose- and Galactose-utilizing Pathways from Lactic Acid Bacteria in Corynebacterium glutamicum for Production of Lysine in Whey. Appl. Environ. Microbiol. 2004, 70, 2861–2866. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, P.; Gray, J.I.; Asghar, A. Synthesis of Lipids by Certain Yeast Strains Grown on Whey Permeate. J. Food Lipids 1998, 5, 283–297. [Google Scholar] [CrossRef]
- Banat, I.M.; Nigam, P.; Marchant, R. Isolation of Thermotolerant, Fermentative Yeasts Growing at 52 °C and Producing Ethanol at 45 °C and 50 °C. World J. Microbiol. Biotechnol. 1992, 8, 259–263. [Google Scholar] [CrossRef]
- Groeneveld, P.; Stouthamer, A.H.; Westerhoff, H.V. Super Life—How and Why ‘Cell Selection’ Leads to the Fastest-Growing Eukaryote. FEBS J. 2009, 276, 254–270. [Google Scholar] [CrossRef]
- Cernak, P.; Estrela, R.; Poddar, S.; Skerker, J.M.; Cheng, Y.-F.; Carlson, A.K.; Chen, B.; Glynn, V.M.; Furlan, M.; Ryan, O.W.; et al. Engineering Kluyveromyces marxianus as a Robust Synthetic Biology Platform Host. MBio 2018, 9, e01410-18. [Google Scholar] [CrossRef]
- Ibdah, M.; Muchlinski, A.; Yahyaa, M.; Nawade, B.; Tholl, D. Carrot Volatile Terpene Metabolism: Terpene Diversity and Biosynthetic Genes; Springer: Cham, Switzerland, 2019; pp. 279–293. [Google Scholar]
- Duarte, S.H.; dos Santos, P.; Michelon, M.; de Pinho Oliveira, S.M.; Martínez, J.; Maugeri, F. Recovery of yeast lipids using Different Cell Disruption Techniques and Supercritical CO2 Extraction. Biochem. Eng. J. 2017, 125, 230–237. [Google Scholar] [CrossRef]
- Reverchon, E. Supercritical Fluid Extraction and Fractionation of Essential Oils and Related Products. J. Supercrit. Fluids 1997, 10, 1–37. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).