Structural Optimization of Alkylbenzenes as Graphene Dispersants
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Exfoliation of Graphite and Evaluation of Graphene Dispersion
2.3. Recovery and Reuse of Dispersants
3. Results and Discussion
3.1. Evaluation of Graphene Dispersant
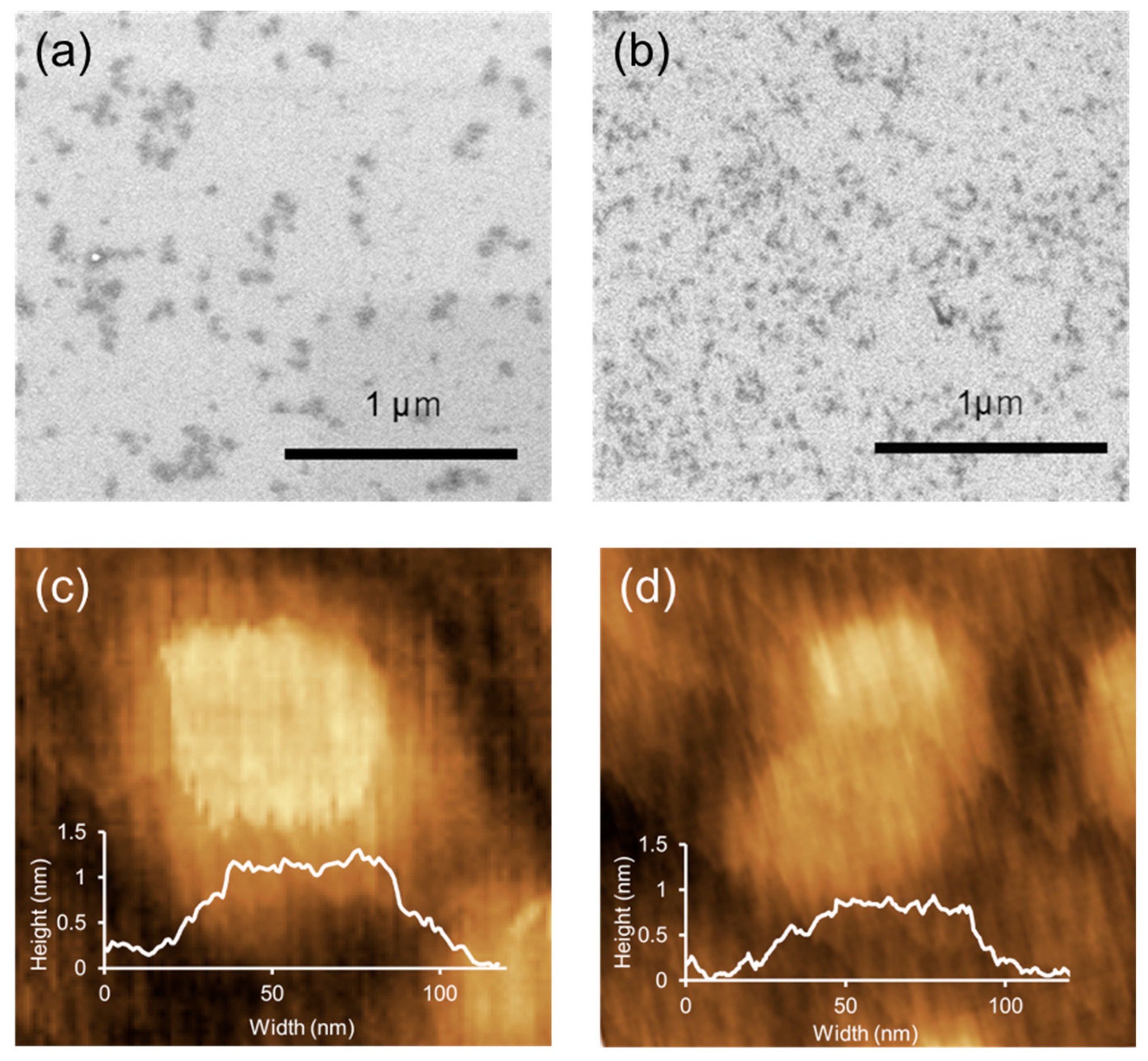
3.2. Structure and Morphology Analysis
3.3. Recovery and Reuse of Dispersants
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef]
- Mayorov, A.S.; Gorbachev, R.V.; Morozov, S.V.; Britnell, L.; Jalil, R.; Ponomarenko, L.A.; Blake, P.; Novoselov, K.S.; Watanabe, K.; Taniguchi, T.; et al. Micrometer-scale ballistic transport in encapsulated graphene at room temperature. Nano. Lett. 2011, 11, 2396–2399. [Google Scholar] [CrossRef] [PubMed]
- Balandin, A.A. Thermal properties of graphene and nanostructured carbon materials. Nat. Mater. 2011, 10, 569–581. [Google Scholar] [CrossRef]
- Yang, Z.; Gao, R.; Hu, N.; Chai, J.; Cheng, Y.; Zhang, L.; Wei, H.; Kong, E.S.; Zhang, Y. The Prospective Two-Dimensional Graphene Nanosheets: Preparation, Functionalization and Applications. Nano-Micro. Lett. 2012, 4, 1–9. [Google Scholar] [CrossRef]
- Bae, S.; Lee, Y.; Sharma, B.K.; Lee, H.; Kim, J.; Ahn, J. Graphene-based transparent strain sensor. Carbon 2013, 51, 236–242. [Google Scholar]
- Reddy, A.L.M.; Srivastava, A.; Gowda, S.R.; Gullapalli, H.; Dubey, M.; Ajayan, P.M. Synthesis of nitrogen-doped graphene films for lithium battery application. ACS Nano. 2010, 4, 6337–6342. [Google Scholar] [CrossRef] [PubMed]
- Shboul, A.A.; Trudeau, C.; Cloutier, S.; Siaj, M.; Claverie, J.P. Graphene dispersions in alkanes: Toward fast drying conducting inks. Nanoscale 2017, 9, 9893–9901. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhu, Y.; Cai, W.; Borysiak, M.; Han, B.; Chen, D.; Piner, R.D.; Colombo, L.; Ruoff, R.S. Transfer of Large-Area Graphene Films for High-Performance Transparent Conductive Electrodes. Nano Lett. 2009, 9, 4359–4363. [Google Scholar] [CrossRef]
- Li, X.; Cai, W.; An, J.; Kim, S.; Nah, J.; Yang, D.; Piner Velamakanni, A.; Jung, I.; Tutuc, E.; Banerjee, S.K.; et al. Large-area synthesis of high-quality and uniform graphene films on copper foils. Science 2009, 324, 1312–1314. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef]
- Hu, Y.; Song, S.; Lopez-Valdivieso, A. Effects of oxidation on the defect of reduced graphene oxides in graphene preparation. J. Colloid. Interface Sci. 2015, 450, 68–73. [Google Scholar] [CrossRef]
- Johnson, D.W.; Dobson, B.P.; Coleman, K.S. A manufacturing perspective on graphene dispersions. Curr. Opin. Colloid Interface Sci. 2015, 20, 367–382. [Google Scholar] [CrossRef]
- Konios, D.; Stylianakis, M.M.; Stratakis, E.; Kymakis, E. Dispersion behaviour of graphene oxide and reduced graphene oxide. J. Colloid Interface Sci. 2014, 430, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Price, E.K.; Bansala, T.; Achee, T.C.; Sun, W.; Green, M.J. Tunable dispersibility and wettability of graphene oxide through one-pot functionalization and reduction. J. Colloid Interface Sci. 2019, 552, 771–780. [Google Scholar] [CrossRef] [PubMed]
- Guardia, L.; Fernández-Merino, M.J.; Paredes, J.I.; Solís-Fernández, P.; Villar-Rodil, S.; Martínez-Alonso, A.; Tascón, J.M.D. High-throughput production of pristine graphene in an aqueous dispersion assisted by non-ionic surfactants. Carbon 2011, 49, 1653–1662. [Google Scholar] [CrossRef]
- Khan, U.; O’Neill, A.; Porwal, H.; May, P.; Nawaz, K.; Coleman, J.N. Size selection of dispersed, exfoliated graphene flakes by controlled centrifugation. Carbon 2012, 50, 470–475. [Google Scholar] [CrossRef]
- Alaferdov, A.V.; Gholamipour-Shirazi, A.; Canesqui, M.A.; Danilov, Y.A.; Moshkalev, S.A. Size-controlled synthesis of graphite nanoflakes and multi-layer graphene by liquid phase exfoliation of natural graphite. Carbon 2014, 69, 525–535. [Google Scholar] [CrossRef]
- Hernandez, Y.; Nicolosi, V.; Lotya, M.; Blighe, F.M.; Sun, Z.; De, S.; Holland, B.; Byrne, M.; Gun, Y.; Boland, J.; et al. High-yield production of graphene by liquid-phase exfoliation of graphite. Nat. Nanotechnol. 2008, 3, 563–568. [Google Scholar] [CrossRef]
- Lotya, M.; Hernandez, Y.; King, P.J.; Smith, R.J.; Nicolosi, V.; Karlsson, L.S.; Blighe, F.M.; De, S.; Wang, Z.; McGovern, I.T.; et al. Liquid phase production of graphene by exfoliation of graphite in surfactant/water solutions. J. Am. Chem. Soc. 2009, 131, 3611–3620. [Google Scholar] [CrossRef]
- Xu, L.; McGraw, J.W.; Gao, F.; Grundy, M.; Ye, Z.; Gu, Z.; Shepherd, J.L. Production of High-Concentration Graphene Dispersions in Low-Boiling-Point Organic Solvents by Liquid-Phase Noncovalent Exfoliation of Graphite with a Hyperbranched Polyethylene and Formation of Graphene/Ethylene Copolymer Composites. J. Phys. Chem. C 2013, 117, 10730–10742. [Google Scholar] [CrossRef]
- Ciesielski, A.; Samorì, P. Supramolecular Approaches to Graphene: From Self-Assembly to Molecule-Assisted Liquid-Phase Exfoliation. Adv. Mater. 2016, 28, 6030–6051. [Google Scholar] [CrossRef]
- Haar, S.; El Gemayel, M.; Shin, Y.; Melinte, G.; Squillaci, M.A.; Ersen, O.; Casiraghi, C.; Ciesielski, A.; Samorì, P. Enhancing the Liquid-Phase Exfoliation of Graphene in Organic Solvents upon Addition of n-Octylbenzene. Sci. Rep. 2015, 5, 16684. [Google Scholar] [CrossRef] [PubMed]
- Haar, S.; Ciesielski, A.; Clough, J.; Yang, H.; Mazzaro, R.; Richard, F.; Conti, S.; Merstorf, N.; Cecchini, M.; Morandi, V.; et al. A supramolecular strategy to leverage the liquid-phase exfoliation of graphene in the presence of surfactants: Unraveling the role of the length of fatty acids. Small 2015, 11, 1691–1702. [Google Scholar] [CrossRef] [PubMed]
- Haar, S.; Bruna, M.; Lian, J.X.; Tomarchio, F.; Olivier, Y.; Mazzaro, R.; Morandi, V.; Moran, J.; Ferrari, A.C.; Beljonne, D.; et al. Liquid-Phase Exfoliation of Graphite into Single- and Few-Layer Graphene with α-Functionalized Alkanes. J. Phys. Chem. Lett. 2016, 7, 2714–2721. [Google Scholar] [CrossRef] [PubMed]
- Díez-Pascual, A.M.; Vallés, C.; Mateos, R.; Vera-López, S.; Kinloch, I.A.; Andrés, M.P.S. Influence of surfactants of different nature and chain length on the morphology, thermal stability and sheet resistance of graphene. Soft Matter 2018, 14, 6013–6023. [Google Scholar] [CrossRef]
- Miranda, P.B.; Pflumio, V.; Saijo, H.; Shen, Y.R. Chain−Chain Interaction between Surfactant Monolayers and Alkanes or Alcohols at Solid/Liquid Interfaces. J. Am. Chem. Soc. 1998, 120, 12092–12099. [Google Scholar] [CrossRef]
- Liang, Y.; Wu, D.; Feng, X.; Müllen, K. Dispersion of Graphene Sheets in Organic Solvent Supported by Ionic Interactions. Adv. Mater. 2009, 21, 1679–1683. [Google Scholar] [CrossRef]
- Parviz, D.; Das, S.; Ahmed, H.S.T.; Irin, F.; Bhattacharia, S.; Green, M.J. Dispersions of Non-Covalently Functionalized Graphene with Minimal Stabilizer. ACS Nano. 2012, 6, 8857–8867. [Google Scholar] [CrossRef]
- Bose, S.; Kuila, T.; Mishra, A.K.; Kim, N.H.; Lee, J.H. Preparation of non-covalently functionalized graphene using 9-anthracene carboxylic acid. Nanotechnology 2011, 22, 405603. [Google Scholar] [CrossRef]
- Su, Q.; Pang, S.; Alijani, V.; Li, C.; Feng, X.; Müllen, K. Composites of Graphene with Large Aromatic Molecules. Adv. Mater. 2009, 21, 3191. [Google Scholar] [CrossRef]
- Ghosh, A.; Rao, K.V.; George, S.J.; Rao, C.N.R. Noncovalent Functionalization, Exfoliation, and Solubilization of Graphene in Water by Employing a Fluorescent Coronene Carboxylate. Chem. Eur. J. 2010, 16, 2700. [Google Scholar] [CrossRef]
- Bourlinos, A.B.; Georgakilas, V.; Zboril, R.; Steriotis, T.A.; Stubos, A.K.; Trapalis, C. Aqueous-phase exfoliation of graphite in the presence of polyvinylpyrrolidone for the production of water-soluble graphenes. Solid State Commun. 2009, 149, 2172. [Google Scholar] [CrossRef]
- Skaltsas, T.; Karousis, N.; Yan, H.J.; Wang, C.R.; Pispas, S.; Tagmatarchis, N. Graphene exfoliation in organic solvents and switching solubility in aqueous media with the aid of amphiphilic block copolymers. J. Mater. Chem. 2012, 22, 21507–21512. [Google Scholar] [CrossRef]
- Xu, J.; Dang, D.K.; Tran, V.T.; Liu, X.; Chung, J.S.; Hur, S.H.; Choi, W.M.; Kim, E.J.; Kohl, P.A. Liquid-phase exfoliation of graphene in organic solvents with addition of naphthalene. J. Colloid. Interface Sci. 2014, 418, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Narayan, R.; Lim, J.; Jeon, T.; Li, D.J.; Kim, S.O. Perylene tetracarboxylate surfactant assisted liquid phase exfoliation of graphite into graphene nanosheets with facile re-dispersibility in aqueous/organic polar solvents. Carbon 2017, 119, 555–568. [Google Scholar] [CrossRef]
- Imani, R.; Shao, W.; Emami, S.H.; Faghihi, S.; Prakash, S. Improved dispersibility of nano-graphene oxide by amphiphilic polymer coatings for biomedical applications. RSC Adv. 2016, 6, 77818–77829. [Google Scholar] [CrossRef]
- Dai, Z.Q.; Zhang, W.W.; Zhang, Z.Y.; Wei, B.M.; Yang, Y.K. Cuprous-Catalyzed Cross-Coupling Reaction of Grignard Reagents with Alkyl Halides. Asian J. Chem. 2011, 23, 4087–4089. [Google Scholar]
- Huang, W.Y.; Gao, W.; Kwei, T.K.; Okamoto, Y. Synthesis and Characterization of Poly(alkyl-substituted p-phenylene ethynylene)s. Macromolecules 2001, 34, 1570–1578. [Google Scholar]
- Castro Neto, A.H.; Guinea, F.; Peres, N.M.R.; Novoselov, K.S.; Geim, A.K. The electronic properties of graphene. Rev. Mod. Phys. 2009, 81, 109. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S.; et al. Raman Spectrum of Graphene and Graphene Layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef]
- Eckmann, A.; Felten, A.; Mishchenko, A.; Britnell, L.; Krupke, R.; Novoselov, K.S.; Casiraghi, C. Probing the Nature of Defects in Graphene by Raman Spectroscopy. Nano. Lett. 2012, 12, 3925–3930. [Google Scholar] [CrossRef]

| Entry | Dispersant | Concentration of Graphene | |
|---|---|---|---|
| 1 | None | 22 µg/mL | |
| 2 | Octylbenzene | 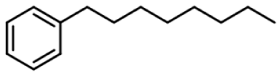 | 92 µg/mL |
| 3 | Butylbenzene |  | 101 µg/mL |
| 4 | Hexadecylbenzene | 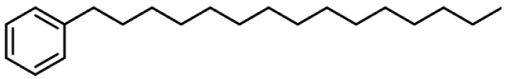 | (128 µg/mL) b |
| 5 | Octylnaphthalene | 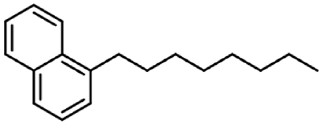 | 35 µg/mL |
| 6 | Octylphenanthrene | 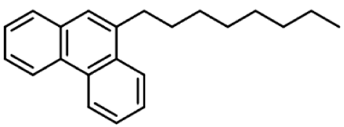 | 10 µg/mL |
| 7 | 1,4-Dioctylbenzene | 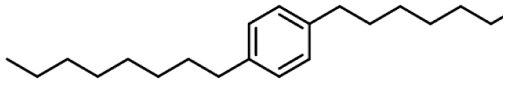 | 306 µg/mL |
| 8 | 1,3,5-Trioctylbenzene |  | - c |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takeda, S.; Nishina, Y. Structural Optimization of Alkylbenzenes as Graphene Dispersants. Processes 2020, 8, 238. https://doi.org/10.3390/pr8020238
Takeda S, Nishina Y. Structural Optimization of Alkylbenzenes as Graphene Dispersants. Processes. 2020; 8(2):238. https://doi.org/10.3390/pr8020238
Chicago/Turabian StyleTakeda, Shimpei, and Yuta Nishina. 2020. "Structural Optimization of Alkylbenzenes as Graphene Dispersants" Processes 8, no. 2: 238. https://doi.org/10.3390/pr8020238
APA StyleTakeda, S., & Nishina, Y. (2020). Structural Optimization of Alkylbenzenes as Graphene Dispersants. Processes, 8(2), 238. https://doi.org/10.3390/pr8020238






