Thermodynamic Performance Analysis of Hydrofluoroolefins (HFO) Refrigerants in Commercial Air-Conditioning Systems for Sustainable Environment
Abstract
1. Introduction
2. Materials and Methods
2.1. Selection of Refrigerants
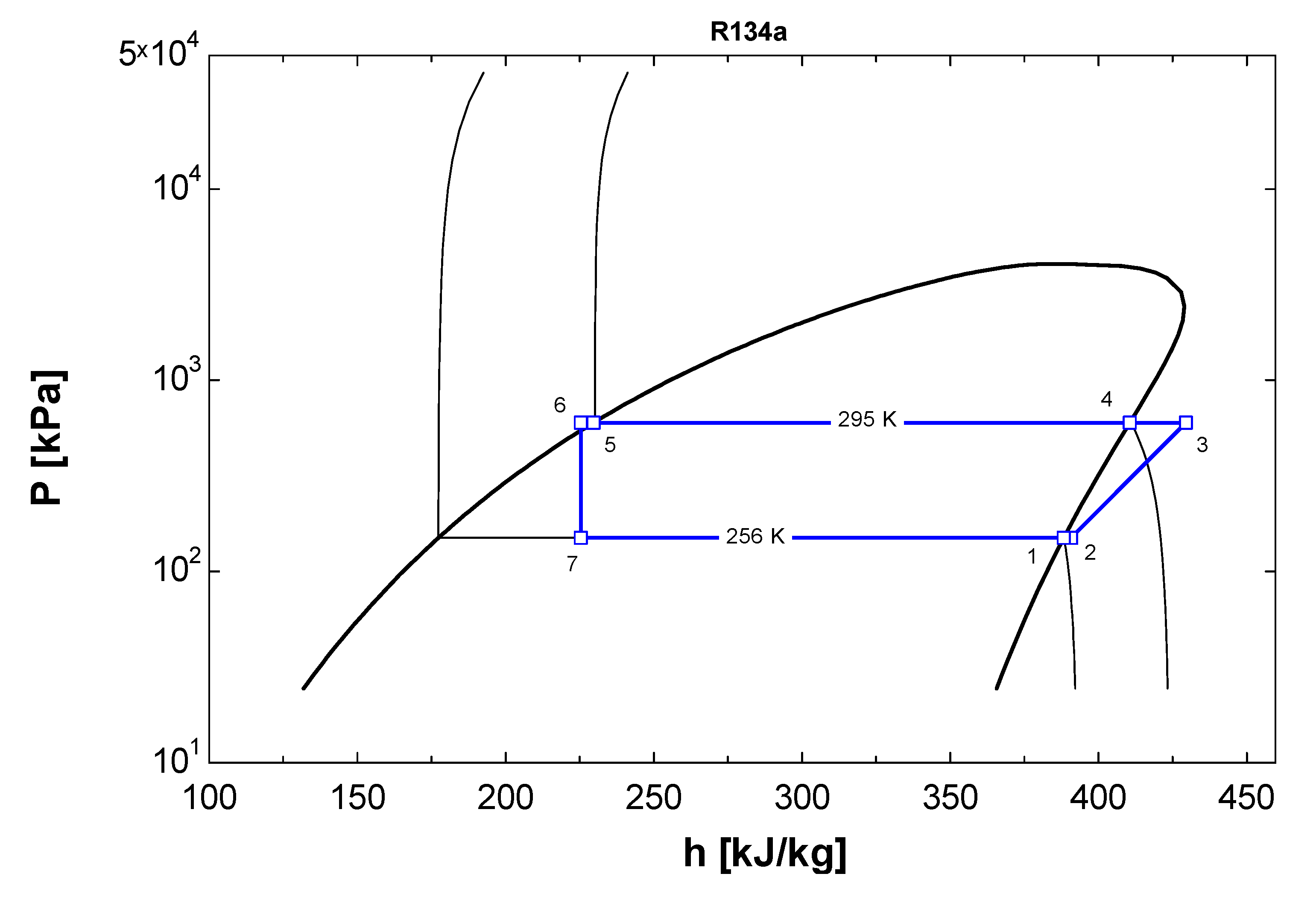
2.2. Numerical Modeling of VCRC-Based Refrigeration System
2.3. Comparison of EES Model Using Vapor Compression Refrigeration System
2.4. Error Analysis of Results
2.5. Comparison of VCRC for HFO refrigerants
3. Results and Discussions
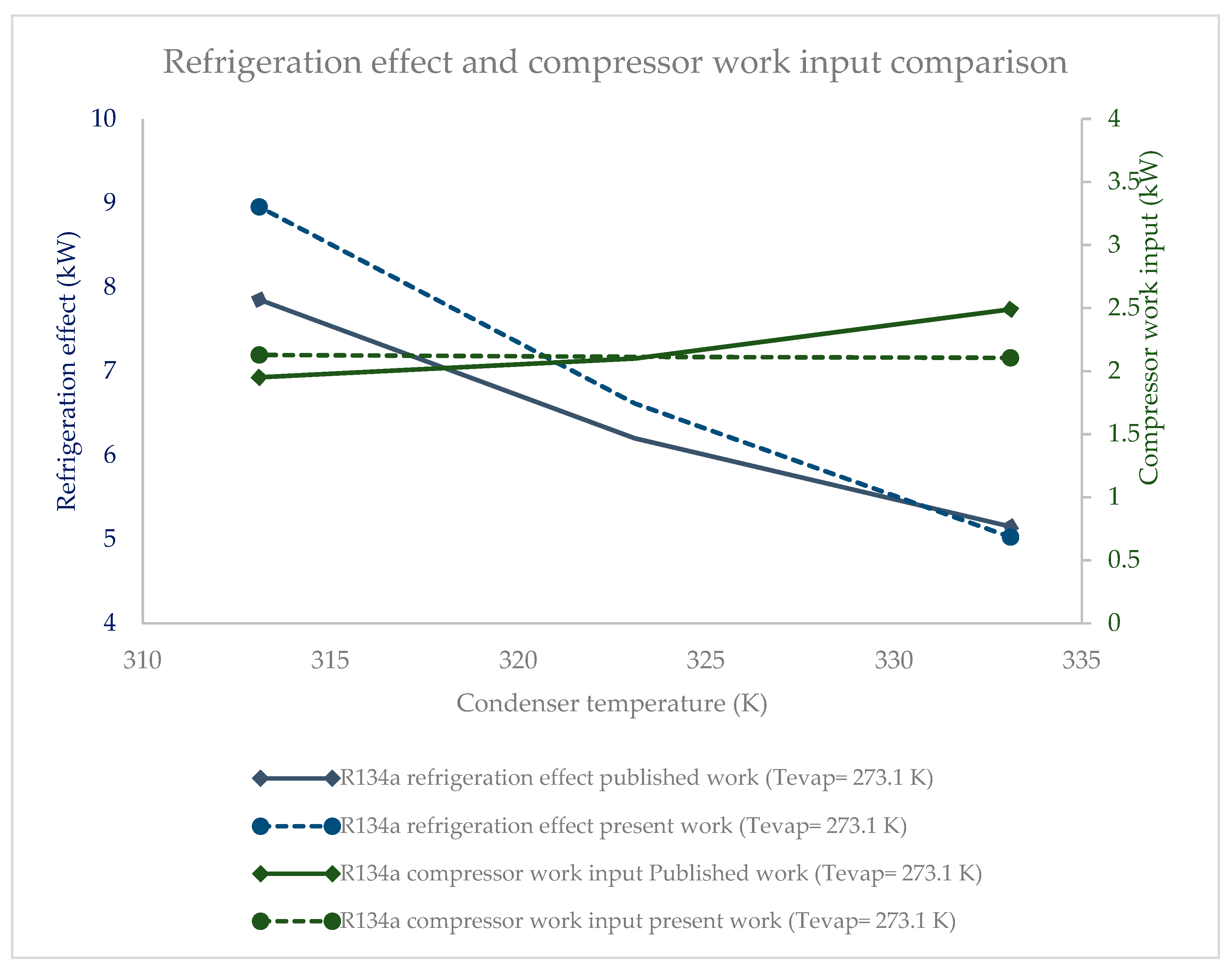
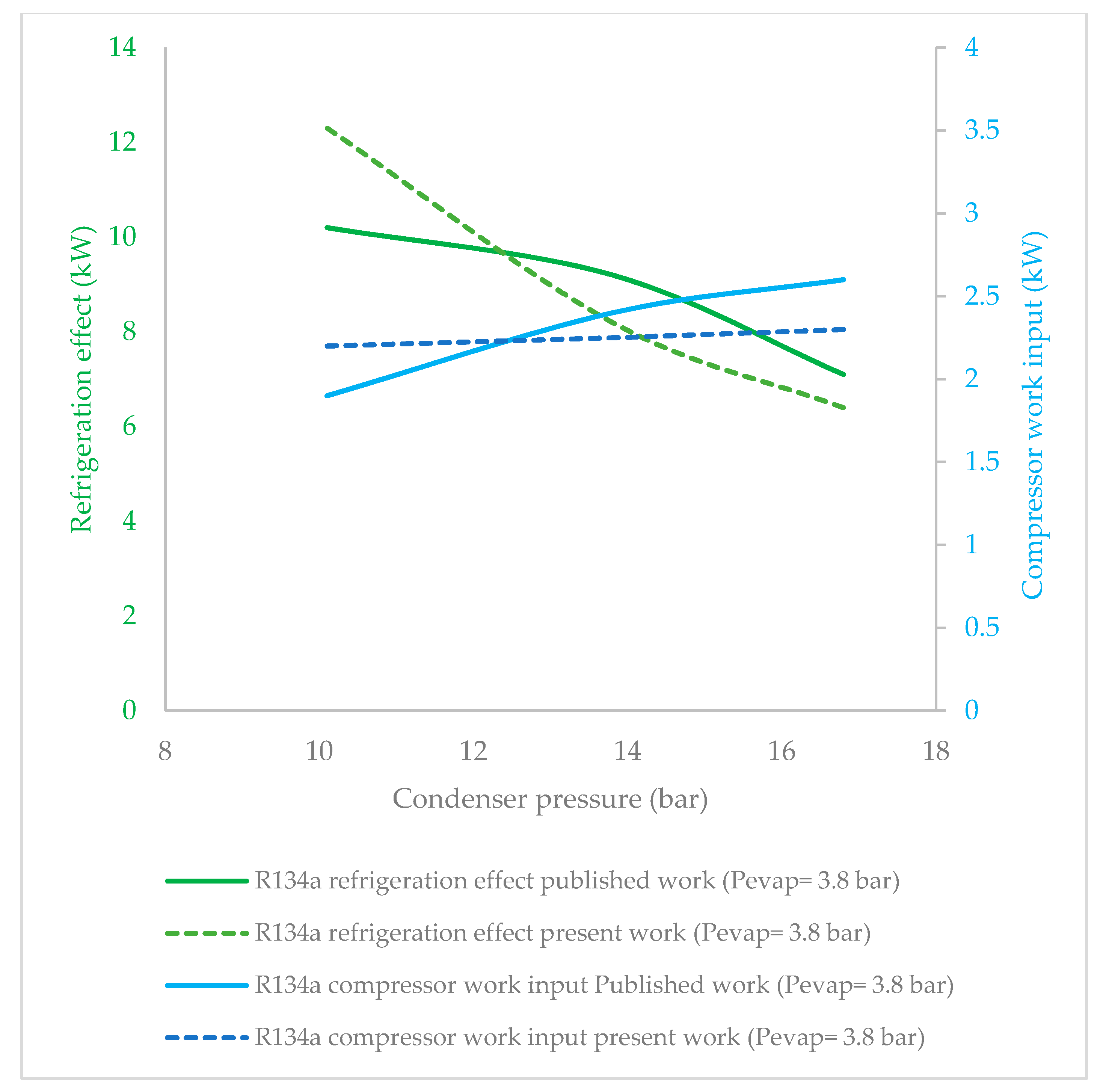
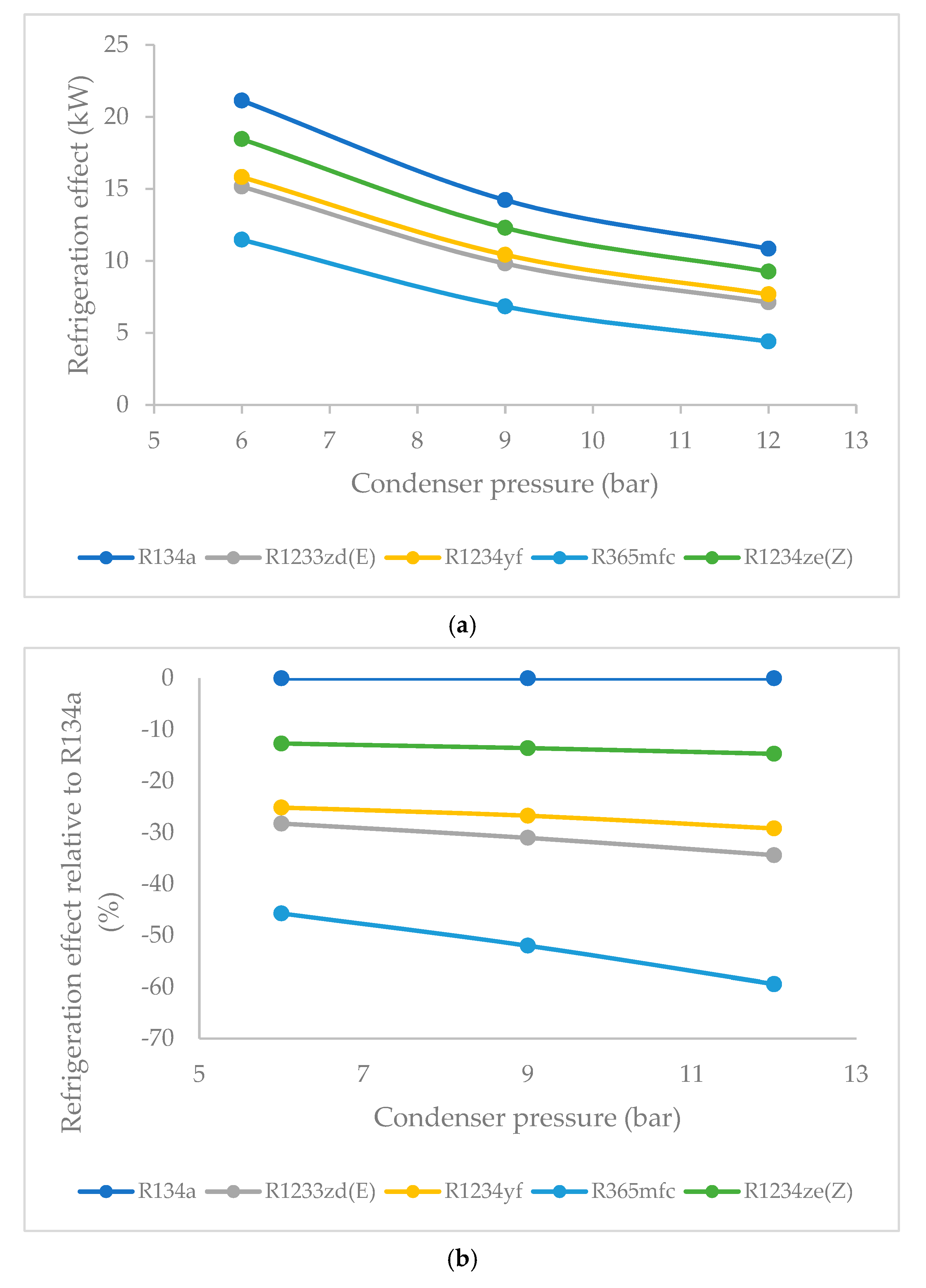
3.1. Effect of Condenser Pressure on Refrigeration Effect
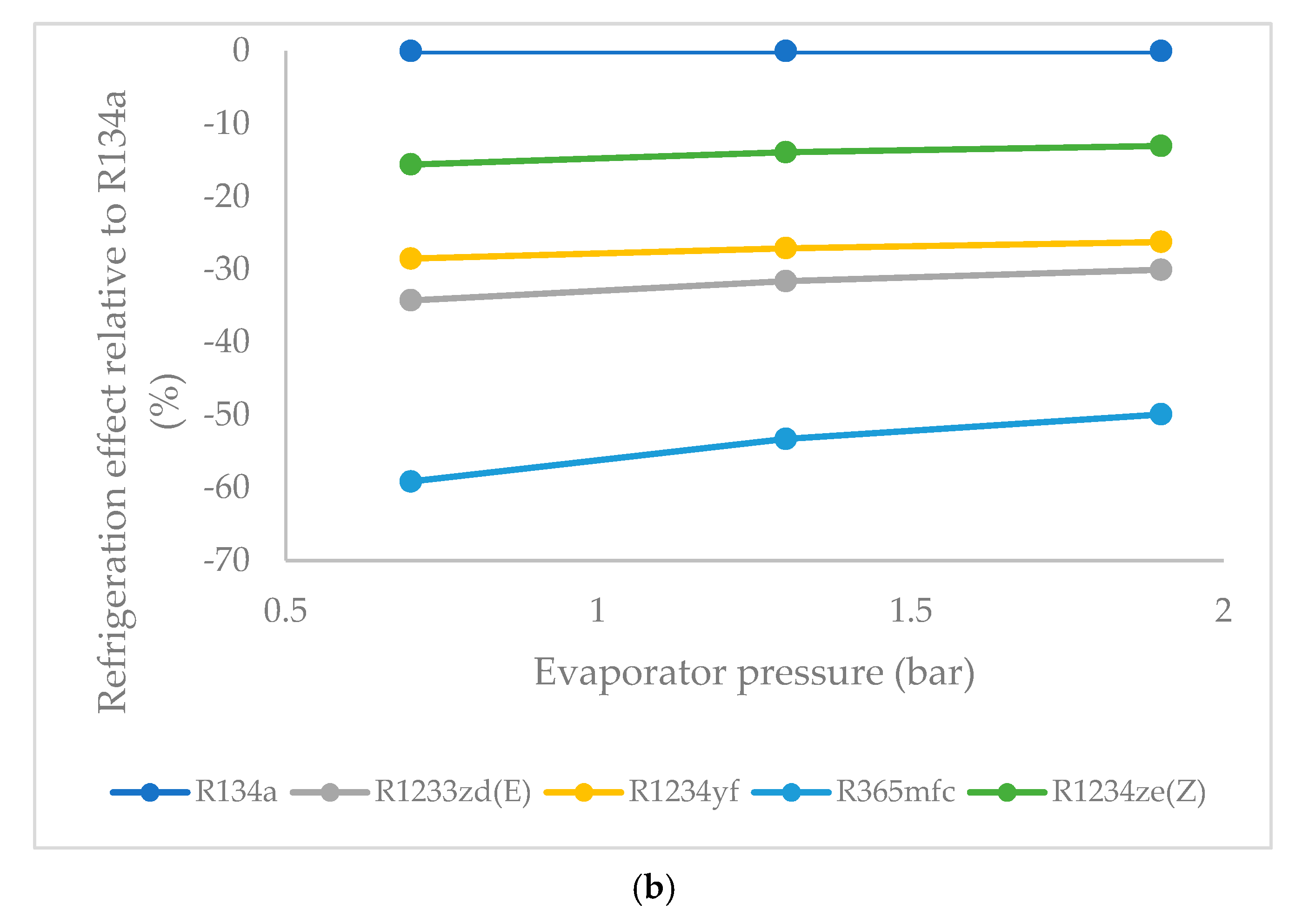
3.2. Refrigeration Effect as a Function of Evaporator Pressure
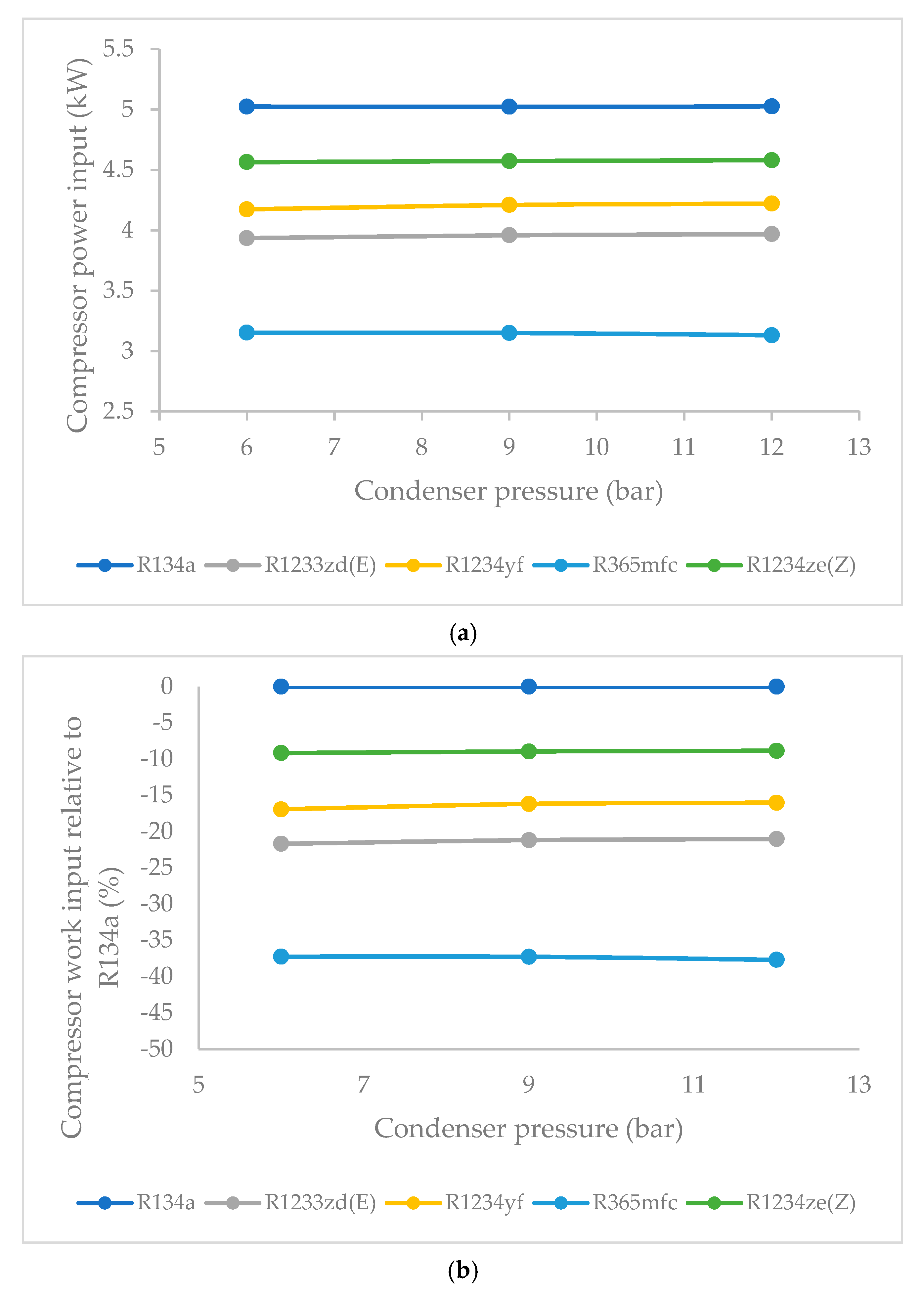
3.3. Effect of Condenser Pressure on Compressor Work Input
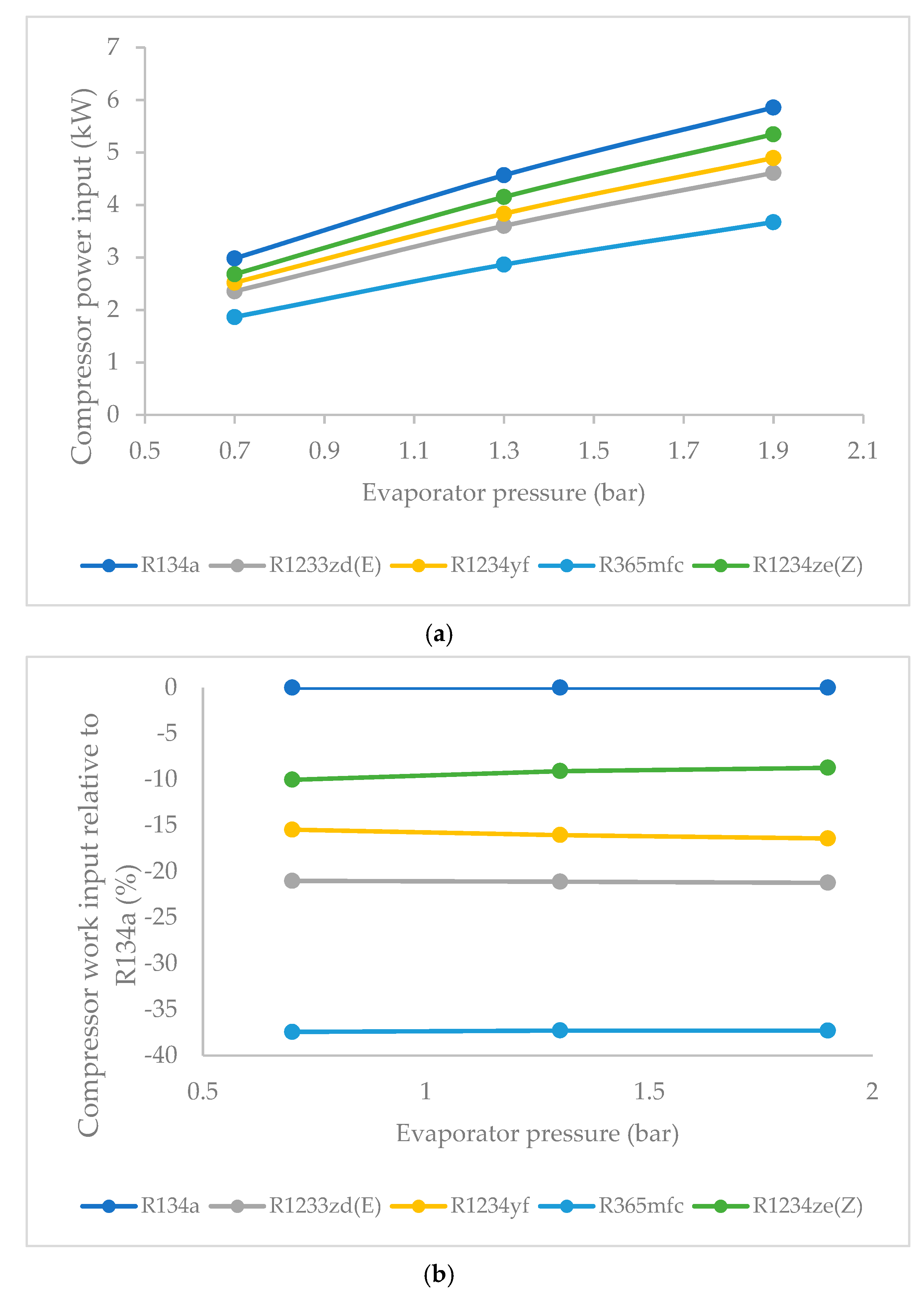
3.4. Effect of Evaporator Pressure on Compressor Work Input
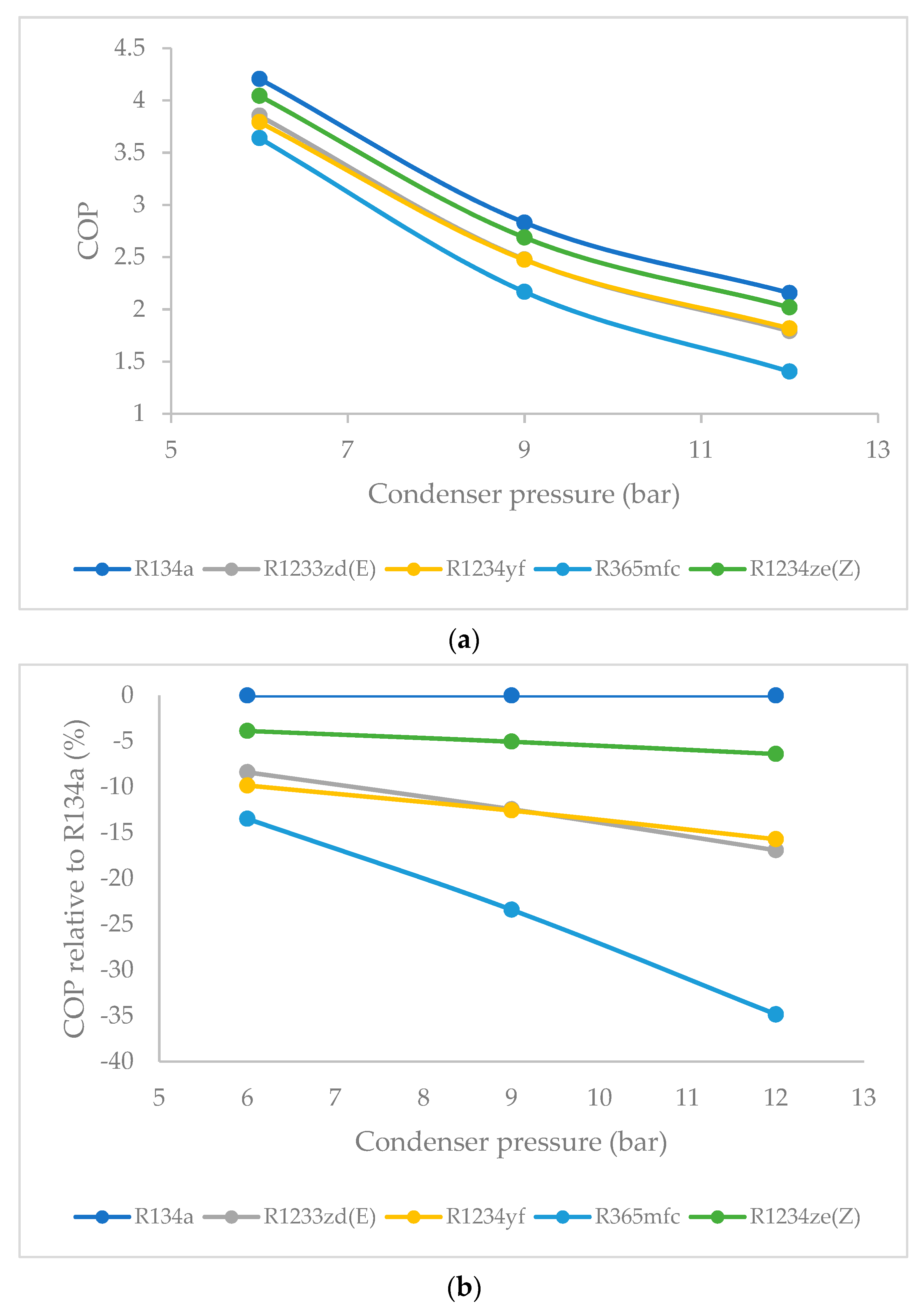
3.5. Effect of Condenser Pressure on Coefficient of Performance (COP)
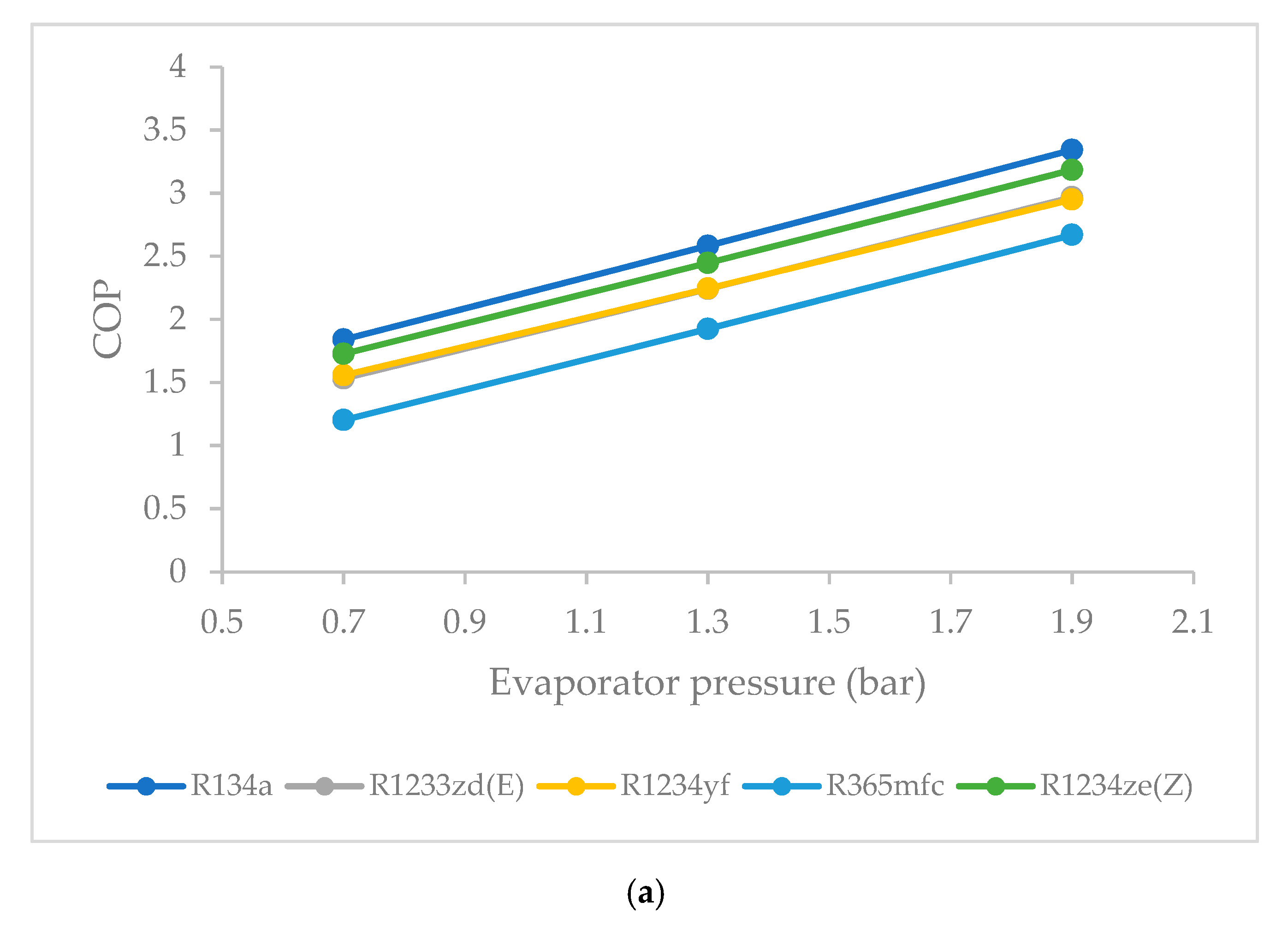
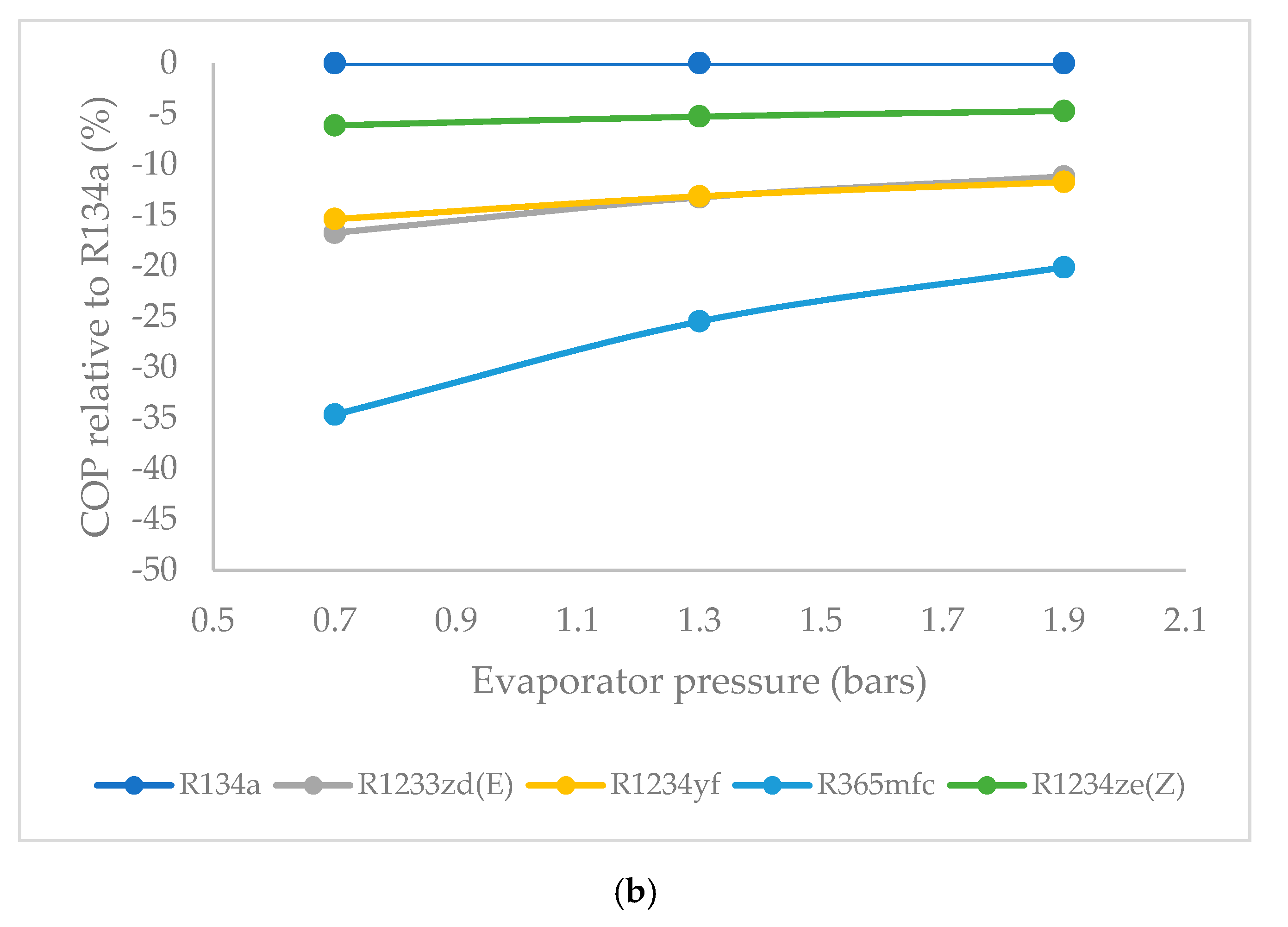
3.6. Effect of Evaporator Pressure on Coefficient of Performance (COP)
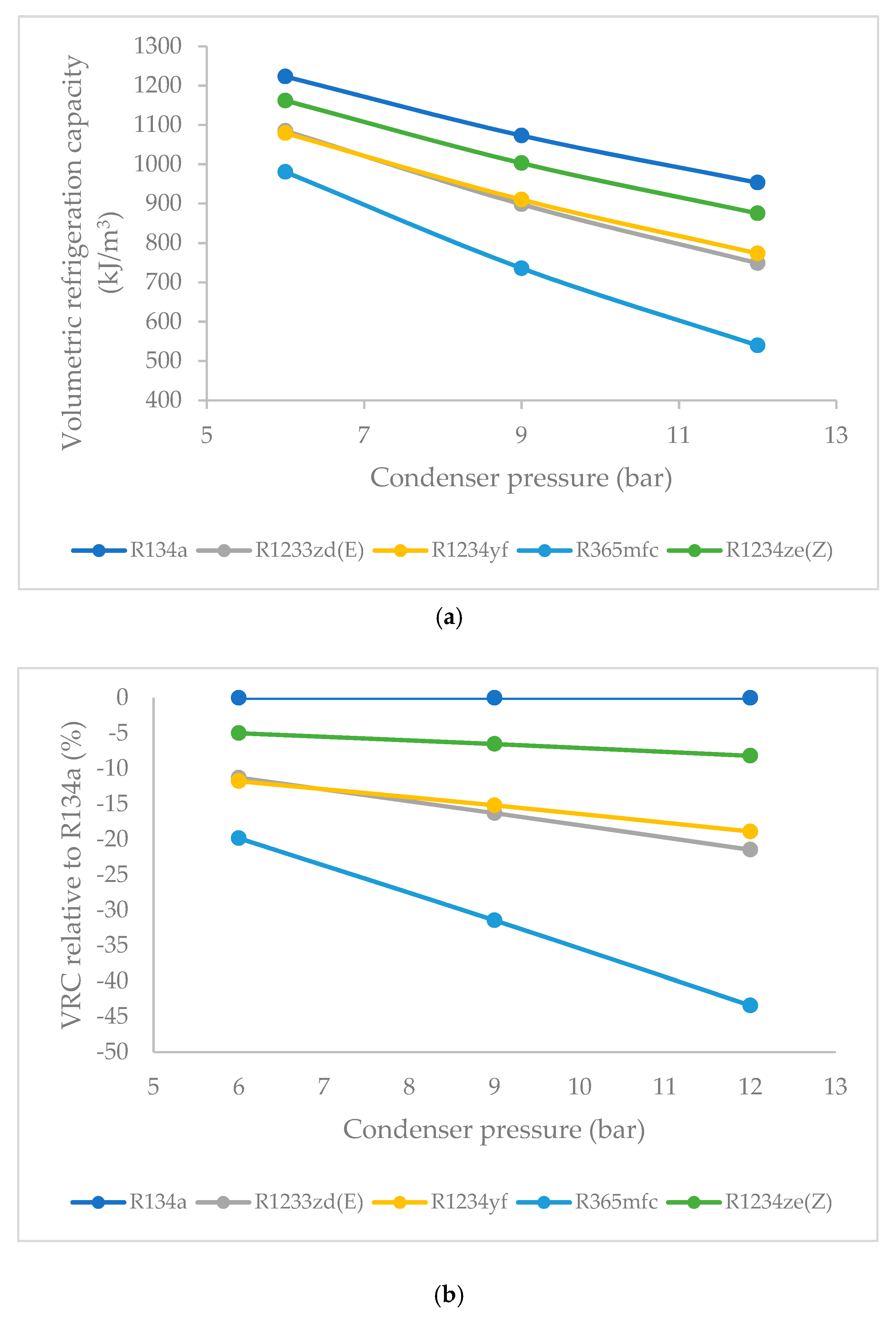
3.7. Effect of Condenser Pressure on Volumetric Refrigeration Capacity (VRC)
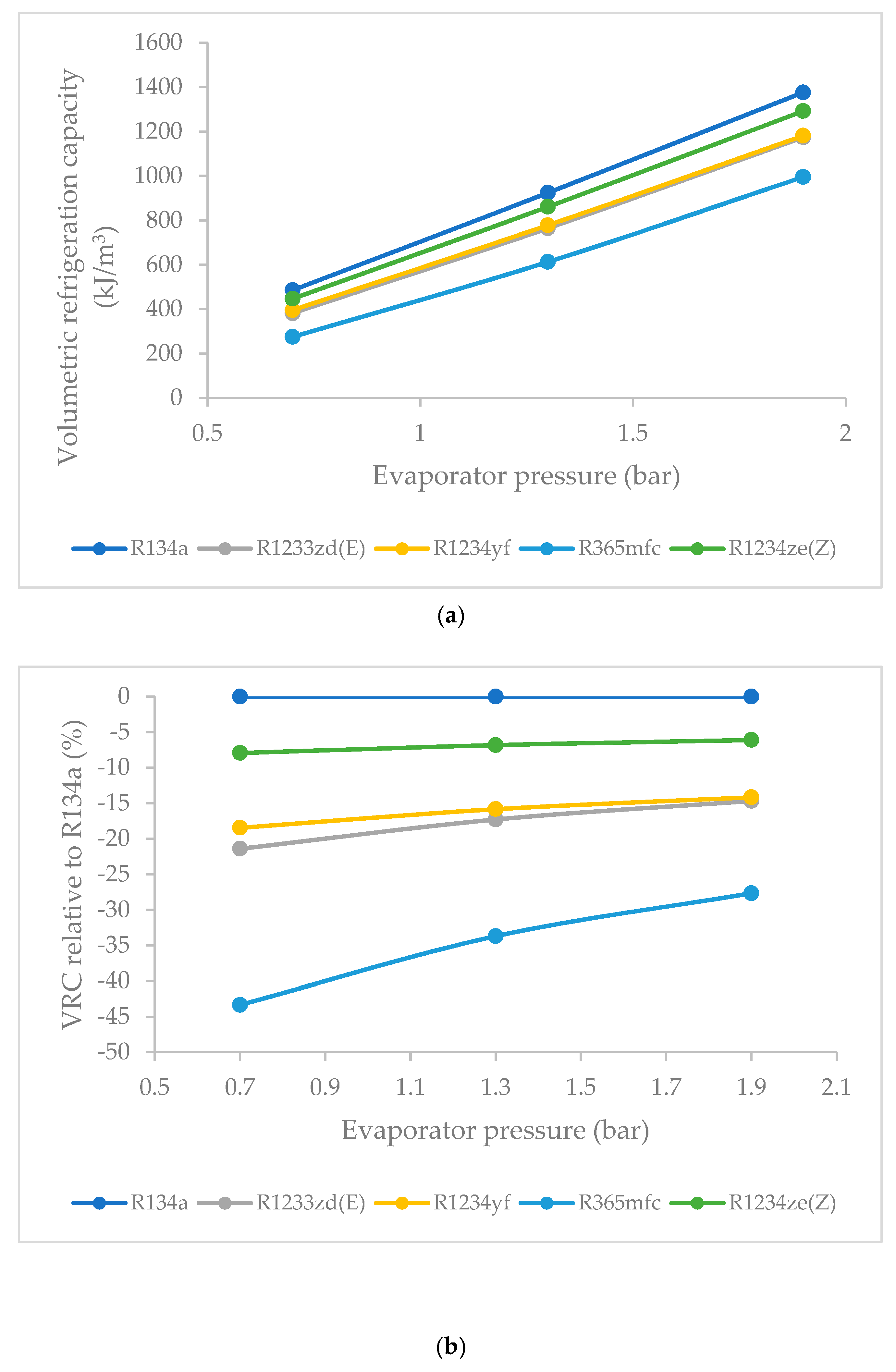
3.8. Effect of Evaporator Pressure on Volumetric Refrigeration Capacity (VRC)
4. Conclusions
- R134a clearly possessed the highest refrigeration effect, overall, at all condenser pressures. The second refrigerant that performed almost as well as R134a was R1234ze(Z), which is an HFO refrigerant. The refrigeration effect of R1234ze(Z) was less than that of R134a and greater than that of all of the other HFO refrigerants at all condenser pressures. R1233zd(E) fell midway between R1234ze(Z) and R365mfc. As condenser pressure was increased, the refrigeration effect decreased in general. The lowest decrease in refrigeration effect was observed in the case of R1234ze(Z), and the refrigeration effect of R365mfc decreased drastically at higher condenser pressures.
- At a lower evaporator pressure, the highest refrigeration effect among the HFO refrigerants was observed for R1234ze(Z), followed by those of R1234yf and R1233zd(E). R365mfc had the lowest refrigeration effect among all refrigerants. This trend remained the same at a higher evaporator pressure. As evaporator pressure increased, the refrigeration effect of all refrigerants approached that of the reference refrigerant R134a. The refrigeration effect of R1234ze(Z) increased steadily, while it increased rapidly for R365mfc, though it lagged behind all refrigerants at all evaporator pressures.
- The compressor work input did not significantly vary against condenser pressure. The base refrigerant R134a clearly drew highest compressor work input at all condenser pressures, followed by R1234ze(Z), R134yf and R1233zd(E). The lowest power consumption among the HFO refrigerants was observed in the case of R365mfc. When condenser pressure was increased, the compressor work input increased and approached that of R134a.
- The compressor work input was highest for R1234ze(Z) among the HFO refrigerants, followed by those of R1234yf R1233zd(E). R365mfc offered the lowest compressor work input among the HFO refrigerants, but its refrigeration effect was also the lowest of all. As the evaporator pressure was increased, the compressor work input of all refrigerants increased.
- Overall, the COP is highest for R134a, and, among all the HFO refrigerants, the COP is highest for R1234ze(Z) and decreased steadily at higher condenser pressures. When the condenser pressure was increased, the COP decreased for all refrigerants in general. This decrease was quite slow for R1234ze(Z), and it was relatively rapid for R1233zd(E) and R1234yf. For R365mfc, the COP dropped drastically at higher condenser pressures.
- The COP was highest for R1234ze(Z) among the HFO refrigerants, and lowest for R365mfc at all evaporator pressures. The COP of all refrigerants tended to increase when the evaporator pressure was increased. The COP of R365mfc increased rapidly but still lagged behind those of all other refrigerants at all evaporator pressures.
- The VRC of R1234ze(Z) was highest at 6 bars of condenser pressure, the VRC of R1234yf and R1233zd(E) were nearly equal, and the lowest values were obtained in the case of R365mfc. As the condenser pressure was increased, the VRC decreases linearly. The decrease was lowest for R1234ze(Z) and the highest for R365mfc at higher condenser pressures.
- R1234ze(Z) had the highest VRC among the HFO refrigerants at all evaporator pressures, followed by those of R1234yf, R1233zd(E) and R365mfc. Though R1234ze(Z) required a higher compressor work input, it also had higher refrigeration effects. The VRC of refrigerants increased rapidly as the evaporator pressure was increased. This increase was sharp for R365mfc and relatively slow for R1234yf and R1233zd(E).
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| GWP | Global warming potential |
| CFCs | Chlorofluorocarbons |
| HCFCs | Hydrochlorofluorocarbons |
| HFCs | Hydrofluorocarbons |
| HFOs | Hydrofluoroolefens |
| VCRC | Vapor compression refrigeration cycle |
| VRC | Volumetric refrigeration capacity |
| EES | Engineering equation solver |
| NBP | Normal boiling point |
| ASH | Reference state for ASHRAE |
| IIR | International Institute of Refrigeration |
| COP | Coefficient of performance |
| HVAC | Heating ventilation and air-conditioning system |
| List of symbols | |
| ρ | Density of refrigerant |
| η | Isentropic efficiency of compressor |
| Qevap | Refrigeration effect of evaporator |
| Pcomp | Compressor power input |
| COP | Improvement in coefficient of performance |
| m· | Mass flow rate of refrigerant |
| h | Refrigerant specific enthalpy |
| N | Compressor speed |
| Dcomp | Compressor displacement |
| P | Refrigerant pressure |
| Additional subscripts | |
| S | Isentropic process |
| 1, 2, 3, … | State points on property diagram |
References
- Usman, M.; Hayat, N. Lubrication, emissions, and performance analyses of LPG and petrol in a motorbike engine: A comparative study. J. Chin. Inst. Eng. 2020, 43, 47–57. [Google Scholar] [CrossRef]
- Farooq, M.; Saeed, M.; Imran, M.; Uddin, G.; Asim, M.; Bilal, H.; Younas, M.; Andresen, J.M. CO2 capture through electro-conductive adsorbent using physical adsorption system for sustainable development. Environ. Geochem. Health 2019, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kashif, M.; Awan, M.; Nawaz, S.; Amjad, M.; Talib, B.; Farooq, M.; Nizami, A.; Rehan, M. Untapped renewable energy potential of crop residues in Pakistan: Challenges and future directions. J. Environ. Manag. 2020, 256, 109924. [Google Scholar] [CrossRef] [PubMed]
- Abas, N.; Kalair, A.R.; Khan, N.; Haider, A.; Saleem, Z.; Saleem, M.S. Natural and synthetic refrigerants, global warming: A review. Renew. Sustain. Energy Rev. 2018, 90, 557–569. [Google Scholar] [CrossRef]
- Kharal, H.S.; Kamran, M.; Ullah, R.; Saleem, M.Z.; Alvi, M.J. Environment-Friendly and Efficient Gaseous Insulator as a Potential Alternative to SF6. Processes 2019, 7, 740. [Google Scholar] [CrossRef]
- Zhang, D.; Gao, Z. Improvement of Refrigeration Efficiency by Combining Reinforcement Learning with a Coarse Model. Processes 2019, 7, 967. [Google Scholar] [CrossRef]
- Usman, M.; Hayat, N. Use of CNG and Hi-octane gasoline in SI engine: A comparative study of performance, emission, and lubrication oil deterioration. Energy Sources Part A Recovery Util. Environ. Eff. 2019, 1–15. [Google Scholar] [CrossRef]
- The Effects of Climate Change; NASA: Washington, DC, USA. Available online: https://climate.nasa.gov/effects/ (accessed on 30 December 2019).
- ASHRAE. New Developments in Lower GWP Refrigerants (MENA)—HVACR Expo; ASHRAE: Dubai, UAE, 2018; Available online: https://www.hvacrexpodubai.com/media/1681/ashrae.pdf (accessed on 30 December 2019).
- Steffen, J.; Bernath, P.F.; Boone, C.D. Trends in halogen-containing molecules measured by the Atmospheric Chemistry Experiment (ACE) satellite. J. Quant. Spectrosc. Radiat. Transf. 2019, 238, 106619. [Google Scholar] [CrossRef]
- Protocol, M. Montreal Protocol on Substances that Deplete the Ozone Layer; US Government Printing Office: Washington, DC, USA, 1987; Volume 26, pp. 128–136.
- Nielsen, S.; Christensen, S.W.; Thorsen, R.S.; Elmegaard, B. Comparison of Heat Pump Design and Performance for Modern Modern Refrigerants. In Proceedings of the 13th IIR Gustav Lorentzen Conference on Natural Refrigerants (GL2018), Valencia, Spain, 18–20 June 2018; pp. 307–314. [Google Scholar]
- Reddy, V.S.; Panwar, N.; Kaushik, S.C. Exergetic analysis of a vapour compression refrigeration system with R134a, R143a, R152a, R404A, R407C, R410A, R502 and R507A. Clean Technol. Environ. Policy 2012, 14, 47–53. [Google Scholar] [CrossRef]
- Mussati, S.F.; Mansouri, S.S.; Gernaey, K.V.; Morosuk, T.; Mussati, M.C. Model-Based Cost Optimization of Double-Effect Water-Lithium Bromide Absorption Refrigeration Systems. Processes 2019, 7, 50. [Google Scholar] [CrossRef]
- Wang, Y.; Velswamy, K.; Huang, B. A long-short term memory recurrent neural network based reinforcement learning controller for office heating ventilation and air conditioning systems. Processes 2017, 5, 46. [Google Scholar] [CrossRef]
- James, M.; Calm, P.E. Refrigerant options for centrifugal chillers. In Proceedings of the 2004 International Workshop on Ozone Depleting Substance Institute Technologies Xian China, Xi’an, China, 17 September 2004. [Google Scholar]
- New Refrigerants Impact Standards and Codes; Carrier Engineering Newsletter; Volume 3. Issue 2. Available online: https://dms.hvacpartners.com/docs/1001/Public/0E/ENG_NEWS_3_2.pdf (accessed on 30 December 2019).
- Poole, J.E.; Powell, R. Non Ozone Depleting and Low Global Warming Potential Refrigerants for Low Temperature Refrigeration. U.S. Patent 9,023,231, 5 May 2015. [Google Scholar]
- Calm, J.M. The next generation of refrigerants—Historical review, considerations, and outlook. Int. J. Refrig. 2008, 31, 1123–1133. [Google Scholar] [CrossRef]
- Mota-Babiloni, A.; Navarro-Esbrí, J.; Barragán-Cervera, Á.; Molés, F.; Peris, B. Experimental study of an R1234ze (E)/R134a mixture (R450A) as R134a replacement. Int. J. Refrig. 2015, 51, 52–58. [Google Scholar] [CrossRef]
- The Kyoto Protocol and Greenhouse Gas Emissions; United Nations: Kyoto, Japan, 1999. Available online: https://www.aph.gov.au/Help/404?item=%2f...%2fhouse_of_representatives_committees&user=extranet%5cAnonymous&site=website (accessed on 30 December 2019).
- Alahmer, A.; Alsaqoor, S. Simulation and optimization of multi-split variable refrigerant flow systems. Ain Shams Eng. J. 2018, 9, 1705–1715. [Google Scholar] [CrossRef]
- Finally, a Replacement for R123? Available online: https://www.coolingpost.com/world-news/finally-a-replacement-for-r123/ (accessed on 30 December 2019).
- Environmental Protection Agency. Summary Guide to the HFC Phase Down. 2015. Available online: https://www.epa.ie/pubs/advice/air/ods/6%20%20IRL%20Summary%20Guide%20to%20the%20HFC%20Phase%20Down%20V1.0.pdf (accessed on 30 December 2019).
- Usman, M.; Farooq, M.; Naqvi, M.; Saleem, M.W.; Hussain, J.; Naqvi, S.R.; Jahangir, S.; Usama, J.; Muhammad, H.; Idrees, S.; et al. Use of Gasoline, LPG and LPG-HHO Blend in SI Engine: A Comparative Performance for Emission Control and Sustainable Environment. Processes 2020, 8, 74. [Google Scholar] [CrossRef]
- Lavrenchenko, G.K.; Ruvinskij, G.Y.; Iljushenko, S.V.; Kanaev, V.V. Thermophysical properties of refrigerant R134a. Int. J. Refrig. 1992, 15, 386–392. [Google Scholar] [CrossRef]
- Thermodynamic Properties of HCFC-123; SUVA 123 Refrigerant. Germany. Available online: https://www.chemours.com/Refrigerants/en_US/assets/downloads/h47753_hcfc123_thermo_prop_eng.pdf (accessed on 30 December 2019).
- Hulse, R.J.; Basu, R.S.; Singh, R.R.; Thomas, R.H. Physical properties of HCFO-1233zd (E). J. Chem. Eng. Data 2012, 57, 3581–3586. [Google Scholar] [CrossRef]
- Thermodynamic Properties of DuPont Opteon-yf SI Units; France, 2019. Available online: http://www.gasco-france.com/fichs/12683.pdf (accessed on 30 December 2019).
- Marrucho, I.M.; Oliveira, N.S.; Dohrn, R. Vapor-phase thermal conductivity, vapor pressure, and liquid density of R365mfc. J. Chem. Eng. Data 2002, 47, 554–558. [Google Scholar] [CrossRef]
- Solstice® ze Refrigerant (HFO-1234ze). Available online: https://www.honeywell-refrigerants.com/europe/product/solstice-1234ze/ (accessed on 30 December 2019).
- Qamar, A.; Farooq, M.; Amjad, M.; Bilal, H.; Sultan, M.M. Experimental Investigation of Heat Sink Configuration Effect on the COP of Thermoelectric Refrigeration System for Storage of Polio Vaccine. J. Faculty Eng. Technol. 2016, 23, 1–10. [Google Scholar]
- Eyerer, S.; Wieland, C.; Vandersickel, A.; Spliethoff, H. Experimental study of an ORC (Organic Rankine Cycle) and analysis of R1233zd-E as a drop-in replacement for R245fa for low temperature heat utilization. Energy 2016, 103, 660–671. [Google Scholar] [CrossRef]
- Gomaa, A. Performance characteristics of automotive air conditioning system with refrigerant R134a and its alternatives. Int. J. Energy Power Eng. 2015, 4, 168. [Google Scholar] [CrossRef]
- Mota-Babiloni, A.; Navarro-Esbrí, J.; Barragán, Á.; Molés, F.; Peris, B. Drop-in energy performance evaluation of R1234yf and R1234ze(E) in a vapor compression system as R134a replacements. Appl. Therm. Eng. 2014, 71, 259–265. [Google Scholar] [CrossRef]
- Aized, T.; Hamza, A. Thermodynamic Analysis of Various Refrigerants for Automotive Air Conditioning System. Arab. J. Sci. Eng. 2019, 44, 1697–1707. [Google Scholar] [CrossRef]
- Aprea, C.; Greco, A.; Maiorino, A.; Masselli, C.; Metallo, A. HFO1234ze as Drop-in Replacement for R134a in Domestic Refrigerators: An Environmental Impact Analysis. Energy Procedia 2016, 101, 964–971. [Google Scholar] [CrossRef]
- Bolaji, B.O.; Komolafe, O.D.; Ajayi, F.O. Performance Assessment of three Eco-Friendly Hydro-fluorocarbon and Hydrocarbon refrigerant mixtures as R22 alternatives in Refrigeration Systems. Middle East J. Sci. Res. 2015, 23, 1677–1684. [Google Scholar]
- McLinden, M.O.; Brown, J.S.; Brignoli, R.; Kazakov, A.F.; Domanski, P.A. Limited options for low-global-warming-potential refrigerants. Nat. Commun. 2017, 8, 14476. [Google Scholar] [CrossRef] [PubMed]
- Raveendran, P.S.; Sekhar, S.J. Energy and exergy analysis on hydrofluoroolefin/hydrofluorocarbon (HFO/HFC) refrigerant mixtures in low and medium temperature small-scale refrigeration systems. Proc. Inst. Mech. Eng. Part E J. Process Mech. Eng. 2019. [Google Scholar] [CrossRef]
- Sánchez, D.; Cabello, R.; Llopis, R.; Arauzo, I.; Catalán-Gil, J.; Torrella, E. Energy performance evaluation of R1234yf, R1234ze (E), R600a, R290 and R152a as low-GWP R134a alternatives. Int. J. Refrig. 2017, 74, 269–282. [Google Scholar] [CrossRef]
- Sethi, A.; Becerra, E.V.; Motta, S.Y. Low GWP R134a replacements for small refrigeration (plug-in) applications. Int. J. Refrig. 2016, 66, 64–72. [Google Scholar] [CrossRef]
- McLinden, M.O.; Kazakov, A.F.; Steven Brown, J.; Domanski, P.A. A thermodynamic analysis of refrigerants: Possibilities and tradeoffs for Low-GWP refrigerants. Int. J. Refrig. 2014, 38, 80–92. [Google Scholar] [CrossRef]
- Domanski, P.A.; Brignoli, R.; Brown, J.S.; Kazakov, A.F.; McLinden, M.O. Low-GWP refrigerants for medium and high-pressure applications. Int. J. Refrig. 2017, 84, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Uddin, K.; Saha, B.B.; Thu, K.; Koyama, S. Low GWP Refrigerants for Energy Conservation and Environmental Sustainability. In Advances in Solar Energy Research; Tyagi, H., Agarwal, A.K., Chakraborty, P.R., Powar, S., Eds.; Springer: Singapore, 2019; pp. 485–517. [Google Scholar]
- ASHRAE. ANSI/ASHRAE Standard 34-2019, Designation and Safety Classification of Refrigerants; ASHRAE: Atlanta, GA, USA, 2019; Available online: https://www.ashrae.org/technical-resources/standards-and-guidelines/standards-addenda/addenda-to-standard-34-2019 (accessed on 30 December 2019).
- Shahzad, M.K.; Rehan, M.A.; Ali, M.; Mustafa, A.; Abbas, Z.; Mujtaba, M.; Akram, M.I.; Yousaf, M. Cooling Performance Assessment of a Slinky Closed Loop Lake Water Heat Pump System under the Climate Conditions of Pakistan. Processes 2019, 7, 553. [Google Scholar] [CrossRef]
- Moshfeghian, D.M. How to Estimate Compressor Efficiency? Available online: http://www.jmcampbell.com/tip-of-the-month/2015/07/how-to-estimate-compressor-efficiency/ (accessed on 30 December 2019).
- Imran, M.; Pambudi, N.A.; Farooq, M. Thermal and hydraulic optimization of plate heat exchanger using multi objective genetic algorithm. Case Stud. Therm. Eng. 2017, 10, 570–578. [Google Scholar] [CrossRef]
- Navarro-Esbrí, J.; Mendoza-Miranda, J.M.; Mota-Babiloni, A.; Barragán-Cervera, A.; Belman-Flores, J.M. Experimental analysis of R1234yf as a drop-in replacement for R134a in a vapor compression system. Int. J. Refrig. 2013, 36, 870–880. [Google Scholar] [CrossRef]
- Trott, A.R.; Welch, T. 3—Refrigerants. In Refrigeration and Air Conditioning, 3rd ed.; Trott, A.R., Welch, T., Eds.; Butterworth-Heinemann: Oxford, UK, 2000; pp. 28–35. [Google Scholar]
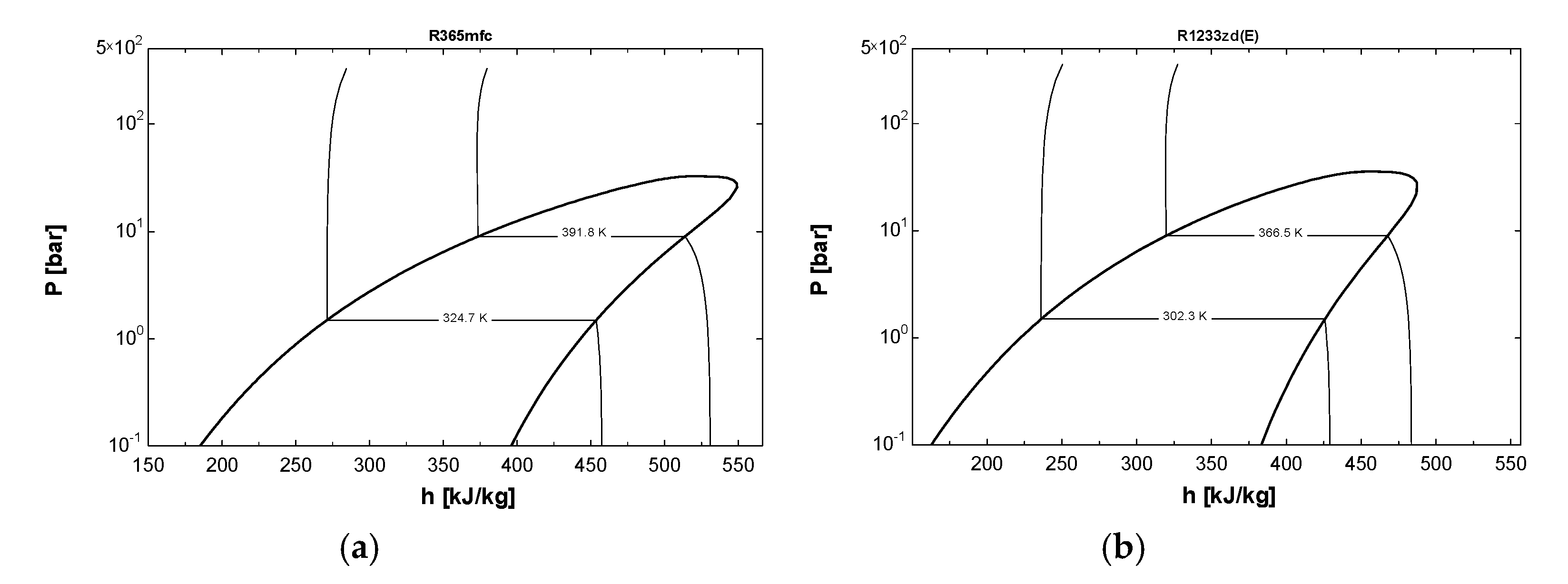
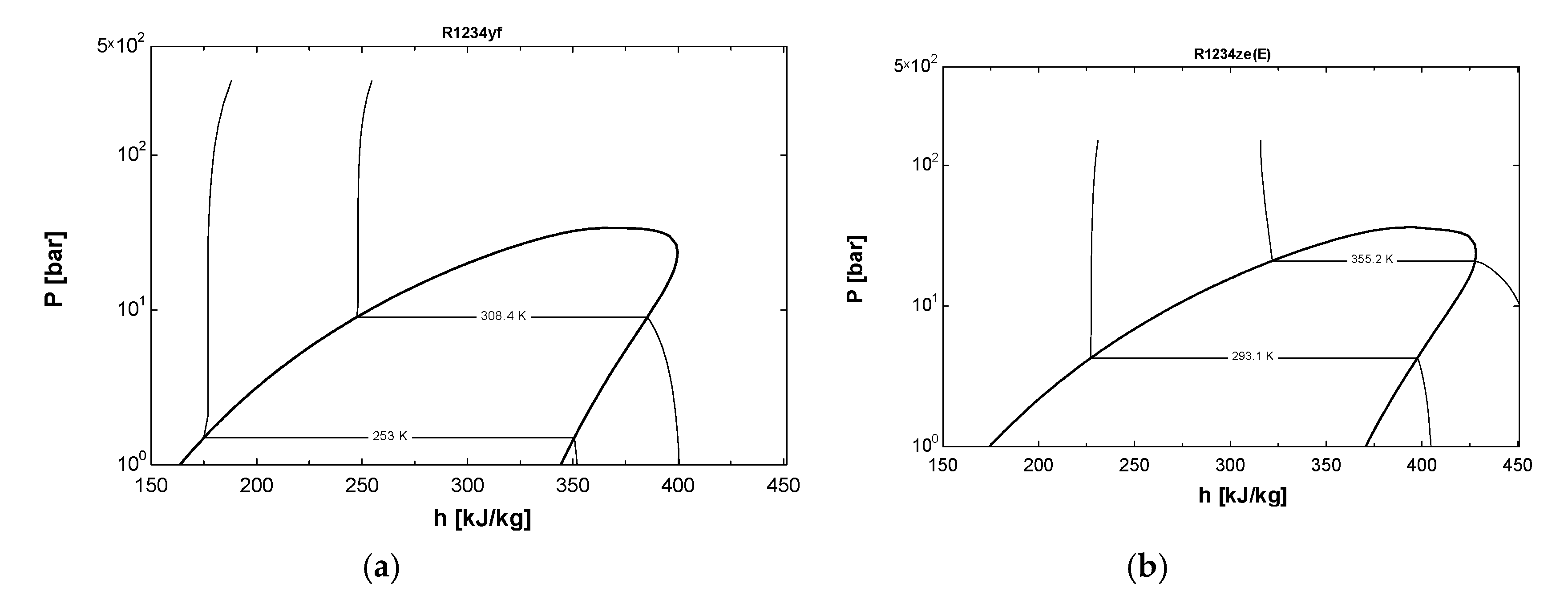

| Thermodynamic Properties of Refrigerants | Refrigerant Category | Molar Mass (g/mol) | Normal Boiling Point (NBP) (°C) | Melting Point (°C) | Vapor Pressure at 20 °C (kPa) | Critical Temperature (°C) | Global Warming Potential (GWP) |
|---|---|---|---|---|---|---|---|
| R134a [26] | HFCs | 102.3 | −26.3 | −142.3 | 571.7 | 101 °C | 1320 |
| R123 [27] | HCFC | 152.93 | 27.85 | −107 | 258.5 | 183.68 | 77 |
| R1233zd(E) [28] | HCFO | 130 | 19 | −107 | 106.3 | 165.7 | <5 |
| R1234yf [29] | HFO | 114.04 | −29.45 | −150 | 592 | 94.7 | 4 |
| R365mfc [30] | HFO | 148 | 40 | <−20 | 47 | 187 | 825 |
| R1234ze(Z) [31] | HFO | 114 | −19.0 | −156 | - | 109 | 6 |
| Toxicity Level | No Flame Propagation | Lower Flammability | High Flammability |
|---|---|---|---|
| Low toxicity | A1 R-22 R-134a R-410A R-404A R1233zd (E) R-407C R/507A R744 CO2 | A2 R-152a A2L * R-32 R1234yf R-1234ze(E) | A3 R-290 Propane R-600a Isobutane |
| High toxicity | B1 R-123 | B2 B2L R-717 Ammonia | B3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farooq, M.; Hamayoun, A.; Naqvi, M.; Nawaz, S.; Usman, M.; Naqvi, S.R.; Imran, M.; Nadeem, R.; Razi, A.; Turan, A.; et al. Thermodynamic Performance Analysis of Hydrofluoroolefins (HFO) Refrigerants in Commercial Air-Conditioning Systems for Sustainable Environment. Processes 2020, 8, 187. https://doi.org/10.3390/pr8020187
Farooq M, Hamayoun A, Naqvi M, Nawaz S, Usman M, Naqvi SR, Imran M, Nadeem R, Razi A, Turan A, et al. Thermodynamic Performance Analysis of Hydrofluoroolefins (HFO) Refrigerants in Commercial Air-Conditioning Systems for Sustainable Environment. Processes. 2020; 8(2):187. https://doi.org/10.3390/pr8020187
Chicago/Turabian StyleFarooq, Muhammad, Ahsan Hamayoun, Muhammad Naqvi, Saad Nawaz, Muhammad Usman, Salman Raza Naqvi, Muhammad Imran, Rida Nadeem, Allah Razi, Ahmet Turan, and et al. 2020. "Thermodynamic Performance Analysis of Hydrofluoroolefins (HFO) Refrigerants in Commercial Air-Conditioning Systems for Sustainable Environment" Processes 8, no. 2: 187. https://doi.org/10.3390/pr8020187
APA StyleFarooq, M., Hamayoun, A., Naqvi, M., Nawaz, S., Usman, M., Naqvi, S. R., Imran, M., Nadeem, R., Razi, A., Turan, A., Pettinau, A., & Andresen, J. M. (2020). Thermodynamic Performance Analysis of Hydrofluoroolefins (HFO) Refrigerants in Commercial Air-Conditioning Systems for Sustainable Environment. Processes, 8(2), 187. https://doi.org/10.3390/pr8020187












