Improved Removal of Quinoline from Wastewater Using Coke Powder with Inorganic Ions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Batch Adsorption Tests
2.2.2. Zeta Potential Measurement
3. Results and Discussion
3.1. Coke Powder Characteristics
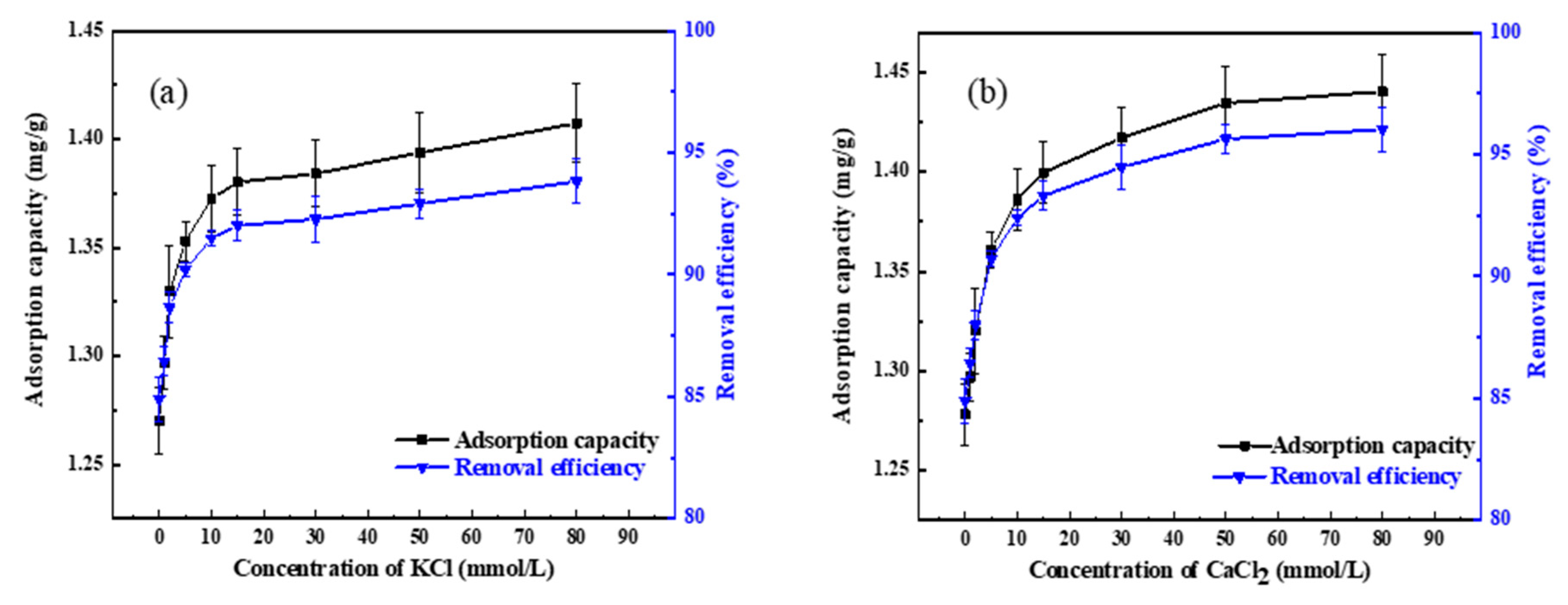
3.2. Quinoline Adsorption by Coke Powder with Inorganic Ions
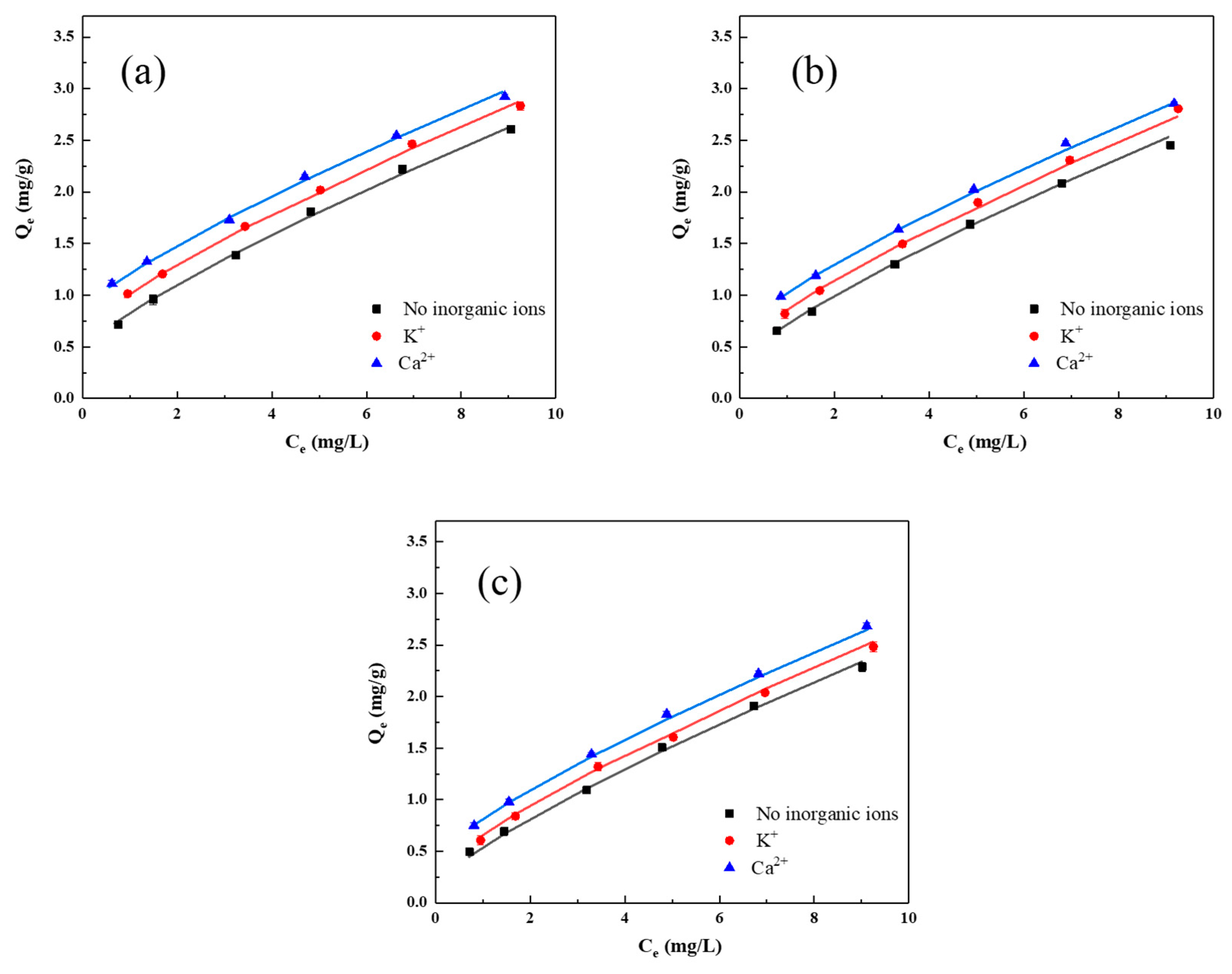
3.3. Adsorption Isotherm Modelling
3.4. Adsorption Thermodynamic Parameters
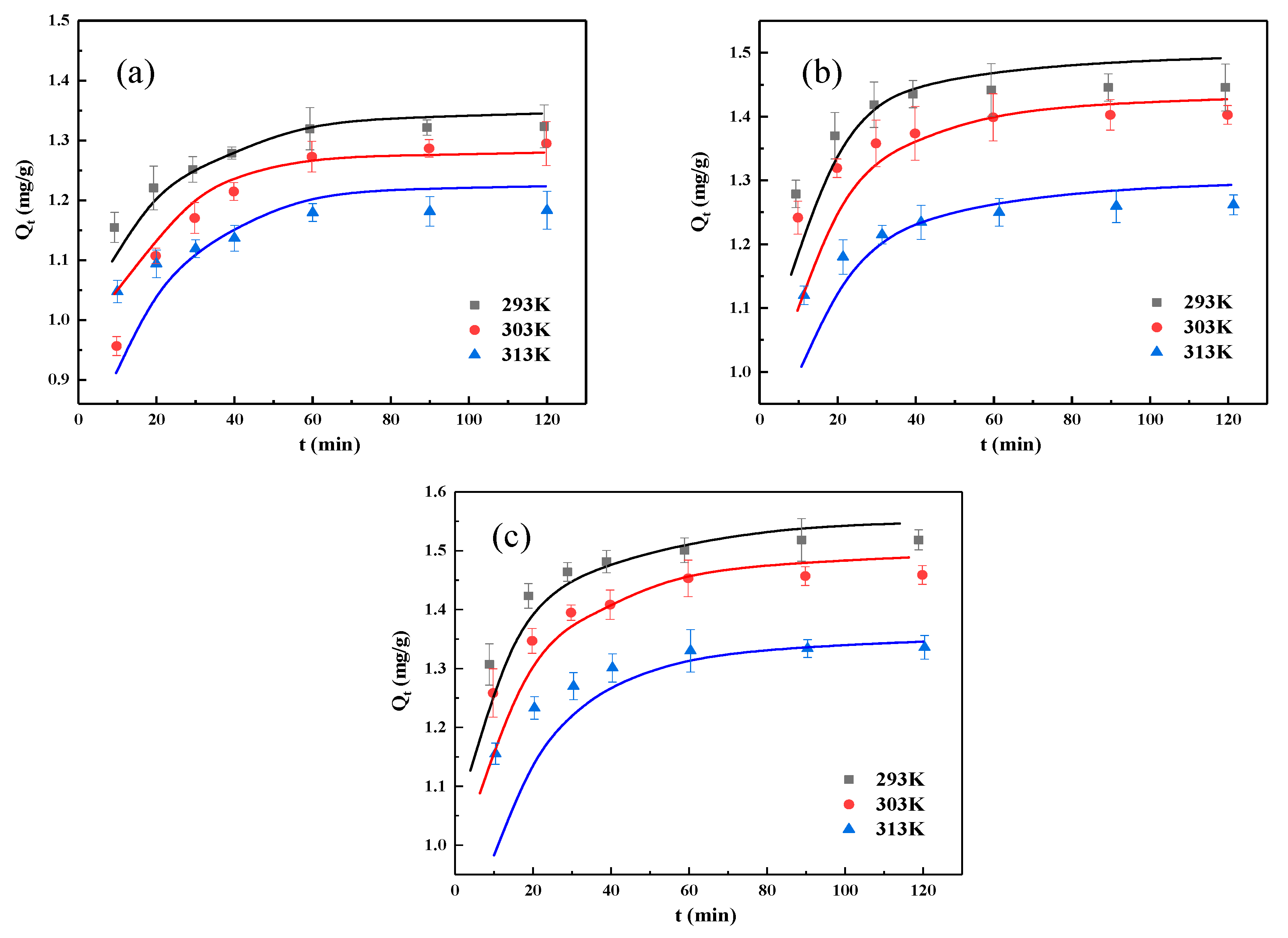
3.5. Adsorption Kinetics
4. Conclusions
- (1)
- Coke powder exhibited a reasonably good adsorption performance in the treatment of aqueous solution containing quinoline due to its pore structure and surface characteristics, and this adsorption could be further improved by the presence of K+ and Ca2+.
- (2)
- Freundlich isotherm was found to best fit the experimental data obtained from the adsorption tests in the absence and presence of inorganic ions. The adsorption thermodynamic analysis for ΔG°, ΔH°, ΔS°, and Ea suggested that adsorption of quinoline onto coke powder was spontaneous and exothermic, which was dominated by physical adsorption. The addition of inorganic ions would increase the absolute values of ΔG° and ΔH°, and decrease the ΔS° and activation energy Ea for qunoline adsorption, suggesting a beneficial effect of K+ and Ca2+ on the quinoline adsorption.
- (3)
- The adsorption of quinoline onto coke powder in the absence and presence of inorganic ions was found to correspond to the pseudo-second-order model, and the presence of K+ and Ca2+ increased the rate of quinoline adsorbed onto coke powder.
- (4)
- K+ and Ca2+ were found to reduce the surface potential of coke powder particles and the thickness of water film surrounding them, facilitating the motion of quinoline molecules onto the coke powder surface. In addition, the reduced surface potential of coke powder particles would also decrease the electrostatic repulsion between coal particles and quinoline molecules, which was favorable for the adsorption of quinoline onto coke powder.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jing, J.Y.; Li, W.Y.; Boyd, A.; Zhang, Y.; Colvin, V.L.; Yu, W.W. Photocatalytic degradation of quinoline in aqueous TiO2 suspension. J. Hazard. Mater. 2012, 237, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Padoley, K.V.; Mudliar, S.N.; Pandey, R.A. Heterocyclic nitrogenous pollutants in the environment and their treatment options—An overview. Bioresour. Technol. 2008, 99, 4029–4043. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.R.; Ma, K.K.; Wu, T.T.; Ye, M.; Tan, P.; Yan, K.C. Electrochemical mineralization pathway of quinoline by boron-Doped diamond anodes. Chemosphere 2016, 149, 219–223. [Google Scholar] [CrossRef]
- Zhu, H.; Ma, W.C.; Han, H.J.; Han, Y.X.; Ma, W.W. Catalytic ozonation of quinoline using Nano-MgO: Efficacy, pathways, mechanisms and its application to real biologically pretreated coal gasification wastewater. Chem. Eng. J. 2017, 327, 91–99. [Google Scholar] [CrossRef]
- Erdogan, S.; Safi, Z.S.; Kaya, S.; Isin, D.O.; Guo, L.; Kaya, C. A computational study on corrosion inhibition performances of novel quinoline derivatives against the corrosion of iron. J. Mol. Strcut. 2017, 1134, 751–761. [Google Scholar] [CrossRef]
- Ferreira, M.E.D.; Vaz, B.G.; Borba, C.E.; Alonso, C.G.; Ostroski, I.C. Modified activated carbon as a promising adsorbent for quinoline removal. Micropor. Mesopor. Mater. 2019, 277, 208–216. [Google Scholar] [CrossRef]
- Burgos, W.; Nipon, P.; Mazzarese, M.C.; Chorover, J. Adsorption of quinoline to kaolinite and montmorillonite. Environ. Eng. Sci. 2002, 19, 59–68. [Google Scholar] [CrossRef]
- Kuang, L.K.; Ming, L.I. Recovering pyridine from industrial waste water by means of continuous distillation. Pesticides 2005, 02, 69–71. [Google Scholar]
- Lataye, D.H.; Mishra, I.M.; Mall, I.D. Removal of pyridine from aqueous solution by adsorption on bagasse fly ash. Ind. Eng. Chem. Res. 2006, 45, 3934–3943. [Google Scholar] [CrossRef]
- Gosu, V.; Gurjar, B.R.; Rao, Y.S.; Zhang, T.C. Treatment of pyridine-Bearing wastewater by nano zero-Valent iron supported on activated carbon derived from agricultural waste. Desalin. Water Treat. 2016, 57, 1–11. [Google Scholar] [CrossRef]
- Padoley, K.V.; Mudliar, S.N.; Banerjee, S.K. Fenton oxidation: A pretreatment option for improved biological treatment of pyridine and 3-Cyanopyridine plant wastewater. Chem. Eng. J. 2011, 166, 1–9. [Google Scholar] [CrossRef]
- Xu, H.X.; Huang, G.; Li, X.B.; Gao, L.H.; Wang, Y.T. Removal of Quinoline from aqueous solutions by lignite, coking coal and anthracite. Adsorption asotherms and thermodynamics. Physicochem. Probl. Miner. 2016, 52, 214–227. [Google Scholar]
- Khasaeva, F.; Vasilyuk, N.; Terentyev, P.; Troshina, M.; Lebedev, A.T. A novel soil bacterial strain degrading pyridines. Environ. Chem. Lett. 2011, 9, 439–445. [Google Scholar] [CrossRef]
- Kim, M.K.; Singleton, I.; Yin, C.R.; Quan, Z.X.; Lee, M.; Lee, S.T. Influence of phenol on the biodegradation of pyridine by freely suspended and immobilized pseudomonas putida mk1. Lett. Appl. Microbiol. 2010, 42, 495–500. [Google Scholar] [CrossRef]
- Mohan, S.V.; Sistla, S.; Guru, R.K.; Prasad, K.K.; Kumar, C.S.; Ramakrishna, S.V. Microbial degradation of pyridine using pseudomonas sp. and isolation of plasmid responsible for degradation. Waste Manag. 2003, 23, 167–171. [Google Scholar] [CrossRef]
- Watson, G.K.; Cain, R.B. Microbial metabolism of the pyridine ring. metabolic pathways of pyridine biodegradation by soil bacteria. Biochem. J. 1975, 146, 157–172. [Google Scholar] [CrossRef]
- Kalebaila, K.K.; Kenneth, J.M.; Misheck, M. Selected Adsorbents for Removal of Contaminants from Wastewater: Towards Engineering Clay Minerals. Open J. Appl. Sci. 2018, 08, 355–369. [Google Scholar]
- Aksu, Z.; Yener, J. A comparative adsorption/biosorption study of mono-Chlorinated phenols onto various sorbents. Waste Manag. 2001, 21, 695–697. [Google Scholar] [CrossRef]
- Badmus, M.A.O.; Audu, T.O.K. Periwinkle shell based granular activated carbon for treatment of chemical oxygen demand (COD) in industrial wastewater. Can. J. Chem. Eng. 2009, 87, 69–71. [Google Scholar] [CrossRef]
- Crini, G. Selected adsorbents for removal of contaminants from wastewater: Towards engineering clay minerals non-Conventional low-Cost adsorbents for dye removal: A review. Bioresour. Technol. 2006, 97, 1061–1085. [Google Scholar] [CrossRef]
- Moon, J.K.; Keum, D.K.; Lee, W.K. Adsorption equilibria for m-Cresol, quinoline, and 1-Naphthol onto silica gel. Korean J. Chem. Eng. 1989, 6, 172–178. [Google Scholar] [CrossRef]
- Zhu, S.; Bell, P.R.F.; Greenfield, P.F. Adsorption of pyridine onto spent Rundle oil shale in dilute aqueous solution. Water Res. 1988, 22, 1331–1337. [Google Scholar] [CrossRef]
- Zhu, S.; Bell, P.R.F.; Greenfield, P.F. Quinoline adsorption onto combusted rundle spent shale in dilute aqueous solution at the natural pH 8. Water Res. 1995, 29, 1393–1400. [Google Scholar] [CrossRef]
- Vico, L.I.; Acebal, S.G. Some aspects about the adsorption of quinoline on fibrous silicates and Patagonian saponite. Appl. Clay Sci. 2006, 33, 142–148. [Google Scholar] [CrossRef]
- Santarossa, G.; Iannuzzi, M.; Vargas, A.; Baiker, A. Adsorption of Naphthalene and quinoline on Pt, Pd and Rh: A DFT study. Chem. Phys. 2008, 9, 401–413. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, G.; Jia, Y.H.; Liu, H.O. Adsorptive removal of nitrogen-Containing compounds from fuel. J. Chem. Eng. Data 2010, 55, 173–177. [Google Scholar] [CrossRef]
- Wang, N.N.; Zhao, Q.; Xu, H.; Niu, W.Y.; Ma, L.; Lan, D.C.; Hao, L.L. Adsorptive treatment of coking wastewater using raw coal fly ash: Adsorption kinetic, thermodynamics and regeneration by fenton process. Chemosphere 2018, 210, 624–632. [Google Scholar] [CrossRef]
- Gao, Q.Y.; Wang, L.; Li, Z.P.; Xie, Y.Q.; He, Q.Q.; Wang, Y.T. Adsorptive removal of pyridine in simulation wastewater using coke powder. Processes 2019, 7, 459. [Google Scholar] [CrossRef]
- Luo, H.M.; Yu, H.M.; Feng, H.X.; Zhang, J.Q.; Zhao, X.; Wang, Y. The mechanism and properties of methylene blue adsorption on modified coke powder. J. China Coal Soc. 2009, 34, 971–976. [Google Scholar]
- Shen, L.F.; Shen, Y.P.; Liu, W.X.; Niu, H.F.; Li, Y. Treatment of coking wastewater by physicochemical-Hydrolytic acidification-A/O combined process. Technol. Water Treat. 2007, 9, 90–93. [Google Scholar]
- Bian, Y.; Sun, H.; Luo, Y.X.; Gao, Q.Y.; Li, G.S.; Wang, Y.T. Effect of inorganic salt ions on the adsorption of quinoline using coal powder. Water Sci. Technol. 2018, 78, 496–505. [Google Scholar] [CrossRef] [PubMed]
- Oldham, K.B. A Gouy-Chapman-Stern model of the double layer at a (metal)/(ionic liquid) interface. J. Electroanal. Chem. 2008, 613, 131–138. [Google Scholar] [CrossRef]
- Zhang, R.; Guo, F.Y.; Xia, Y.C.; Tan, J.L.; Xing, Y.W.; Gui, X.H. Recovering unburned carbon from gasification fly ash using saline water. Waste Manag. 2019, 98, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Arafat, H.A.; Marcus, F.A.; Pinto, N.G. Effect of Salt on the Mechanism of Adsorption of Aromatics on Activated Carbon. Langmuir 1999, 15, 5997–6003. [Google Scholar] [CrossRef]
- Freundlich, H.M.F. Over the adsorption in solution. J. Phys. Chem. 1906, 57, 385–471. [Google Scholar]
- Langmuir, I. The Adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Temkin, M.J.; Pyzhev, V. Kinetics of ammonia synthesis on promoted iron catalysts. Acta Physiochim. URSS. 1940, 12, 217–222. [Google Scholar]
- Kim, Y.S.; Kim, J.H. Isotherm, kinetic and thermodynamic studies on the adsorption of paclitaxel onto Sylopute. J. Chem. Thermodyn. 2019, 130, 104–113. [Google Scholar] [CrossRef]
- Rameshraja, D.; Srivastava, V.C.; Kushwaha, J.P.; Mall, I.D. Quinoline adsorption onto granular activated carbon and bagasse fly ash. Chem. Eng. J. 2012, 181, 343–351. [Google Scholar] [CrossRef]
- Khan, A.A.; Singh, R.P. Adsorption thermodynamics of carbofuran on Sn(IV) arsenosilicate in H+, Na+, and Ca2+ forms. Colloids Surf. 1987, 24, 33–42. [Google Scholar] [CrossRef]
- Shu, Y.H.; Jia, X.S. The mechanisms for CTMAB-Bentonites to adsorb CBs from water in the adsorption kinetics and thermodynamics view. Acta Sci. Circumstantiae 2005, 25, 1530–1536. [Google Scholar]
- Chandra, T.C.; Mirna, M.M.; Suaryanto, Y.; Ismadji, S. Adsorption of basic dye onto activated carbon prepared from durian shell: Studies of adsorption equilibrium and kinetics. Chem. Eng. J. 2007, 127, 121–129. [Google Scholar] [CrossRef]
- Ho, Y.S.; Mckay, G.A. Comparison of chemisorption kinetic models applied to pollutant removal on various sorbents. Process Saf. Environ. Prot. 1998, 76, 332–340. [Google Scholar] [CrossRef]




| Compound | SiO2 | Al2O3 | CaO | Fe2O3 | MgO | TiO2 | K2O |
|---|---|---|---|---|---|---|---|
| (%) | 2.60 | 2.08 | 1.31 | 0.42 | 0.79 | 0.341 | 0.05 |
| Specific Surface Area (m2/g) | Average Pore Size (nm) | Mesoporous Volume (cm3/g) | Total Pore Volume (cm3/g) |
|---|---|---|---|
| 55.65 | 7.45 | 0.029 | 0.030 |
| Isotherms | Equations | Parameters |
|---|---|---|
| Langmuir | KL (L/mg): Langmuir adsorption constant qm (mg/g): monolayer adsorption capacity | |
| Freundlich | KF (L/mg): Freundlich adsorption constant 1/n: parameter for evaluating the level of adsorption | |
| Temkin | T (K): absolute temperature R (8.314*10−3 KJ/(mol*K)): the ideal gas constant KT (L/mg): adsorption constant bT (KJ/mol): a parameter related to adsorption heat |
| Isotherms | Parameters | No Inorganic Ions | KCl | CaCl2 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Temperature (K) | Temperature (K) | Temperature (K) | ||||||||
| 293 | 303 | 313 | 293 | 303 | 313 | 293 | 303 | 313 | ||
| Langmuir | KL (L/mg) | 0.2160 ± 0.0092 | 0.0932 ± 0.0064 | 0.0295 ± 0.0030 | 0.2916 ± 0.0130 | 0.0797 ± 0.0017 | 0.0418 ± 0.0042 | 0.4998 ± 0.0055 | 0.1881 ± 0.0041 | 0.0656 ± 0.0026 |
| qm (mg/g) | 3.6030 ± 0.0498 | 4.7583 ± 0.1846 | 9.7203 ± 0.8711 | 3.3258 ± 0.0882 | 5.7627 ± 0.0778 | 7.7392 ± 0.4763 | 3.0436 ± 0.0185 | 3.9499 ± 0.0610 | 6.3187 ± 0.0918 | |
| R2 | 0.9539 | 0.9933 | 0.9988 | 0.9354 | 0.9908 | 0.9983 | 0.8851 | 0.9664 | 0.9975 | |
| Freundlich | KF (L/mg) | 0.7708 ± 0.0059 | 0.4775 ± 0.0128 | 0.3006 ± 0.0092 | 0.8688 ± 0.0092 | 0.5060 ± 0.0078 | 0.3469 ± 0.0174 | 1.0949 ± 0.0123 | 0.7475 ± 0.0012 | 0.4565 ± 0.0127 |
| 1/n | 0.5281 ± 0.0013 | 0.6974 ± 0.0126 | 0.8745 ± 0.0107 | 0.4843 ± 0.0109 | 0.7148 ± 0.0050 | 0.8261 ± 0.0180 | 0.4025 ± 0.0021 | 0.5594 ± 0.0029 | 0.7482 ± 0.0081 | |
| R2 | 0.9892 | 0.9985 | 0.9970 | 0.9852 | 0.9988 | 0.9983 | 0.9718 | 0.9937 | 0.9998 | |
| Temkin | KT (L/mg) | 5.1109 ± 0.4431 | 1.9851 ± 0.0477 | 1.0946 ± 0.0040 | 5.3997 ± 0.5527 | 1.5638 ± 0.0134 | 1.2089 ± 0.0152 | 9.6520 ± 0.2926 | 2.7686 ± 0.0873 | 1.1937 ± 0.0075 |
| bT (KJ/mol) | 42.5716 ± 1.6799 | 36.6732 ± 0.3924 | 31.9177 ± 0.4899 | 41.3435 ± 1.7288 | 29.8692 ± 0.0695 | 32.0165 ± 0.3123 | 44.5572 ± 0.4651 | 34.3601 ± 0.6169 | 27.8543 ± 0.2673 | |
| R2 | 0.8857 | 0.9283 | 0.9449 | 0.8938 | 0.9351 | 0.9488 | 0.8743 | 0.9257 | 0.9592 | |
| Parameters | No Inorganic Ions | KCl | CaCl2 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Temperature (K) | Temperature (K) | Temperature (K) | |||||||
| 293 | 303 | 313 | 293 | 303 | 313 | 293 | 303 | 313 | |
| lnK | 3.83 | 3.71 | 3.42 | 4.59 | 4.12 | 3.86 | 5.82 | 4.77 | 4.47 |
| ΔH° (kJ/mol) | −6.45 | −13.13 | −16.25 | ||||||
| ΔS° (J/mol·K) | −9.36 | −28.75 | −37.27 | ||||||
| ΔG° (kJ/mol) | −3.50 | −3.62 | −3.73 | −4.56 | −4.72 | −4.87 | −4.79 | −4.96 | −5.12 |
| Kinetic Model | Parameters | No Inorganic Ions | KCl | CaCl2 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Temperature (K) | Temperature (K) | Temperature (K) | ||||||||
| 293 | 303 | 313 | 293 | 303 | 313 | 293 | 303 | 313 | ||
| Pseudo-first-order model | Qe(exp) (mg/g) | 1.26 | 1.24 | 1.22 | 1.38 | 1.35 | 1.31 | 1.42 | 1.41 | 1.37 |
| Qe(cal) (mg/g) | 0.17 | 0.15 | 0.13 | 0.10 | 0.13 | 0.14 | 0.17 | 0.17 | 0.17 | |
| k1 (min−1) | 0.0297 | 0.0290 | 0.0218 | 0.0294 | 0.0274 | 0.0297 | 0.0253 | 0.0301 | 0.0322 | |
| R2 | 0.8746 | 0.8812 | 0.9126 | 0.7947 | 0.8538 | 0.9445 | 0.8973 | 0.8922 | 0.8755 | |
| Pseudo-second-order model | Qe(cal) (mg/g) | 1.27 | 1.11 | 1.21 | 1.31 | 1.37 | 1.32 | 1.45 | 1.43 | 1.39 |
| k2 (g/(mg·min)) | 0.4405 | 0.7232 | 0.7678 | 0.4423 | 0.4662 | 0.5472 | 0.3494 | 0.3670 | 0.4104 | |
| h (mg/(g·min)) | 0.719 | 0.892 | 1.135 | 0.887 | 0.880 | 0.966 | 0.732 | 0.751 | 0.797 | |
| R2 | 0.9641 | 0.9308 | 0.8446 | 0.9802 | 0.9941 | 0.9892 | 0.9973 | 0.9873 | 0.9840 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Gao, Q.; Li, Z.; Wang, Y. Improved Removal of Quinoline from Wastewater Using Coke Powder with Inorganic Ions. Processes 2020, 8, 156. https://doi.org/10.3390/pr8020156
Wang L, Gao Q, Li Z, Wang Y. Improved Removal of Quinoline from Wastewater Using Coke Powder with Inorganic Ions. Processes. 2020; 8(2):156. https://doi.org/10.3390/pr8020156
Chicago/Turabian StyleWang, Lei, Qieyuan Gao, Zhipeng Li, and Yongtian Wang. 2020. "Improved Removal of Quinoline from Wastewater Using Coke Powder with Inorganic Ions" Processes 8, no. 2: 156. https://doi.org/10.3390/pr8020156
APA StyleWang, L., Gao, Q., Li, Z., & Wang, Y. (2020). Improved Removal of Quinoline from Wastewater Using Coke Powder with Inorganic Ions. Processes, 8(2), 156. https://doi.org/10.3390/pr8020156





