Mechanistic Approach to Thermal Production of New Materials from Asphaltenes of Castilla Crude Oil
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Determination of the Molecular Weight of the Asphaltenes
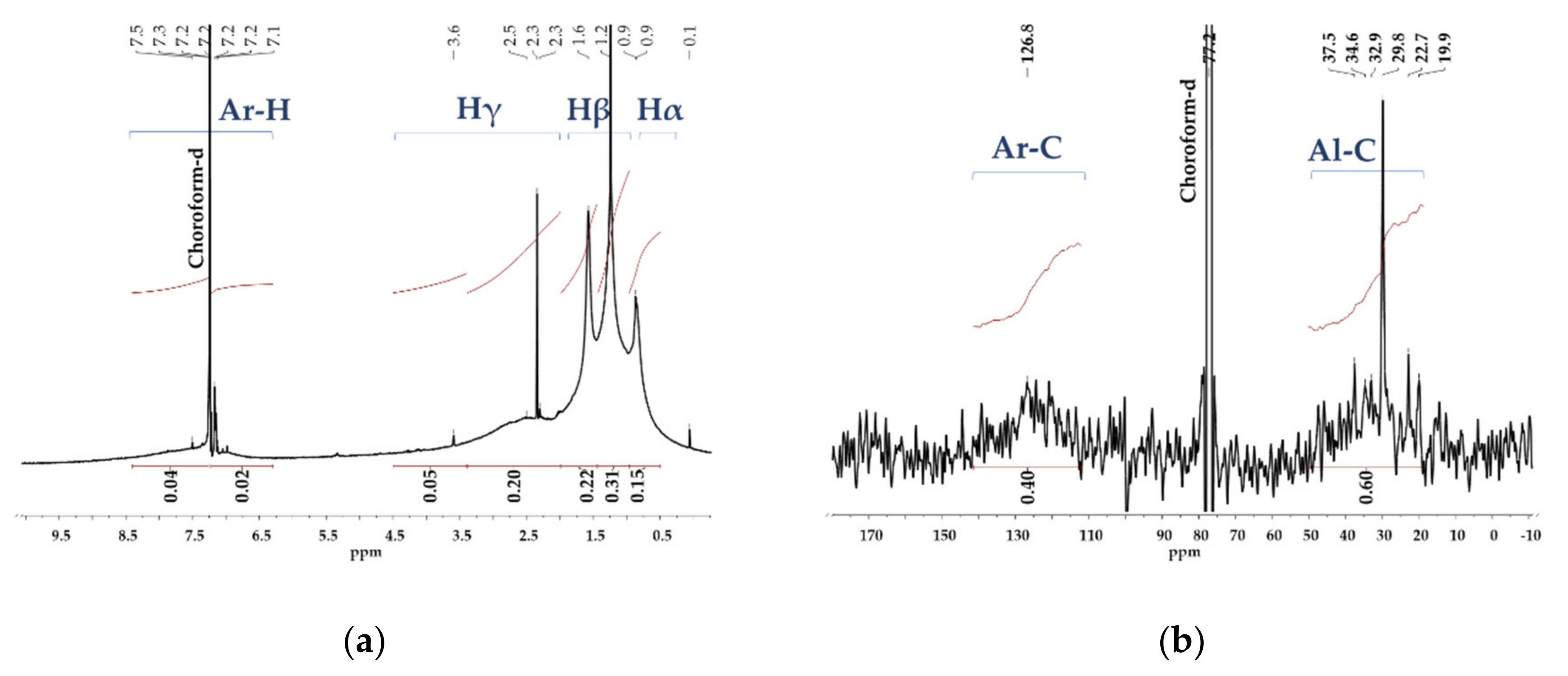
2.3. NMR Analysis of Asphaltene Structure
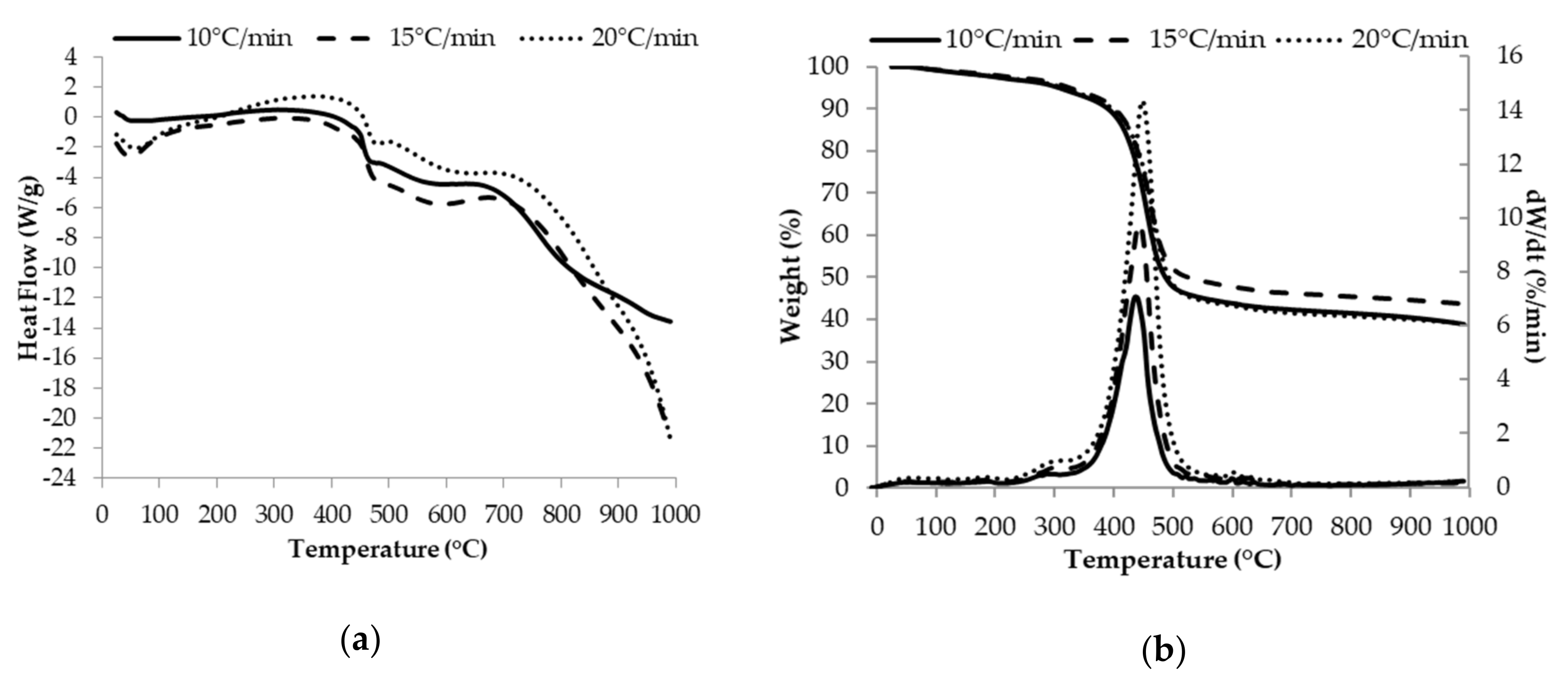
2.4. Thermal Analysis of Asphaltenes
2.5. Pyrolysis of Asphaltenes
2.6. Chemical Composition
2.7. FTIR Analysis
2.8. X-ray Diffraction
2.9. Elemental and SARA Analysis of the Liquid Product
2.10. Gas Chromatography-Mass Spectrometry
3. Results and Discussion
3.1. NMR Analysis of Asphaltene Structure
3.2. Thermal Analysis of Asphaltenes
3.3. Chemical Composition of the Asphaltenes and Cokes
3.4. FTIR Analysis
3.5. XRD Analysis
3.6. Analysis of the Liquid Products
3.7. Analysis of the Gaseous Products
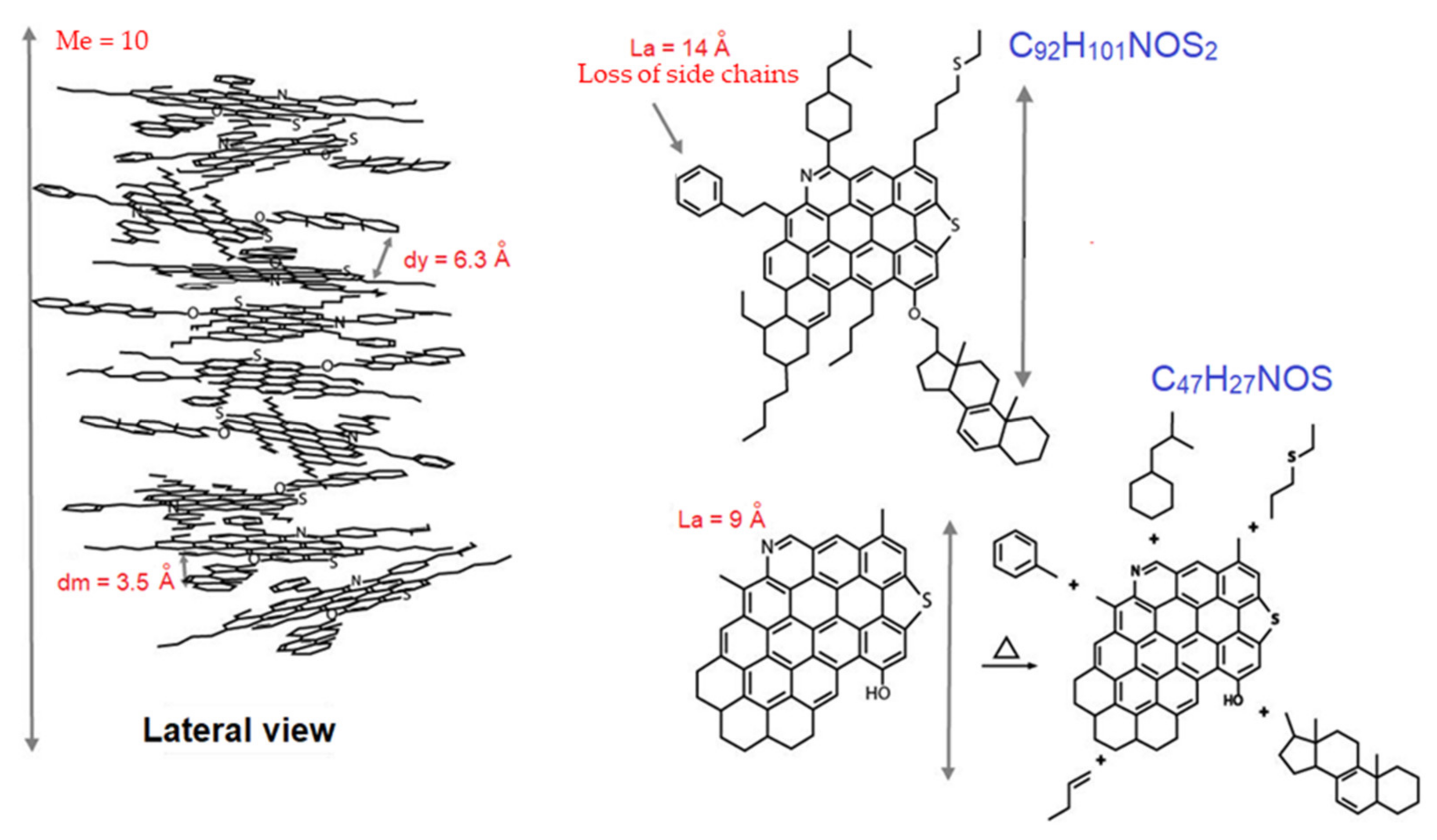
3.8. Hypothetical Structures and Transformation of Asphaltenes during Pyrolysis
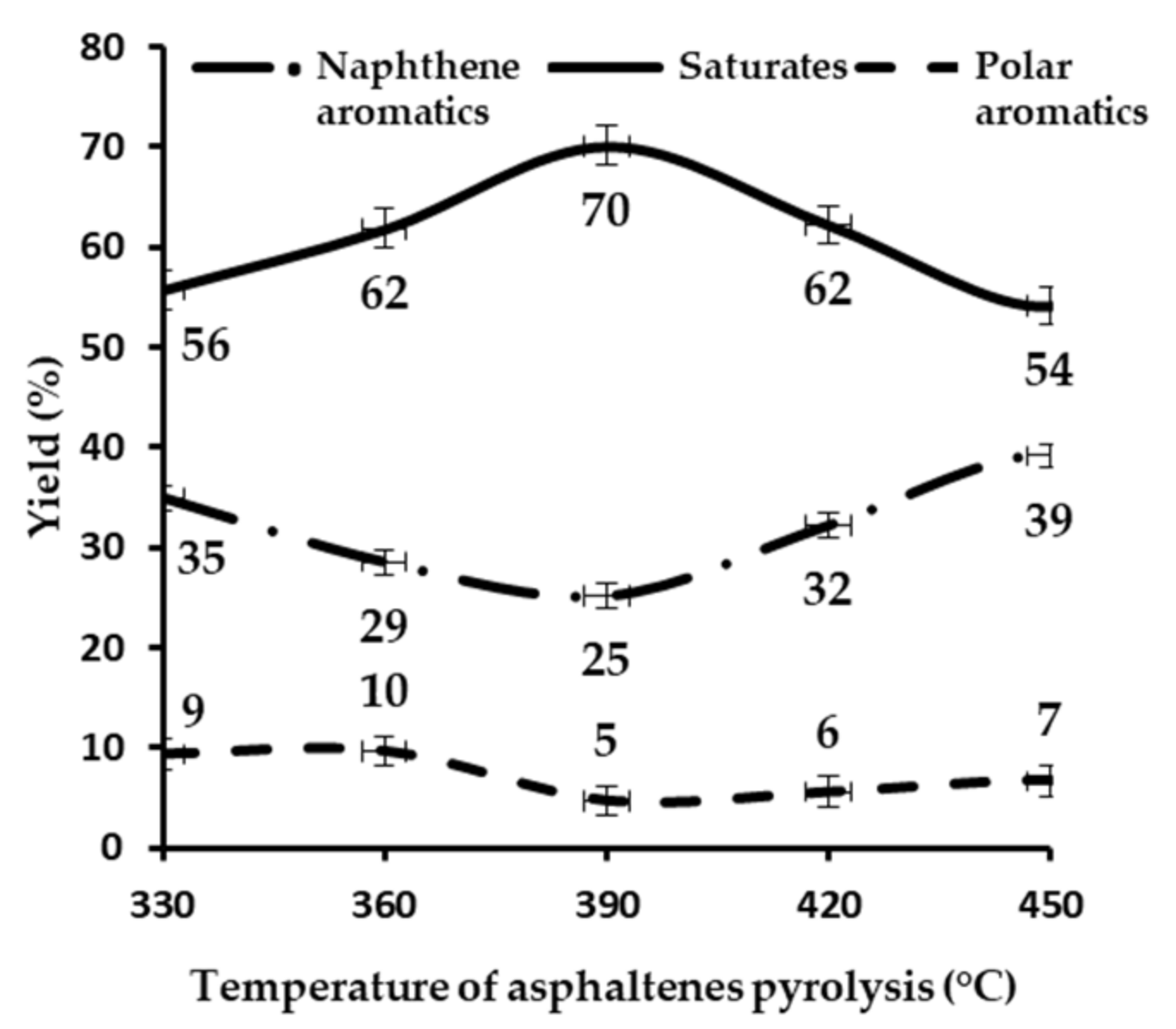
3.9. Pyrolysis of Asphaltenes
3.10. Perspectives
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Liu, Y.J.; Li, Z.F. Structural characterisation of asphaltenes during residue hydrotreatment with light cycle oil as an additive. J. Chem. 2015, 2015, 580950. [Google Scholar] [CrossRef]
- Mullins, O.C.; Sabbah, H.; Pomerantz, A.E.; Andrews, A.B.; Ruiz-morales, Y.; Mostow, F.; Mcfarlane, R.; Goual, L.; Lepkowicz, R.; Cooper, T.; et al. Advances in Asphaltene Science and the Yen—Mullins Model. Energy Fuels 2012, 26, 3986–4003. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, Z.; Zhang, S.; Wang, F.; Yan, Y. Molecular Structure Models of Asphaltene in Crude and Upgraded Bio-Oil. Chem. Eng. Technol. 2014, 37, 1198–1204. [Google Scholar] [CrossRef]
- Shutkova, S.A.; Dolomatov, M.Y.; Dezortsev, S.V. Structural and Chemical Characteristics of Model Molecular Fragments of Petroleum Asphaltenes. Pet. Chem. 2012, 52, 267–271. [Google Scholar] [CrossRef]
- Mullins, O.C. The Modified Yen Model. Energy Fuels 2010, 24, 2179–2207. [Google Scholar] [CrossRef]
- Yen, T.F.; Chilingarian, G.V. Asphaltenes and Asphalts, 2; Elsevier: Amsterdam, The Netherlands, 2000; p. 64. [Google Scholar]
- Zuo, P.; Qu, S.; Shen, W. Asphaltenes: Separations, structural analysis and applications. J. Energy Chem. 2019, 34, 186–207. [Google Scholar] [CrossRef]
- Li, D.D.; Greenfield, M.L. High Internal Energies of Proposed Asphaltene Structures. Energy Fuels 2011, 25, 3698–3705. [Google Scholar] [CrossRef]
- Chacón-Patiño, M.L.; Rowland, S.M.; Rodgers, R.P. Advances in Asphaltene Petroleomics. Part 3. Dominance of Island or Archipelago Structural Motif Is Sample Dependent. Energy Fuels 2018, 32, 9106–9120. [Google Scholar] [CrossRef]
- Díaz-Sánchez, H.; Rojas-Trigos, J.B.; Leyva, C.; Trejo-Zárraga, F. An approach for determination of asphaltene crystallite by X-ray diffraction analysis: A case of study. Pet. Sci. Technol. 2017, 35, 1415–1420. [Google Scholar] [CrossRef]
- Hemmati-Sarapardeh, A.; Ameli, F.; Ahmadi, M.; Dabir, B.; Mohammadi, A.H.; Esfahanizadeh, L. Effect of asphaltene structure on its aggregation behavior in toluene-normal alkane mixtures. J. Mol. Struct. 2020, 1220, 1–10. [Google Scholar] [CrossRef]
- Fergoug, T.; Bouhadda, Y. Determination of Hassi Messaoud Asphaltene Aromatic Structure from 1H & 13C NMR Analysis. Fuel 2014, 115, 521–526. [Google Scholar] [CrossRef]
- Speight, J.G. The Chemistry and Technology of Petroleum, 5th ed.; CRC Press: Boca Raton, FL, USA, 2014; pp. 299–302, 334–339. [Google Scholar]
- Morgan, T.J.; Alvarez-Rodriguez, P.; George, A.; Herod, A.A.; Kandiyoti, R. Characterization of Maya Crude Oil Maltenes and Asphaltenes in Terms of Structural Parameters Calculated from Nuclear Magnetic Resonance (NMR) Spectroscopy and Laser Desorption−Mass Spectroscopy (LD−MS). Energy Fuels 2010, 24, 3977–3989. [Google Scholar] [CrossRef]
- Dickinson, E.M. Structural Comparison of Petroleum Fractions Using Proton and 13C n.m.r. Spectroscopy. Fuel 1980, 59, 290–294. [Google Scholar] [CrossRef]
- Speight, J.G. A Structural Investigation of the Constituents of Athabasca Bitumen by Proton Magnetic Resonance Spectroscopy. Fuel 1970, 49, 76–90. [Google Scholar] [CrossRef]
- Ancheyta, J.; Centeno, G.; Trejo, F.; Speight, J.G. Asphaltene Characterization as Function of Time On-Stream during Hydroprocessing of Maya Crude. Catal. Today 2005, 109, 162–166. [Google Scholar] [CrossRef]
- Savage, P.E.; Klein, M.T.; Kukes, S.G. Asphaltene Reaction Pathways. 1. Thermolysis. Ind. Eng. Chem. Process Des. Dev. 1985, 24, 1169–1174. [Google Scholar] [CrossRef]
- Ruiz-morales, Y. Molecular Orbital Calculations and Optical Transitions of PAHs and Asphaltenes. In Asphaltenes, Heavy Oils, and Petroleomics; Mullins, O.C., Sheu, E.Y., Hammami, A., Marshall, A.G., Eds.; Springer: New York, NY, USA, 2007; pp. 95–137. [Google Scholar] [CrossRef]
- Acevedo, S.; Escobar, G.; Ranaudo, M.A.; Rizzo, A. Molecular Weight Properties of Asphaltenes Calculated from GPC Data for Octylated Asphaltenes. Fuel 1998, 77, 853–858. [Google Scholar] [CrossRef]
- Yasar, M.; Akmaz, S.; Ali Gurkaynak, M. Investigation of glass transition temperatures of Turkish asphaltenes. Fuel 2007, 86, 1737–1748. [Google Scholar] [CrossRef]
- Liu, Y.; Hodek, W.; Heek, K.H. Characterization of tar, char and gas from pyrolysis of coal asphaltenes. Fuel 1998, 77, 1099–1105. [Google Scholar] [CrossRef]
- Pramana, A.; Rachmat, S.; Abdassah, D.; Abdullah, M. A Study of Asphalttene Content of Indonesian. Heavy Oil 2012, 6, 64–73. [Google Scholar] [CrossRef][Green Version]
- Morantes, L.R.; Percebom, A.M.; Mejía-Ospino, E. On the Molecular Basis of Aggregation and Stability of Colombian Asphaltenes and Their Subfractions. Fuel 2019, 241, 542–549. [Google Scholar] [CrossRef]
- AlHumaidan, F.S.; Hauser, A.; Rana, M.S.; Lababidi, H.M.S.; Behbehani, M. Changes in Asphaltene Structure during Thermal Cracking of Residual Oils: XRD Study. Fuel 2015, 150, 558–564. [Google Scholar] [CrossRef]
- Liao, Z.; Zhao, J.; Creux, P.; Yang, C. Discussion on the Structural Features of Asphaltene Molecules. Energy Fuels 2009, 23, 6272–6274. [Google Scholar] [CrossRef]
- Mullins, O.; Sheu, E. Structures and Dynamics of Asphaltenes; Springer: New York, NY, USA, 1999; pp. 87–89. [Google Scholar]
- Bouhadda, Y.; Florian, P.; Bendedouch, D.; Fergoug, T.; Bormann, D. Determination of Algerian Hassi-Messaoud Asphaltene Aromaticity with Different Solid-State NMR Sequences. Fuel 2010, 89, 522–526. [Google Scholar] [CrossRef]
- Sjöblom, J.; Simon, S.; Xu, Z. Model Molecules Mimicking Asphaltenes. Adv. Colloid Interface Sci. 2015, 218, 1–16. [Google Scholar] [CrossRef]
- Zhao, Y.; Wei, F.; Yu, Y. Effects of Reaction Time and Temperature on Carbonization in Asphaltene Pyrolysis. J. Pet. Sci. Eng. 2010, 74, 20–25. [Google Scholar] [CrossRef]
- Gray, M.R.; McCaffrey, W.C. Role of Chain Reactions and Olefin Formation in Cracking, Hydroconversion, and Coking of Petroleum and Bitumen Fractions. Energy Fuels 2002, 16, 756–766. [Google Scholar] [CrossRef]
- Akmaz, S.; Gurkaynak, M.A.; Yasar, M. The Effect of Temperature on the Molecular Structure of Raman Asphaltenes during Pyrolysis. J. Anal. Appl. Pyrolysis 2012, 96, 139–145. [Google Scholar] [CrossRef]
- Alshareef, A.H.; Scherer, A.; Tan, X.; Azyat, K.; Stryker, J.M.; Tykwinski, R.R.; Gray, M.R. Effect of Chemical Structure on the Cracking and Coking of Archipelago Model Compounds Representative of Asphaltenes. Energy Fuels 2012, 26, 1828–1843. [Google Scholar] [CrossRef]
- Savage, P.E. Mechanisms and Kinetics Models for Hydrocarbon Pyrolysis. J. Anal. Appl. Pyrolysis 2000, 54, 109–126. [Google Scholar] [CrossRef]
- Douda, J.; Llanos, M.E.; Alvarez, R.; Navarrete, J. Structure of Maya Asphaltene—Resin Complexes through the Analysis of Soxhlet Extracted Fractions. Energy Fuels 2004, 18, 736–742. [Google Scholar] [CrossRef]
- Yasar, M.; Trauth, D.M.; Klein, M.T. Asphaltene and Resid Pyrolysis. 2. The Effect of Reaction Environment on Pathways and Selectivities. Energy Fuels 2001, 15, 504–509. [Google Scholar] [CrossRef]
- Trauth, D.M.; Yasar, M.; Neurock, M.; Nigam, A.; Klein, M.T.; Kukes, S.G. Asphaltene and Resid Pyrolysis. Effect of Reaction Environment. Fuel Sci. Technol. Int. 1992, 10, 1161–1179. [Google Scholar] [CrossRef]
- Grin’ko, A.A.; Min, R.S.; Sagachenko, T.A.; Golovko, A.K. Aromatic Sulfur-Containing Structural Units of Resins and Asphaltenes in Heavy Hydrocarbon Feedstock. Pet. Chem. 2012, 52, 221–227. [Google Scholar] [CrossRef]



| Parameter | Standard Method | Value |
|---|---|---|
| Humidity (wt %) | American Society for Testing and Materials (ASTM) D5142-09 | 1.76 ± 0.01 |
| Volatile matter (wt %) | ASTM D5142-09 | 57.29 ± 0.01 |
| Ash (wt %) | ASTM D5142-09 | 0.59 ± 0.01 |
| Fixed carbon (wt %) | ASTM D3172-13 | 40.36 ± 0.01 |
| Nickel (mg/kg) | ASTM D5863-00a | 382 ± 1 |
| Vanadium (mg/kg) | ASTM D5863-00a | 1851 ± 1 |
| Average molecular weight (g/mol) | ASTM D5296-19 | 1492 ± 206 |
| Polydispersity index, Mw/Mn | ASTM D5296-19 | 3.8 ± 0.3 |
| Sample Value (%) | Domain (ppm) | Original Asphaltenes |
|---|---|---|
| Aromatic proton, Ar-H (%) | 6.30–9.30 | 6 |
| Aliphatic proton, Al-H (%) | 0.50–4.50 | 94 |
| Aliphatic proton linked to a α carbon, Hα (%) | 0.50–1.00 | 25 |
| Aliphatic proton linked to a β carbon, Hβ (%) | 1.00–1.85 | 54 |
| Aliphatic proton linked to a γ carbon, Hγ (%) | 1.85–4.50 | 15 |
| Aromatic carbons, Ar-C (%) | 118–130.0 | 40 |
| Aliphatic carbons, Al-C (%) | 0.0–70.0 | 60 |
| Peripheric carbons, p-C | 129–137.0 | 26 |
| Internal aromatic carbon, Cin | 118–130.0 | 10 |
| Ratio p-C/Ar-C | - | 0.73 |
| Aromaticity factor, A | - | 0.4 |
| Average chain carbons, n | - | 4 |
| Substitution percentage of the aromatic ring, Arsus (%) | - | 71 |
| T (°C) | C | H | N | O | S | H/C | N/C | O/C | S/C | HHV (J/kg) | CEF | FW (g/mol) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (wt %) * | ||||||||||||
| Asphaltenes | ||||||||||||
| 25 1 | 84 | 7.8 | 1.5 | 1.2 | 4.7 | 1.1 | 0.02 | 0.01 | 0.02 | 39,649 | C91H101NOS2 | 1289 |
| 330 | 85 | 7.6 | 1.5 | 2.0 | 4.2 | 1.1 | 0.02 | 0.02 | 0.02 | 39,336 | C65H70NOS | 913 |
| 360 | 82 | 6.8 | 1.6 | 4.9 | 4.3 | 1.0 | 0.02 | 0.04 | 0.02 | 37,049 | C61H60NOS | 855 |
| 390 | 77 | 4.9 | 1.8 | 7.7 | 4.2 | 0.8 | 0.02 | 0.07 | 0.02 | 32,066 | C50H38NO2S | 717 |
| 420 | 77 | 4.2 | 2.0 | 12.7 | 3.8 | 0.6 | 0.02 | 0.12 | 0.02 | 30,301 | C46H30NO2S | 661 |
| 450 | 64 | 3.2 | 2.0 | 27.7 | 3.7 | 0.6 | 0.03 | 0.32 | 0.02 | 22,474 | C46H27NO3S | 674 |
| Coke | ||||||||||||
| 360 | 84 | 7.1 | 1.7 | 2.8 | 4.4 | 1.0 | 0.02 | 0.03 | 0.05 | 30,286 | C58H58NOS | 817 |
| 390 | 85 | 4.6 | 2.1 | 3.6 | 4.7 | 0.6 | 0.02 | 0.04 | 0.06 | 31,072 | C48H31NO2S | 686 |
| 420 | 85 | 3.9 | 2.1 | 4.6 | 4.4 | 0.5 | 0.02 | 0.05 | 0.05 | 30,889 | C52H28NO2S | 731 |
| 450 | 86 | 3.2 | 1.9 | 5.8 | 3.1 | 0.4 | 0.02 | 0.07 | 0.04 | 30,612 | C53H23NO3S | 754 |
| T (°C) | RA | ACD | DD | ASD | RD |
|---|---|---|---|---|---|
| 25 1 | 0.3 | 0.9 | 1.1 | 0.9 | 0.7 |
| 330 | 0.3 | 0.8 | 0.9 | 0.9 | 0.3 |
| 360 | 0.3 | 0.9 | 1.1 | 0.8 | 0.5 |
| 390 | 1.2 | 0.4 | 0.9 | 0.5 | 1.3 |
| 420 | 2.1 | 0.9 | 0.7 | 1.4 | 1.5 |
| 450 | 2.0 | 1.5 | 1.7 | 0.9 | 1.2 |
| T (°C) | fA | dm (Å) | dγ (Å) | Lc (Å) | Me | La (Å) | WAD * |
|---|---|---|---|---|---|---|---|
| 25 1 | 0.4 | 3.5 | 6.3 | 34 | 10 | 14 | 577 |
| 330 | 0.7 | 3.6 | 6.4 | 29 | 8 | 15 | 694 |
| 360 | 0.8 | 3.6 | 5.9 | 36 | 10 | 13 | 531 |
| 390 | 1.0 | 3.5 | - | 36 | 10 | 9 | 293 |
| 420 | 1.0 | 3.5 | - | 32 | 9 | 9 | 293 |
| T (°C) | C | H | N | O | S | H/C | N/C | O/C | S/C | HHV (J/kg) |
|---|---|---|---|---|---|---|---|---|---|---|
| (wt %) * | ||||||||||
| 330 | 78 | 9.8 | 0.1 | 9.2 | 2.9 | 1.5 | 0.001 | 0.12 | 0.04 | 39,308 |
| 360 | 81 | 11.0 | 0.3 | 4.9 | 2.8 | 1.6 | 0.003 | 0.07 | 0.03 | 42,686 |
| 390 | 82 | 11.0 | 0.3 | 3.7 | 3.0 | 1.6 | 0.003 | 0.05 | 0.04 | 43,199 |
| 420 | 82 | 9.5 | 0.2 | 5.8 | 2.5 | 1.4 | 0.002 | 0.07 | 0.03 | 40,603 |
| 450 | 79 | 8.7 | 0.2 | 9.1 | 3.0 | 1.3 | 0.002 | 0.12 | 0.04 | 38,006 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Afanasjeva, N.; González-Córdoba, A.; Palencia, M. Mechanistic Approach to Thermal Production of New Materials from Asphaltenes of Castilla Crude Oil. Processes 2020, 8, 1644. https://doi.org/10.3390/pr8121644
Afanasjeva N, González-Córdoba A, Palencia M. Mechanistic Approach to Thermal Production of New Materials from Asphaltenes of Castilla Crude Oil. Processes. 2020; 8(12):1644. https://doi.org/10.3390/pr8121644
Chicago/Turabian StyleAfanasjeva, Natalia, Andrea González-Córdoba, and Manuel Palencia. 2020. "Mechanistic Approach to Thermal Production of New Materials from Asphaltenes of Castilla Crude Oil" Processes 8, no. 12: 1644. https://doi.org/10.3390/pr8121644
APA StyleAfanasjeva, N., González-Córdoba, A., & Palencia, M. (2020). Mechanistic Approach to Thermal Production of New Materials from Asphaltenes of Castilla Crude Oil. Processes, 8(12), 1644. https://doi.org/10.3390/pr8121644





