Abstract
Continuous manufacturing of biologics (biopharmaceuticals) has been an area of active research and development for many reasons, ranging from the demand for operational streamlining to the requirement of achieving obvious economic benefits. At the same time, biopharma strives to develop systems and concepts that can operate at similar scales for clinical and commercial production—using flexible infrastructures, such as single-use flow paths and small surge vessels. These developments should simplify technology transfer, reduce footprint and capital investment, and will allow to react readily to changing market pressures while maintaining quality attributes. Despite a number of clearly identified benefits compared to traditional batch processes, continuous bioprocessing is still not widely adopted for commercial manufacturing. This paper details how industry-specific technological, organizational, economic, and regulatory barriers that exist in biopharmaceutical manufacturing are hindering the adoption of continuous production processes. Based on this understanding, the roles of process systems engineering (PSE), process analytical technologies, and process modeling and simulation are highlighted as key enabling tools in overcoming these multi-faceted barriers in today’s manufacturing environment. Of course, we do recognize that there is also a need for a clear set of regulations to guide a transition of biologics manufacturing towards continuous processing. Furthermore, the role played by the emerging fields of process integration and automation as well as digitalization is explored, as these are the tools of the future to facilitate this transition from batch to continuous production. Finally, an outlook focusing on technology, management, and regulatory aspects is presented to identify key concerted efforts required to drive the broad adaptation of continuous manufacturing in biopharmaceutical processes.
1. Introduction: Where Are We Now
There is a growing interest in continuous biologics manufacturing in the pharmaceutical sector, encouraged by regulatory entities such as the Food and Drug Administration (FDA) and the International Council for Harmonization (ICH) [1,2,3,4]. Continuous manufacturing (CM) constitutes the critical aspect of process intensification which endeavors to reduce the time, cost, and complexities of bioprocess development and manufacturing. Several potential benefits are to be expected from its implementation, such as sustained and steady-state operation, the promise of more consistent product quality and high productivity, reduced equipment size, and streamlined process flow, thus lowering operating and capital costs [5,6]. An overall increase in sustainability can, in principle, be achieved by switching to continuous manufacturing, in line with the UN’s Sustainable Development Goals to adopt better and more sustainable processes. However, despite the immense opportunities of continuous over batch operation, technical and operational challenges need to be addressed and overcome for integrated continuous bioprocessing to become a reality. Like any other disruptive “new” technology, besides the scientific element, there are also business and human aspects involved in decision-making that need attention. Of course, some changes are already happening, and people are currently working to develop more of the continuous biomanufacturing technology. However, such development is generally occurring at a much slower pace than expected. The main question to be answered is then why adopting continuous biomanufacturing technology is, in general, extremely slow, despite the obvious potential benefits that can be achieved by its implementation.
This manuscript aims to explore this unknown conundrum behind the slow adoption of continuous processing in commercial biologics manufacturing despite known business benefits, innovations, technical support, and regulatory push (Figure 1).
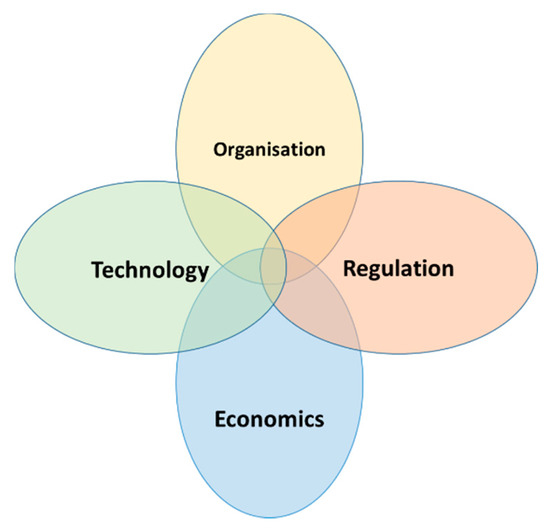
Figure 1.
Key forces that must be taken into consideration when introducing process technologies to biologics manufacturing.
1.1. Critical Views on Current Practices
On the one hand, the manufacturing of biologics is the epitome of the contemporary industry with cutting-edge research and development, resulting in the steady discovery of novel molecules. On the other hand, there are the manufacturing processes of these biologics, which are, even today, to a large extent, based on inefficient batch production platforms. Since biotech production started developing gradually, the first reactors were typically relatively small, inspired, e.g., by the dairy industry. However, throughout the years, the need for reactor capacity and control has been growing steadily, which has resulted in bioreactor vessels that are often up to several hundred cubic meters in capacity. Operating these processes in a batch mode has its advantages. Namely, batch processes require less precise and robust controls to make the desired product than continuous operation. At the same time, the key disadvantages are the inherent inefficiencies of batch fermentation processes. For example, several studies [7,8] have shown that continuous, or for that matter, even semi-continuous, fermentation processes are more efficient than batch processes due to promoting gains both in space-time yield and raw material usage.
To this end, both academia and industry have been deliberating the adoption of continuous biologics manufacturing for over half a century. Challenges with regards to various aspects of operation and control of the biologics process in a continuous mode were identified in this period. A significant number of mechanistic insights translated into several technological advancements. On the other hand, regulatory agencies have also been evolving in support of continuous manufacturing from the FDA guidance on process analytical technologies (PATs) in 2004 for the adoption of a science- and risk-based approach allowing quality by design (QbD) [9] to the recent draft guidance dedicated to continuous manufacturing [2,10]. Despite explicit knowledge, technological advancement, and regulatory support, the implementation of continuous bioprocessing is and has been slow [11]. At best, few unit operations/steps in upstream and downstream have been implemented as continuous processes by a minority of facilities. This, too, is limited to some large-scale biopharmaceutical products, such as antibodies [12], insulin [13], Factor VIII, and coagulation factors [14], which essentially require moderate processing conditions in perfusion systems and external alternating tangential flow (ATF) filtration systems for cell retention [5,15]. Some bio-manufacturers, such as Genzyme, Bayer, and Amgen, with considerable experience using perfusion and single-use systems for commercial products manufacture are leaders in continuous bioprocessing implementation. Several others are still evaluating the commercial feasibility of the technology. Commercial continuous downstream processing, particularly chromatography operations, still remains rare. Optimistically, only one or a few out of the multiple chromatographic steps in multi-step downstream processing having been implemented as continuous operations. It is noteworthy that some processes claim to be “continuous” manufacturing operations. Upon closer inspection, they are found to be parallelized upstream and downstream unit operations that give only the “illusion” of being continuous operations. These processes are cyclical operations with multiple batch units being sequentially scheduled to provide a consistent process flow. In contrast, continuous bioprocessing is expected to contribute to process intensification by offering an opportunity to simplify the processes and, thus, replace complex cyclical batch operations.
1.2. Academic Engagements Supporting the Advancement
The first challenges towards adopting continuous manufacturing in biologics were posed in terms of knowledge gaps—pointing towards long-term stable and sterile cultivation of cells. While continuous processing is beneficial for unstable products that cannot be left in the culture medium over the entire cultivation period, it is not suitable for the vectors’ genetic stability and the producer cell lines. Studies to understand cells’ microenvironment, functioning, and long-term genetic stability within the cells were performed in great detail [16,17]. Similarly, several technological developments lowered the risk of contamination during in situ product removal as in continuous bioprocessing [18,19]. Besides resolving the risks during continuous processing, increasing the historically low upstream drug substance titer in batch biologics manufacturing (<1 g/L) has been an important underlying driver for the exploration of continuous processing in biologics [20]. A solution came with perfusion processes that kept cell counts constant while exchanging culture supernatants with fresh media at regular intervals. However, this solution came with several process systems engineering challenges such as (i) inhomogeneity in the continuous bioreactor vessel, including nutrient shortages or regions of cell accumulation; (ii) maintenance of sterility in the long run; (iii) increase in process development cost and time due to complex continuous culture systems in labs; and (iv) regulatory expectation towards developing a complex control strategy addressing time invariance in a continuous run.
As mentioned, the presence of heterogeneities in the continuous bioreactor vessel continues to be one of the main challenges in the manufacturing of biologics. However, not easy to tackle, new technologies are being developed to investigate and improve this. For example, recently, computational fluid dynamics (CFD) has been the focus of growing interest in the study of mixing, mass transfer, and substrate gradients in (bio)reactors [21]. Furthermore, CFD, when coupled with compartmental modeling, has been shown to be a powerful tool for process design and optimization [22,23]. Another promising approach for the monitoring of real gradients inside the bioreactor is, for example, the use of free-floating sensors [24]. Since in long perfusion processes, membrane fouling and blocking were observed, newer technologies for external cell retention such as ATF filtration were developed and widely adopted [15]. In an ATF system, a feed stream through hollow fibers is regularly reversed to wash off material that can clog the pores. Similarly, with respect to agility in process development and handling the complexity of continuous equipment, innovative PAT techniques (Section 3.2) and process control strategies (including process modeling and advanced process control approaches) (Section 3.3) and robotics and automation (Section 3.2) have been the key focus in academic research bringing new solutions [25,26]. These innovations are expected to simplify operation, improve process robustness, and reduce handling efforts significantly.
Furthermore, due to the inherent multi-step nature of the process, the implementation of continuous operations is complicated in downstream processing. However, downstream processing of biologics usually represents 50–80% of the manufacturing cost, making commercial batch manufacturing very inefficient and expensive [26]. Thus, significant research efforts on the development of continuous downstream processes were made in recent decades to offer improved use of the column capacity and increased resolution efficiency. A better resin capacity use and column lifetime exploitation are particularly essential when expensive protein and resins are involved in the protein capture step of downstream processing. Most of the current studies on continuous downstream processing have been carried out on monoclonal antibodies (mAbs) for two key reasons: first, mAbs comprise the most significant segment of biopharmaceutical products under development and getting ready for commercial manufacturing; second, the use of three chromatography steps—Protein A affinity, cation-exchange (CEX), and anion-exchange (AEX)—already provides a standardized route for achieving high purities and high recoveries, but it is in need of a fresh intensification focus. Therefore, several methods and multi-column systems for continuous liquid chromatography have been developed in recent years, several of which translated into commercially available solutions, such as ÄKTA PCC (GE Healthcare), BioSC® (Novasep), BioSMB® (Pall Life Sciences), and Contichrom® (ChromaCon® AG) [27]. Next to innovation in the established production route, innovative separation technologies have been proposed using computational modeling and simulation. For example, Castilho et al. [28] developed Protein A membrane adsorbers and utilized computational fluid dynamics to design a rotating disk filter.
Besides individual research in academia and industry, several collaborative platforms also aim to address these challenges. A non-exhaustive list of such collaborative platforms enabling development and adoption of continuous manufacturing technologies in bioprocessing is given in Table 1. Some of the key focus points for these groups include accelerating the biopharmaceutical innovation, developing adaptive process control and advanced sensing for robust quality in biomanufacturing, demonstrating the commercial feasibility of new technologies, and enabling the biopharmaceutical manufacturing workforce for new and future manufacturing technologies. Such collaborations are playing an increasingly important role in pushing the accepted state of the art on technology and its knowledge, as well as helping in developing a well-educated workforce that can address the challenges related to the introduction of novel production technology.

Table 1.
Some of the collaborative platforms that enable the development and adoption of new manufacturing technologies such as continuous bioprocessing.
2. What Is Creating This Disjoint
The initial implementation of continuous bioprocessing was slow due to technical challenges. Similar to other technological advancements and new solutions development, it could be argued that the benefits of continuous processing outweigh its challenges and constraints. Hence, even though many biologics firms have been successfully operating continuous manufacturing plants for years, it is esoteric that continuous bioprocessing has not implemented a typical technology lifecycle paradigm. The negative assessment from within the industry, despite known business and quality benefits, lies in many different dimensions of decision making for technology adoption (Figure 1). From a practical point of view, a migration to continuous manufacturing, both during clinical trials and mass manufacturing, requires approval at a senior executive level. However, with the need to maintain quarterly and yearly performance figures, it means that a drive towards continuous manufacturing, which requires a more long-term time horizon to be truly beneficial, becomes a tough sell. As expected, business priorities and decision making are often driven by short-term goals, visible challenges, and deeply entrenched mental models rooted in batch manufacturing technologies (Figure 2).
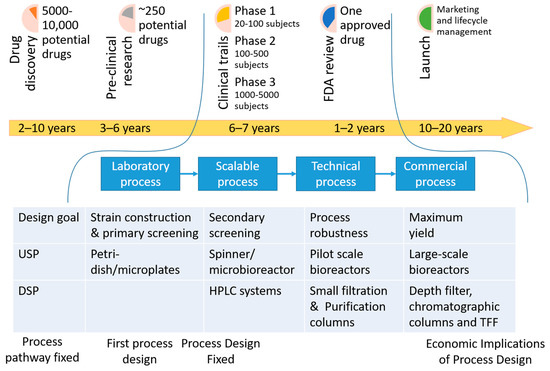
Figure 2.
Lifecycle of a drug from discovery to mass market availability. Abbreviations: DSP (Downstream processing), HPLC (High performance liquid chromatography), TFF (Tangential-flow filtration system), USP (Upstream processing).
In the following section, we look into various dimensions as the cause for the slow adoption of industry’s continuous biologics manufacturing.
2.1. Are Costs or Regulators to Blame
It is often argued that the biopharmaceutical industry will eventually evolve towards continuous processing, similar to the trends observed in other industrial sectors such as oil, food, and paper manufacturing. That is, however, not really happening. Such arguments ignore the considerable differences in the required processing capacity and product value in the manufacturing of biologics. It is safe to say that a biologics plant that processes anywhere near the per-day processing capacity of plants in the aforementioned industrial sectors will never be needed. Thus, a business case never truly stands if driven by the need to be continuous on a capacity basis [5]. In general, much of the cost advantage from continuous processing comes from intensifying the process, resulting in smaller equipment footprints (compared with batch and fed-batch systems) and, consequently, lower capital investments. Simultaneously, smaller facilities are more comfortable to expand or replicate as back-up systems at multiple sites. Moreover, it is essential to realize that biopharmaceutical manufacturing is currently challenged by questions that are very different from the questions faced by the biopharma industry of the past, built on the heritage of blockbuster drugs, and faced during its development and manufacturing. Under development, the current biopharma portfolio is a collection of smaller targeted treatments, requiring a flexible and economical production option for these products instead of hitting a capacity dead-end. Thus, it is crucial to make a correct comparison while building the business case for the continuous manufacturing of biologics.
The early discourse presented regulatory aspects as the biggest hurdle on the road toward the actual implementation of continuous bioprocessing. However, over the past few years, regulatory agencies such as the FDA appeared to be a strong supporter of continuous bioprocessing as it reduces manual interference with product streams and improves process control and quality. In this context, the agency also recommends using QbD principles to establish sufficient process robustness [9]. However, it is still challenging to develop proper scale-down models, which are prerequisites for this approach. While running experiments for this purpose, the prohibitively expensive experimental campaigns associated with media costs alone restrict this [32]. Different strategies are being investigated to avert these costs potentially (Figure 3). For example, Tajsoleiman et al. (2019) [22] explored the use of compartmental modeling by the automatic conversion of fully developed CFD (bio)reactor models into compartment models. This approach is highly efficient and can be used as a strategy to develop a scale-down model of the process/equipment [33].
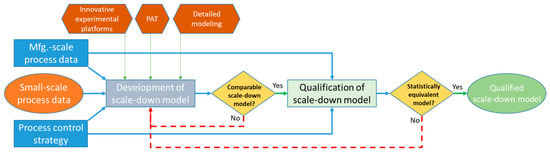
Figure 3.
Scale-down modeling workflow supported by innovative solutions.
Furthermore, for the same purpose, the application of continuous hollow-fiber bioreactors and miniaturized stirred bioreactors (MSBRs) (or small scale reactors in general) as well as microfluidics is currently being explored [34]. While a hollow-fiber system can be as small as 2.5 mL, it is not easy to measure process parameters. On the other side, although easier to measure parameters, the minimum volume for commercially available MSBRs is finally scaling-down to actual low volumes, such as 15 mL and 250 mL volume Ambr® systems [35]. Similarly, a few commercial microfluidic perfusion systems without active mixing are also currently available. Most of these systems struggle with challenges such as the inability to incorporate control of pH and dissolved gases. Tajsoleiman et al. [33] reviewed opportunities, critical parameters, and challenges of implementing MSBRs for scale-down purposes.
2.2. Realization of Flexible and Intensified Manufacturing
Success for a modern biopharma company strongly depends on operational flexibility to adjust production capacity in response to rapidly changing demand forecast, unexpected failures in late-stage clinical trials, or faster than expected clinical adoption. With smart batch definitions, scale-up could be enabled simply by extending a perfusion process’ duration in continuous processing. Capacity use is improved when only a small number of long-duration campaigns are performed, reducing the number of required product change-overs [36]. Moreover, continuous bioprocessing plants are often made specifically for a single process/product, with little or no intention of adapting the same equipment for a different process. However, this translates into an inherently low facility flexibility level with long continuous operation run times. It also limits the degree of flexibility a company has to switch quickly between different products. Process intensification to achieve final titers similar to conventional upstream processes in a shorter timeframe and a modular biomanufacturing platform have been proposed as strategies to regain the degree of flexibility.
Early-stage process development and clinical manufacturing are often critical to getting a new product into clinical trials. Clinical delays due to process development or manufacturing issues can be very costly. Although many firms have successfully operated continuous perfusion for years, it is generally not seen as a more reliable production method than (fed-)batch, especially at the early stage. On the other hand, standard fed-batch culture services are offered by many biologics contract manufacturers. Perfusion culture services are far less common and, even when found, are readily available only with the particular cell retention method already in use by that vendor. The compatibility of that method for a specific process may take time to resolve. Such early-stage challenges often impact early decisions about the most appropriate way to use the available manufacturing technology to meet the demand and timeline. Even at the commercial scale, two significant biopharmaceutical shortages occurred due to commercial production problems using continuous perfusion systems. Therefore, it is crucial to improve design, characterization, and protocol standardization methods to improve continuous bioprocessing predictability and confidence.
2.3. The Dilemma of Technology Evolution—Continuous Stainless Steel vs. Single-Use
Continuous processing equipment manufacturers and users report that many of the problems long associated with perfusion and continuous bioprocessing have been resolved in recent years by applying innovative technologies, including new developments in single-use equipment. All the necessary building blocks for a fully disposable continuous value stream exist—bioreactors, cell-retention devices, prepacked columns, and filters. However, for very high cell densities aimed for titer increase, disposable/single-use bioreactors are not recommended. At some point, in single-use bioreactors, the dissolved oxygen (DO) level might become a limiting factor for high cell densities. In such cases, continuous processing at a sufficient scale is not possible due to limits of oxygen transfer rates and pressure limits of welded seams in the plastic bags. A high cell concentration also generates heat that is difficult to transfer across insulating plastic layers.
While single-use or disposable systems provide much more flexibility, an inevitable concern appears regarding these systems’ environmental sustainability. In a world where people are worried about grocery bags and disposable cups, justifying an industry’s transition from multi-use stainless steel to disposable plastic adds another dimension of concern. Although the waste output from biomanufacturing is only a fraction, appropriate waste disposal procedures are crucial to adopting these systems, especially towards scale-up uses. The process intensification supported by continuous manufacturing can, in fact, help to reduce the footprint and improve sustainability. Thus, although the two technology trends look to be competing, they are mutually enabling the future of bioprocessing. Table 2 compares the benefits and challenges of the two manufacturing platforms—continuous stainless steel vs. single-use systems.

Table 2.
Benefits and challenges that single-use systems can offer compared to stainless steel-based manufacturing platforms.
2.4. Organisational Readiness
From a management perspective, it should be noted that multiple stakeholders are involved in successfully implementing continuous biologics manufacturing—for both clinical trials and, more importantly, consumers. One key stakeholder that is often overlooked is the plant operations department. With the inherent variations present in raw materials and processes, an argument can be made that plant operations would instead prefer to operate batch units (where these variations can be more easily dealt with) instead of dealing with the precise operations required for continuous manufacturing. Besides, operating a new type of process requires plant operations to develop appropriate strategies and train operators to understand and respond to a new set of operational challenges. Thus, onboarding plant operations at an early stage of process design and reducing their burden of a late-stage switch from batch to continuous operations play a major role in gathering a critical mass of support to implement continuous production technologies.
Considering an alternative perspective, it can be argued that for biologic firms focused on drug discovery as the main form of innovation, the introduction of novel production methodologies can be seen as a distraction. This is because the primary “value creation”, i.e., the economic benefit, happens through drug discovery. From an organization’s point of view, the production process is just the “vehicle” used to mass-produce these drugs. It may be argued in such a case that any “economic benefit” that can be achieved by improved production is minor in comparison to drug discovery, and the pursuit of such improvements can “jeopardize” the focus on more significant sources of economic benefit. To this end, generic and control biologic manufacturers might have a greater willingness to consider novel technologies such as continuous manufacturing processes, as their core business model is founded on “cost-efficient production”.
3. What Can Be Done to Improve the Situation
3.1. Need for More PSE Case Studies to Build Confidence
There have been significant knowledge-driven advancements in continuous bioprocessing technologies. Perfusion processing is now significantly less complex, less prone to contamination, and more readily scalable than previously. Similarly, multiple solutions for continuous downstream processing have intensified the process to accommodate the increased titers and processing rate. A cost-of-goods analysis on consumables has shown a 6–10-fold cost reduction from the conventional batch to the intensified continuous process [8]. Thus, the negative assessments from within the industry of continuous perfusion and downstream processing may, overall, reflect a lack of direct exposure or experience with continuous technology combined with a relative lack of case studies documenting success [11]. Case studies and reports of superior performance, when compared to existing batch platforms, will further help the rapid adoption of continuous bioprocessing. Moreover, such case studies can reflect business drivers’ alignment during research and development of a biologics drug and its commercial manufacturing to create a supportive environment (Figure 4). The business drivers during research and development are focused on maximizing knowledge acquisition with minimal expenditure. As “kiss” or “kill” is an approach regarding either continuation or termination heavily used in early stage of drug development, a knowledge spill-over across projects is a supporting driver. In fact, nine times out of 10, candidate molecules that show promise at the early stage of development fail at later trials. Therefore, while accuracy in such a decision is critical for ultimate success, a delay in search of accuracy comes with a heavy cost due to the fact that there are many candidate molecules at the early stage. At the later stages, the speed and flexibility in process technology for achieving capacity for clinical supplies and later-stage market demand drive the development strategy. On the commercial side, business drivers focus on maximizing supplies and savings while maintaining the promised quality. This involves improving the process’ robustness by continued process validation programs, continuous improvement projects for lean manufacturing, and efficient product facility change-overs.
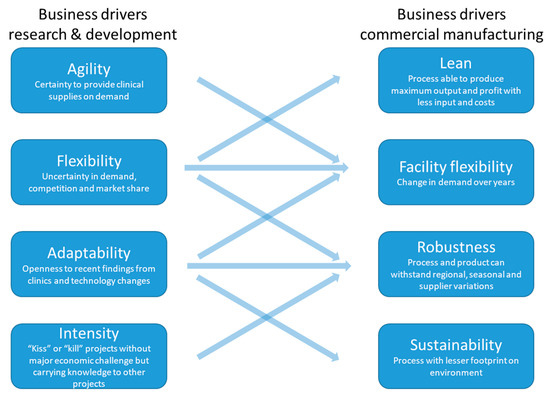
Figure 4.
Alignment between interlinked business drivers in industrial research and development (R&D) unit and commercial manufacturing is key to success.
Furthermore, analyzing the inherently negative perception of new process technologies by plant operations and management, in general, can be traced to the nature in which the performance of these individuals is assessed. In particular, the primary objective of management and plant operations is to achieve a high level of plant “uptime” while guaranteeing “on-specification” production, with minimal operator intervention. In other words, the obvious choice is, then, to operate technologies that are robust and well understood by the operators. From a systems thinking standpoint, these two risk factors can be categorized into (i) technology risk (the likelihood that a new technology fails); and (ii) plant-wide operational risk (the reduction in operational flexibility due to the new technology).
Process systems engineering methods can be employed to systematically assess (and thus manage) these two risks during technology development and implementation. One such method is the concept of technology readiness level (TRL). The TRL was originally developed by National Aeronautics and Space Administration (NASA) to assess space technology development but has now been adopted in other areas, including bio-based production [37]. The TRL provides a systematic framework in which technology readiness can be easily estimated based on literature and publicly available information. This framework also provides a guide on how to factor in the TRL’s economic consequences of new technology. While these frameworks are not explicitly developed to rate the technology development in biologics production, they can be adopted to make informed decisions based on “facts and figures” rather than on perception. Thus, the TRL allows technology developers to identify key areas of improvements throughout the design process.
Similarly, the plant-wide operational risk introduced by new technology can be systematically evaluated (and thus managed) based on a systematic assessment. For example, the framework presented in [38], which was initially intended to rate process control configurations, can be adopted to systematically assess the economic consequences of reducing operational flexibility due to the implementation of new technologies. In this particular framework, the concepts of layer of protection analysis (LOPA) and net present value (NPV) analysis are employed to quantify the consequences. This type of analysis allows both the technology developer and plant operations to chart a minimal risk path to implementation. This can include steps such as the development of validated plant-wide process models or the use of modified benchmark simulations to demonstrate the plant-wide ramification that a new process technology will bring. Moreover, the development of built-in redundancies, as well as implementation strategies, minimize plant-wide ramifications.
3.2. PAT Solutions and Robotics for Better Control
From a pure process control point of view, both continuous and batch production processes have unique characteristics that make their control difficult. One of the most important differences between continuous bioprocessing and batch or even fed-batch operations is in their required level of control sophistication [39]. For a fed-batch process, the objective of process control is mainly to achieve a given feeding profile while ensuring that variables such as pH and temperature are kept in check. The fact that the content within a vessel (e.g., fermentation) changes over time means that there is a need to adjust the controls’ behavior to account for these variations. In academia, an extensive body of work can be found on batch process optimization and control, including the use of machine learning techniques, such as Kalman filters, neural networks [40], adaptive neuro-fuzzy inference systems (ANFISs) [41], or evolutionary algorithms [42]. However, in practice, industrial batch and fed-batch operations imply a minimum degree of automation and, in many instances, require operators to carry out manual tasks (e.g., nutrient addition). For example, in fermentation operations, pH and temperature controllers are present, while fed-batch operations may follow a pre-determined feeding regime. In batch bioprocessing, this is an acceptable practice as operators can prolong or shorten the fermentation processes to counter all other variations.
On the other hand, continuous bioprocesses have a simpler technical requirement as the objective of the control structure is to maintain a set point where, in principle, the content within the vessel/unit operation stays the same as raw materials are added continuously and products are removed continuously. Nonetheless, in practice, the control of continuous processes is far more complex. First of all, available measurement devices are unable to measure state variables of interest directly. Thus, it requires inferences to be made to determine key variables (e.g., the concentration of product in a continuous fermentation). In situations where key state variables can be measured, there is a limitation in the number of measurements that can be performed in a given time frame and in the fact that significant time is needed to carry out the analysis. Furthermore, unlike batch processes, where variables (e.g., pH and temperature) must be tightly controlled, in continuous manufacturing, process variations must be detected by monitoring key state variables, and appropriate real-time control action is to be taken to counteract these variations. While these objectives can be accomplished from a base layer control structure, a hierarchical advanced regulatory control structure must be ensured for the purpose of within-specification operations. To achieve this and, thus, introduce an integrated process control approach, one of the strategies is to embed novel sensors to monitor the critical control parameters of key processes in real-time.
As emphasized by the FDA [43], novel control and monitoring approaches are necessary to improve and ensure product quality in continuous biologics manufacturing. These strategies, such as PAT solutions [44] and the introduction of an industrial automation hierarchy, play a detrimental role in adapting to future demands and are, thus, essential for the robust and dependable monitoring and control of bioprocesses. However, depending on the TRL of advanced sampling methods, the time needed for process monitoring and control might be reduced, which is highly desired.
Among other developments in terms of sensing tools, the development and application of smart sensors seem to pave the way for the future, facilitating the adoption of continuous manufacturing and promoting development towards the factory of the future (smart manufacturing, also known as Industry 4.0). Free-floating wireless sensors, advanced image analysis, spectroscopic and soft sensors, and the use of chemometrics are some examples of smart sensing technology. They facilitate collecting more and improved data (information-rich), thus enhancing monitoring and control tasks. In particular, these developments break away from the traditional pressure, flow, temperature, and pH sensors that are usually available in a manufacturing process and rather focus on generating data related to the state of development of a process. Free-floating wireless sensors are a quite novel and bold sensing technology, based upon non-invasive instrumented particles [45], which can provide access to process data in a harsh environment inside an agitated bioreactor [46]
Another recent development is the use of advanced image analysis for process monitoring. For example, current studies have demonstrated that imaging and advanced image analysis, coupled with chemometrics and state-of-the-art machine learning algorithms, are promising to monitor fermentation [47].
On the other hand, spectroscopic sensors have been used and tested for a longer period. In theory, they can detect several compounds simultaneously without time delay by processing the spectra with chemometrics [48]. For example, new studies include developing infrared spectrometers [49] and Raman spectroscopy [50], which are being modified to allow for application in the demanding manufacturing environment.
Soft sensors are another sensing technology that has shown great promise for on-line monitoring. They are an alternative to traditional approaches which allow for the monitoring of state variables that affect the bioprocesses but cannot be sensed in real time otherwise [51,52,53,54]. As an advanced process monitoring technology, they use algorithms to perform an on-line analysis to collect information on unmeasured process state variables [44,55]. The interest in using these sensors has spiked in recent years due to the growing computer capacity and the numerous advances in signal processing (artificial intelligence and machine learning algorithms), which are, without a doubt, the great enablers of their successful implementation. The opportunities they bring align with the PAT initiatives and, thus, agree with the intended shift towards smart manufacturing and, consequently, the implementation of continuous manufacturing [49].
The use of robotics is also regarded as a promising strategy to facilitate the transition to continuous production lines. Great examples are the use of mechanical arms for the final production steps, such as filling millions of vials per year in big pharmaceutical companies’ filling operations. Furthermore, robots are also being used to transport samples across factories for analysis and gradually replace repetitive manual tasks in the production line, tasks that were, until recently, mainly carried out by process operators. However, despite the enticing and demonstrated usefulness, we are still in the first stages of adopting this technology.
3.3. Modeling and Simulation
The successful shift from batch to continuous operation calls for a systematic modeling and simulation framework to explore, test, and evaluate a set of scenarios to narrow down the list of process candidates. Testing different scenarios on a full scale, pilot, or even lab bench is extremely resource-demanding, especially in the biopharma industry. For example, integrated continuous API production is barely investigated due to challenges such as the lack of process understanding and technology readiness [56]. Thus, it seems necessary and highly beneficial to apply modeling and simulations tools to design, control, and optimize processes when testing continuous process alternatives. These tools have been evaluated in the chemical and fine chemical industry and academia for many years.
Furthermore, looking at the many inherent challenges, the development of reliable in silico plant-wide simulation models that represent, as well as make possible, the potential continuous production of biopharmaceuticals would tremendously empower and encourage this industry to take the next steps. Plant-wide simulation models are a comprehensive modeling approach that requires a plug-and-play type of model development [56]. There are different types of modeling strategies currently available [23] which vary in their degree of accuracy, from first principles models to data-driven and even hybrid modeling. To the interested readers, Gargalo et al. (2020) [23] presented a detailed review of the different types of modeling approaches that are available, especially for bioprocess modeling.
Besides process understanding, optimization, and control, modeling has a significant role in the techno-economic and sustainability evaluation of emerging platforms. For example, in this regard, several studies have compared the impact on the overall sustainability, economics, and environmental aspects of switching from classic stainless steel batch options to single-use equipment in continuous biomanufacturing [57,58,59].
3.4. The Clarity in Regulation for Continuous Biologics Manufacturing
Beside scientific, technical, and business drivers, the adoption of industrial-scale continuous biologics manufacturing strongly demands clear scientific and regulatory considerations for their development, implementation, operation, and lifecycle management. The scientific guidelines, both by the FDA and ICH, ever since the publication of FDA’s PAT guidance in 2004, have been providing principles and concepts which apply to continuous manufacturing. Specifically, building upon the principles and concepts described in the already published ICH guidelines, this guideline offers clarification on CM concepts, describes scientific approaches, and presents regulatory considerations unique to CM of drug substances and drug products. However, the need for a more clearly identified path was more recently identified by regulators. The FDA established an Emerging Technology Program in 2014 that works collaboratively with companies to support the adoption of a continuous manufacturing platform. In 2019, a draft guidance on Quality Considerations for Continuous Manufacturing was presented to clarify the FDA’s current thinking regarding innovative CM approaches and to resolve potential issues some companies have as they consider implementation. However, the guidance only provides recommendations for small-molecule, oral, solid drug products linked with (abbreviated-)new drug application (ANDA/NDA) and explicitly notes that the recommendations do not apply to biological products submitted under a biologics license application (BLA). The ICH is, at the same time, working on the Q13 guideline on continuous manufacturing in a broader sense. This guideline will apply to CM of drug substances and drug products for both chemical entities and therapeutic proteins. Moreover, this guideline will apply to CM processes for new products (e.g., new drugs, generic drugs, and biosimilars) and the conversion of batch manufacturing to CM processes for existing products. While such regulatory efforts are very encouraging, it is also essential to ensure that continuous manufacturing standards are harmonized.
4. Sustainability Considerations of Continuous Manufacturing of Biopharmaceuticals: Process Integration and Automation
Currently, all companies are pursuing viable strategies to stand by green chemistry principles and achieve overall sustainability. As early as 2007, the pharmaceutical roundtable founded by the American Chemical Society Green Chemistry Institute (GCI) and global pharmaceutical companies recommended continuous manufacturing as the number one key green research field to boost green and sustainable production [60,61]. Continuous bioprocessing requires automation, instrumentation, and process integration, for example, by using process intensification. Integrated analytics and automation systems open the door for on-line detection and deviation handling to make corrections without human intervention. However, a fully integrated system would require high analytical precision, robust control, execution, and well-defined protocols to handle the potential deviations. The next generation of automation for bioprocessing platforms should be capable of connecting upstream and downstream, creating an integrated continuous biomanufacturing platform. Nonetheless, a clear disadvantage is that the more we connect and automate, the lower the systems’ flexibility is. Thus, supervisory automation with the flexibility to switch or bypass unit operations in a lab setting to evaluate different and experimental bioprocessing approaches is desired [62]. In a Good Manufacturing Practices (GMP) environment where such flexibility within an approved workflow is not needed, such features can be controlled through an access control system, allowing operational flexibility in new manufacturing facilities. Thus, automation, instrumentation, and process integration enable more effective scale-up, better product quality, safety, and greater efficiency in terms of economy and productivity. This leads to higher raw material utilization levels, waste minimization, energy efficiency, and better facility utilization compared to a similar batch process.
Furthermore, it is important to note that continuous manufacturing processes are more suited for implementing smart manufacturing concepts (Industry 4.0). These concepts also promise further gains in process efficiency and sustainability. Batch processes, due to the dynamic nature, are more dependent on human operators to carry out complex tasks and are limited in their capabilities to carry out timely actions. In contrast, continuous bioprocessing, due to the steady-state nature, allows employing a higher degree of process automation for streamlining operations by continuous monitoring of the state of control and, thus, overall sustainability.
However, one should also acknowledge that continuous production in the biomanufacturing industry usually comes with introducing single-use and/or disposable systems. Even though they provide flexibility, an inevitable consequence is compromising the environmental sustainability of such processes. In a world where we try to avoid single-use plastics, such as plastic bags and bottles, justifying the transition from multi-use stainless steel to disposable systems is a real concern. Hence, appropriate waste disposal procedures are vital in adopting these systems, especially towards scale-up uses [63,64].
5. Digitalization
Biologics manufacturing, like many other manufacturing industries, is being influenced by the Industry 4.0 push. However, from the numerous concepts currently explored under the digitalization umbrella, which spans across multiple sectors, three key advances are likely to impact the biologics industry and create further incentives to adopt continuous manufacturing. These are smart sensors, Big Data, and Digital Twins.
5.1. From Smart Sensors to Big Data
Smart sensors, as discussed in Section 3.2, as part of the general digitalization drive, are indeed a promising tool to obtain large and improved datasets that better reflect the state of development of a process. Leveraging these data is the other half of the digitalization movement. In this case, the objective is to take the large amount of data collected and convert it into actionable information. Initially, the aim is to monitor a process with the intention of later using this information to change/control the process [65]. In batch production, these concepts have already been employed to predict batch end-time from seemingly information-poor variables, such as temperature and pH. Concepts such as predictive maintenance also leverage data to predict and schedule maintenance operations [65].
5.2. Digital Twins
Like Big Data, Digital Twins form one of the key pillars in Industry 4.0 and the digitalization drive [23,49]. While the Digital Twins concept is somewhat vaguely defined for bio-based manufacturing, there are two types of digital twins that can aid future development. This first type of digital twins deals with operational support and control. For example, the use of digital twins to forecast the evolution of a fermentation process, such as in [66]. On the other hand, a digital twin can be a digital representation of a future production process, where it acts as a validated test-bed which can be used to refine and build confidence in a process design prior to construction.
All these developments directly impact further strengthening of the case of continuous production and its business case. The development of novel sensors enables collecting datasets that are needed for “real-time” tracking of key state variables in continuous production. Simultaneously, the big data-based process monitoring and control methods are required to ensure that the data gathered can be turned into actionable information for the process operators and/or perform closed-loop control action. Digital twins, on the one hand, play a similar role in producing actionable information and providing the ability to implement closed-loop controls.
A knock-on effect of the operation supports that these elements provide, together with the high degree of process automation, is needed for continuous production processes. This brings the need for reduced staff for given production output. In addition, the operators who are working on continuous production processes would operate the process through more automated operations, avoiding manual tasks as much as possible, which are the norm in batch production.
5.3. Application of Modeling in Regulatory Decision
A unique and challenging aspect of introducing changes to pharmaceutical processes’ design and operation is the need for regulatory bodies to approve any specific changes. To this end, a concept such as “Digital Twins”, which, in essence, opens up towards shifting validations and testing on a process into an in-silico environment, needs to be accepted by the regulatory bodies. Quality by design is one such framework. It has been adopted and endorsed by the FDA, which indicates their willingness to acknowledge the need for more in-silico-based studies to improve the precision and speed at which process designs can be created and tested. However, the question remains whether multiple designs (process paths) can be validated by employing the digital twin concept. At the very least, these digital twins can be applied for building a multivariate design space and scale-down models for commercial-scale systems, reducing the economic burden on the R&D department without compromising the quality.
5.4. Leveraging Process Data
With the development and pilot-scale operation of hundreds of process designs in a year and dozens of commercial production processes, pharmaceutical companies can collect a large amount of diverse process data from operations. With the recent advances made in data-driven analytics and the availability of “big data”, pharmaceutical companies can leverage these data to identify common failures and successes rapidly. In turn, these situations can be further analyzed by subject matter experts to develop process insights for an improved design and operation of future production processes. As such, data analytics will allow pharmaceutical companies to rapidly improve process designs as opposed to the more subdued pace at which changes usually occur. It is noteworthy that data analytics acts as an enabler for experts to analyze hundreds, if not thousands, of datasets effectively, thus facilitating the gathering of insights and pro-active planning of operational changes.
5.5. Hybrid Facilities: Acknowledging the Best of Both Worlds
Depending on the demand and manufacturing stage, it becomes easier or more challenging to work with single-use/disposable systems or multi-use stainless steel systems. A hybrid facility that uses disposables and reusable systems potentially combines both systems’ benefits and could be the path forward depending on the required capacity. This has often come to mean single-use technologies in seed-train development. However, for upstream production, the use of stainless steel-based perfusion bioreactors is more common, and then again using single-use systems in downstream processing for in-process holding and filtration units. Such flexibility will automatically enable the flexibility of connected unit operations and, thus, continuous operations while keeping the risk of contamination to a minimal level [36].
6. Summary and Outlook
Despite the benefits of continuous over batch bioprocessing, its adoption has lagged, with few exceptions. However, the batch manufacturing paradigm’s dominance in the industry for reasons such as “batch processing is familiar and works very well” cannot be sustained in the long term given the new biomanufacturing challenges. The industry-held perception of complexity in continuous bioprocessing is becoming obsolete, as more and more new technologies and solutions are continually improving the situation. Several academic- and industry-led consortia are working to improve the perception regarding continuous bioprocessing by bringing the questions to the correct stakeholders who can address them. The training provided by these initiatives to the top management of the companies is playing an essential role in changing the perception and, at the same time, also creating new scientists and operators that can understand and respond to a new set of operational challenges. However, wider adoption of continuous bioprocessing will only be possible if the gaps at the technical, management, and regulatory levels are acknowledged. As discussed in this paper, concerted efforts are being made to abridge them. These include:
- Technical:
- Improvement in automation to allow flexibility in the design and control of continuous processes;
- Adoption of a “digital twin” of processes to reduce the costs and risks linked with a decision made based on a limited set of experimental results;
- Application of detailed modeling and expert systems to support the development and regulatory requirements, such as scale-down modeling;
- Working on hybrid approaches such as single-use and multi-use to obtain best-of-all outcomes, thus enabling continuous manufacturing;
- Application of “big data” to support process development, control, and regulatory filing of a project.
- Management:
- Training on realistic situations highlighting risks and benefits of continuous manufacturing will allow removing the barrier caused by preexisting perceptions;
- Acquisition of trained staff who can support the adoption of new technology;
- Identifying key stakeholders from across the organization and getting them involved in the migration processes;
- Early alignment of R&D and commercial manufacturing business drivers to realize the extensive benefits of continuous biologics manufacturing.
- Regulatory:
- Clarity on the regulation of continuous vs. batch definitions under newer integrated and hybrid biomanufacturing process designs will be very useful;
- Increase in acceptability of digital twins as evidence for regulatory clearance;
- A joint effort by regulatory agencies and industries to develop a possible roadmap for the integrated continuous manufacturing will be highly beneficial for the biologics sector;
- Harmonization of continuous manufacturing standards and regulations.
Author Contributions
Conceptualization, A.K., C.L.G., I.A.U. and K.V.G.; writing—original draft preparation, A.K., C.L.G., I.A.U.; writing—review and editing, A.K., C.L.G., I.A.U. and K.V.G.; visualization, A.K., C.L.G., I.A.U.; supervision, K.V.G.; project administration, K.V.G.; funding acquisition, K.V.G.; All authors have read and agreed to the published version of the manuscript.
Funding
This work was partly sponsored by the Novo Nordisk Foundation in the frame of the “Accelerated Innovation in Manufacturing Biologics” (AIMBio) project (Grant number NNF19SA0035474).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chatterjee, S. FDA Perspective on Continuous Manufacturing. In Proceedings of the IFPAC Annual Meeting, Baltimore, MD, USA, 22–25 January 2012. [Google Scholar]
- ICH Expert Working Group. Q13 Continuous Manufacturing of Drug Substances and Drug Products. Available online: https://database.ich.org/sites/default/files/Q13_EWG_Concept_Paper.pdf (accessed on 11 December 2020).
- Lee, S.L. Current FDA Perspective for Continuous Manufacturing. In Proceedings of the MIT-CMAC 2nd International Symposium on Continuous Manufacturing of Pharmaceuticals, Cambridge, MA, USA, 26–27 September 2016. [Google Scholar]
- Nasr, M.; Krumme, M.; Matsuda, Y.; Trout, B.L.; Badman, C.; Mascia, S.; Cooney, C.; Jensen, K.D.; Florence, A.; Johnston, C.; et al. Regulatory Perspectives on Continuous Pharmaceutical Manufacturing: Moving From Theory to Practice. J. Pharm. Sci. 2017, 106, 3199–3206. [Google Scholar] [CrossRef] [PubMed]
- Croughan, M.S.; Konstantinov, K.B.; Cooney, C. The future of industrial bioprocessing: Batch or continuous? Biotechnol. Bioeng. 2015, 112, 648–651. [Google Scholar] [CrossRef] [PubMed]
- Konstantinov, K.B.; Cooney, C. White Paper on Continuous Bioprocessing 20–21 May 2014 Continuous Manufacturing Symposium. J. Pharm. Sci. 2015, 104, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Gernaey, K.V.; Gani, R. A model-based systems approach to pharmaceutical product-process design and analysis. Chem. Eng. Sci. 2010, 65, 5757–5769. [Google Scholar] [CrossRef]
- Schaber, S.D.; Gerogiorgis, D.I.; Ramachandran, R.; Evans, J.M.B.; Barton, P.I.; Trout, B.L. Economic Analysis of Integrated Continuous and Batch Pharmaceutical Manufacturing: A Case Study. Ind. Eng. Chem. Res. 2011, 50, 10083–10092. [Google Scholar] [CrossRef]
- Department of Health and Human Services, Food and Drug Administration. PAT Guidance for Industry-Framework for Innovative Pharmaceutical Development, Manufacturing and Quality Assurance. Available online: https://www.fda.gov/media/71012/download (accessed on 11 December 2020).
- FDA. Quality Considerations for Continuous Manufacturing Guidance for Industry. Food Drug Adm. Available online: https://www.fda.gov/media/121314/download (accessed on 11 December 2020).
- Langer, E. Biomanufacturing: Demand for Continuous Bioprocessing Increasing. BioPharm Int. 2020, 33, 5. [Google Scholar]
- Godawat, R.; Konstantinov, K.; Rohani, M.; Warikoo, V. End-to-end integrated fully continuous production of recombinant monoclonal antibodies. J. Biotechnol. 2015, 213, 13–19. [Google Scholar] [CrossRef]
- Aakesson, M.; Heitmann, M.; Tiainen, P. Integrated Continuous Biomanufacturing Process. U.S. Patent Application No. 15/306,938, 2 March 2017. [Google Scholar]
- Desai, S.G. Continuous and semi-continuous cell culture for production of blood clotting factors. J. Biotechnol. 2015, 213, 20–27. [Google Scholar] [CrossRef]
- Farid, S.S.; Thompson, B.; Davidson, A. Continuous bioprocessing: The real thing this time? In Proceedings of the10th Annual bioProcessUK Conference, London, UK, 3–4 December 2013. [Google Scholar]
- Jones, S.; Castillo, F.; Levine, H. Advances in the development of therapeutic monoclonal antibodies. BioPharm. Int. 2007, 20, 96–114. [Google Scholar]
- Agrawal, V.; Bal, M. Strategies for rapid production of therapeutic proteins in mammalian cells. BioProcess Int. 2012, 10, 32–48. [Google Scholar]
- Ebeler, M.; Lind, O.; Norrman, N.; Palmgren, R.; Franzreb, M. One-step integrated clarification and purification of a monoclonal antibody using Protein A Mag Sepharose beads and a cGMP-compliant high-gradient magnetic separator. New Biotechnol. 2018, 42, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Käppler, T.; Cerff, M.; Ottow, K.E.; Hobley, T.J.; Posten, C. In situ magnetic separation for extracellular protein production. Biotechnol. Bioeng. 2009, 102, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Xu, X.; Huang, C.; Angelo, J.; Oliveira, C.L.; Xu, M.; Xu, X.; Temel, D.; Ding, J.; Ghose, S.; et al. Biomanufacturing evolution from conventional to intensified processes for productivity improvement: A case study. mAbs 2020, 12, 1770669. [Google Scholar] [CrossRef] [PubMed]
- Haringa, C.; Mudde, R.F.; Noorman, H.J. From industrial fermentor to CFD-guided downscaling: What have we learned? Biochem. Eng. J. 2018, 140, 57–71. [Google Scholar] [CrossRef]
- Tajsoleiman, T.; Spann, R.; Bach, C.; Gernaey, K.V.; Huusom, J.K.; Krühne, U. A CFD based automatic method for compartment model development. Comput. Chem. Eng. 2019, 123, 236–245. [Google Scholar] [CrossRef]
- Gargalo, C.L.; Heras, S.C.; de Las Jones, M.N.; Mansouri, S.S.; Krühne, U.; Gernaey, K.V. Towards the Development of Digital Twins for the Bio-Manufacturing Industry. Available online: https://doi.org/10.1007/10_2020_142 (accessed on 11 December 2020).
- Busse, C.; Biechele, P.; de Vries, I.; Reardon, K.F.; Solle, D.; Scheper, T. Sensors for disposable bioreactors. Eng. Life Sci. 2017, 17, 940–952. [Google Scholar] [CrossRef]
- Kornecki, M.; Schmidt, A.; Lohmann, L.; Huter, M.; Mestmäcker, F.; Klepzig, L.S.; Mouellef, M.; Zobel-Roos, S.; Strube, J. Accelerating Biomanufacturing by Modeling of Continuous Bioprocessing—Piloting Case Study of Monoclonal Antibody Manufacturing. Processes 2019, 7, 495. [Google Scholar] [CrossRef]
- Huter, M.; Strube, J. Model-Based Design and Process Optimization of Continuous Single Pass Tangential Flow Filtration Focusing on Continuous Bioprocessing. Processes 2019, 7, 317. [Google Scholar] [CrossRef]
- Carvalho, R.J.; Castilho, L.R.; Subramanian, G. Tools Enabling Continuous and Integrated Upstream and Downstream Processes in the Manufacturing of Biologicals. In Continuous Biomanufacturing-Innovative Technologies and Methods; Wiley: Hoboken, NJ, USA, 2017; pp. 31–68. [Google Scholar]
- Castilho, L.; Anspach, F. CFD-aided design of a dynamic filter for mammalian cell separation. Biotechnol. Bioeng. 2003, 83, 514–524. [Google Scholar] [CrossRef]
- Biomanufacturing Consortium (BioMAN)|MIT Center for Biomedical Innovation. Available online: http://cbi.mit.edu/research-overview/bioman/ (accessed on 31 August 2020).
- BioPhorum. Available online: https://www.biophorum.com/ (accessed on 31 August 2020).
- Udugama, I.A.; Feldman, H.; de las Heras, S.C.; Kizhedath, A.; Bryde-Jacobsen, J.; van den Berg, F.; Mansouri, S.S.; Gernaey, K.V. Biopro World Talent Campus: A week of real world challenge for biotechnology post-graduate students. Educ. Chem. Eng. 2018, 25, 1–8. [Google Scholar] [CrossRef]
- DiCesare, C.; Yu, M.; Yin, J.; Zhou, W.; Hwang, C.; Tengtrakool, J.; Konstantinov, K. Development, qualification, and application of a bioreactor scale-down process: Modeling large-scale microcarrier perfusion cell culture. Bioprocess. Int. 2016, 14, 18–29. [Google Scholar]
- Tajsoleiman, T.; Mears, L.; Krühne, U.; Gernaey, K.V.; Cornelissen, S. An Industrial Perspective on Scale-Down Challenges Using Miniaturized Bioreactors. Trends Biotechnol. 2019, 37, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Ram, R.J. Tools for Continuous Bioprocess Development. BioPharm Int. 2016, 29, 18–25. [Google Scholar]
- Sartorius Ambr® 15 Fermentation-High throughput Automated System|Sartorius. Available online: https://www.sartorius.com/us-en/products/fermentation-bioreactors/ambr-multi-parallel-bioreactors/ambr-15-fermentation (accessed on 9 October 2020).
- Scott, C. Large-Scale Capacity Strategies: Single Use, Multiuse, or Both? Bioprocess. Int. 2019. Available online: https://bioprocessintl.com/manufacturing/facility-design-engineering/large-scale-capacity-strategies-single-use-multiuse-or-both/ (accessed on 11 December 2020).
- Goji, T.; Hayashi, Y.; Sakata, I. Evaluating “startup readiness” for researchers: Case studies of research-based startups with biopharmaceutical research topics. Heliyon 2020, 6, e04160. [Google Scholar] [CrossRef] [PubMed]
- Udugama, I.A.; Taube, M.A.; Mansouri, S.S.; Kirkpatrick, R.; Gernaey, K.V.; Yu, W.; Young, B.R. A Systematic Methodology for Comprehensive Economic Assessment of Process Control Structures. Ind. Eng. Chem. Res. 2018, 57, 13116–13130. [Google Scholar] [CrossRef]
- Thiess, H.; Gronemeyer, P.; Ditz, R.; Strube, J.; Zobel-Roos, S.; Subramanian, G. Engineering Challenges of Continuous Biomanufacturing Processes (CBP). In Continuous Biomanufacturing-Innovative Technologies and Methods; Wiley: Hoboken, NJ, USA, 2017; pp. 69–106. [Google Scholar]
- Montes, F.; Gernaey, K.V.; Sin, G. Implementation of a Radial Basis Function control strategy for the crystallization of Ibuprofen under uncertainty. Comput. Aided Chem. Eng. 2018, 44, 565–570. [Google Scholar] [CrossRef]
- O’Mahony, N.; Murphy, T.; Panduru, K.; Riordan, D.; Walsh, J. Adaptive process control and sensor fusion for process analytical technology. In Proceedings of the 2016 27th Irish Signals and Systems Conference (ISSC), Londonderry, UK, 21–22 June 2016; pp. 1–6. [Google Scholar]
- Allmendinger, R.; Simaria, A.S.; Turner, R.; Farid, S.S. Closed-loop optimization of chromatography column sizing strategies in biopharmaceutical manufacture. J. Chem. Technol. Biotechnol. 2013, 89, 1481–1490. [Google Scholar] [CrossRef]
- FDA. PAT Guidance for Industry—A Framework for Innovative Pharmaceutical Development; Manufacturing and Quality Assurance: Rockville, MD, USA, 2004. [Google Scholar]
- Randek, J.; Mandenius, C.-F. On-line soft sensing in upstream bioprocessing. Crit. Rev. Biotechnol. 2017, 38, 106–121. [Google Scholar] [CrossRef]
- Zimmermann, R.; Fiabane, L.; Gasteuil, Y.; Volk, R.; Pinton, J.-F. Measuring Lagrangian accelerations using an instrumented particle. Phys. Scr. 2013, 2013, 014063. [Google Scholar] [CrossRef]
- Freesense. Available online: https://www.freesense.dk (accessed on 11 December 2020).
- Pontius, K.; Junicke, H.; Gernaey, K.V.; Bevilacqua, M. Monitoring yeast fermentations by nonlinear infrared technology and chemometrics—Understanding process correlations and indirect predictions. Appl. Microbiol. Biotechnol. 2020, 104, 5315–5335. [Google Scholar] [CrossRef] [PubMed]
- Landgrebe, D.; Haake, C.; Höpfner, T.; Beutel, S.; Hitzmann, B.; Scheper, T.; Rhiel, M.; Reardon, K.F. On-line infrared spectroscopy for bioprocess monitoring. Appl. Microbiol. Biotechnol. 2010, 88, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Gargalo, C.L.; Udugama, I.A.; Pontius, K.; Lopez, P.C.; Nielsen, R.F.; Hasanzadeh, A.; Mansouri, S.S.; Bayer, C.; Junicke, H.; Gernaey, K.V. Towards smart manufacturing: A perspective on recent developments in industrial measurement and monitoring technologies for bio-based production processes. J. Ind. Microbiol. Biotechnol. 2020, 47, 947–964. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.-K.; Yoo, S.J.; Jeong, D.H.; Lee, J.M. Real-time estimation of glucose concentration in algae cultivation system using Raman spectroscopy. Bioresour. Technol. 2013, 142, 131–137. [Google Scholar] [CrossRef]
- De Assis, A.J.; Filho, R.M. Soft sensors development for on-line bioreactor state estimation. Comput. Chem. Eng. 2000, 24, 1099–1103. [Google Scholar] [CrossRef]
- Veloso, A.C.A.; Rocha, I.; Ferreira, E.C. Monitoring of fed-batch E. coli fermentations with software sensors. Bioprocess. Biosyst. Eng. 2009, 32, 381–388. [Google Scholar] [CrossRef]
- Sharma, S.; Tambe, S.S. Soft-sensor development for biochemical systems using genetic programming. Biochem. Eng. J. 2014, 85, 89–100. [Google Scholar] [CrossRef]
- Krause, D.; Hussein, M.; Becker, T. Online monitoring of bioprocesses via multivariate sensor prediction within swarm intelligence decision making. Chemom. Intell. Lab. Syst. 2015, 145, 48–59. [Google Scholar] [CrossRef]
- Luttmann, R.; Bracewell, D.G.; Cornelissen, G.; Gernaey, K.V.; Glassey, J.; Hass, V.C.; Kaiser, C.; Preusse, C.; Striedner, G.; Mandenius, C.-F. Soft sensors in bioprocessing: A status report and recommendations. Biotechnol. J. 2012, 7, 1040–1048. [Google Scholar] [CrossRef]
- Ramin, P.; Mansouri, S.S.; Udugama, I.; Benyahia, B.; Gernaey, K.V. Modelling continuous pharmaceutical and bio-based processes at plant-wide level: A roadmap towards efficient decision-making. Chim. Oggi Chem. Today 2018, 36, 26–30. [Google Scholar]
- Jacquemart, R.; VanderSluis, M.; Zhao, M.; Sukhija, K.; Sidhu, N.; Stout, J. A Single-use Strategy to Enable Manufacturing of Affordable Biologics. Comput. Struct. Biotechnol. J. 2016, 14, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Zürcher, P.; Shirahata, H.; Badr, S.; Sugiyama, H. Multi-stage and multi-objective decision-support tool for biopharmaceutical drug product manufacturing: Equipment technology evaluation. Chem. Eng. Res. Des. 2020, 161, 240–252. [Google Scholar] [CrossRef]
- Shirahata, H.; Hirao, M.; Sugiyama, H. Multiobjective decision-support tools for the choice between single-use and multi-use technologies in sterile filling of biopharmaceuticals. Comput. Chem. Eng. 2019, 122, 114–128. [Google Scholar] [CrossRef]
- Dallinger, D.; Kappe, C.O. Why flow means green–Evaluating the merits of continuous processing in the context of sustainability. Curr. Opin. Green Sustain. Chem. 2017, 7, 6–12. [Google Scholar] [CrossRef]
- Jiménez-González, C.; Poechlauer, P.; Broxterman, Q.B.; Yang, B.-S.; Ende, D.A.; Baird, J.; Bertsch, C.; Hannah, R.E.; Dell’Orco, P.; Noorman, H.; et al. Key Green Engineering Research Areas for Sustainable Manufacturing: A Perspective from Pharmaceutical and Fine Chemicals Manufacturers. Org. Process. Res. Dev. 2011, 15, 900–911. [Google Scholar] [CrossRef]
- May, M. Modular Bioprocessing Makes Adaptability a Snap: By swapping out and adding bioprocessing modules, biomanufacturers can modify functionality and adjust capacity quickly and economically. Genet. Eng. Biotechnol. News 2019, 39, 38–40. [Google Scholar] [CrossRef]
- Scott, C. Sustainability in Bioprocessing. BioProcess Int. 2011, 9. Available online: https://bioprocessintl.com/manufacturing/monoclonal-antibodies/sustainability-in-bioprocessing-323438/ (accessed on 11 December 2020).
- Lonza Innovations in Pharma Biotech & Nutrition. Available online: https://annualreport.lonza.com/2019/segments/pharma-biotech-nutrition/innovations.html (accessed on 11 December 2020).
- Udugama, I.A.; Gargalo, C.L.; Yamashita, Y.; Taube, M.A.; Palazoglu, A.; Young, B.R.; Gernaey, K.V.; Kulahci, M.; Bayer, C. The Role of Big Data in Industrial (Bio)chemical Process Operations. Ind. Eng. Chem. Res. 2020, 59, 15283–15297. [Google Scholar] [CrossRef]
- Lopez, P.C.; Udugama, I.A.; Thomsen, S.T.; Roslander, C.; Junicke, H.; Mauricio-Iglesias, M.; Gernaey, K.V. Towards a digital twin: A hybrid data-driven and mechanistic digital shadow to forecast the evolution of lignocellulosic fermentation. Biofuels, Bioprod. Biorefining 2020, 14, 1046–1060. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).