Sorption of Organic Pollutants onto Soils: Surface Diffusion Mechanism of Congo Red Azo Dye
Abstract
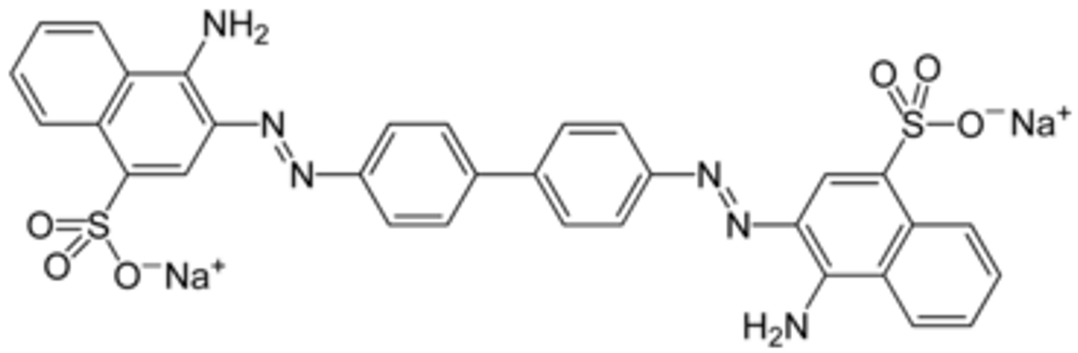
1. Introduction
2. Experimental
2.1. Materials
2.2. Soil Characterization
- 0–25 cm, periurban soil sampled from Iași city, denoted as the IS-T sample (northeastern Romania);
- 0–25 cm, suburban soil sampled from Dancu village near the Iaşi area, denoted as the IS-65 sample (northeastern Romania);
- subsurface soil profile (20–40 cm), sampled from Harghita county, denoted as the MH-13 sample (central Romania).
2.3. Sorption Studies
3. Results and Discussions
3.1. Soil Characterization
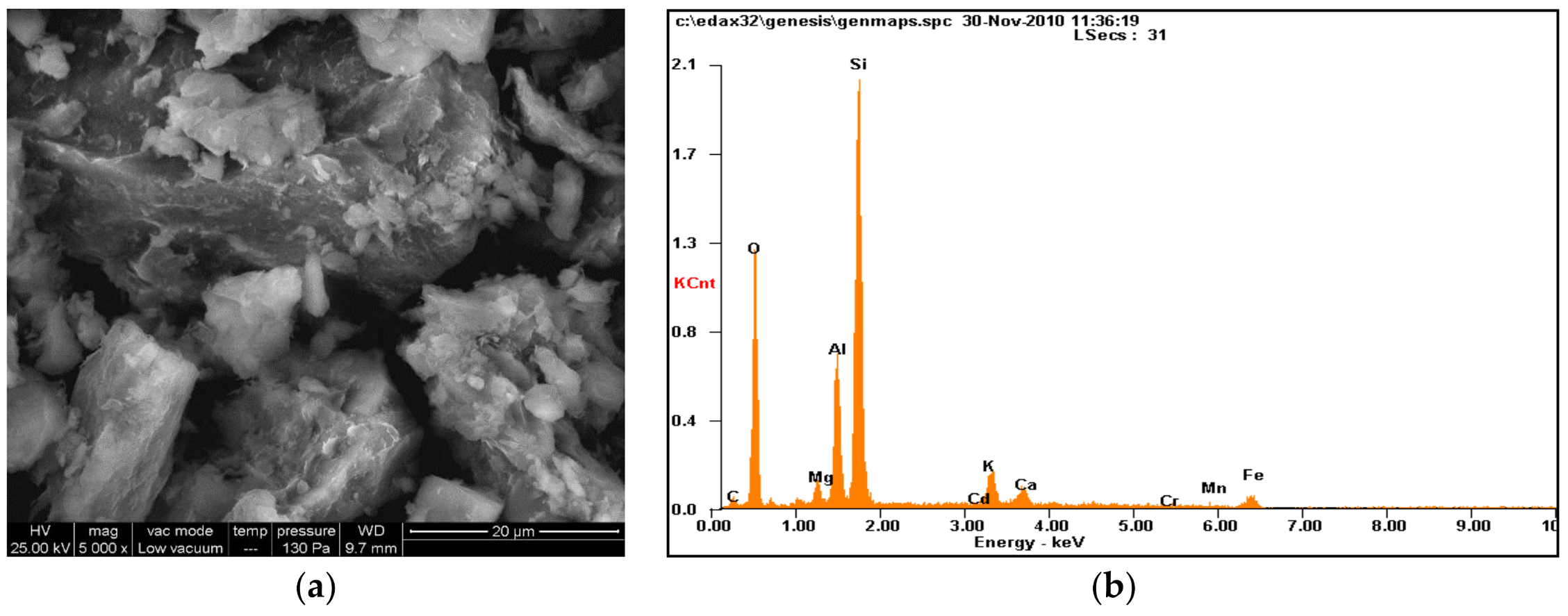
3.2. Surface Characterization
3.3. Diffusion Studies
- Diffusion of sorbate from the bulk inside the initial phase to the interface by transport in solution;
- Diffusion through the external liquid boundary layer (film diffusion);
- Diffusion inside the particle pores and the penetration of the solid along the pore walls (pore diffusion);
- Sorption and desorption inside the particles, on the internal surface.
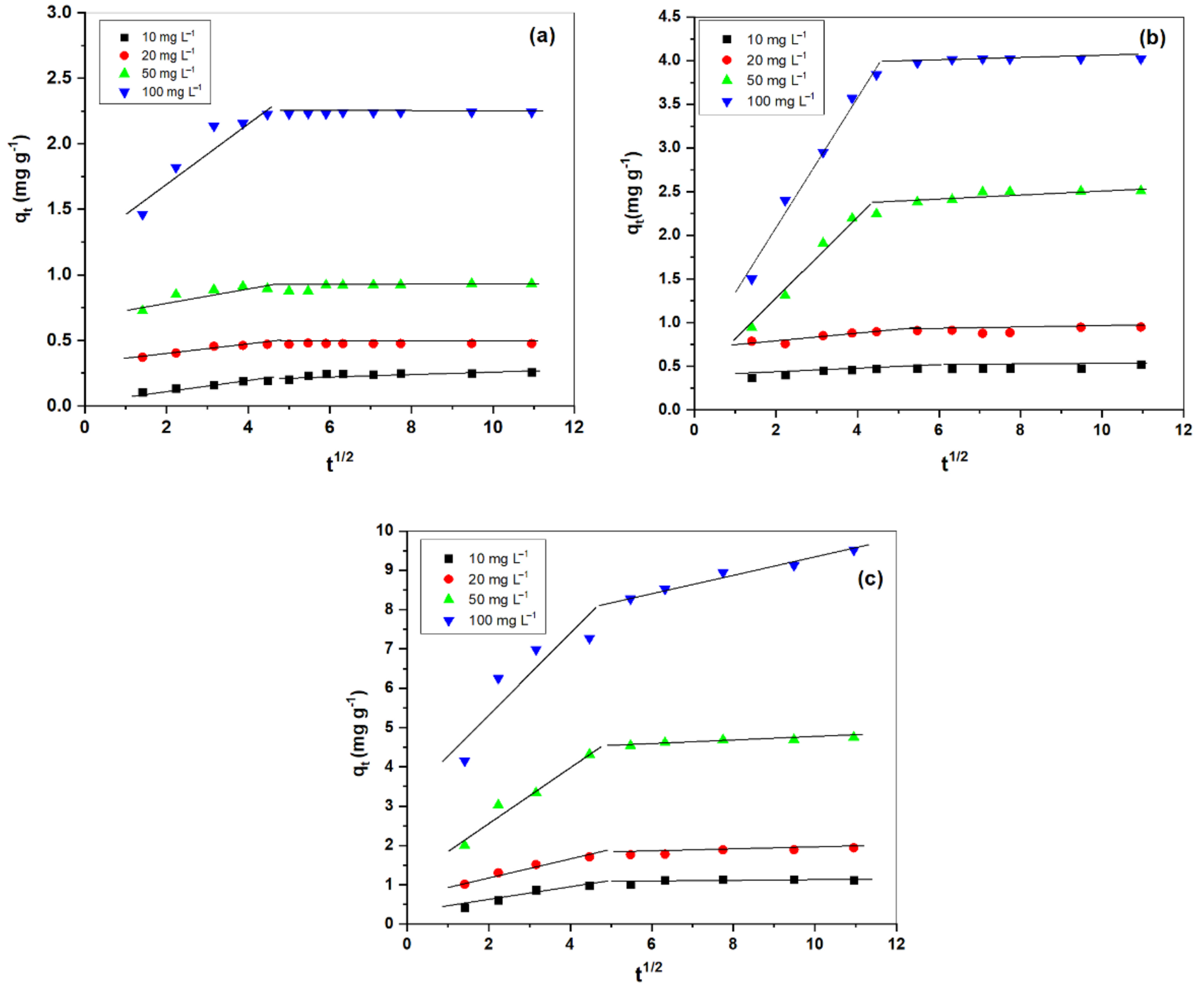
3.3.1. Intraparticle Diffusion
3.3.2. Diffusion Model
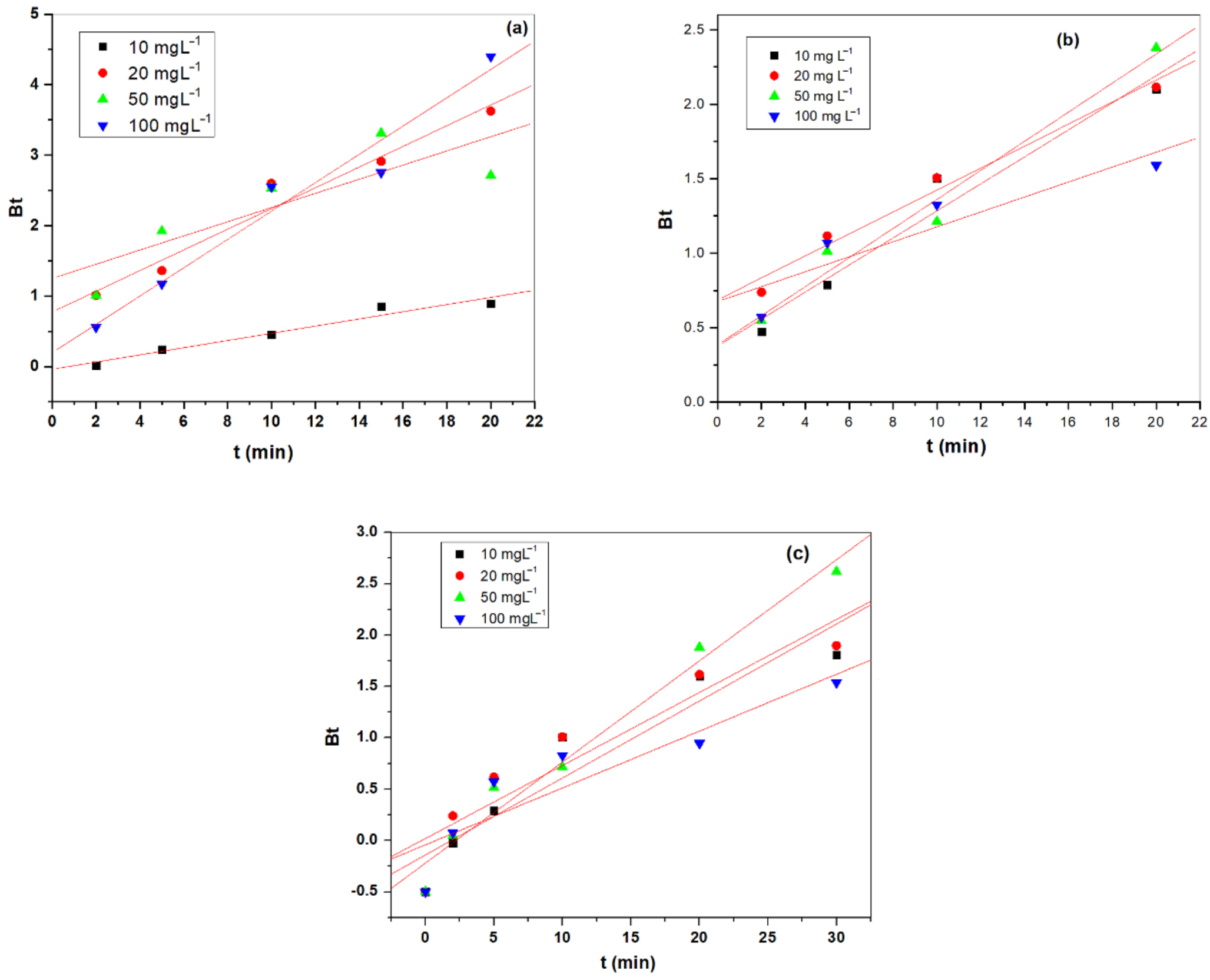
3.3.3. External Diffusion Model
3.3.4. Film and Pores Diffusion
3.3.5. Mass Transfer Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bajocco, S.; De Angelis, A.; Perini, L.; Ferrara, A.; Salvati, L. The impact of land use/land cover changes on land degradation dynamics: A Mediterranean case study. Environ. Manag. 2012, 49, 980–989. [Google Scholar] [CrossRef] [PubMed]
- Naseer, A.; Pandey, P. Assessment and monitoring of land degradation using geospatial technology in Bathinda district, Punjab, India. Solid Earth 2018, 9, 75–90. [Google Scholar] [CrossRef]
- Tetteh, R.N. Chemical soil degradation as a result of contamination: A review. J. Soil Sci. Environ. Manag. 2015, 6, 301–308. [Google Scholar] [CrossRef]
- FAO. Status of the World’s Soil Resources—Technical Summary. 2015. Available online: http://www.fao.org/3/i5199e/i5199e.pdf (accessed on 20 September 2020).
- Niculiță, M.; Bălan, M.G.; Andrei, A.; Rusu, E. Digital soil mapping in a mountainous area with mixed land use (Humor catchment—Eastern Carpathians, Romania) using soil-landscape systems, fuzzy logic and environmental covariates. Environ. Eng. Manag. J. 2019, 18, 479–489. [Google Scholar] [CrossRef]
- Prince, S.; Von Maltitz, G.; Zhang, F. Status and trends of land degradation and restoration and associated changes in biodiversity and ecosystem functions. In The IPBES Assessment Report on Land Degradation and Restoration; Montanarella, L., Scholes, R., Brainich, A., Eds.; Secretariat of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services: Bonn, Germany, 2018; pp. 221–338. [Google Scholar] [CrossRef]
- EEA. The State of Soil in Europe A Contribution of the JRC to the EEA Environment State and Outlook Report—SOER; Publications Office of the European Union: Luxembourg, 2012. [Google Scholar] [CrossRef]
- Coman, A.M.; Lacatusu, G.; Macsim, A.M.; Lazar, G. Assessment of soil erosion using Fournier indexes to estimate rainfall erosivity. Environ. Eng. Manag. J. 2019, 18, 1739–1745. [Google Scholar] [CrossRef]
- Panagos, P.; Van Liedekerke, M.; Yigini, Y.; Montanarella, L. Contaminated sites in Europe: Review of the current situation based on data collected through an European Network. J. Environ. Public Health 2013. [Google Scholar] [CrossRef]
- Gomiero, T. Soil degradation, land scarcity and food security: Reviewing a complex challenge. Sustainability 2016, 8, 281. [Google Scholar] [CrossRef]
- Bünemann, E.K.; Bongiorno, G.; Baic, Z.; Creamer, R.E.; de Deyn, G.; de Goede, R.; Fleskens, L.; Geissen, V.; Kuyper, T.W.; Mäder, P.; et al. Soil quality—A critical review. Soil Biol. Biochem. 2018, 120, 105–125. [Google Scholar] [CrossRef]
- Galli, E.; Muzzini, V.G.; Finizio, A.; Fumagalli, P.; Grenni, P.; Caracciolo, A.B.; Rauseo, J.; Patrolecco, L. Ecotoxicity of foaming agent conditioned soils tested on two terrestrial organisms. Environ. Eng. Manag. J. 2019, 18, 1703–1710. [Google Scholar] [CrossRef]
- Gavrilescu, M. Fate of pesticides in the environment and its bioremediation. Eng. Life Sci. 2005, 5, 497–526. [Google Scholar] [CrossRef]
- Rodríguez-Eugenio, N.; McLaughlin, M.; Pennock, D. Soil Pollution: A Hidden Reality; Food and Agriculture Organization of the United Nations: Rome, Italy, 2018; Available online: http://www.fao.org/3/I9183EN/i9183en.pdf (accessed on 20 September 2020).
- Căliman, F.A.; Robu, B.; Smaranda, C.; Pavel, V.L.; Gavrilescu, M. Soil and groundwater cleanup: Benefits and limits of emerging technologies. Clean Technol. Environ. Policy 2011, 13, 241–268. [Google Scholar] [CrossRef]
- Sparks, D.L. (Ed.) Environmental soil chemistry: An overview. In Environmental Soil Chemistry, 2nd ed.; Academic Press: San Diego, CA, USA, 2002; pp. 1–42. [Google Scholar] [CrossRef]
- Srivastava, M.; Srivastava, A.; Yadav, A.; Rawat, V. Source and control of hydrocarbon pollution. In Hydrocarbon Pollution and Its Effect on the Environment; Ince, M., Ince, O.M., Eds.; Intech: Rijeka, Croatia, 2019; pp. 1–21. [Google Scholar] [CrossRef]
- Hassaan, M.A.; Nemr, A.E. Health and environmental impacts of dyes: Mini review. Am. J. Environ. Sci. 2017, 3, 64–67. [Google Scholar] [CrossRef]
- Singh, L.; Singh, V.P. Decolourization of azo (acid red) and anthraquinonic (basic blue) dyes by the fungus Aspergillus Flavus. Int. J. Med. Eng. Inform. 2017, 3, 1–5. [Google Scholar] [CrossRef]
- Jamee, R.; Siddique, R. Biodegradation of synthetic dyes of textile effluent by microorganisms: An environmentally and economically sustainable approach. Eur. J. Microbiol. Immunol. 2019, 9, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Raval, N.P.; Shah, P.U.; Shah, N.K. Adsorptive amputation of hazardous azo dye Congo red from wastewater: A critical review. Environ. Sci. Pollut. Res. 2016, 23, 14810–14853. [Google Scholar] [CrossRef]
- Durães, N.; Novo, L.A.B.; Candeias, C.; Ferreirada da Silva, E. Distribution, transport and fate of pollutants. In Soil Pollution: From Monitoring to Remediation; Duarte, A.C., Cachada, A., Rocha-Santos, T., Eds.; Academic Press: New York, NY, USA, 2018; pp. 29–57. [Google Scholar] [CrossRef]
- Smaranda, C.; Gavrilescu, M.; Bulgariu, D. Studies on sorption of Congo Red from aqueous solution onto soil. Int. J. Environ. Res. 2011, 5, 177–188. [Google Scholar] [CrossRef]
- Loffredo, E.; Senesi, N. Fate of anthropogenic organic pollutants in soils with emphasis on adsorption/desorption processes of endocrine disruptor compounds. Pure Appl. Chem. 2006, 78, 947–961. [Google Scholar] [CrossRef]
- Florea, N.; Munteanu, I.; Băcăinţan, N.; Bălăceanu, V.; Cârstea, S. Romanian Soil Taxonomy System: SRTS; Estfalia Publishing House: Bucureşti, Romania, 2003. (In Romanian) [Google Scholar]
- Pansu, M.; Gautheyrou, J. Handbook of Soil Analysis Mineralogical, Organic and Inorganic Methods; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 2006. [Google Scholar]
- Bulgariu, D.; Rusu, C.; Breabăn, I.G.; Bulgariu, L.; Aştefanei, D. Instrumental Methods of Analysis in Geosciences; Sampling Demiurg Publishing House: Iaşi, Romania, 2005; Volume I. (In Romanian) [Google Scholar]
- Adriano, D.C. Trace Elements in Terrestrial Environments: Biogeochemistry, Bioavailability, and Risks of Metals, 2nd ed.; Springer: New York, NY, USA, 2001. [Google Scholar] [CrossRef]
- Kabata-Pendias, A.; Mukherjee, A.B. Trace Elements from Soil to Human; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar] [CrossRef]
- Schnitzer, M.; Schuppli, P. Method for the sequential extraction of organic matter from soil and soil fractions. Soil Sci. Soc. Am. J. 1989, 53, 1418–1424. [Google Scholar] [CrossRef]
- Swift, R.S. Organic matter characterization. In Methods of Soil Analysis. Part 3. Chemical Methods; Sparks, D.S., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnston, C.T., Sumner, M.E., Eds.; Soil Science Society of America: Madison, WI, USA, 1996; pp. 1018–1020. [Google Scholar] [CrossRef]
- Stevenson, F.J. Humus Chemistry; Wiley: New York, NY, USA, 1982. [Google Scholar]
- Smaranda, C.; Bulgariu, D.; Măluţan, T.; Gavrilescu, M. The evaluation of factors affecting sorption of pentachlorophenol in soil. Sci. Pap. Agron. Ser. 2014, 57, 133–138. [Google Scholar]
- Smaranda, C.; Popescu, M.-C.; Bulgariu, D.; Măluţan, T.; Gavrilescu, M. Adsorption of organic pollutants onto a Romanian soil: Column dynamics and transport. Process Saf. Environ. Prot. 2017, 108, 108–120. [Google Scholar] [CrossRef]
- Ho, Y.S.; Ng, J.C.Y.; McKay, G. Kinetics of pollutant sorption by biosorbents: Review separation and purification methods. Sep. Purif. Technol. 2000, 29, 189–232. [Google Scholar] [CrossRef]
- Choy, K.K.H.; Ko, D.C.K.; Cheung, C.W.; Porter, J.F.; McKay, G. Film and intraparticle mass transfer during the adsorption of metal ions onto bone char. J. Colloid Interface Sci. 2004, 271, 284–295. [Google Scholar] [CrossRef] [PubMed]
- Hameed, B.H.; El-Khaiary, M.I. Sorption kinetics and isotherm studies of a cationic dye using agricultural waste: Broad bean peels. J. Hazard. Mater. 2008, 154, 639–648. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, S.; Sivakumar, B.; Sivakumar, N. Film and pore diffusion modeling for adsorption of reactive red 2 from aqueous solution on to activated carbon prepared from bio-diesel industrial waste. J. Chem. 2010, 7, 175–184. [Google Scholar] [CrossRef]
- Sahmounea, M.N.; Ouazene, N. Mass-transfer processes in the adsorption of cationic dye by sawdust. Environ. Prog. Sustain. Energy 2012, 31, 597–603. [Google Scholar] [CrossRef]
- Vimonses, V.; Lei, S.; Jin, B.; Chow, C.W.K.; Saint, C. Adsorption of Congo red by three Australian kaolins. Appl. Clay Sci. 2009, 43, 465–472. [Google Scholar] [CrossRef]
- Coşkun, Y.İ.; Aksuner, N.; Yanik, J. Sandpaper wastes as adsorbent for the removal of brilliant green and malachite green dye. Acta Chim. Slov. 2019, 66, 402–413. [Google Scholar] [CrossRef]
- Lian, L.; Guo, L.; Guo, C. Adsorption of Congo red from aqueous solutions onto Ca-bentonite. J. Hazard. Mater. 2009, 161, 126–131. [Google Scholar] [CrossRef]
- Litefti, K.; Freire, M.S.; Stitou, M.; González-Álvarez, J. Adsorption of an anionic dye (Congo red) from aqueous solutions by pine bark. Sci. Rep. 2019, 9, 16530. [Google Scholar] [CrossRef]
- Mall, I.D.; Srivastava, V.C.; Agarwal, N.K.; Mishra, I.M. Removal of Congo red from aqueous solution by bagasse fly ash and activated carbon: Kinetic study and equilibrium isotherm analyses. Chemosphere 2005, 61, 492–501. [Google Scholar] [CrossRef]
- Ayoob, S.; Gupta, A.K.; Bhakat, P.B.; Bhat, V.T. Investigations on the kinetics and mechanisms of sorptive removal of fluoride from water using alumina cement granules. Chem. Eng. J. 2008, 140, 6–14. [Google Scholar] [CrossRef]
- Periyaraman, P.M.; Karan, S.; Ponnusamy, S.K.; Vaidyanathan, V.; Vasanthakumar, S.; Dhanasekaran, A.; Subramanian, S. Adsorption of an anionic dye onto native and chemically modified agricultural waste. Environ. Eng. Manag. J. 2019, 18, 257–270. [Google Scholar] [CrossRef]
- Smaranda, C.; Bulgariu, D.; Gavrilescu, M. Equilibrium and kinetic studies on acid dye adsorption onto soils from Iasi area. Environ. Eng. Manag. J. 2010, 9, 57–66. [Google Scholar] [CrossRef]
- Maiyalagan, T.; Karthikeyan, S. Film-pore diffusion modeling for sorption of azo dye on to exfoliated graphitic nanoplatelets. Indian J. Chem. Technol. 2013, 20, 7–14. Available online: http://nopr.niscair.res.in/bitstream/123456789/15857/1/IJCT%2020%281%29%207-14.pdf (accessed on 10 May 2020).
- Naiya, T.K.; Bhattacharya, A.K.; Das, S.K. Adsorption of Cd(II) and Pb(II) from aqueous solutions on activated alumina. J. Colloid Interface Sci. 2009, 333, 14–26. [Google Scholar] [CrossRef]
- Viegas, R.M.C.; Campinas, M.; Costa, H.; Rosa, M.J. How do the HSDM and Boyd’s model compare for estimating intraparticle diffusion coefficients in adsorption processes. Adsorption 2014, 20, 737–746. [Google Scholar] [CrossRef]
- Li, D.; Yan, J.; Liu, Z.; Liu, Z. Adsorption kinetic studies for removal of methylene blue using activated carbon prepared from sugar beet pulp. Int. J. Environ. Sci. Technol. 2016, 13, 1815–1822. [Google Scholar] [CrossRef]
- Zhu, W.; Liu, J.; Li, M. Fundamental studies of novel zwitterionic hybrid membranes: Kinetic model and mechanism insights into strontium removal. Sci. World J. 2014. [Google Scholar] [CrossRef]
- Gupta, V.K.; Mittal, A.; Gajbe, V. Adsorption and desorption studies of a water soluble dye, Quinoline Yellow, using waste materials. J. Colloid Interface Sci. 2005, 284, 89–98. [Google Scholar] [CrossRef]
- Banerjee, S.; Chattopadhyaya, M.C. Adsorption characteristics for the removal of a toxic dye, tartrazine from aqueous solutions by a low cost agricultural by-product. Arab. J. Chem. 2017, 10, S1629–S1638. [Google Scholar] [CrossRef]
- Podder, M.S.; Majumder, C.B. Biosorption of As(III) and As(V) on the surface of TW/MnFe2O4 composite from wastewater: Kinetics, mechanistic and thermodynamics. J. Appl. Water Eng. Res. 2017, 7, 2689–2715. [Google Scholar] [CrossRef]
- Gedamy, Y.R. Successive consecutive removal of some heavy metals from groundwater of the quaternary aquifer in El-Minya governorate using rice straw. J. Appl. Sci. 2013, 9, 4299–4317. Available online: http://www.aensiweb.com/old/jasr/jasr/2013/4299-4317.pdf (accessed on 20 May 2020).
- Ejikeme, P.M.; Okoye, A.I.; Onukwul, O.D. Kinetics and isotherm studies of Cu2+ and Pb2+ ions removal from simulated waste water by Gambeya albida seed shell activated carbon. Afr. Rev. Phys. 2011, 6, 143–152. Available online: http://lamp.ictp.it/index.php/aphysrev/article/view/451/208 (accessed on 25 May 2020).
- Shanthi, P.; Tamilarasan, G.; Anitha, K.; Karthikeyan, S. Film and pore diffusion modeling for adsorption of Reactive Red-4 onto Sterculia quadrifida seed shell waste as activated carbon. Rasayan J. Chem. 2014, 7, 229–240. Available online: https://rasayanjournal.co.in/vol-7/issue_3/4_%20Vol.7_3_,%20229-240,%20%202014,%20RJC-1150.pdf (accessed on 29 May 2020).
- Gupta, G.S.; Prasad, G.; Panday, K.K.; Singh, V.N. Removal of chrome dye from aqueous solutions by fly ash. Water Air Soil Pollut. 1988, 37, 13–24. [Google Scholar] [CrossRef]




| Sample Characteristics | IS-65 | IS-T | MH-13 |
|---|---|---|---|
| Area | Dancu village-Iaşi (IS) county | Tudor Vladimirescu-Iaşi (IS) city | Harghita Mountains (MH)/Orientals Carpathian (1370 m altitude) |
| Sampling depth (cm) | 0–25 | 0–25 | 20–40 |
| Soil type/subtype * | Cernisoil/cambic chernozem | Protisoil/Entiantrosoil urbic associated with aluviosol molic-gleic/pelic | Andosoil cambic-histic |
| Soil horizon * | Am/Bv | Apk/Bvk | 2Aou |
| Soil utilization | Agricultural soil (crop—Medicago sativa) | Green area (ruderal plant—Gramineae) | Coniferous forest |
| Anthropic soil transformation | Weak | Intense | Very weak |
| Pollution degree ** | Non-polluted | Weak to medium | Non-polluted |
| pH | 7.11 | 5.88 | 6.38 |
| Eh | 535.21 | 580.13 | 611.81 |
| CTSC | 25.63 | 10.61 | 15.74 |
| Chemical–Mineralogical Composition | IS-65 | IS-T | MH-13 | ||
|---|---|---|---|---|---|
| Clay minerals (%, w/w) | Smectite | 13.85 | 15.29 | 6.33 | |
| Illite | 4.20 | 9.87 | 8.86 | ||
| Kaolinite | 12.66 | 19.20 | 9.67 | ||
| Amorphous | 1.81 | 3.24 | 18.52 | ||
| Total | 32.53 | 47.62 | 43.38 | ||
| Carbonates (%, w/w) | Crystalline | 1.75 | 3.17 | 3.38 | |
| Amorphous | 0.40 | 1.73 | 0.71 | ||
| Total | 2.15 | 4.91 | 4.10 | ||
| Iron oxides and oxihydroxides (%, w/w) | Crystalline | 4.25 | 1.50 | 1.61 | |
| Amorphous | 2.39 | 2.04 | 4.03 | ||
| Total | 6.65 | 3.54 | 5.65 | ||
| Silica (SiO2) (%. w/w) | Crystalline | 3.41 | 6.64 | 4.96 | |
| Amorphous | 2.15 | 4.42 | 2.89 | ||
| Total | 5.57 | 11.06 | 7.86 | ||
| Other minerals ** (%, w/w) | 6.08 | 6.13 | 7.36 | ||
| Organic matter (%. w/w) | Humic substances | Humic acids | 2.32 | 3.19 | 20.21 |
| Fulvic acids | 0.25 | 1.72 | 7.75 | ||
| Humin | 0.15 | 0.42 | 1.43 | ||
| Total | 2.74 | 5.33 | 29.40 | ||
| Others non-humic organic compounds # | 0.63 | 1.86 | 4.51 | ||
| Total | 3.37 | 7.19 | 33.91 | ||
| Undecomposed vegetal matter % (w/w) | 0.48 | 0.73 | 0.12 | ||
| Coarse fraction *** % (w/w) | 0.21 | 1.51 | 0.03 | ||
| Chemical Composition | IS-65 | IS-T | MH-13 |
|---|---|---|---|
| Major elements (%) | |||
| SiO2 | 59.67 | 53.95 | 52.06 |
| Al2O3 | 20.63 | 20.21 | 29.15 |
| Fe2O3 | 1.17 | 2.85 | 5.98 |
| TiO2 | 0.1 | 0.06 | 0.03 |
| Na2O | 2.25 | 1.69 | 1.24 |
| K2O | 3.41 | 4.20 | 2.27 |
| MgO | 1.13 | 1.26 | 1.61 |
| CaO | 4.84 | 8.06 | 2.97 |
| P2O5 | 0.19 | 0.37 | 1.08 |
| SO3 | 0.21 | 0.22 | 0.34 |
| CO2 | 2.94 | 4.83 | 2.28 |
| ∑ | 96.54 | 97.70 | 98.81 |
| Minor elements (μg/g) | |||
| Cd | 0.06 | 1.13 | 0.01 |
| Cr * | 6.44 | 17.02 | 0.02 |
| Pb | 10.59 | 78.05 | 0.14 |
| As | 0.83 | 1.37 | 0.17 |
| Zn | 57.55 | 93.74 | 0.54 |
| Cu | 20.15 | 48.26 | 0.05 |
| Mn | 72.03 | 217.85 | 0.18 |
| BET Parameters | IS-65 | IS-T | MH-13 |
|---|---|---|---|
| BET surface area (m2/g) | 29.36 | 26.26 | 19.66 |
| Total pore volume (cm3/g) | 4.329 × 10−2 | 4.805 × 10−2 | 5.174 × 10−2 |
| Average pore diameter (Å) | 58.98 | 73.17 | 105.5 |
| Micro-pore volume (cm3 /g) | 3.996 × 10−3 | 3.087 × 10−3 | 2.353 × 10−3 |
| Micro-pore surface area (m2/g) | 9.146 | 7.015 | 4.671 |
| External surface area (m2/g) | 20.22 | 19.25 | 14.99 |
| Kinetic Model Parameters | Initial Concentration of CR (mg L−1) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IS-65 | IS-T | MH-13 | |||||||||||
| 10 | 20 | 50 | 100 | 10 | 20 | 50 | 100 | 10 | 20 | 50 | 100 | ||
| Experimental | qexp | 0.2565 | 0.4756 | 0.9323 | 2.2415 | 0.5231 | 0.9513 | 2.5075 | 4.0215 | 1.1230 | 1.9444 | 4.7557 | 9.5171 |
| Intraparticle diffusion model Weber–Morris | C (mg/g) | 0.0550 | 0.318 | 0.6508 | 1.1167 | 0.3306 | 0.7021 | 0.3418 | 0.5584 | 0.1929 | 0.7607 | 1.1646 | 3.4983 |
| kid1 (mg/g min1/2) | 0.0390 | 0.0339 | 0.0722 | 0.2910 | 0.0334 | 0.0441 | 0.4555 | 0.7574 | 0.1880 | 0.2218 | 0.7116 | 0.9461 | |
| R2 | 0.9870 | 0.9506 | 0.8744 | 0.9221 | 0.9271 | 0.8855 | 0.9647 | 0.9838 | 0.9436 | 0.9600 | 0.9598 | 0.8763 | |
| RMSD | 0.0643 | 0.0037 | 0.0051 | 0.0098 | 0.409 | 0.0099 | 0.0424 | 0.0031 | 0.0738 | 0.0424 | 0.0787 | 0.8151 | |
| kid2 (mg/g min1/2) | 0.0082 | 0.0005 | 0.0008 | 0.0024 | 0.0068 | 0.0100 | 0.0218 | 0.0057 | 0.0143 | 0.0326 | 0.0341 | 0.2162 | |
| C (mg/g) | 0.1770 | 0.4707 | 0.855 | 2.211 | 0.3306 | 0.33418 | 0.5584 | 0.7021 | 0.9910 | 1.5948 | 4.3859 | 7.1475 | |
| RMSD | 228.84 | 18.2893 | 0.0013 | 0.1110 | 0.0285 | 0.2372 | 0.2141 | 0.2372 | 0.0489 | 0.0091 | 0.0089 | 0.0893 | |
| Type of Soil | CR Concentration (mg L−1) | Def (cm2 s−1) |
|---|---|---|
| IS-65 | 10 | 1.293 × 10−7 |
| 20 | 3.722 × 10−7 | |
| 50 | 2.548 × 10−7 | |
| 100 | 5.094 × 10−7 | |
| IS-T | 10 | 8.270 × 10−6 |
| 20 | 6.727 × 10−6 | |
| 50 | 8.904 × 10−6 | |
| 100 | 4.573 × 10−6 | |
| MH-13 | 10 | 1.678 × 10−7 |
| 20 | 1.509 × 10−7 | |
| 50 | 1.767 × 10−7 | |
| 100 | 1.015 × 10−7 |
| Soil | CR (mg L−1) | t½ (s) | Dp (cm2 s−1) | Df (cm2 s−1) |
|---|---|---|---|---|
| IS-65 | 10 | 987.858 | 7.592 × 10−10 | 3.282 × 10−10 |
| 20 | 632.049 | 1.186 × 10−9 | 5.522 × 10−10 | |
| 50 | 679.556 | 1.103 × 10−9 | 4.424 × 10−10 | |
| 100 | 461.073 | 1.626 × 10−9 | 1.002 × 10−9 | |
| IS-T | 10 | 2376.504 | 1.136 × 10−8 | 1.565 × 10−8 |
| 20 | 1026.884 | 2.629 × 10−8 | 1.164 × 10−8 | |
| 50 | 531.147 | 5.083 × 10−8 | 3.521 × 10−8 | |
| 100 | 365.776 | 7.381 × 10−8 | 7.752 × 10−8 | |
| MH-13 | 10 | 529.794 | 1.415 × 10−9 | 3.532 × 10−8 |
| 20 | 1103.152 | 6.798 × 10−10 | 1.044 × 10−9 | |
| 50 | 852.230 | 8.800 × 10−10 | 3.688 × 10−9 | |
| 100 | 1311.950 | 5.716 × 10−10 | 3.522 × 10−9 |
| Soil | CR Concentration (mg L−1) | lnD | K0 (min−1) |
|---|---|---|---|
| IS-65 | 10 | 1.160 | 0.0058 |
| 20 | 2.167 | 0.0011 | |
| 50 | 2.834 | 0.0011 | |
| 100 | 3.677 | 0.0017 | |
| IS-T | 10 | 2.144 | 0.0018 |
| 20 | 2.808 | 0.0013 | |
| 50 | 3.499 | 0.005 | |
| 100 | 4.009 | 0.004 | |
| MH-13 | 10 | 1.958 | 0.005 |
| 20 | 2.630 | 0.003 | |
| 50 | 3.514 | 0.004 | |
| 100 | 1.718 | 0.006 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smaranda Bețianu, C.; Cozma, P.; Roșca, M.; Comăniță Ungureanu, E.-D.; Mămăligă, I.; Gavrilescu, M. Sorption of Organic Pollutants onto Soils: Surface Diffusion Mechanism of Congo Red Azo Dye. Processes 2020, 8, 1639. https://doi.org/10.3390/pr8121639
Smaranda Bețianu C, Cozma P, Roșca M, Comăniță Ungureanu E-D, Mămăligă I, Gavrilescu M. Sorption of Organic Pollutants onto Soils: Surface Diffusion Mechanism of Congo Red Azo Dye. Processes. 2020; 8(12):1639. https://doi.org/10.3390/pr8121639
Chicago/Turabian StyleSmaranda Bețianu, Camelia, Petronela Cozma, Mihaela Roșca, Elena-Diana Comăniță Ungureanu, Ioan Mămăligă, and Maria Gavrilescu. 2020. "Sorption of Organic Pollutants onto Soils: Surface Diffusion Mechanism of Congo Red Azo Dye" Processes 8, no. 12: 1639. https://doi.org/10.3390/pr8121639
APA StyleSmaranda Bețianu, C., Cozma, P., Roșca, M., Comăniță Ungureanu, E.-D., Mămăligă, I., & Gavrilescu, M. (2020). Sorption of Organic Pollutants onto Soils: Surface Diffusion Mechanism of Congo Red Azo Dye. Processes, 8(12), 1639. https://doi.org/10.3390/pr8121639







