Development of Circularly Polarized Luminescence (CPL) Peptides Containing Pyrenylalanines and 2-Aminoisobutyric Acid
Abstract
1. Introduction
2. Materials and Methods
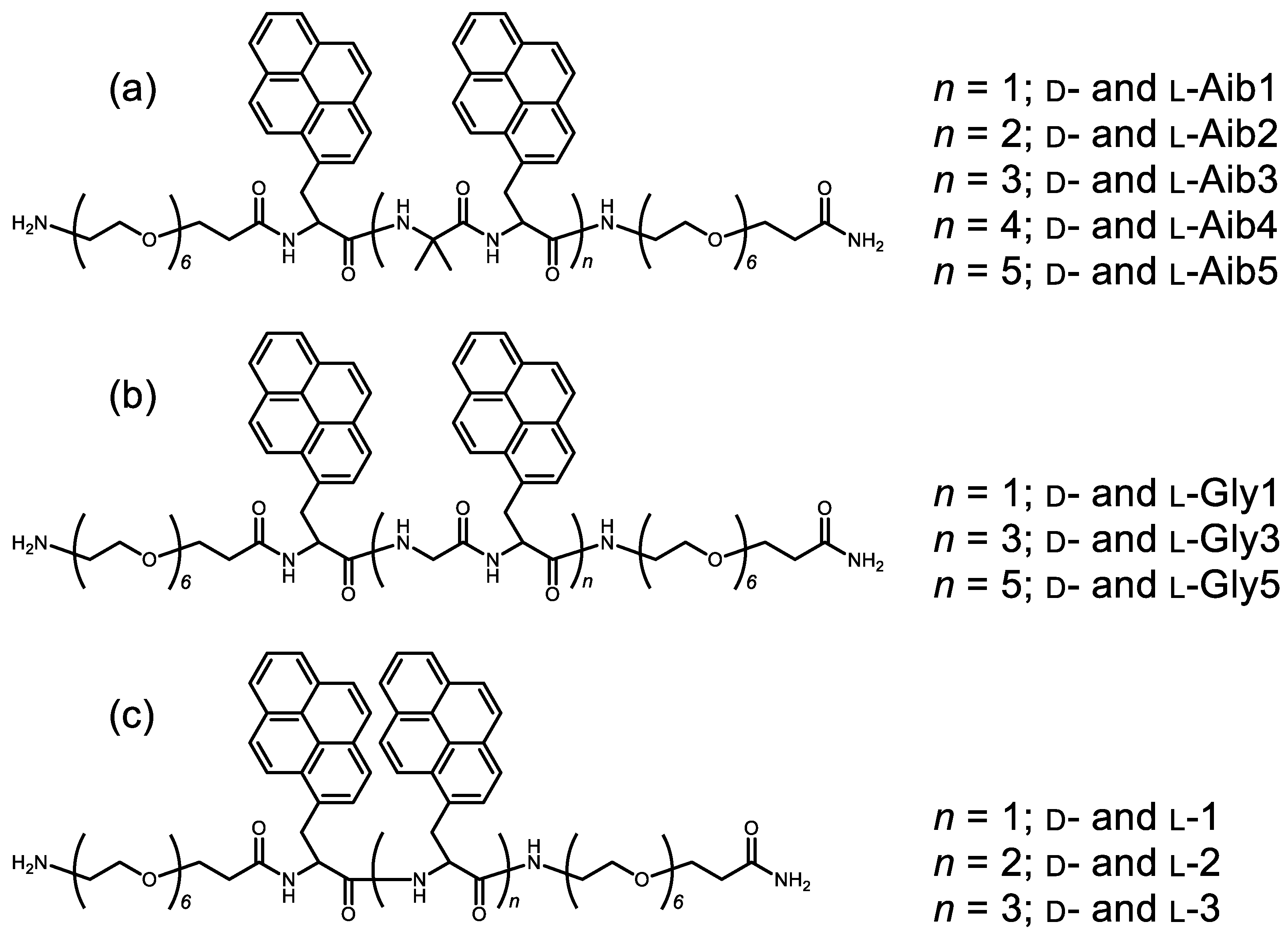
2.1. Synthesis of Chiral Aib-Pyrenyl Oligopeptides
2.2. Measurement of Circularly Polarized Luminescence (CPL) and Photoluminescence (PL) Spectra
2.3. Measurement of Circular Dichroism (CD) and UV–Vis Absorption Spectra
3. Results and Discussion
3.1. Measurement of Aib1–Aib5 Circularly Polarized Luminescence (CPL) and Photoluminescence (PL) Spectra and Circular Dichroism (CD) and UV–Vis Absorption Spectra in CHCl3 Solution
3.2. Measurement of Gly1, Gly3, and Gly5 Circularly Polarized Luminescence (CPL) and Photoluminescence (PL) Spectra and Circular Dichroism (CD) and UV–Vis Absorption Spectra in CHCl3 Solution
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Field, J.E.; Muller, G.; Riehl, J.P.; Venkataraman, D. Circularly Polarized Luminescence from Bridged Triarylamine Helicenes. J. Am. Chem. Soc. 2003, 125, 11808–11809. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Bando, Y. Recent progress in research on stimuliresponsive circularly polarized luminescence based on π-conjugated molecules. Pure Appl. Chem. 2013, 85, 1967–1978. [Google Scholar] [CrossRef]
- Sanchez-Carnerero, E.M.; Agarrabeitia, A.R.; Moreno, F.; Maroto, B.L.; Muller, G.; Ortiz, M.J.; Moya, S. Circularly Polarized Luminescence from Simple Organic Molecules. Chem. Eur. J. 2015, 21, 13488–13500. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.; Nakashima, T.; Kawai, T. Circularly Polarized Luminescence in Chiral Molecules and Supramolecular Assemblies. J. Phys. Chem. Lett. 2015, 6, 3445–3452. [Google Scholar] [CrossRef] [PubMed]
- Longhi, G.; Castiglioni, E.; Kosyoubu, J.; Mazzeo, G.; Sergio, A. Circularly Polarized Luminescence: A Review of Experimental and Theoretical Aspects. Chirality 2016, 28, 696–707. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Suenaga, T.; Sarkar, P.; Sato, S.; Kotani, M.; Isobe, H. Stereoisomerism, crystal structures, and dynamics of belt-shaped cyclonaphthylenes. Proc. Natl. Acad. Sci. USA 2016, 113, 8109–8114. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Inoue, Y.; Mori, T. Circularly Polarized Luminescence and Circular Dichroisms in Small Organic Molecules: Correlation between Excitation and Emission Dissymmetry Factors. ChemPhotoChem 2018, 2, 386–402. [Google Scholar] [CrossRef]
- Pop, F.; Zigon, N.; Avarvari, N. Main-Group-Based Electro- and Photoactive Chiral Materials. Chem. Rev. 2019, 119, 8435–8478. [Google Scholar] [CrossRef]
- Ma, J.-L.; Peng, Q.; Zhao, C.-H. Circularly Polarized Luminescence Switching in Small Organic Molecules. Chem. Eur. J. 2019, 25, 15441–15454. [Google Scholar] [CrossRef] [PubMed]
- Ohishi, Y.; Inouye, M. Circularly polarized luminescence from pyrene excimers. Tetrahedron Lett. 2019, 60, 151232. [Google Scholar] [CrossRef]
- Gao, J.; Zhang, W.Y.; Wu, Z.G.; Zheng, Y.X.; Fu, D.W. Enantiomorphic Perovskite Ferroelectrics with Circularly Polarized Luminescence. J. Am. Chem. Soc. 2020, 142, 4756–4761. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, T.; Tajima, N.; Kitamatsu, M.; Fujiki, M.; Imai, Y. Circularly polarised luminescence and circular dichroism of l- and d-oligopeptides with multiple pyrenes. Org. Biomol. Chem. 2015, 13, 11426–11431. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, T.; Kitamura, S.; Kitamatsu, M.; Fujiki, M.; Imai, Y. Peptide Magic: Interdistance-Sensitive Sign Inversion of Excimer Circularly Polarized Luminescence in Bipyrenyl Oligopeptides. ChemistrySelect 2016, 4, 831–835. [Google Scholar] [CrossRef]
- Yuan, W.Z.; Lu, P.; Chen, S.; Lam, J.W.Y.; Wang, Z.; Liu, Y.; Kwok, H.S.; Ma, Y.; Tang, B.Z. Changing the Behavior of Chromophores from Aggregation-Caused Quenching to Aggregation-Induced Emission: Development of Highly Efficient Light Emitters in the Solid State. Adv. Mater. 2010, 22, 2159–2163. [Google Scholar] [CrossRef] [PubMed]
- The absolute CD magnitude using the dimensionless Kuhn’s anisotropy factor in the ground state is defined as gCD = Δε/ε.
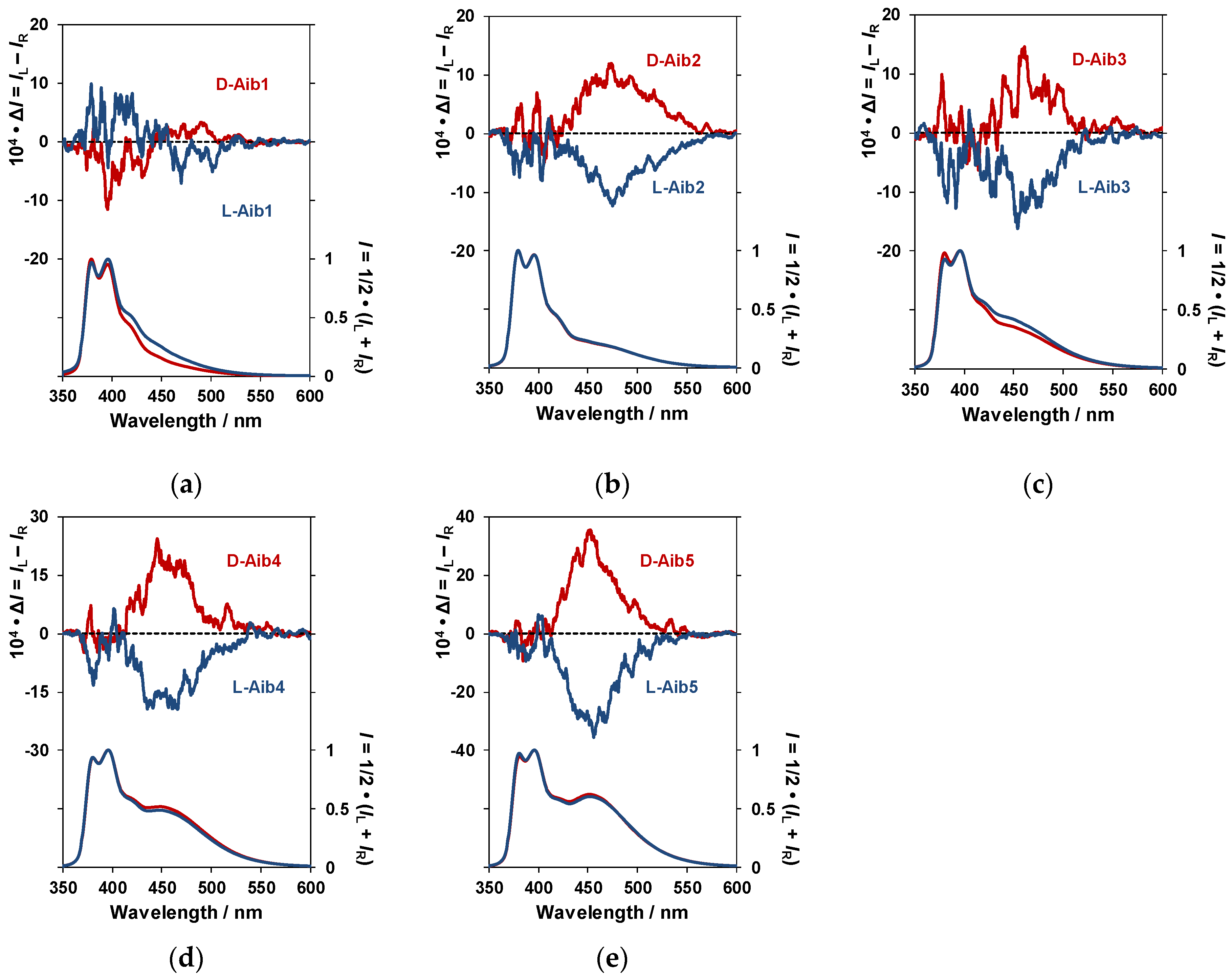
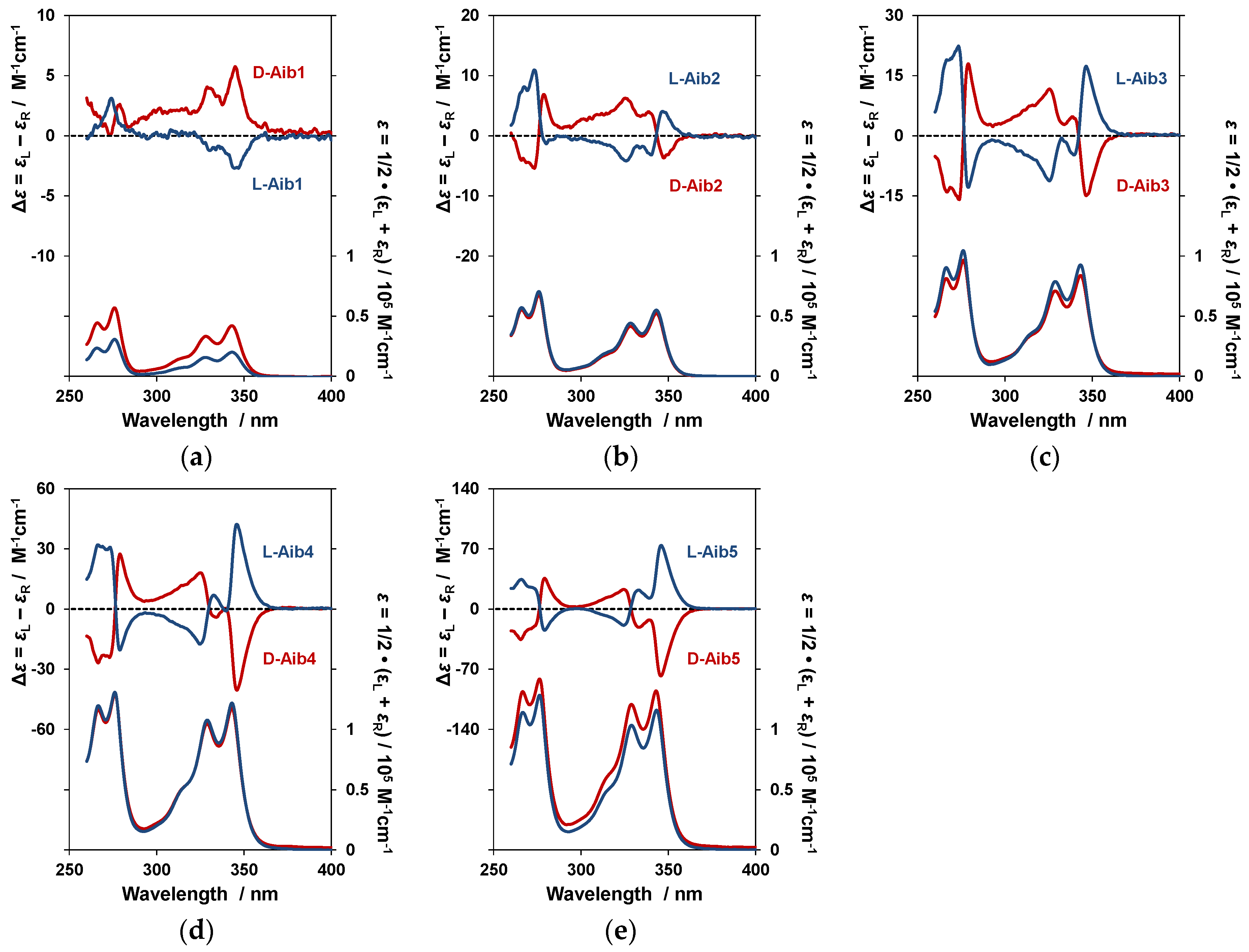


| Name | Monomer PL (nm) | Excimer PL (nm) | ΦF | gCPL (×10−3) | λCD (nm) | gCD (×10−4) | Note |
|---|---|---|---|---|---|---|---|
| d-Aib1 | 379 | ND | 0.11 | ND | 345 | +1.5 | |
| l-Aib1 | 379 | ND | ND | −1.7 | |||
| d-Aib2 | 395.5 | 473.5 | 0.12 | +6.7 | 347.5 | −1.1 | |
| l-Aib2 | 395.5 | 474.5 | −7.0 | +1.3 | |||
| d-Aib3 | 395.5 | 460.5 | 0.12 | +4.6 | 346.5 | −2.3 | |
| l-Aib3 | 395.5 | 453.5 | −4.0 | +2.4 | |||
| d-Aib4 | 395.5 | 449 | 0.14 | +4.7 | 346 | −1.6 | |
| l-Aib4 | 395.5 | 448 | −4.4 | +1.6 | |||
| d-Aib5 | 395.5 | 451.5 | 0.17 | +5.7 | 346 | −7.3 | |
| l-Aib5 | 395.5 | 451.5 | −5.9 | +7.7 | |||
| d-Gly1 [13] | 398 | 481.5 | 0.12 | ±2.0 | 346 | ±0.90 | |
| l-Gly1 [13] | 398 | 481.5 | |||||
| d-Gly3 | ND | ND | 0.08 | ND | 361.5 | +20 | Aggregated |
| l-Gly3 | ND | ND | ND | −18 | Aggregated | ||
| d-Gly5 | ND | ND | 0.11 | ND | 361 | +5.2 | Aggregated |
| l-Gly5 | ND | ND | ND | −7.8 | Aggregated | ||
| d-1 [12] | 395.5 | 463 | 0.10 | +11 | 349 | ∓0.91 | |
| l-1 [12] | 379 | 455 | −8.6 | ||||
| d-2 [12] | 396 | 454 | 0.12 | +8.5 | 351 | ∓1.2 | |
| l-2 [12] | 396 | 460 | −8.4 | ||||
| d-3 [12] | 396 | 454 | 0.15 | +2.7 | 351 | ∓1.1 | |
| l-3 [12] | 396 | 456 | −3.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mimura, Y.; Motomura, Y.; Kitamatsu, M.; Imai, Y. Development of Circularly Polarized Luminescence (CPL) Peptides Containing Pyrenylalanines and 2-Aminoisobutyric Acid. Processes 2020, 8, 1550. https://doi.org/10.3390/pr8121550
Mimura Y, Motomura Y, Kitamatsu M, Imai Y. Development of Circularly Polarized Luminescence (CPL) Peptides Containing Pyrenylalanines and 2-Aminoisobutyric Acid. Processes. 2020; 8(12):1550. https://doi.org/10.3390/pr8121550
Chicago/Turabian StyleMimura, Yuki, Yuki Motomura, Mizuki Kitamatsu, and Yoshitane Imai. 2020. "Development of Circularly Polarized Luminescence (CPL) Peptides Containing Pyrenylalanines and 2-Aminoisobutyric Acid" Processes 8, no. 12: 1550. https://doi.org/10.3390/pr8121550
APA StyleMimura, Y., Motomura, Y., Kitamatsu, M., & Imai, Y. (2020). Development of Circularly Polarized Luminescence (CPL) Peptides Containing Pyrenylalanines and 2-Aminoisobutyric Acid. Processes, 8(12), 1550. https://doi.org/10.3390/pr8121550





