Carbonaceous Adsorbent Derived from Sulfur-Impregnated Heavy Oil Ash and Its Lead Removal Ability from Aqueous Solution
Abstract
1. Introduction
2. Materials and Methods
2.1. Heavy Oil Ash
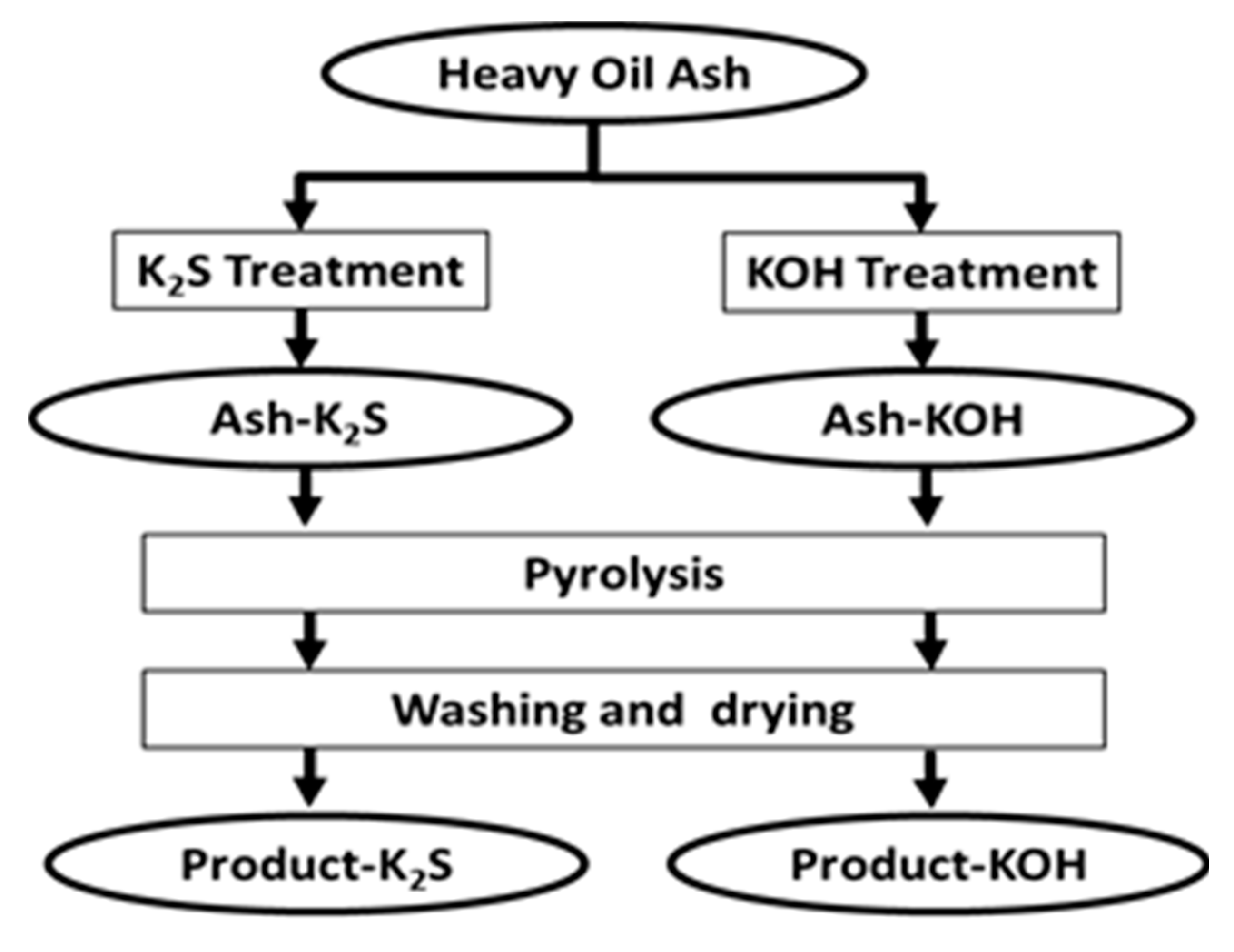
2.2. Preparation of Adsorbent
2.3. Lead Removal
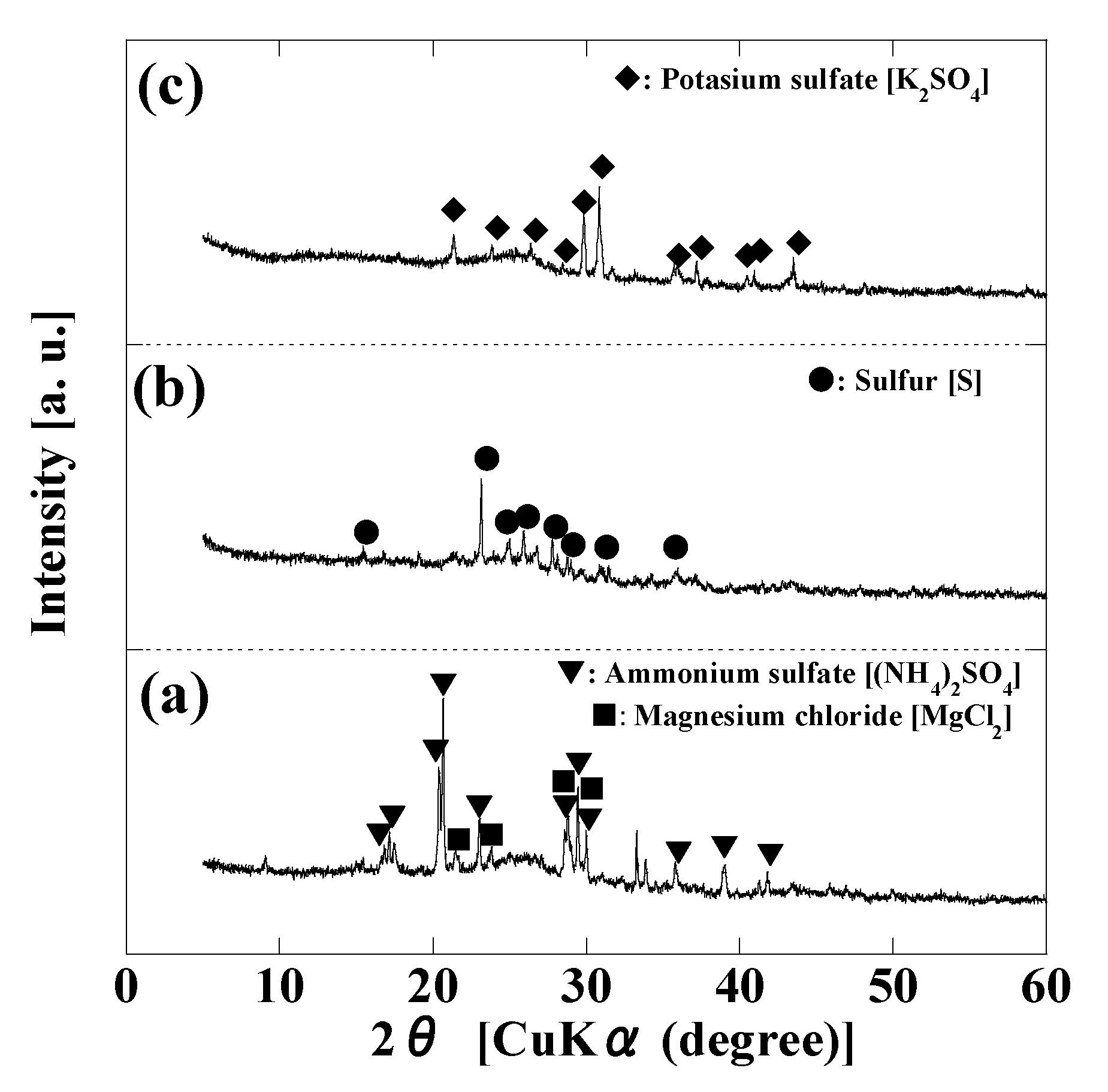
2.4. Characterization
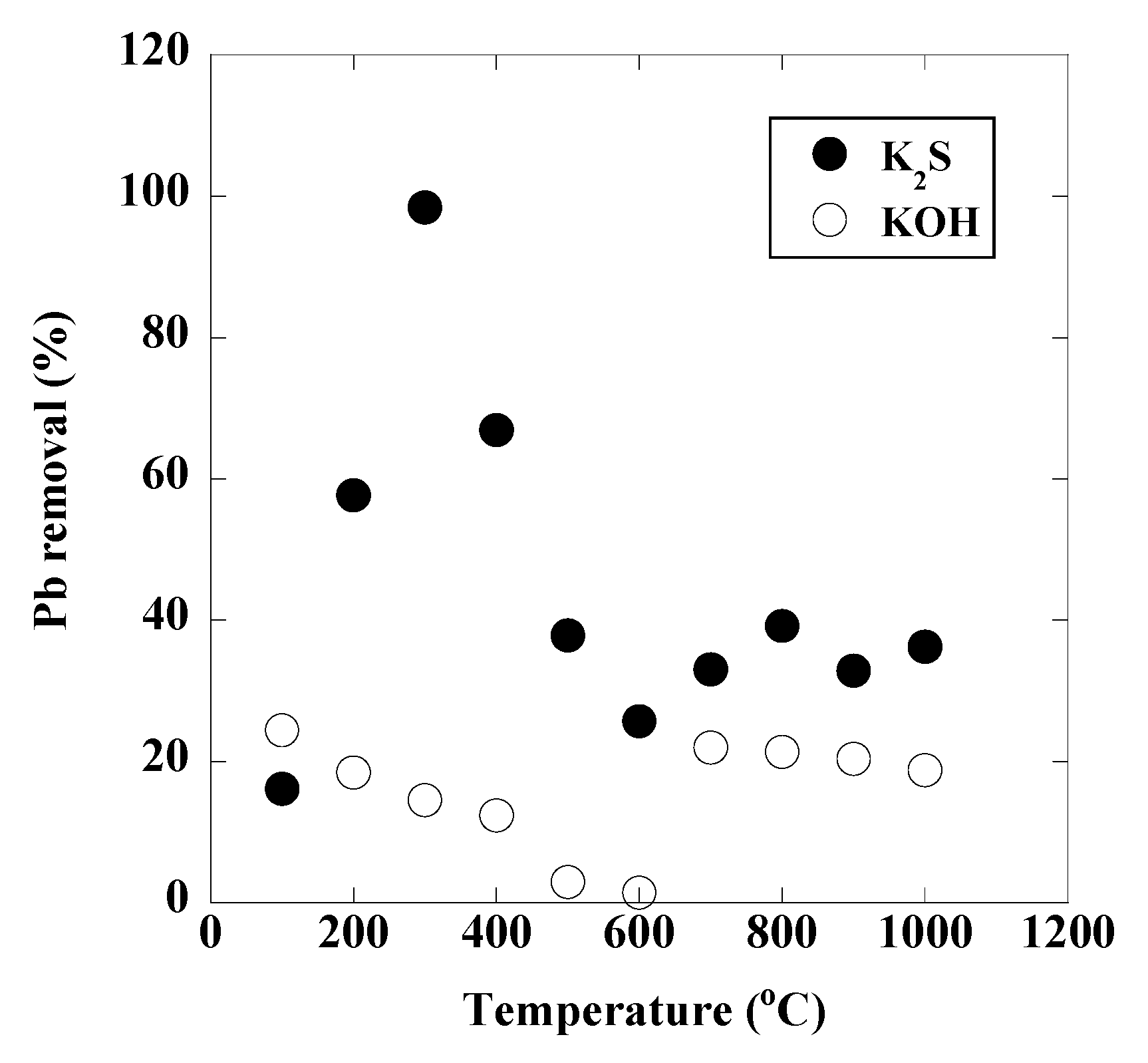
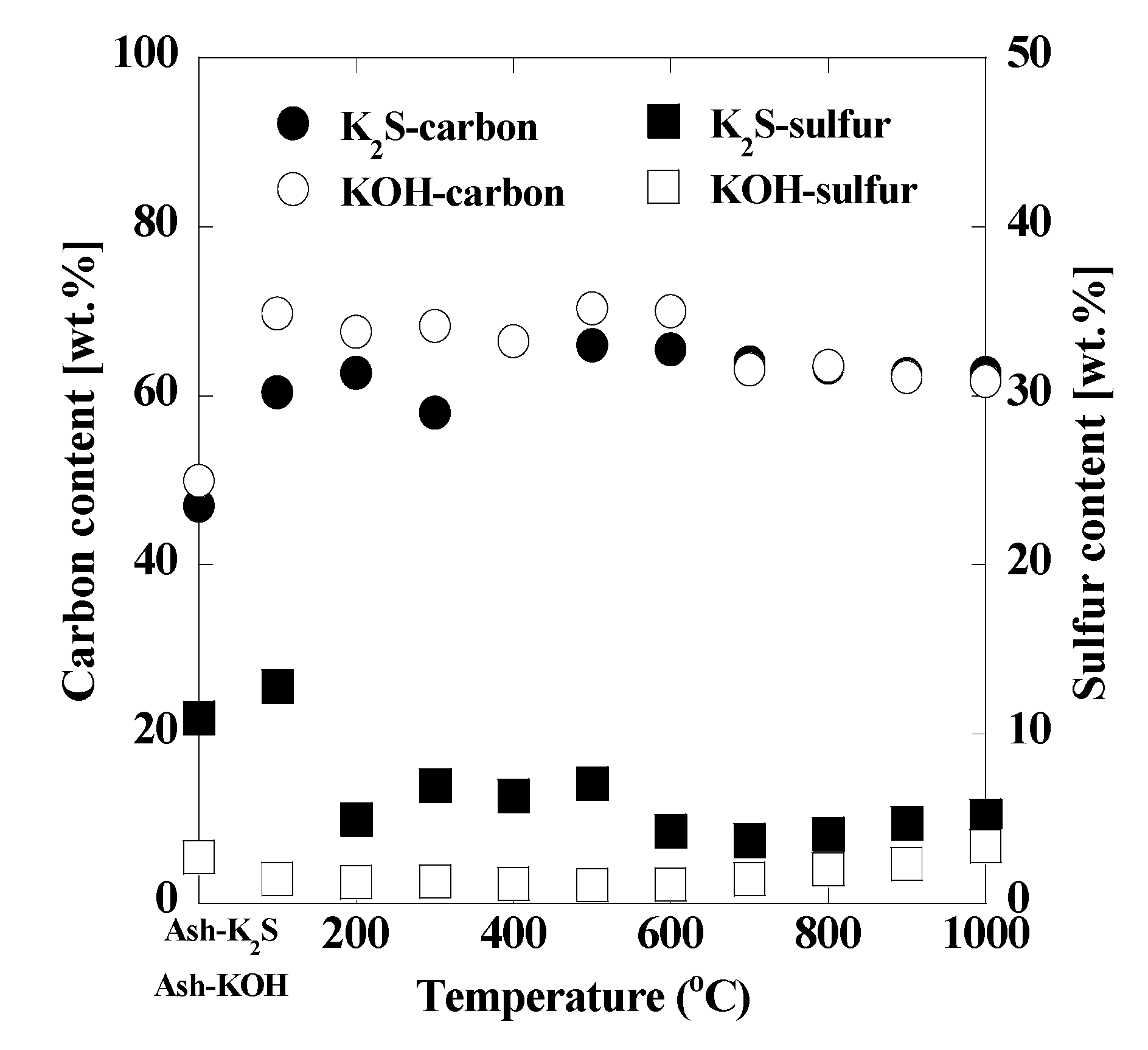
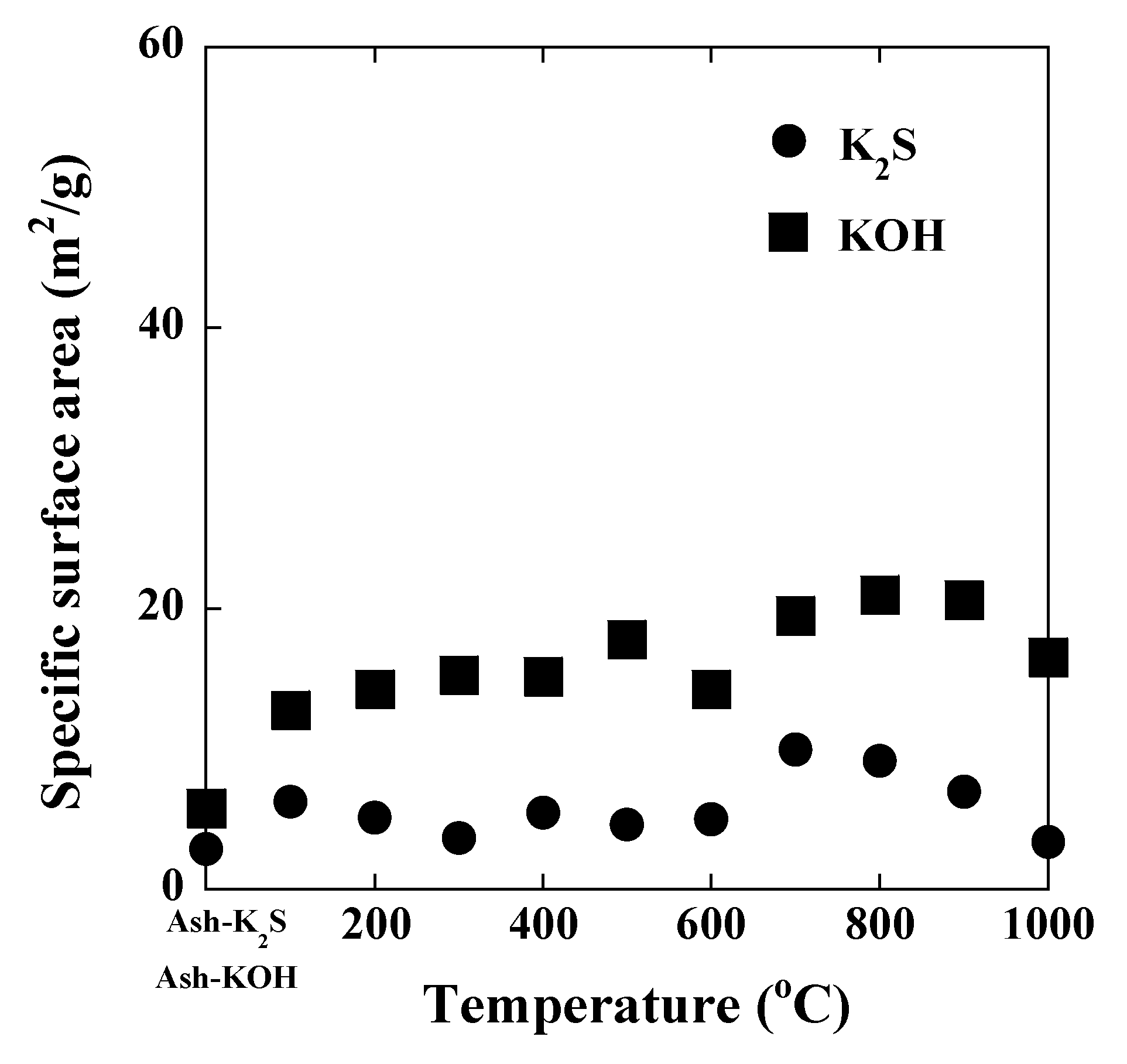
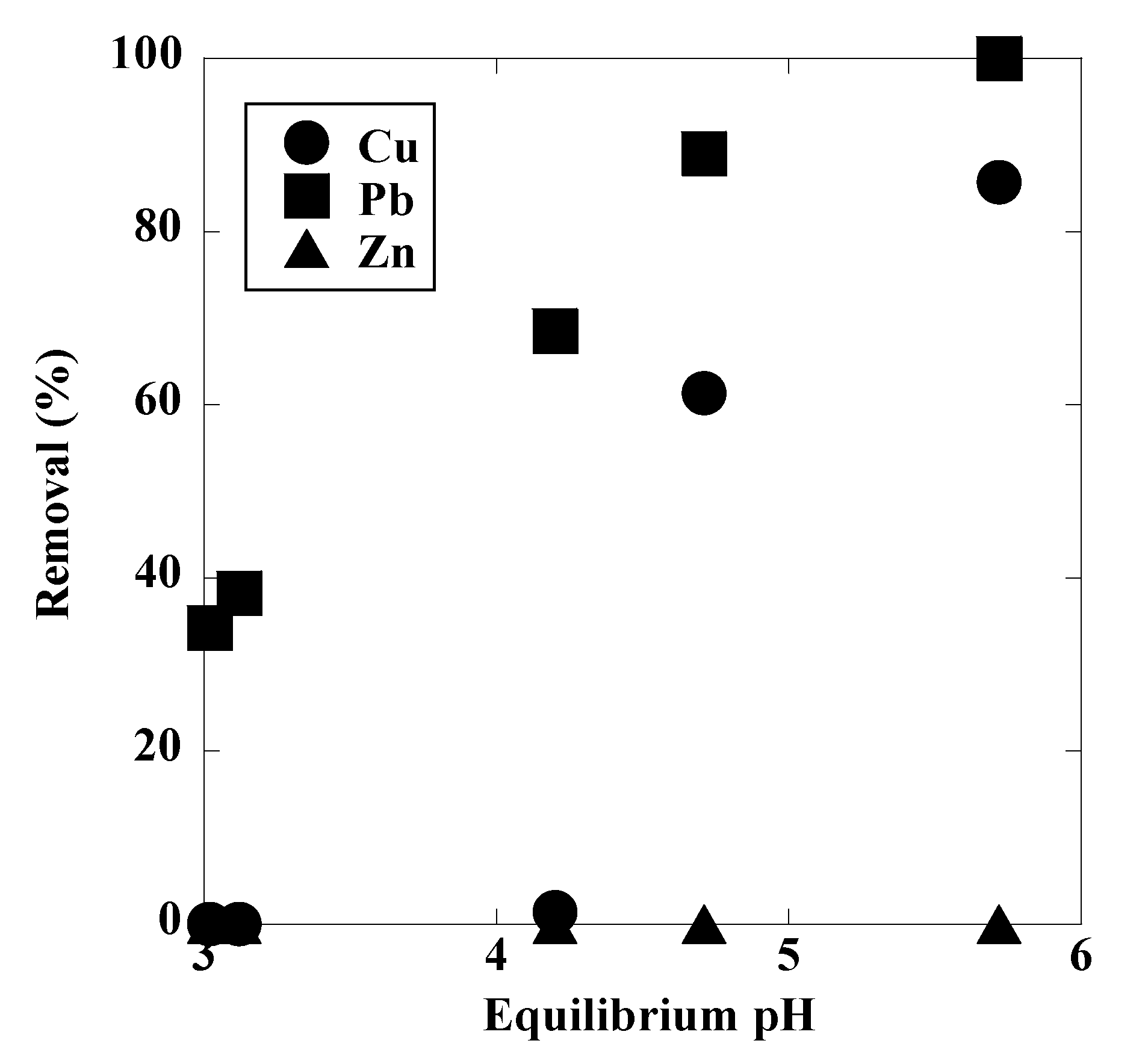
3. Results and Discussion
4. Conclusions
Funding
Conflicts of Interest
References
- Amer, A.M. Processing of Egyptian boiler-ash for extraction of vanadium and nickel. Waste Manag. 2002, 22, 515–520. [Google Scholar] [CrossRef]
- Tsai, S.-L.; Tsai, M.-S. A study of the extraction of vanadium and nickel in oil-fired fly ash. Resour. Conserv. Recycl. 1998, 22, 163–176. [Google Scholar] [CrossRef]
- Hsieh, Y.-M.; Tsai, M.-S. Physical and chemical analyses of unburned carbon from oil-fired fly ash. Carbon 2003, 41, 2317–2324. [Google Scholar] [CrossRef]
- Caramuscio, P.; De Stefano, L.; Seggiani, M.; Vitolo, S.; Narducci, P. Preparation of activated carbons from heavy-oil ash. Waste Manag. 2003, 23, 345–351. [Google Scholar] [CrossRef]
- Seggiani, M.; Vitolo, S.; Narducci, P. Investigation on the porosity development by CO2 activation in heavy oil ashes. Fuel 2003, 82, 1441–1450. [Google Scholar] [CrossRef]
- Seggiani, M.; Vitolo, S.; Filippis, P.D. Effect of pre-oxidation on the porosity development in a heavy oil fly ash by CO2 activation. Fuel 2005, 84, 1593–1596. [Google Scholar] [CrossRef]
- Adhoum, N.; Monser, L. Removal of cyanide from aqueous solution using impregnated activated carbon. Chem. Eng. Process. 2002, 41, 17–21. [Google Scholar] [CrossRef]
- Sadraei, R.; Paganini, M.C.; Calza, P.; Magnacca, G. An easy synthesis for preparing bia-based hybrid adsorbent useful for fast adsorption of polar pollutants. Nanomaterials 2019, 9, 731. [Google Scholar] [CrossRef]
- Magnacca, G.; Santos, F.N.D.; Sadraei, R. Bio-based substances from compost as reactant and active phase for selective capture of cationic pollutants from waste water. Front. Chem. 2020, 8, 550. [Google Scholar] [CrossRef]
- Volkan, M.; Ataman, A.; Howard, A. Pre-concentration of some trace metals from sea water on a mercapto-modified silica gel. Analyst 1987, 112, 1409–1412. [Google Scholar] [CrossRef]
- Krishnan, K.A.; Anirudhan, T.S. Removal of cadmium(II) from aqueous solutions by steam-activated sulphurised carbon prepared from sugar-cane bagasse pith: Kinetics and equilibrium studies. Water SA 2003, 29, 147–156. [Google Scholar] [CrossRef]
- Gomez-Serrano, V.; Macias-Garcia, A.; Espinosa-Mansilla, A.; Valenzuela-Calahorroc, C. Adsorption of mercury, cadmium and lead from aqueous solution on heat-treated and sulphurized activated carbon. Water Res. 1998, 32, 1–4. [Google Scholar] [CrossRef]
- Wajima, T. A new carbonaceous adsorbent for heavy metal removal from aqueous solution prepared from paper sludge by sulfur-impregnation and pyrolysis. Process Saf. Environ. Prot. 2017, 112, 342–352. [Google Scholar] [CrossRef]
- Pearson, R.G. Hard and soft acids and bases. J. Am. Chem. Soc. 1963, 85, 3533–3539. [Google Scholar] [CrossRef]
- Pearson, R.G. Recent advances in the concept of hard and soft acids and bases. J. Chem. Educ. 1987, 64, 561–567. [Google Scholar] [CrossRef]
- Zhao, D.; Gao, X.; Wu, C.; Xie, R.; Feng, S.; Chen, C. Facile preparation of amino functionalized graphene oxide decorated with Fe2O3 nanoparticles for the adsorption of Cr (IV). Appl. Surf. Sci. 2016, 384, 1–9. [Google Scholar] [CrossRef]
- Erdem, M.; Ozverdi, A. Lead adsorption from aqueous solution onto siderite. Sep. Purif. Technol. 2005, 42, 259–264. [Google Scholar] [CrossRef]
- Adebowale, K.O.; Unuabonah, I.E.; Olu-Owolabi, B.I. The effect of some operating variables on the adsorption of lead and cadmium ions on kaolinite clay. J. Hazard. Mater. 2006, 134, 130–139. [Google Scholar] [CrossRef]
- Feng, Q.; Lin, Q.; Gong, F.; Sugita, S.; Shoya, M. Adsorption of lead and mercury by rice husk ash. J. Colloid Interface Sci. 2004, 278, 1–8. [Google Scholar] [CrossRef]
- Huang, Y.H.; Hsueh, C.L.; Huang, C.P.; Su, L.C.; Chen, C.Y. Adsorption thermodynamic and kinetic studies of Pb (II) removal from water onto a versatile Al2O3-supported iron oxide. Sep. Purif. Technol. 2007, 55, 23–29. [Google Scholar] [CrossRef]
- Vuković, G.D.; Marinković, A.D.; Škapin, S.D.; Ristić, M.T.; Aleksić, R.; Perić-Grujić, A.A.; Uskoković, P.S. Removal of lead from water by amino modified multi-walled carbon nanotubes. Chem. Eng. J. 2011, 173, 855–865. [Google Scholar] [CrossRef]
- Saha, D.; Barakat, S.; Bramer, S.E.V.; Nelson, K.A.; Hensley, D.K.; Chen, J. Noncompetitive and competitive adsorption of heavy metals in sulfur-functionalized ordered mesoporous carbon. ACS Appl. Mater. Interfaces 2016, 8, 34132–34142. [Google Scholar] [CrossRef] [PubMed]
- Imamoglu, M.; Tekir, O. Removal of copper (II) and lead (II) ions from aqueous solutions by adsorption on activated carbon from a new precursor hazelnut husks. Desalination 2008, 228, 108–113. [Google Scholar] [CrossRef]
- Abdulkarim, M.; Al-Rub, F.A. Adsorption of lead ions from aqueous solution onto activated carbon and chemically-modified activated carbon prepared from date pits. Adsorp. Sci. Technol. 2004, 22, 119–134. [Google Scholar] [CrossRef]
- Brown, P.; Jefcoat, I.A.; Parrish, D.; Gil, S.; Graham, E. Evaluation of the adsorptive capacity of peanut hull pellets for heavy metals in solution. Adv. Environ. Res. 2000, 4, 19–29. [Google Scholar] [CrossRef]
- Johns, M.M.; Marshall, W.E.; Toles, C.A. Agricultural by-products as granular activated carbons for adsorbing dissolved metals and organics. J. Chem. Technol. Biotechnol. 1998, 71, 131–140. [Google Scholar] [CrossRef]
- Vilar, V.J.P.; Botelho, C.M.S.; Boaventura, R.A.R. Influence of pH, ionic strength and temperature on lead biosorption by Gelidium and agar extraction algal waste. Process Biochem. 2005, 40, 3267–3275. [Google Scholar] [CrossRef]
- Bansode, R.R.; Losso, J.N.; Marshall, W.E.; Rao, R.M.; Portier, R.J. Adsorption of metal ions by pecan shell-based granular activated carbons. Bioresour. Technol. 2003, 89, 115–119. [Google Scholar] [CrossRef]
- Kikuchi, Y.; Qian, Q.; Machida, M.; Tatsumoto, H. Effect of ZnO loading to activated carbon on Pb (II) adsorption from aqueous solution. Carbon 2006, 44, 195–202. [Google Scholar] [CrossRef]
- Issabayeva, G.; Aroua, M.K.; Sulaiman, N.M.N. Removal of lead from aqueous solutions on palm shell activated carbon. Bioresour. Technol. 2006, 97, 2350–2355. [Google Scholar] [CrossRef]
- Li, Q.; Yu, J.; Zhou, F.; Jiang, X. Synthesis and characterization of dithiocarbamate carbon nanotubes for the removal of heavy metal ions from aqueous solutions. Colloids Surf. A 2015, 482, 306–314. [Google Scholar] [CrossRef]
- Jing, X.; Liu, F.; Yang, X.; Ling, P.; Li, L.; Long, C.; Li, A. Adsorption performances and mechanisms of the newly synthesized N, N′-di(carboxymethyl) dithiocabamate chelating resin toward divalent heavy metal ions from aqueous media. J. Hazard. Mater. 2009, 167, 589–596. [Google Scholar] [CrossRef] [PubMed]



| Raw Ash | |
|---|---|
| Proximate analysis (wt% dry basis) | |
| Volatile matter 1 | 19.0 |
| Fixed carbon | 73.3 |
| Ash | 7.7 |
| Ultimate analysis (wt% dry ash free) | |
| Carbon | 79.5 |
| Hydrogen | 1.2 |
| Nitrogen | 4.4 |
| Sulfur | 4.0 |
| Ash composition (wt%) | |
| NiO | 29.9 |
| Fe2O3 | 18.8 |
| SO3 | 14.1 |
| SiO2 | 7.7 |
| CaO | 6.2 |
| V2O5 | 5.6 |
| Na2O | 5.0 |
| Al2O3 | 3.8 |
| ZnO | 3.2 |
| MgO | 1.1 |
| K2O | 0.9 |
| Specific surface area (m2/g) | 6.2 |
| Langmuir | Freundlich | ||||
|---|---|---|---|---|---|
| Qmax | KL | R2 | N | KF | R2 |
| 0.54 | 8.48 | 0.999 | 4.06 | 0.41 | 0.813 |
| Adsorbent | Adsorption Capacity (mmol/g) | Reference |
|---|---|---|
| Siderite | 0.06 | [17] |
| Kaolinite clay | 0.09 | [18] |
| Rice husk ash | 0.06 | [19] |
| Alumina supported iron oxide | 0.14 | [20] |
| Carbon nanotube | 0.01 | [21] |
| Sulfur-functionalized ordered mesoporous carbon | 0.14 | [22] |
| Hazelnut husk activated carbon | 0.06 | [23] |
| Date pits activated carbon | 0.15 | [24] |
| Peanut hull activated carbon | 0.15 | [25] |
| Rice straw activated carbon | 0.17 | [26] |
| Algal waste activated carbon | 0.21 | [27] |
| Pecan shell activated carbon | 0.31 | [28] |
| Coconut shell activated carbon | 0.37 | [29] |
| Palm shell activated carbon | 0.4 | [30] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wajima, T. Carbonaceous Adsorbent Derived from Sulfur-Impregnated Heavy Oil Ash and Its Lead Removal Ability from Aqueous Solution. Processes 2020, 8, 1484. https://doi.org/10.3390/pr8111484
Wajima T. Carbonaceous Adsorbent Derived from Sulfur-Impregnated Heavy Oil Ash and Its Lead Removal Ability from Aqueous Solution. Processes. 2020; 8(11):1484. https://doi.org/10.3390/pr8111484
Chicago/Turabian StyleWajima, Takaaki. 2020. "Carbonaceous Adsorbent Derived from Sulfur-Impregnated Heavy Oil Ash and Its Lead Removal Ability from Aqueous Solution" Processes 8, no. 11: 1484. https://doi.org/10.3390/pr8111484
APA StyleWajima, T. (2020). Carbonaceous Adsorbent Derived from Sulfur-Impregnated Heavy Oil Ash and Its Lead Removal Ability from Aqueous Solution. Processes, 8(11), 1484. https://doi.org/10.3390/pr8111484






