In Vitro Molecular Biology Studies of Spirooxindole Heterocyclic Hybrids
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Ames, B.N.; Gold, L.S.; Willett, W.C. The causes and prevention of cancer. Proc. Natl. Acad. Sci. USA 1995, 92, 5258–5265. [Google Scholar] [CrossRef]
- Seyfried, T.N.; Huysentruyt, L.C. On the origin of cancer metastasis. Crit. Rev. Oncog. 2013, 18, 43–73. [Google Scholar] [CrossRef] [PubMed]
- Blackadar, C.B. Historical review of the causes of cancer. World J. Clin. Oncol. 2016, 7, 54–86. [Google Scholar] [CrossRef] [PubMed]
- Schirrmacher, V. From chemotherapy to biological therapy: A review of novel concepts to reduce the side effects of systemic cancer treatment (Review). J. Oncol. 2019, 54, 407–419. [Google Scholar]
- Mansoori, B.; Mohammadi, A.; Davudian, S.; Shirjang, S.; Baradaran, B. The different mechanisms of cancer drug resistance: A brief review. Adv. Pharm. Bull. 2017, 7, 339–348. [Google Scholar] [CrossRef]
- Roy, S.; Kumar, A.; Islam, M.S.; Rabbi, F.A.; Paul, P.; Mia, M.M.; Islam, A.K.M.K.; Ray, A.K. Drug resistance and its future perspectives in cancer treatment. Asian Oncol. Res. J. 2020, 3, 26–46. [Google Scholar]
- Cotter, T.G. Apoptosis and cancer: The genesis of a research field. Nat. Rev. Cancer. 2009, 9, 501–507. [Google Scholar] [CrossRef]
- Plati, J.; Bucur, O.; Khosravi-Far, R. Dysregulation of apoptotic signaling in cancer: Molecular mechanisms and therapeutic opportunities. J. Cell. Biochem. 2008, 104, 1124–1149. [Google Scholar] [CrossRef]
- Chande, M.S.; Verma, R.S.; Barve, P.A.; Khanwelkar, R.R.; Vaidya, R.B.; Ajaikumar, K.B. Facile synthesis of active antitubercular, cytotoxic and antibacterial agents: A Michael addition approach. Eur. J. Med. Chem. 2005, 40, 1143–1148. [Google Scholar] [CrossRef]
- Lundahl, K.; Schut, J.; Schlatmann, J.L.; Paerels, G.B.; Peters, A. Synthesis and antiviral activities of adamantane spiro compounds. 1. Adamantane and analogous spiro-3’-pyrrolidines. J. Med. Chem. 1972, 15, 129–132. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Liu, X.; Wang, D.; Tian, M.; Han, S.; Feng, T.; Liu, X.; Mei, R.; Zhou, Y. Diversity-oriented one-pot multicomponent synthesis of spirooxindole derivatives and their biological evaluation for anticancer activities. Tetrahedron 2016, 72, 8523–8536. [Google Scholar] [CrossRef]
- Lotfy, G.; Said, M.M.; Ashry, E.E.; Tamany, E.S.; Al-Dhfyan, A.; Aziz, Y.M.; Barakat, A. Synthesis of new spirooxindole-pyrrolothiazole derivatives: Anti-cancer activity and molecular docking. Bioorg. Med. Chem. 2017, 254, 1514–1523. [Google Scholar] [CrossRef] [PubMed]
- Barakat, A.; Islam, M.S.; Ghawas, H.M.; Al-Majid, A.; El-Senduny, F.F.; Badria, F.; Elshaier, Y.A.; Ghabbour, H. Substituted spirooxindole derivatives as potent anticancer agents through inhibition of phosphodiesterase 1. RSC Adv. 2018, 8, 14335–14346. [Google Scholar] [CrossRef]
- Kornet, M.J.; Thio, A.P. Oxindole-3-spiropyrrolidines and -piperidines. Synthesis and local anesthetic activity. J. Med. Chem. 1976, 19, 892–898. [Google Scholar] [CrossRef] [PubMed]
- Maclean, D.; Schullek, J.R.; Murphy, M.M.; Ni, Z.J.; Gordon, E.M.; Gallop, M.A. Encoded combinatorial chemistry: Synthesis and screening of a library of highly functionalized pyrrolidines. Proc. Natl. Acad. Sci. USA 1997, 94, 2805–2810. [Google Scholar] [CrossRef]
- Lo, M.M.-C.; Neumann, C.S.; Nagayama, S.; Perlstein, E.O.; Schreiber, S.L. A Library of Spirooxindoles Based on a Stereoselective Three-Component Coupling Reaction. J. Am. Chem. Soc. 2004, 126, 16077–16086. [Google Scholar] [CrossRef]
- Jossang, A.; Jossang, P.; Hadi, H.A.; Sevenet, T.; Bodo, B. Horsfiline, an oxindole alkaloid from Horsfieldia superba. J. Org. Chem. 1991, 56, 6527–6530. [Google Scholar] [CrossRef]
- Pellegrini, C.; Weber, M.; Borschberg, H.-J. Total Synthesis of (+)-Elacomine and (−)-Isoelacomine, Two Hitherto Unnamed Oxindole Alkaloids from Elaeagnus commutate. Helvitica 1996, 79, 151–168. [Google Scholar]
- Galliford, C.V.; Scheidt, K.A. Pyrrolidinyl-spirooxindole natural products as inspirations for the development of potential therapeutic agents. Angew. Chem. Int. Ed. 2007, 46, 8748–8758. [Google Scholar] [CrossRef]
- Roeder, E.; Wiedenfeld, H. Pyrrolizidine alkaloids in plants used in the traditional medicine of Madagascar and the Mascarene islands. Pharmazie 2011, 66, 637–647. [Google Scholar] [PubMed]
- Wang, S.; Zhao, Y.; Aguilar, A.; Bernard, D.; Yang, C.-Y. Targeting the MDM2–p53 Protein–Protein Interaction for New Cancer Therapy: Progress and Challenges. Cold Spring Harb. Perspect. Med. 2017, 7, a026245. [Google Scholar] [CrossRef] [PubMed]
- Almansour, A.I.; Kumar, R.S.; Beevi, F.; Shirazi, A.N.; Osman, H.; Ismail, R.; Choon, T.S.; Sullivan, B.; McCaffrey, K.; Nahhas, A.; et al. Facile, Regio- and Diastereoselective Synthesis of Spiro-Pyrrolidine and Pyrrolizine Derivatives and Evaluation of Their Antiproliferative Activities. Molecules 2014, 19, 10033–10055. [Google Scholar] [CrossRef]
- Almansour, A.I.; Arumugam, N.; Kumar, R.S.; Subbarayan, P.V.; Alshatwi, A.A.; Ghabbour, H.A. Anticancer Agents. U.S. Patent 9,486,444 B1, 8 November 2016. [Google Scholar]
- Almansour, A.I.; Arumugam, N.; Kumar, R.S.; Subbarayan, P.V.; Alshatwi, A.A.; Athinarayanan, J. Anticancer Agents. U.S. Patent 9,873,699B1, 23 January 2018. [Google Scholar]
- Kia, Y.; Osman, H.; Kumar, R.S.; Basiri, A.; Murugaiyah, V. Synthesis and discovery of highly functionalized mono- and bis-spiro-pyrrolidines as potent cholinesterase enzyme inhibitors. Bioorg. Med. Chem. Lett. 2014, 24, 1815–1819. [Google Scholar] [CrossRef] [PubMed]
- Filosa, R.; Peduto, A.; de Caprariis, P.; Saturnino, C.; Festa, M.; Petrella, A.; Pau, A.; Pinna, G.A.; Colla, P.L.; Busonera, B.; et al. Synthesis and antiproliferative properties ofN3/8-disubstituted3,8-diazabicyclo [3.2.1]octane analogues of 3,8-bis[2-(3,4,5-trimethoxyphenyl)pyridin-4-yl]methyl-piperazine. Eur. J. Med. Chem. 2007, 42, 293–306. [Google Scholar] [CrossRef] [PubMed]
- Carreira, E.M.; Fessard, T.C. Four-membered ring-containing spirocycles: Synthetic strategies and opportunities. Chem. Rev. 2014, 114, 8257–8322. [Google Scholar] [CrossRef]
- Zheng, Y.-J.; Tice, C.M. The utilization of spirocyclic scaffolds in novel drug discovery. Expert Opin. Drug Discov. 2016, 11, 831–834. [Google Scholar] [CrossRef]
- Al-thamili, D.M.; Almansour, A.I.; Arumugam, N.; Mohammad, F.; Kumar, R.S. Functionalized N-pyridinylmethyl engrafted bisarylmethylidenepyridinones as anticancer agents. Processes 2020, 8, 1154. [Google Scholar] [CrossRef]
- Kumar, R.S.; Al-thamili, D.M.; Almansour, A.I.; Arumugam, N.; Dege, N. [Bmim]Br accelerated one-pot three-component cascade protocol for the construction of spirooxindole–pyrrolidine heterocyclic hybrids. Molecules 2020, 25, 4779. [Google Scholar] [CrossRef]





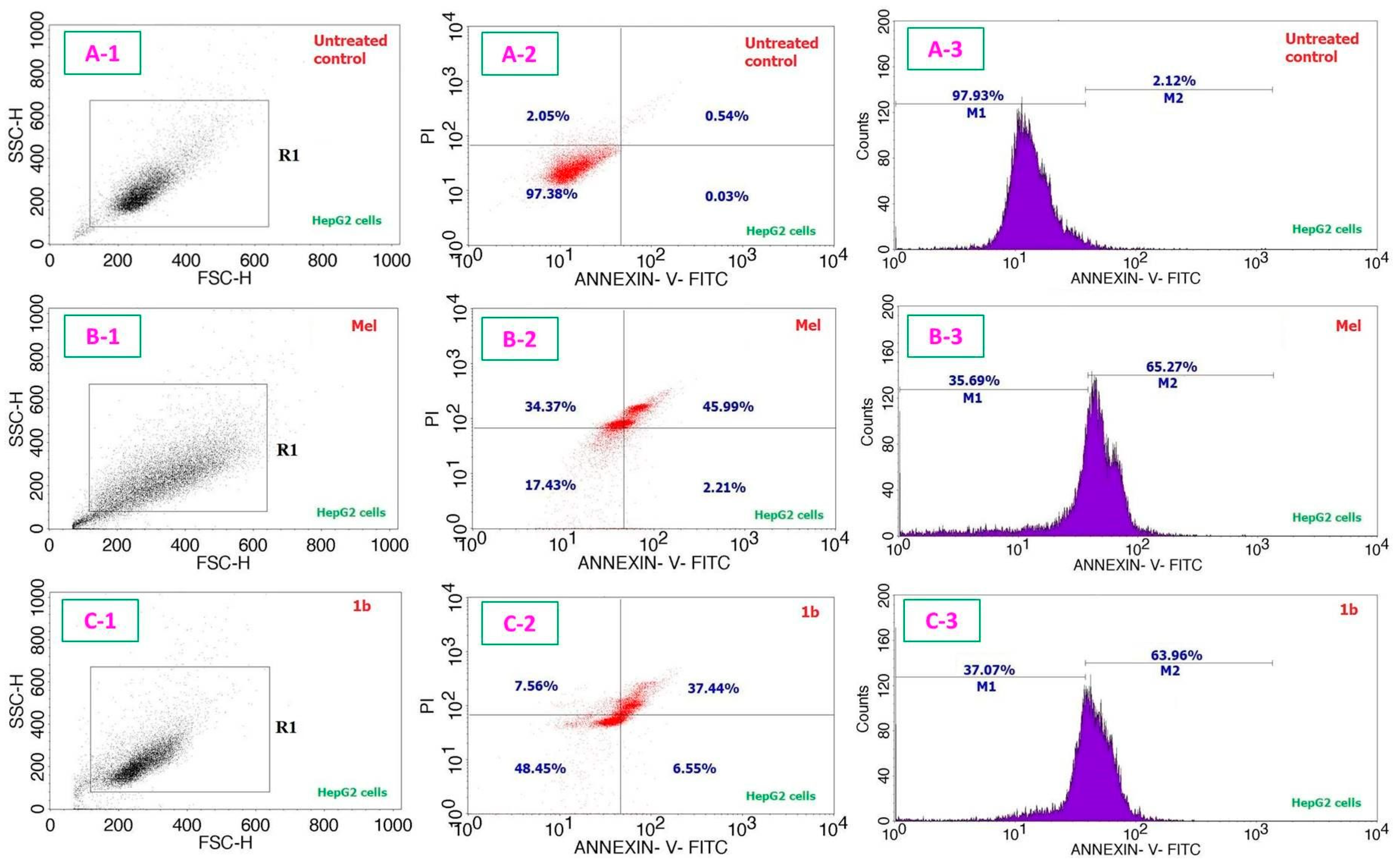


| Entry | Compound | IC50 Values in µg/mL (µM); 24 h | IC50 Values in µg/mL (µM); 48 h | |
|---|---|---|---|---|
| HepG2 Cells | HepG2 Cells | L929 Cells | ||
| 1 |  | 237.59 ± 12.5 (385.23 ± 20.27) | 126.81 ± 10.4 (205.61 ± 16.86) | - |
| 2 |  | 72.24 ± 8.7 (112.03 ± 13.49) | 23.81 ± 2.3 (36.92 ± 3.57) | 301.72 ± 12.51 (467.93 ± 19.40) |
| 3 | 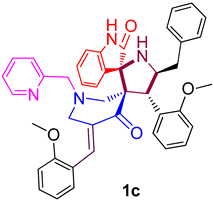 | 265.24 ± 9.4 (391.90 ± 13.89) | 29.83 ± 2.1 (44.07 ± 3.10) | - |
| 4 | 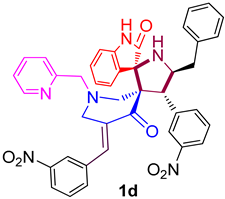 | 107.74 ± 7.5 (152.44 ± 10.62) | 44.1 ± 2.6 (62.40 ± 3.68) | - |
| 5 | 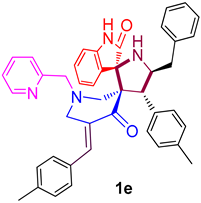 | 237.64 ± 8.9 (368.55 ± 13.80) | 45.86 ± 2.8 (71.12 ± 4.34) | - |
| 6 |  | 90.01 ± 5.6 (131.28 ± 8.17) | 25.01 ± 3.2 (36.48 ± 4.67) | 257.16 ± 9.6 (375.06 ± 14.00) |
| 7 | 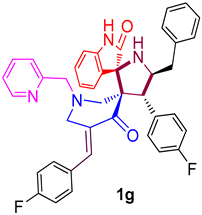 | 509.28 ± 14.3 (780.23 ± 21.91) | 40.51 ± 3.6 (62.06 ± 5.52) | - |
| 8 |  | 157.27 ± 11.3 (208.43 ± 14.98) | 43.17 ± 4.5 (57.21 ± 5.96) | - |
| 9 |  | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
M. Al-thamili, D.; Almansour, A.I.; Arumugam, N.; Mohammad, F.; Suresh Kumar, R. In Vitro Molecular Biology Studies of Spirooxindole Heterocyclic Hybrids. Processes 2020, 8, 1473. https://doi.org/10.3390/pr8111473
M. Al-thamili D, Almansour AI, Arumugam N, Mohammad F, Suresh Kumar R. In Vitro Molecular Biology Studies of Spirooxindole Heterocyclic Hybrids. Processes. 2020; 8(11):1473. https://doi.org/10.3390/pr8111473
Chicago/Turabian StyleM. Al-thamili, Dhaifallah, Abdulrahman I. Almansour, Natarajan Arumugam, Faruq Mohammad, and Raju Suresh Kumar. 2020. "In Vitro Molecular Biology Studies of Spirooxindole Heterocyclic Hybrids" Processes 8, no. 11: 1473. https://doi.org/10.3390/pr8111473
APA StyleM. Al-thamili, D., Almansour, A. I., Arumugam, N., Mohammad, F., & Suresh Kumar, R. (2020). In Vitro Molecular Biology Studies of Spirooxindole Heterocyclic Hybrids. Processes, 8(11), 1473. https://doi.org/10.3390/pr8111473





