A Methodology to Estimate the Sorption Parameters from Batch and Column Tests: The Case Study of Methylene Blue Sorption onto Banana Peels
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Surface Characterization
2.3. Batch Sorption Studies
2.4. Continuous Flow Studies in a Fixed-Bed Column
3. Theoretical Modeling
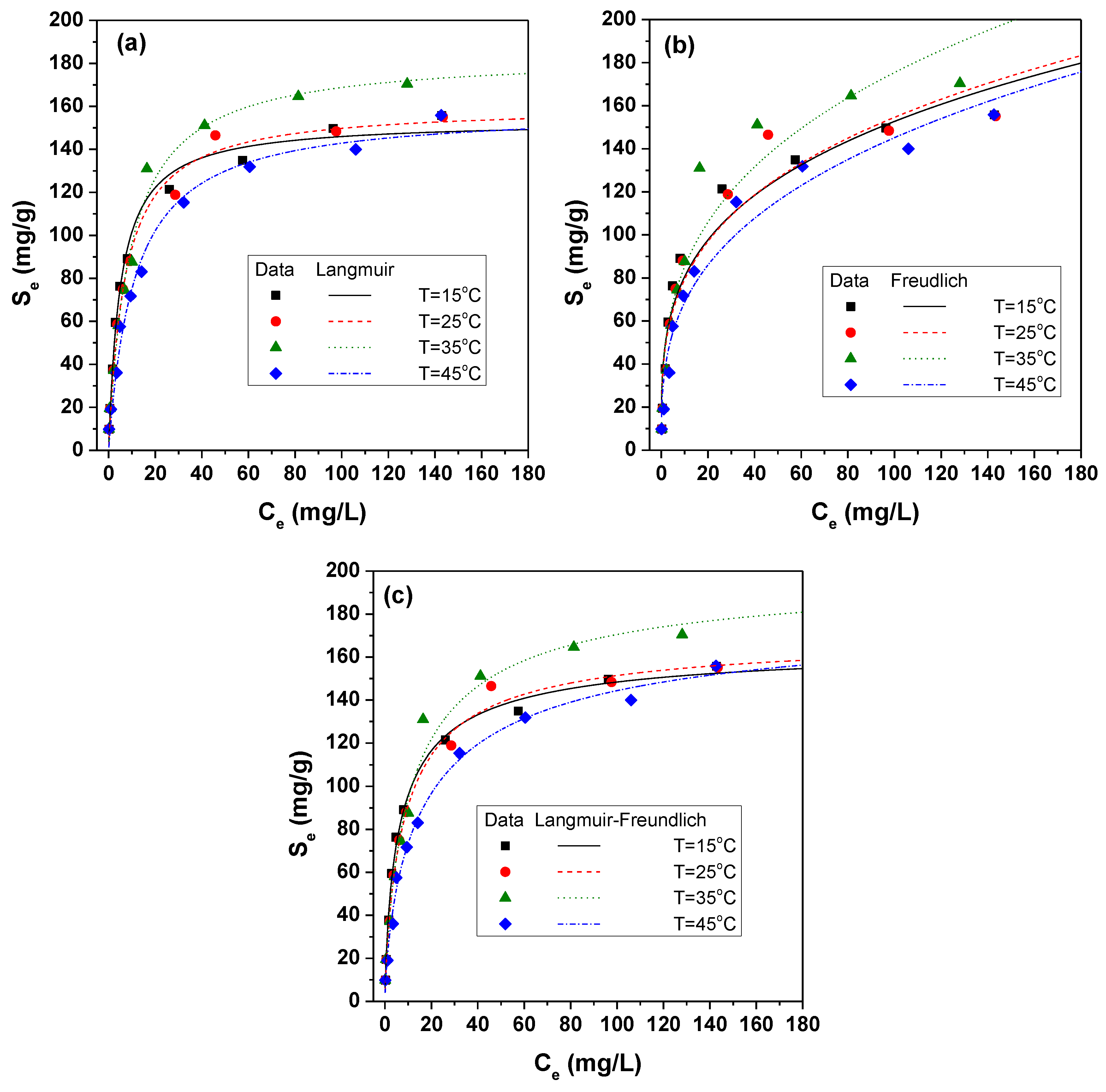
3.1. Sorption Isotherms
3.2. Kinetic Models
- (1)
- In the Langmuir kinetic model, the adsorption rate is regarded proportional to the fraction of vacant sites, and the desorption rate is proportional to the fraction of sites covered by adsorbed molecules. The overall rate is writtenwhere are the kinetic constants of adsorption and desorption steps, respectively. At equilibrium, , and Equation (6) coincides with the Langmuir isotherm with
- (2)
- In the Freundlich kinetic model, the adsorption rate changes nonlinearly with the concentration of dissolved molecules, and the overall sorption rate is given by [48]
- (3)
- In the hybrid (Langmuir–Freundlich) kinetic model, the adsorption rate changes nonlinearly with the concentration of dissolved molecules and is proportional to the concentration of vacant sites. The rate becomes
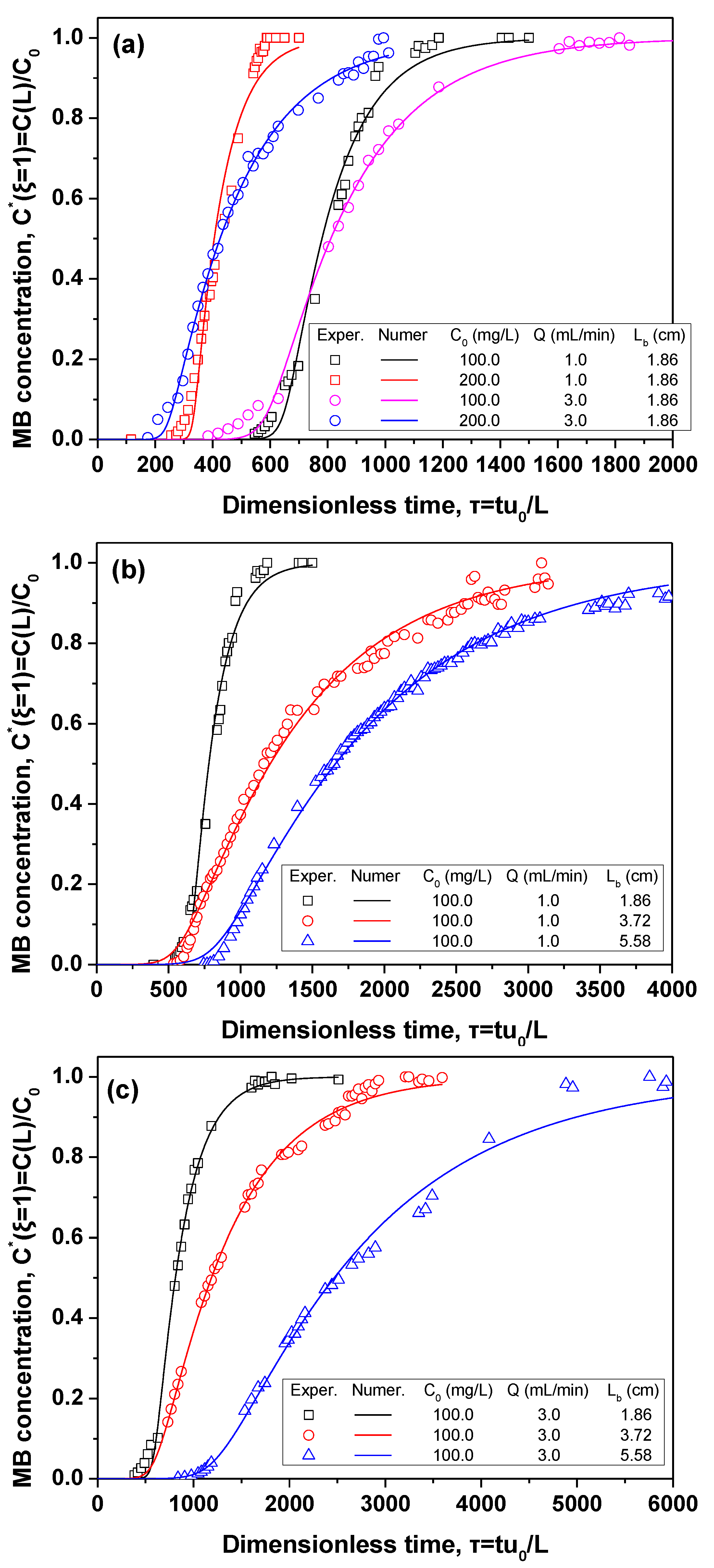
3.3. Modeling the Performance of a Fixed-Bed Column
4. Results
4.1. Surface Chemistry and Sorption Mechanism
4.2. Equilibrium and Kinetic Parameters from Batch Tests
4.3. Parameter Estimation from Column Tests
5. Conclusions
- MB sorption onto BP is an exothermic and reversible process, with a strongly activated desorption step and weakly activated adsorption step.
- Equilibrium and kinetic data are better fitted by the Langmuir model, which is consistent with the monolayer chemisorption of MB cations onto BP solid surface by electrostatic forces.
- The experimental breakthrough curves and MB sorption capacity onto BP grains of the bed are predicted satisfactorily by the numerical model.
- The sorption capacity of adsorbents under continuous flow conditions is comparable to the corresponding one estimated from batch experiments.
- The sorption rate constant estimated from continuous flow tests on a column bed is fully compatible with corresponding parameters estimated from batch tests when the same sorption model is used.
- The kinetics of sorption in column beds is affected by the thickness of the velocity and concentration boundary layers surrounding the grains of sorbent and nonuniformities of the flow field caused by local nonrandom heterogeneities. This parameter is an increasing function of flow velocity and feed concentration and a decreasing function of bed length. It is a decreasing function of , tending to an asymptotic value at high values of this parameter.
- Compared to earlier studies, the highest MB sorption capacity was achieved in the present study with fixed-bed columns of BP operating over relatively low values.
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| Cross-sectional area of fixed-bed column | |
| Pre-exponential factor of Arrhenius rate constant for adsorption step | |
| Pre-exponential factor of Arrhenius rate constant for desorption step | |
| Rate constant of desorption step | |
| Hydrodynamic dispersion length | |
| Integration constant of van’t Hoff equation | |
| Dye concentration in aqueous solution | |
| Initial dye concentration | |
| Dye concentration in solution at equilibrium with adsorbent | |
| Dimensionless dye concentration in aqueous solution | |
| Inside diameter of column | |
| Longitudinal dispersion coefficient | |
| Molecular diffusion coefficient of dye | |
| Damköhler number | |
| Adsorbent grain diameter | |
| Mean grain size | |
| Activation energy for adsorption step | |
| Activation energy for desorption step | |
| Electrical formation factor of packed bed | |
| Freundlich equilibrium constant | |
| Langmuir equilibrium constant | |
| Langmuir–Freundlich equilibrium constant | |
| Permeability of packed bed | |
| Kinetic constant of adsorption step | |
| Kinetic constant of desorption step | |
| Column length | |
| Bed length | |
| Adsorbent mass | |
| Volumetric flow rate | |
| Bed-scale Peclet number | |
| Grain-scale Peclet number | |
| Column-scale Peclet number | |
| Universal gas constant | |
| Adsorbed dye concentration (dye mass per unit mass of adsorbent) | |
| Adsorbed dye concentration at equilibrium | |
| Sorption capacity (maximum dye concentration) | |
| Temperature | |
| time | |
| Mean pore velocity | |
| Axial coordinate | |
| Greek letters | |
| Freundlich exponent | |
| Heat of adsorption | |
| Dimensionless axial coordinate | |
| Dimensionless axial position of the upper bed surface | |
| Bulk density of bed | |
| Dimensionless time | |
| Porosity of packed bed | |
References
- Katheresan, V.; Kansedo, J.; Lau, S.Y. Efficiency of various recent wastewater dye removal methods: A review. J. Environ. Chem. Eng. 2018, 6, 4676–4697. [Google Scholar] [CrossRef]
- Rawat, D.; Mishra, V.; Sharma, R.S. Detoxification of azo dyes in the context of environmental processes. Chemosphere 2016, 155, 591–605. [Google Scholar] [CrossRef] [PubMed]
- Brüschweiler, B.J.; Merlot, C. Azo dyes in clothing textiles can be cleaved into a series of mutagenic aromatic amines which are not regulated yet. Regul. Toxicol. Pharmacol. 2017, 88, 214–226. [Google Scholar] [CrossRef]
- Wainwright, M.; Crossley, K.B. Methylene Blue—A therapeutic dye for all seasons? J. Chemother. 2002, 14, 431–443. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, A.; Sillanpää, M. Utilization of agro-industrial and municipal waste materials as potential adsorbents for water treatment—A review. Chem. Eng. J. 2010, 157, 277–296. [Google Scholar] [CrossRef]
- Alvarez-Torrellas, S.; Boutahala, M.; Boukhalfa, N.; Munoz, M. Effective Adsorption of methylene blue dye onto magnetic nanocomposites. Modeling and Reuse Studies. Appl. Sci. 2019, 9, 4563. [Google Scholar] [CrossRef]
- Djilani, C.; Zaghdoudi, R.; Djazi, F.; Bouchekima, B.; Lallam, A.; Modarressi, A.; Rogalski, M. Adsorption of dyes on activated carbon prepared from apricot stones and commercial activated carbon. J. Taiwan Inst. Chem. Eng. 2015, 53, 112–121. [Google Scholar] [CrossRef]
- Mailler, R.; Gasperi, J.; Coquet, Y.; Derome, C.; Buleté, A.; Vulliet, E.; Bressy, A.; Varrault, G.; Chebbo, G.; Rocher, V. Removal of emerging micropollutants from wastewater by activated carbon adsorption: Experimental study of different activated carbons and factors influencing the adsorption of micropollutants in wastewater. J. Environ. Chem. Eng. 2016, 4, 1102–1109. [Google Scholar] [CrossRef]
- Bhatnagar, A. Applications of Adsorbent for Water Pollution Control; Bentham Science Publishers: Sharjah, UAE, 2012; pp. 382–412. [Google Scholar]
- Cui, L.; Liu, C.; Wu, G. Performance and mechanism of methylene blue biosorption on orange peel. Environ. Technol. 2008, 29, 1021–1030. [Google Scholar] [CrossRef]
- Saeed, A.; Sharif, M.; Iqbal, M. Application potential of grapefruit peel as dye sorbent: Kinetics, equilibrium and mechanism of crystal violet adsorption. J. Hazard. Mater. 2010, 179, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Tanyildizi, M.T. Modeling of adsorption isotherms and kinetics of reactive dye from aqueous solution by peanut hull. Chem. Eng. J. 2011, 168, 1234–1240. [Google Scholar] [CrossRef]
- Deniz, F.; Karaman, S. Removal of Basic Red 46 dye from aqueous solution by pine tree leaves. Chem. Eng. J. 2011, 170, 67–74. [Google Scholar] [CrossRef]
- Rizzi, V.; D’Agostino, F.; Gubitosa, J.; Fini, P.; Petrella, A.; Agostiano, A.; Semeraro, P.; Cosma, P. An alternative use of olive pomace as a wide-ranging bioremediation strategy to adsorb and recover disperse orange and disperse red industrial dyes from wastewater. Separations 2017, 4, 29. [Google Scholar] [CrossRef]
- Rizzi, V.; D’Agostino, F.; Fini, P.; Semeraro, P.; Cosma, P. An interesting environmental frinedly cleanup: The excellent potential of olive pomace for disperse blue adsorption/desoprtion from wastewater. Dyes Pigm. 2017, 140, 480–490. [Google Scholar] [CrossRef]
- Rizzi, V.; Mongiovi, C.; Fini, P.; Petrella, A.; Semeraro, P.; Cosma, P. Operational parameters affecting the removal and recycling of dierct blue industrial dye from wastewater using bleached oil mill waste as alternative adsorbent material. Int. J. Environ. Agric. Biotechnol. 2017, 2, 1560–1572. [Google Scholar]
- Rizzi, V.; Lacalamita, D.; Gubitosa, J.; Fini, P.; Petrella, A.; Romita, R.; Agostiano, A.; Gabaldon, J.A.; Gorbe, M.I.F.; Gomez-Morte, T.; et al. Removal of tetracycline from polluted water by chitosan-olive pomace adsorbing films. Sci. Tot. Environ. 2019, 693, 1–12. [Google Scholar] [CrossRef]
- Palma, C.; Contreras, E.; Urra, J.; Martínez, M.J. Eco-friendly technologies based on banana peel use for the decolourization of the dyeing process wastewater. Waste Biomass Valor. 2011, 2, 77–86. [Google Scholar] [CrossRef]
- Dahiru, M.; Uba Zango, Z.; Haruna, M.A. Cationic Dyes Removal Using Low-Cost Banana Peel Biosorbent. Am. J. Mater. Sci. 2018, 8, 32–38. [Google Scholar]
- Mondal, N.K.; Kar, S. Potentiality of banana peel for removal of Congo red dye from aqueous solution: Isotherm, kinetics and thermodynamics studies. Appl. Water Sci. 2018, 8, 157. [Google Scholar] [CrossRef]
- Abbas, S.H.; Kamar, F.H.; Hossien, Y.K. Methyl Violet 2B Dye from Aqueous Solutions Onto Waste of Banana Peel Using Fixed-Bed Column. Int. J. Civ. Eng. Technol. 2018, 9, 2094–2109. [Google Scholar]
- Djelloul, C.; Hamdaoui, O. Dynamic adsorption of methylene blue by melon peel in fixed-bed columns. Desalin. Water Treat. 2015, 56, 2966–2975. [Google Scholar] [CrossRef]
- Han, R.; Wang, Y.; Yu, W.; Zou, W.; Shi, J.; Liu, H. Biosorption of methylene blue from aqueous solution by rice husk in a fixed-bed column. J. Hazard. Mater. 2007, 141, 713–718. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Zou, W.; Bian, Y.; Su, F.; Han, R. Adsorption characteristics of methylene blue by peanut husk in batch and colmn modes. Desalination 2011, 265, 119–125. [Google Scholar] [CrossRef]
- Afroze, S.; Sen, T.K.; Ang, H.M. Adsorption performance of continuous fixed bed column for the removal of methylene blue (MB) dye using Eucalyptus sheathiana bark biomass. Res. Chem. Interm. 2016, 42, 2343–2364. [Google Scholar] [CrossRef]
- Dardouri, S.; Sghaier, J. Adsorptive removal of methylene blue from aqueous solution using different agricultural wastes as adsorbents. Korean J. Chem. Eng. 2017, 34, 1037–1043. [Google Scholar] [CrossRef]
- Stavrinou, A.; Aggelopoulos, C.A.; Tsakiroglou, C.D. Exploring the adsorption mechanisms of cationic and anionic dyes onto agricultural waste peels of banana, cucumber and potato: Adsorption kinetics and equilibrium isotherms as a tool. J. Environ. Chem. Eng. 2018, 6, 6958–6970. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, J.; Fu, J.; Wang, M.; Wang, X.; Han, R.; Xu, Q. Adsorption of methylene blue onto poly(cyclotriphosphazene-co-4,4′-sulfonyldiphenol) nanotubes: Kinetics, isotherm and thermodynamic analysis. J. Hazard. Mat. 2014, 273, 263–271. [Google Scholar] [CrossRef]
- Inyinbor, A.A.; Adekola, F.A.; Olatunji, G.A. Kinetics, isotherms and thermodynamic modeling of liquid phase adsorption of Rhodamine B dye onto Raphia hookerie fruit epicarp. Water Resour. Ind. 2016, 15, 14–27. [Google Scholar] [CrossRef]
- Li, C.; Xia, H.; Zhang, L.; Peng, J.; Cheng, S.; Shu, J.; Zhang, S. Kinetics, tehrmodynamics, amnd isotherm study on the removal of methylene blue dye by adsorption via copper modified activated carbon. Res. Chem. Intermed. 2018, 44, 2231–2250. [Google Scholar] [CrossRef]
- Varadarajan, G.; Ponnusami, V. Kinetics, equilibrium, and tehrmodynamic studies on adsorption of methylene blue by carbonized plant leaf powder. J. Chem. 2013, 2013, 415280. [Google Scholar] [CrossRef]
- Patel, H. Fixed-bed column adsorption study: A comprehensive review. Appl. Water Sci. 2019, 9, 9–45. [Google Scholar] [CrossRef]
- Al-Degs, Y.S.; Khraisheh, M.A.M.; Allen, S.J.; Ahmad, M.N. Adsorption characteristics of reactive dyes in columns of activated carbon. J. Hazard. Mater. 2009, 165, 944–949. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Thakur, V.; Bhatia, G.; Verma, D.; Singh, K. Eco-friendly and Cost-effective Use of Rice Straw in the Form of Fixed Bed Column to Remove Water Pollutants. J. Biorem. Biodegrad. 2016, 7, 1000374. [Google Scholar] [CrossRef]
- Kojić, P.S.; Vučurović, V.V.; Lukić, N.L.; Karadžić, M.; Popović, S.S. Continuous adsorption of methylene blue dye on the maize stem ground tissue. Acta Periodica Technol. 2017, 48, 127–139. [Google Scholar] [CrossRef]
- Khan, M.R.; Mozumder, S.I.; Islam, A.; Prasad, D.M.R.; Alam, M.M. Methylene blue adsorption onto water hyacinth: Batch and column study. Water Air Soil Pollut. 2012, 223, 2943–2953. [Google Scholar] [CrossRef]
- Kopsidas, O. Fixed-bed-column Studies for Methylene Blue Removal and Recovery by Untreated Coffee Residues. J. Environ. Sci. Eng. B 2016, 5, 412–418. [Google Scholar] [CrossRef]
- Lakshmipathy, R.; Sarada, N.C. Methylene blue adsorption onto native watermelon rind: Batch and fixed bed column studies. Desalin. Water Treat. 2016, 57, 10632–10645. [Google Scholar] [CrossRef]
- Yagub, M.T.; Sen, T.K.; Afroze, S.; Ang, H.M. Fixed-bed dynamic column adsorption study of methylene blue (MB) onto pine cone. Desalin. Water Treat. 2015, 55, 1026–1039. [Google Scholar] [CrossRef]
- Akar, S.T.; Gorgulu, A.; Akar, T.; Celik, S. Decolorization of reactive blue 49 contaminated solutions by capsicum anuum seeds: Batch and continuous mode biosorption applications. Chem. Eng. J. 2011, 168, 125–133. [Google Scholar] [CrossRef]
- Sadaf, S.; Bhatti, H.N. Batch and fixed-bed column studies for the removal of indosol yellow BG dye by peanut husk. J. Taiwan Inst. Chem. Eng. 2014, 45, 541–553. [Google Scholar] [CrossRef]
- Bharathi, K.S.; Ramesh, S.P.T. Fixed-bed column studies on biosorption of crystal violet from aqueous solution by Citrullus lanatus rind and Cyperus rotundus. Appl. Water Sci. 2013, 3, 673–687. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Dynamic adsorption behavior of methylene blue onto oil palm shell granular activated carbon prepared by microwave heating. Chem. Eng. J. 2012, 203, 81–87. [Google Scholar] [CrossRef]
- Ayawei, N.; Ebelegi, A.N.; Wankasi, D. Modelling and Interpretation of adsorption isotherms. J. Chem. 2017, 2017, 3039817. [Google Scholar] [CrossRef]
- Smith, J.M. Chemical Engineering Kinetics, 3rd ed.; McGraw-Hill: Tokyo, Japan, 1981; pp. 9–57. [Google Scholar]
- Ghosal, P.S.; Gupta, A.K. Development of a generalized adsorption isotherm model at solid-liquid interface: A novel approach. J. Mol. Liq. 2017, 240, 21–24. [Google Scholar] [CrossRef]
- Scopp, J. Derivation of the Freundlich Adsorption Isotherm from Kinetics. J. Chem. Educ. 2009, 86, 1341–1343. [Google Scholar] [CrossRef]
- Magga, Z.; Tzovolou, D.N.; Theodoropoulou, M.A.; Dalkarani, T.; Pikios, K.; Tsakiroglou, C.D. Soil column experiments used as a means to assess transport, sorption, and biodegradation of pesticides in groundwater. J Environ. Sci. Health B 2008, 43, 732–741. [Google Scholar] [CrossRef]
- Aggelopoulos, C.A.; Tsakiroglou, C.D. The longitudinal dispersion coefficient of soils as related to the variability of local permeability. Water Air Soil Pollut. 2007, 185, 223–237. [Google Scholar] [CrossRef]
- Jawad, A.H.; Razuan, R.; Appaturi, J.N.; Wilson, L.D. Adsorption and mechanism study for methylene blue dye removal with carbonized watermellon (Citrullus lanatus) rind preparde via one-step liquid phase H2SO4 activation. Surf. Interfaces 2019, 16, 76–84. [Google Scholar] [CrossRef]
- Stewart, W.E.; Caracotsios, M. Computer-Aided Modeling of Reactive Systems; Wiley-Interscience: Hoboken, NJ, USA, 2008; pp. 177–257. [Google Scholar]
- Bird, R.B.; Stewart, W.E.; Lightfoot, E.N. Transport Phenomena; Wiley: New York, NY, USA, 1960; pp. 592–625. [Google Scholar]
- Khalfaoui, M.; Nakhli, A.; Aguir, C.; Omri, A.; M’henni, M.F.; Ben Lamine, A. Statistical thermodynamics of adsorption of dye DR75 onto natural materials and its modifications: Double-layer model with two adsorption energies. Environ. Sci. Pollut. Res. 2014, 21, 3134–3144. [Google Scholar] [CrossRef]

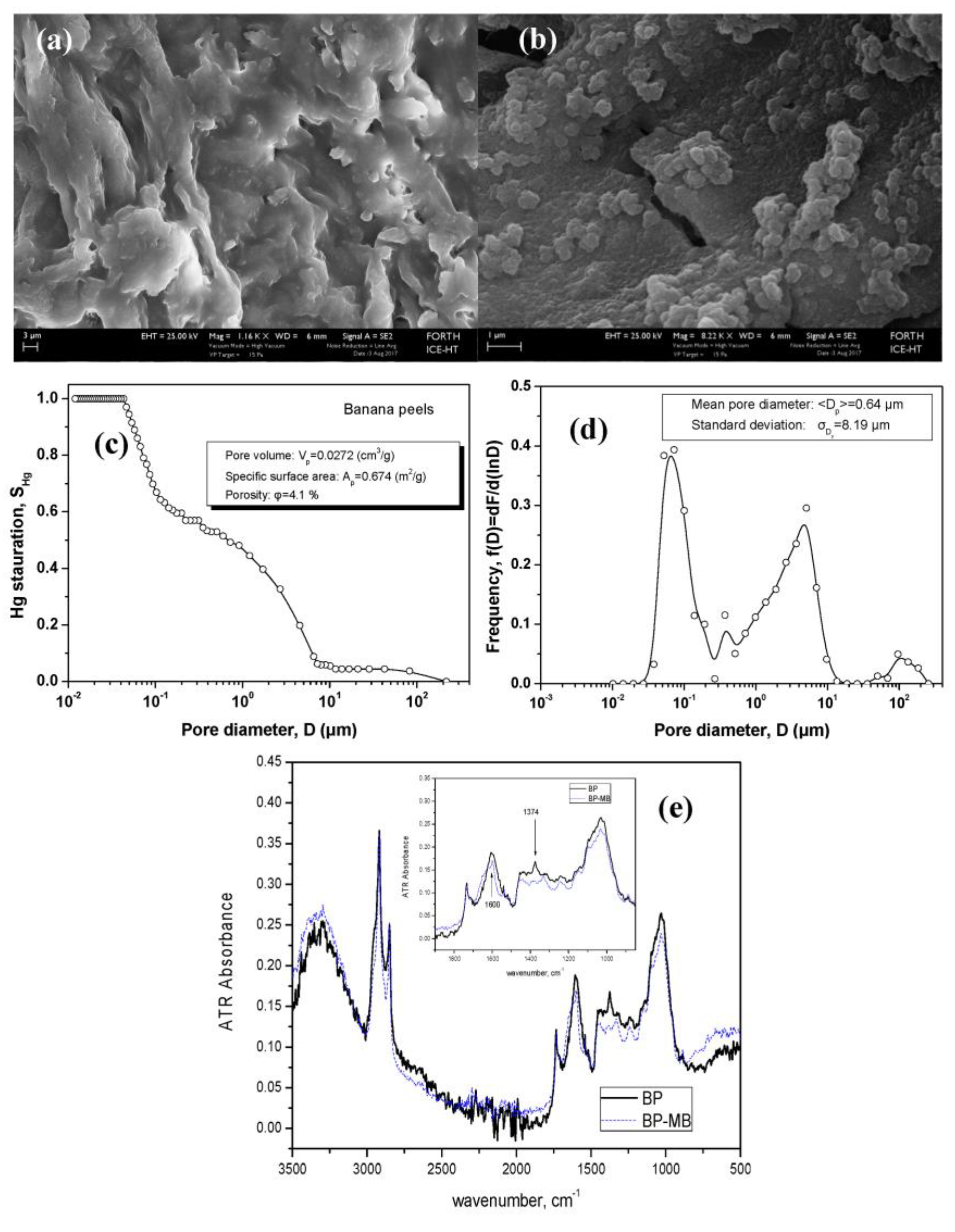



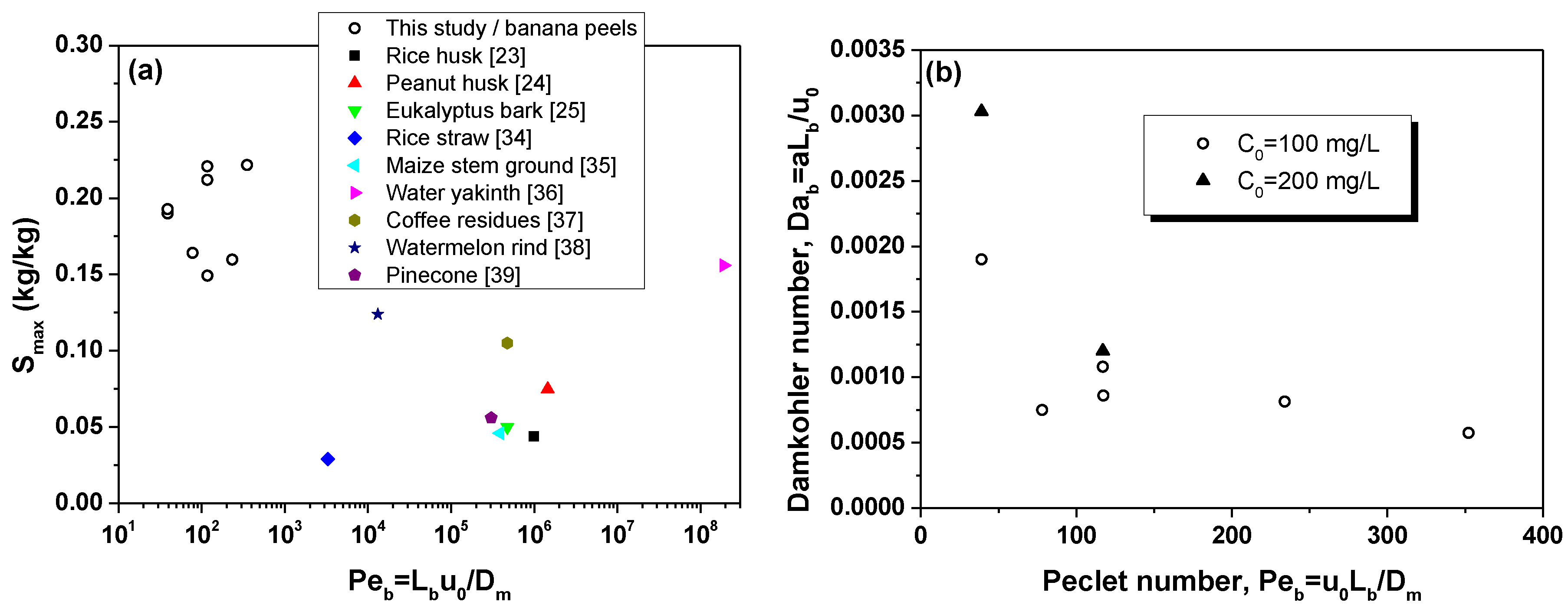
| Adsorbent | Dye | Sorption Capacity (mg/g) | Mode of Study | Reference |
|---|---|---|---|---|
| Banana peel | Methylene blue | 210 | Batch | [27] |
| Cucumber peel | Methylene blue | 170 | Batch | [27] |
| Potato peel | Methylene blue | 110 | Batch | [27] |
| Banana peel | Methylene blue, Malachite green | 120 | Batch | [19] |
| Banana peel | Acid Black 1 | 250 | Batch | [18] |
| Banana peel | Congo red | 1.7 | Batch | [20] |
| Copper-modified activated carbon | Methylene blue | 323 | Batch | [30] |
| Carbonized plant leaf powder | Methylene blue | 61.2 | Batch | [31] |
| Grapefruit peel | Crystal Violet | 254 | Batch | [11] |
| Olive pomace | Direct red, Direct orange | >4, >3.5 | Batch | [14] |
| Oil mill waste | Direct blue | >5 | Batch | [16] |
| Banana peel | Methylene blue | 150–220 | Fixed-bed column | This study |
| Rice husk | Methylene blue | 4.4 | Fixed-bed column | [23] |
| Peanut husk | Methylene blue | 75.1 | Fixed-bed column | [24] |
| Eucalyptus sheathiana bark biomass | Methylene blue | 49.5 | Fixed-bed column | [25] |
| Rice straw | Methylene blue | 29 | Fixed-bed column | [34] |
| Maize stem ground | Methylene blue | 45.9 | Fixed-bed column | [35] |
| Water hyakinth | Methylene blue | 156 | Fixed-bed column | [36] |
| Coffee residues | Methylene blue | 104.5 | Fixed-bed column | [37] |
| Watermelon rind | Methylene blue | 124.4 | Fixed-bed column | [38] |
| Pinecone | Methylene blue | 55.7 | Fixed-bed column | [39] |
| Capsicum annuum seeds | Reactive blue 49 | 65.5 | Fixed-bed column | [40] |
| Peanut husk | Indosol Yellow BG | 25.9 | Fixed-bed column | [41] |
| Citrullus lanatus rind and Cyperus rotundus | Crystal violet | 54.2 | Fixed-bed column | [42] |
| Model | Parameter | Temperature (°C) | |||
|---|---|---|---|---|---|
| 15 | 25 | 35 | 45 | ||
| Langmuir | Smax (mg/g) | 153.4 ± 8.1 | 160.3 ± 9.5 | 184.1 ± 14.0 | 158.7 ± 11.9 |
| KL (L/mg) | 0.196 ± 0.043 | 0.142 ± 0.033 | 0.11 ± 0.029 | 0.090 ± 0.0253 | |
| R2 | 0.991 | 0.990 | 0.988 | 0.987 | |
| Freundlich | β | 0.278 ± 0.0717 | 0.290 ± 0.0817 | 0.314 ± 0.0936 | 0.325 ± 0.069 |
| KF (L/mg)n(mg/g) ((mg/g)(L/g)n) | 42.49 ± 12.44 | 40.72 ± 13.45 | 41.39 ± 15.16 | 32.45 ± 9.25 | |
| R2 | 0.942 | 0.935 | 0.929 | 0.964 | |
| Langmuir–Freundlich | Smax (mg/g) | 166.85 ± 13.91 | 171.34 ± 21.02 | 201.11 ± 19.98 | 184.21 ± 35.63 |
| KL (L/mg) | 0.227 ± 0.037 | 0.169 ± 0.046 | 0.140 ± 0.0405 | 0.122 ± 0.032 | |
| β | 0.775 ± 0.137 | 0.826 ± 0.209 | 0.802 ± 0.157 | 0.737 ± 0.201 | |
| R2 | 0.996 | 0.993 | 0.986 | 0.993 | |
| Model | Parameter | Optimal Value ± Standard Error |
|---|---|---|
| Langmuir | ΔH0 (kJ/mol) | −19.72 ± 1.10 |
| ln(B) | −9.89 ± 0.44 | |
| R2 | 0.991 0.9869 | |
| Freundlich | ΔH0 (kJ/mol) | −5.93 ± 3.06 |
| ln(B) | 1.68 ± 0.94 | |
| R2 | 0.478 | |
| Langmuir–Freundlich | ΔH0 (kJ/mol) | −15.75 ± 1.50 |
| ln(B) | −8.09 ± 0.60 | |
| R2 | 0.973 |
| Adsorbent | Dye | ΔH0 (kJ/mol) | Reference |
|---|---|---|---|
| Banana peel | Methylene blue | −20.0 | This study |
| Banana peel | Congo red | −28.3 | [20] |
| Copper-modified activated carbon | Methylene blue | 6.2 | [30] |
| PZS nanotubes | Methylene blue | 9.8 | [28] |
| Carbonized Plant Leaf Powder | Methylene blue | 23.1 | [31] |
| Model | Parameter | Temperature (°C) | |||
|---|---|---|---|---|---|
| 15 | 25 | 35 | 45 | ||
| Langmuir | kd × 103 (min−1) | 0.397 ± 0.14 | 0.634 ± 0.194 | 0.777 ± 0.182 | 1.328 ± 0.308 |
| R2 | 0.897 | 0.930 | 0.962 | 0.9625 | |
| Freundlich | kd × 103 (min−1) | 6.40 ± 2.61 | 7.68 ± 2.73 | 7.25 ± 2.01 | 10.87 ± 2.84 |
| R2 | 0.853 | 0.896 | 0.939 | 0.947 | |
| Hybrid | kd × 103 (min−1) | 0.814 ± 0.313 | 1.056 ± 0.343 | 1.305 ± 0.337 | 2.617 ± 0.653 |
| R2 | 0.875 | 0.919 | 0.952 | 0.954 | |
| Model | Parameter | Optimal Value ± Standard Error |
|---|---|---|
| Langmuir kinetic model | Ed (kJ/mol) | 29.02 ± 3.58 |
| ln(Ad) | 4.29 ± 1.42 | |
| R2 | 0.956 | |
| Ea (kJ/mol) | 9.30 | |
| ln(Aa) | −5.6 | |
| Freundlich kinetic model | Ed (kJ/mol) | 11.48 ± 4.87 |
| ln(Ad) | −0.28 ± 1.94 | |
| R2 | 0.603 | |
| Ea (kJ/mol) | 5.55 | |
| ln(Aa) | 1.40 | |
| Langmuir–Freundlich (hybrid) kinetic model | Ed (kJ/mol) | 28.04 ± 6.50 |
| ln(Ad) | 4.51 ± 2.58 | |
| R2 | 0.855 | |
| Ea (kJ/mol) | 12.29 | |
| ln(Aa) | −3.59 |
| (m) | 0.0168 | 0.35 | |
| (m) | 0.0665 | 4.0 | |
| (kg/m3) | 0.1 | a (m2) | 0.8 × 10−12 |
| 0.2 | |||
| (m s−1) | 2.15 × 10−4 | (m) | 3.0 × 10−4 |
| 6.45 × 10−4 | |||
| (m) | 0.0186 | (m) | 3.0 × 10−4 |
| 0.0372 | |||
| 0.0558 | |||
| (kg m−3) | 590 | (m2 s−1) | 4.6 × 10−10 |
| Q (mL/min) | Lb/D | C0 (mg/L) | Smax (kg/kg) | × 105 (s−1) | SSR (Sum of Square Residuals) |
|---|---|---|---|---|---|
| 1.0 | 1.107 | 100.0 | 0.190 ± 0.0003 | 2.195 ± 0.0008 | 0.047 |
| 1.0 | 1.107 | 200.0 | 0.193 ± 0.0002 | 3.511 ± 0.0079 | 0.1093 |
| 1.0 | 2.214 | 100.0 | 0.164 ± 0.0020 | 0.433 ± 0.0141 | 0.0512 |
| 1.0 | 3.321 | 100.0 | 0.149 ± 0.0009 | 0.330 ± 0.0057 | 0.0213 |
| 3.0 | 1.107 | 100.0 | 0.212 ± 0.0001 | 3.753 ± 0.0012 | 0.0102 |
| 3.0 | 1.107 | 200.0 | 0.221 ± 0.0029 | 4.184 ± 0.179 | 0.0154 |
| 3.0 | 2.214 | 100.0 | 0.160 ± 0.0019 | 1.412 ± 0.049 | 0.0163 |
| 3.0 | 3.321 | 100.0 | 0.222 ± 0.0053 | 0.666 ± 0.048 | 0.0372 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stavrinou, A.; Aggelopoulos, C.A.; Tsakiroglou, C.D. A Methodology to Estimate the Sorption Parameters from Batch and Column Tests: The Case Study of Methylene Blue Sorption onto Banana Peels. Processes 2020, 8, 1467. https://doi.org/10.3390/pr8111467
Stavrinou A, Aggelopoulos CA, Tsakiroglou CD. A Methodology to Estimate the Sorption Parameters from Batch and Column Tests: The Case Study of Methylene Blue Sorption onto Banana Peels. Processes. 2020; 8(11):1467. https://doi.org/10.3390/pr8111467
Chicago/Turabian StyleStavrinou, Anastasia, Christos A. Aggelopoulos, and Christos D. Tsakiroglou. 2020. "A Methodology to Estimate the Sorption Parameters from Batch and Column Tests: The Case Study of Methylene Blue Sorption onto Banana Peels" Processes 8, no. 11: 1467. https://doi.org/10.3390/pr8111467
APA StyleStavrinou, A., Aggelopoulos, C. A., & Tsakiroglou, C. D. (2020). A Methodology to Estimate the Sorption Parameters from Batch and Column Tests: The Case Study of Methylene Blue Sorption onto Banana Peels. Processes, 8(11), 1467. https://doi.org/10.3390/pr8111467





