Formulation of Piperine Ternary Inclusion Complex Using β CD and HPMC: Physicochemical Characterization, Molecular Docking, and Antimicrobial Testing
Abstract
1. Introduction
2. Material and Methods
2.1. Material
2.2. Methods
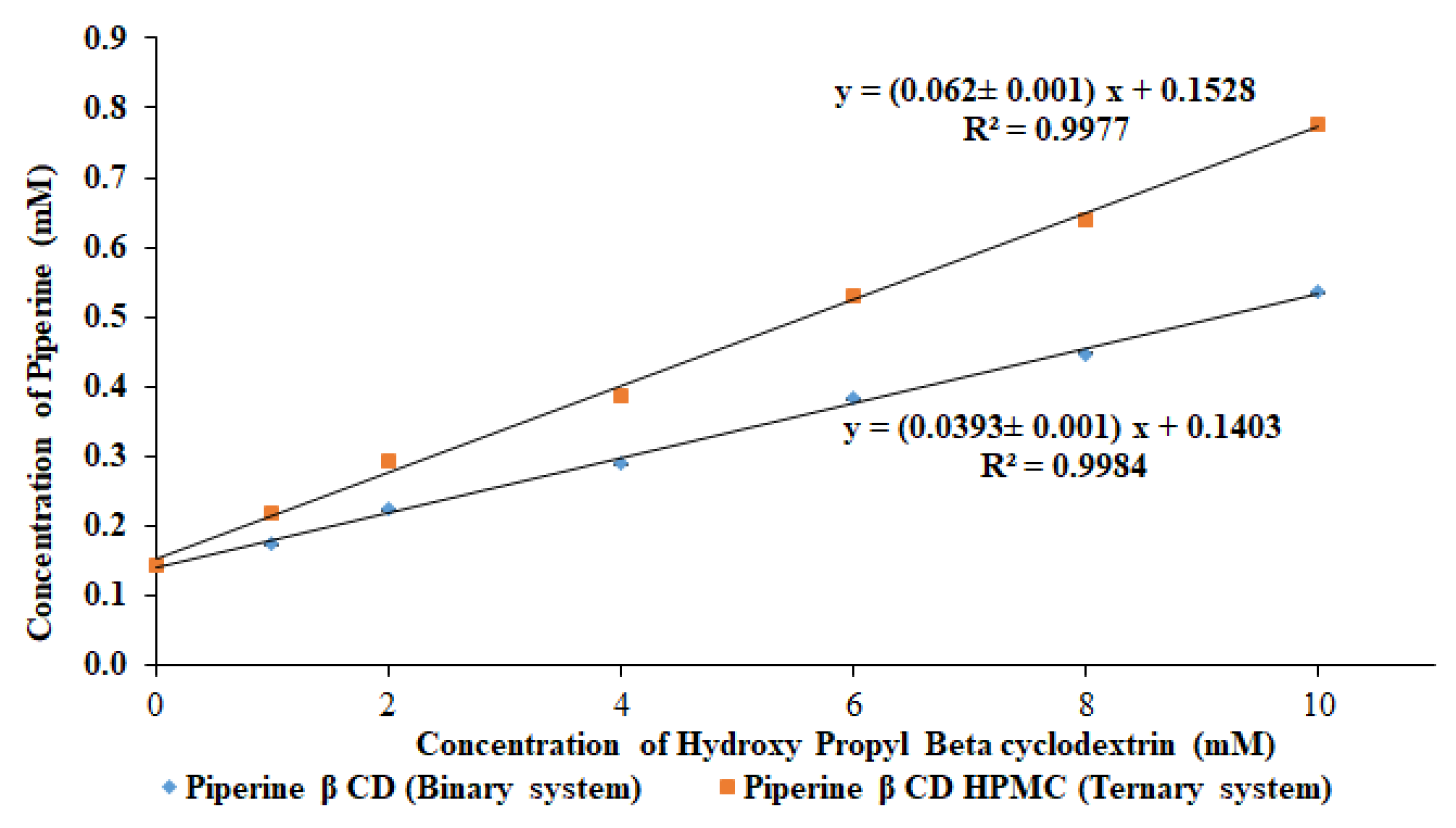
2.2.1. Phase Solubility Study
2.2.2. Formulation of Inclusion Complex
2.2.3. Physical Mixture
2.2.4. Solvent Evaporation Method
2.2.5. Microwave Method
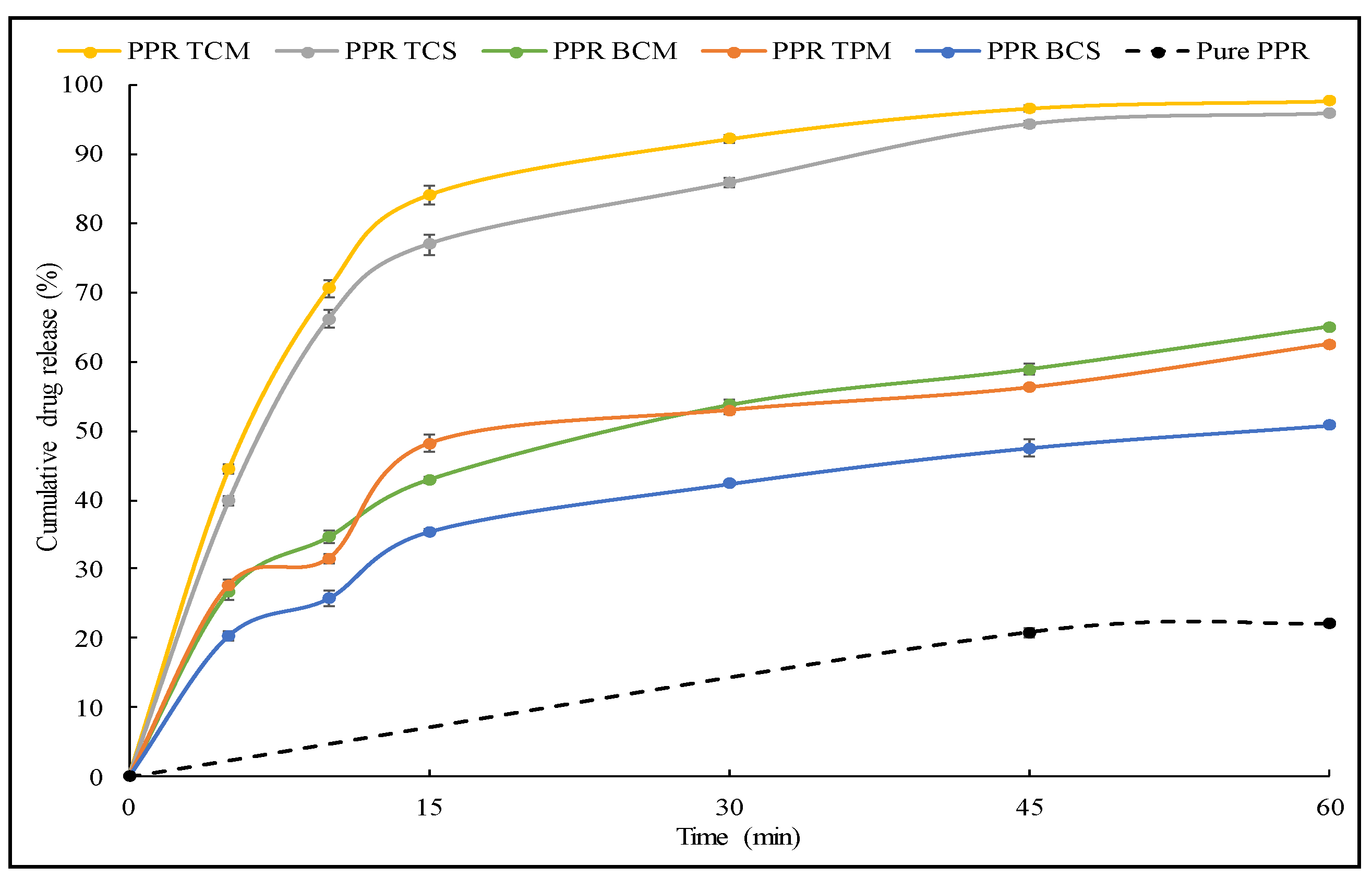
2.2.6. Dissolution Study
2.2.7. X-ray Diffraction
2.2.8. Surface Morphology
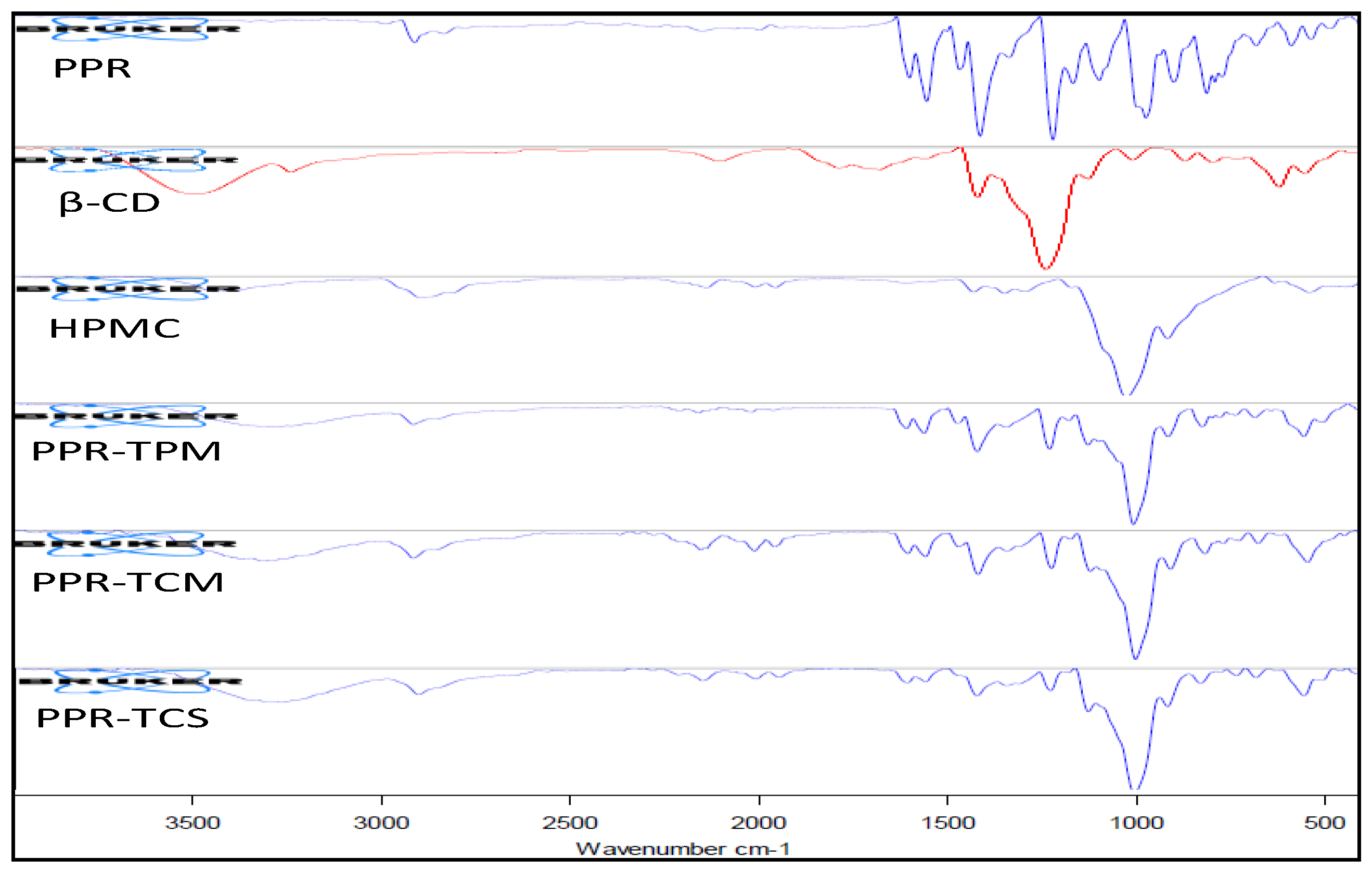
2.2.9. Fourier Transform Infrared (FTIR)
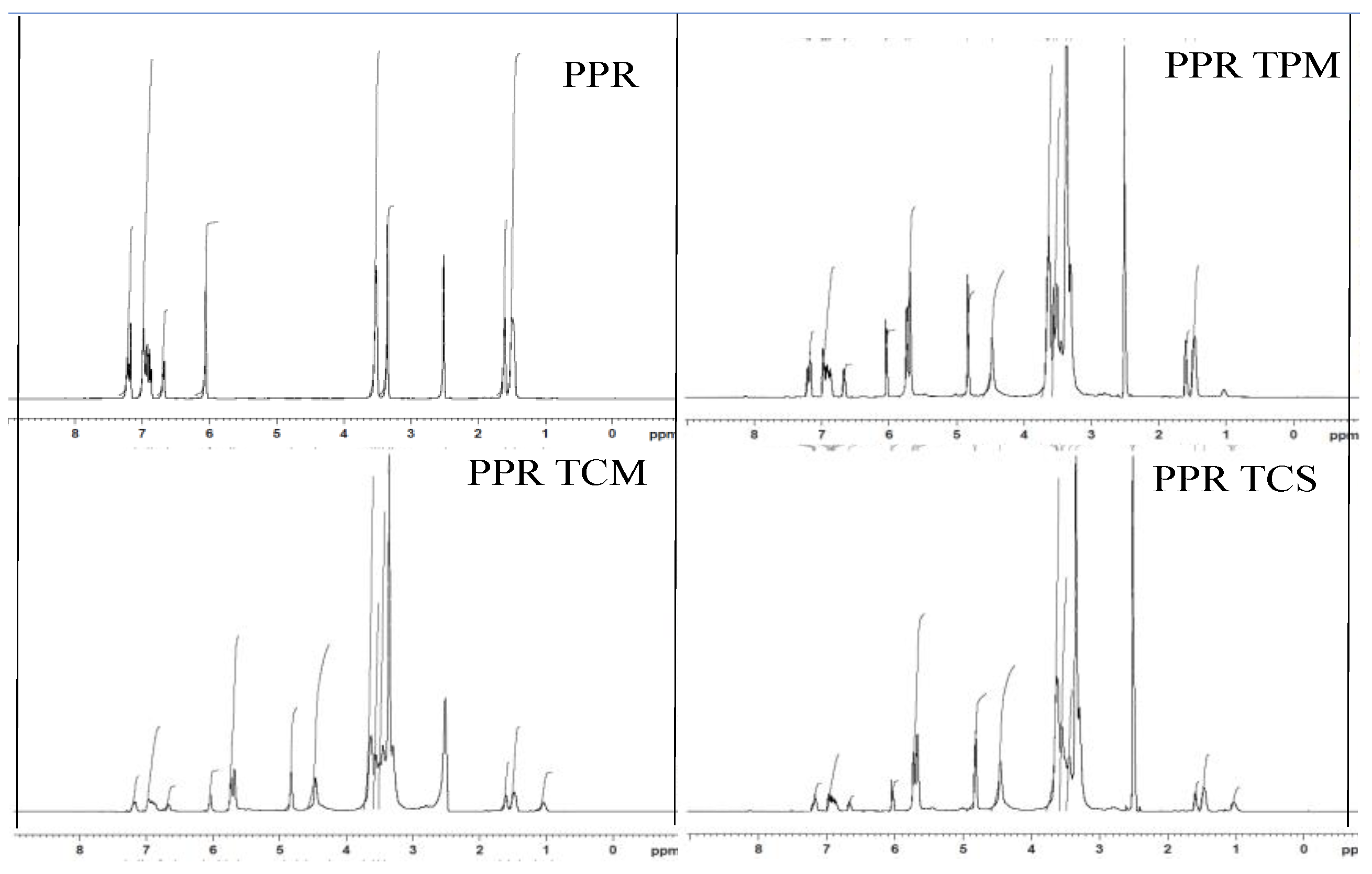
2.2.10. Nuclear Magnetic Resonance (NMR)
2.2.11. Antioxidant Activity
2.2.12. Antimicrobial Study
2.2.13. Molecular Docking Studies
2.2.14. Statistical Analysis
3. Result and Discussion
3.1. Phase Solubility Study
3.2. Dissolution Study
3.3. X-ray Diffraction
3.4. Surface Morphology
3.5. Fourier Transform Infrared (FTIR)
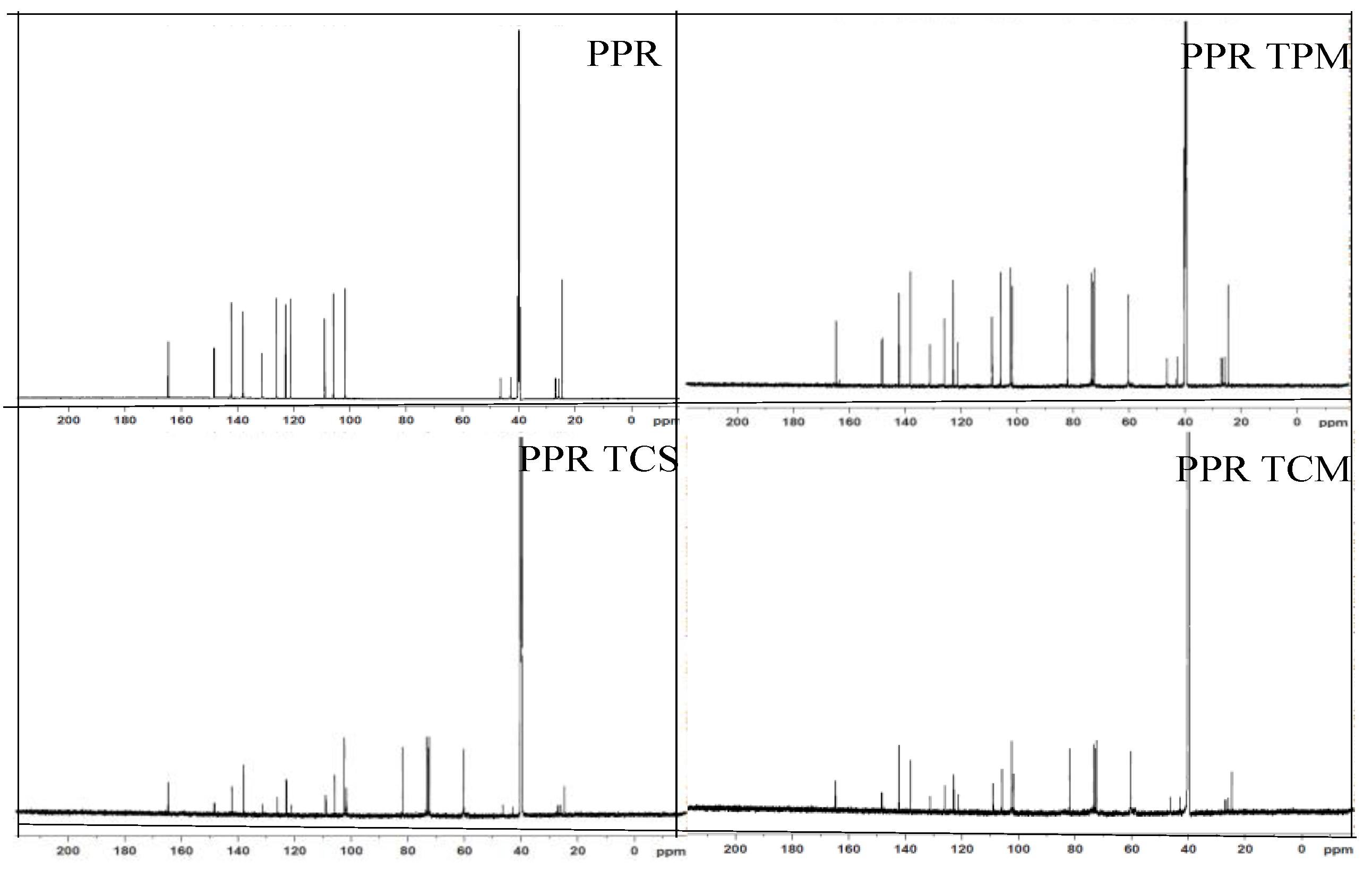
3.6. Nuclear Magnetic Resonance (NMR)
3.7. Antioxidant Activity
3.8. Antimicrobial Activity
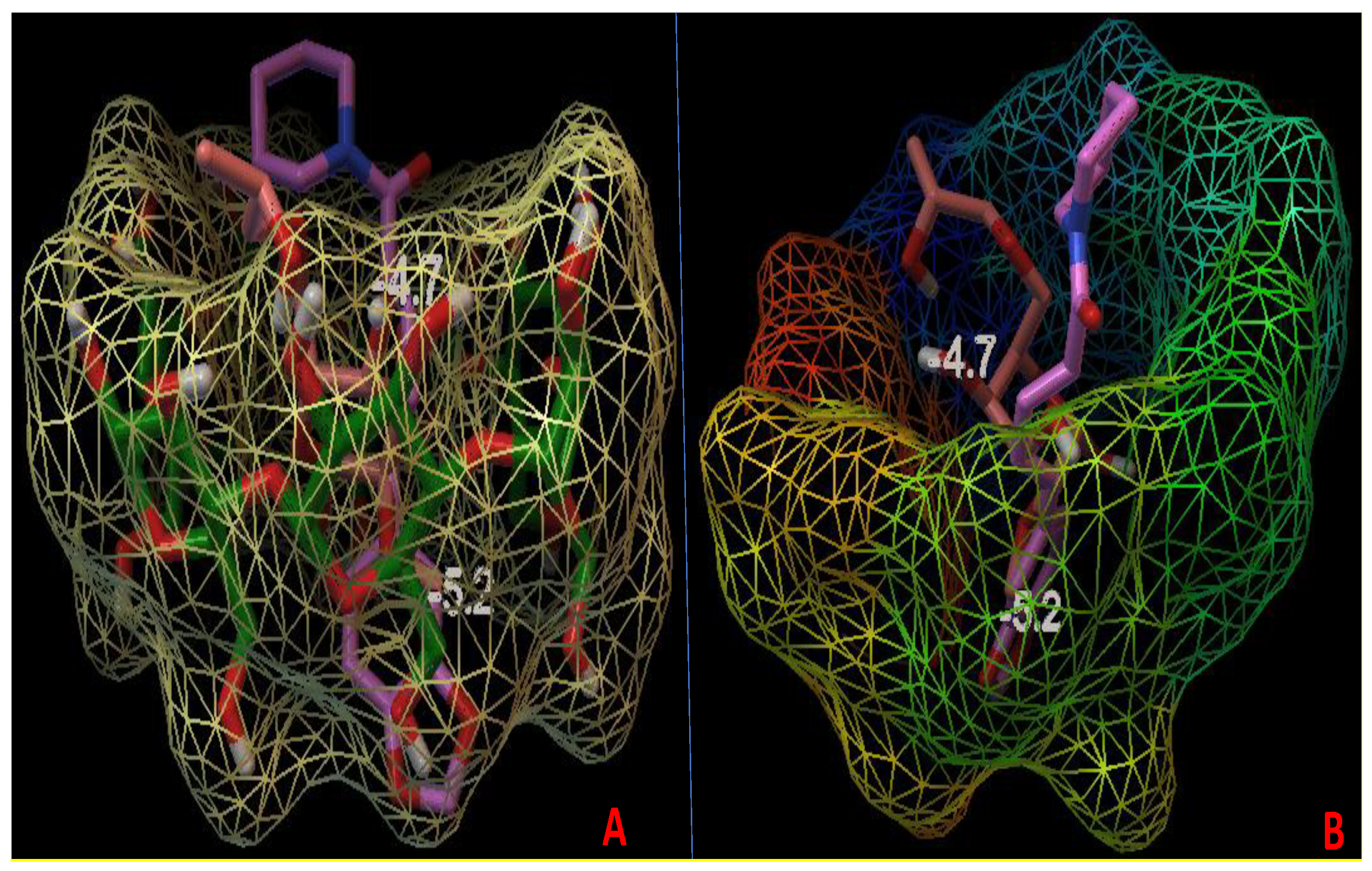
3.9. Molecular Docking
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kurup, M.P.; Nirmal, C.R.; Dusthackeer, A.; Magizhaveni, B.; Kurup, M.R.P.; Balasubramanian, M. Formulation and evaluation of β-cyclodextrin-mediated inclusion complexes of isoniazid scaffolds: Molecular docking and in vitro assessment of antitubercular properties. New J. Chem. 2020, 44, 4467–4477. [Google Scholar] [CrossRef]
- Qiu, N.; Zhao, X.; Liu, Q.; Shen, B.; Liu, J.; Li, X.; An, L. Inclusion complex of emodin with hydroxypropyl-β-cyclodextrin: Preparation, physicochemical and biological properties. J. Mol. Liq. 2019, 289, 111151. [Google Scholar] [CrossRef]
- Gao, S.; Bie, C.; Ji, Q.; Ling, H.; Li, C.; Fu, Y.; Zhao, L.; Ye, F. Preparation and characterization of cyanazine–hydroxypropyl-beta-cyclodextrin inclusion complex. RSC Adv. 2019, 9, 26109–26115. [Google Scholar] [CrossRef]
- Mohandoss, S.; Atchudan, R.; Edison, T.N.J.I.; Mandal, T.K.; Palanisamy, S.; You, S.; Napoleon, A.A.; Shim, J.-J.; Lee, Y.R. Enhanced solubility of guanosine by inclusion complexes with cyclodextrin derivatives: Preparation, characterization, and evaluation. Carbohydr. Polym. 2019, 224, 115166. [Google Scholar] [CrossRef]
- Kurkov, S.V.; Loftsson, T. Cyclodextrins. Int. J. Pharm. 2013, 453, 167–180. [Google Scholar] [CrossRef]
- Ren, T.; Hu, M.; Cheng, Y.; Shek, T.L.; Xiao, M.; Ho, N.J.; Zhang, C.; Leung, S.S.Y.; Zuo, Z. Piperine-loaded nanoparticles with enhanced dissolution and oral bioavailability for epilepsy control. Eur. J. Pharm. Sci. 2019, 137, 104988. [Google Scholar] [CrossRef]
- Liu, G.; Yuan, Q.-J.; Hollett, G.; Zhao, W.; Kang, Y.; Wu, J. Cyclodextrin-based host–guest supramolecular hydrogel and its application in biomedical fields. Polym. Chem. 2018, 9, 3436–3449. [Google Scholar] [CrossRef]
- Ding, X.; Zheng, M.; Lu, J.; Zhu, X. Preparation and evaluation of binary and ternary inclusion complexes of fenofibrate/hydroxypropyl-β-cyclodextrin. J. Incl. Phenom. Macrocycl. Chem. 2018, 91, 17–24. [Google Scholar] [CrossRef]
- AlShehri, S.; Imam, S.S.; Altamimi, M.A.; Jafar, M.; Hassan, M.Z.; Hussain, A.; Ahad, A.; Mahdi, W. Host-guest complex of β-cyclodextrin and pluronic F127 with Luteolin: Physicochemical characterization, anti-oxidant activity and molecular modeling studies. J. Drug Deliv. Sci. Technol. 2020, 55, 101356. [Google Scholar] [CrossRef]
- Mourão, L.C.D.S.; Batista, D.R.M.R.; Honorato, S.B.; Ayala, A.P.; Morais, W.D.A.; Barbosa, E.G.; Raffin, F.N.; Moura, T. Effect of hydroxypropyl methylcellulose on beta cyclodextrin complexation of praziquantel in solution and in solid state. J. Incl. Phenom. Macrocycl. Chem. 2016, 85, 151–160. [Google Scholar] [CrossRef]
- Mohanty, B.; Suvitha, A.; Venkataramanan, N.S. Piperine Encapsulation within Cucurbit[n]uril (n = 6, 7): A Combined Experimental and Density Functional Study. Chem. 2018, 3, 1933–1941. [Google Scholar] [CrossRef]
- Quilaqueo, M.; Millao, S.; Luzardo-Ocampo, I.; Campos-Vega, R.; Acevedo, F.; Shene, C.; Rubilar, M. Inclusion of piperine in β-cyclodextrin complexes improves their bioaccessibility and in vitro antioxidant capacity. Food Hydrocoll. 2019, 91, 143–152. [Google Scholar] [CrossRef]
- Ezawa, T.; Inoue, Y.; Tunvichien, S.; Suzuki, R.; Kanamoto, I. Changes in the Physicochemical Properties of Piperine/β-Cyclodextrin due to the Formation of Inclusion Complexes. Int. J. Med. Chem. 2016, 2016, 1–9. [Google Scholar] [CrossRef]
- Karsha, P.V.; Lakshmi, O.B. Antibacterial activity of black pepper (Piper nigrum Linn.) with special reference to its mode of action on bacteria. Indian J. Nat. Prod. Resour. 2010, 1, 213–215. [Google Scholar]
- Anissian, D.; Ghasemi-Kasman, M.; Khalili-Fomeshi, M.; Akbari, A.; Hashemian, M.; Kazemi, S.; Akbar Moghadamnia, A. Piperine-loaded chitosan-STPP nanoparticles reduce mortal neuronal loss and astrocytes activation in chemical kindling model of epilepsy. Int. J. Biol. Macromol. 2018, 107, 973–983. [Google Scholar] [CrossRef]
- Higuchi, T.; Connors, K.A. Phase solubility techniques. Adv. Anal. Chem. Instrum. 1965, 4, 117–122. [Google Scholar]
- Loh, G.O.K.; Tan, Y.T.F.; Peh, K.K. Effect of HPMC concentration on β-cyclodextrin solubilization of norfloxacin. Carbohydr. Polym. 2014, 101, 505–510. [Google Scholar] [CrossRef]
- Patel, M.; Hirlekar, R. Multicomponent cyclodextrin system for improvement of solubility and dissolution rate of poorly water soluble drug. Asian J. Pharm. Sci. 2019, 14, 104–115. [Google Scholar] [CrossRef]
- Khajuria, A.; Zutshi, U.; Bedi, K.L. Permeability characteristics of piperine on oral absorption--an active alkaloid from peppers and a bioavailability enhancer. Indian J. Exp. Biol. 1998, 36, 46–50. [Google Scholar]
- Zarai, Z.; Boujelbene, E.; Ben Salem, N.; Gargouri, Y.; Sayari, A. Antioxidant and antimicrobial activities of various solvent extracts, piperine and piperic acid from Piper nigrum. LWT 2013, 50, 634–641. [Google Scholar] [CrossRef]
- Adachi, M.; Mikami, B.; Katsube, T.; Utsumi, S. Crystal Structure of Recombinant Soybean β-Amylase Complexed with β-Cyclodextrin. J. Biol. Chem. 1998, 273, 19859–19865. [Google Scholar] [CrossRef] [PubMed]
- De Freitas, M.R.; Rolim, L.A.; Soares, M.F.D.L.R.; Rolim-Neto, P.J.; De Albuquerque, M.M.; Soares-Sobrinho, J.L. Inclusion complex of methyl-β-cyclodextrin and olanzapine as potential drug delivery system for schizophrenia. Carbohydr. Polym. 2012, 89, 1095–1100. [Google Scholar] [CrossRef] [PubMed]
- Imam, S.S.; AlShehri, S.M.; Alzahrani, T.A.; Altamimi, M.A.; Altamimi, M.A. Formulation and Evaluation of Supramolecular Food-Grade Piperine HP β CD and TPGS Complex: Dissolution, Physicochemical Characterization, Molecular Docking, In Vitro Antioxidant Activity, and Antimicrobial Assessment. Molecules 2020, 25, 4716. [Google Scholar] [CrossRef] [PubMed]
- Hirlekar, R.; Sonawane, S.N.; Kadam, V.J. Studies on the Effect of Water-Soluble Polymers on Drug–Cyclodextrin Complex Solubility. AAPS PharmSciTech 2009, 10, 858–863. [Google Scholar] [CrossRef]
- Suvarna, V.; Kajwe, A.; Murahari, M.; Pujar, G.V.; Inturi, B.K.; Sherje, A.P. Inclusion Complexes of Nateglinide with HP–β–CD and L-Arginine for Solubility and Dissolution Enhancement: Preparation, Characterization, and Molecular Docking Study. J. Pharm. Innov. 2017, 12, 168–181. [Google Scholar] [CrossRef]
- AlShehri, S.M.; Imam, S.S.; Altamimi, M.A.; Hussain, A.; Shakeel, F.; AlShehri, A. Stimulatory Effects of Soluplus® on Flufenamic Acid β-Cyclodextrin Supramolecular Complex: Physicochemical Characterization and Pre-clinical Anti-inflammatory Assessment. AAPS PharmSciTech 2020, 21, 1–13. [Google Scholar] [CrossRef]
- Nogueiras-Nieto, L.; Sobarzo-Sánchez, E.; Espinar, F.J.O.; Otero-Espinar, F.J. Competitive displacement of drugs from cyclodextrin inclusion complex by polypseudorotaxane formation with poloxamer: Implications in drug solubilization and delivery. Eur. J. Pharm. Biopharm. 2012, 80, 585–595. [Google Scholar] [CrossRef] [PubMed]
- AlShehri, S.M.; Imam, S.S.; Altamimi, M.A.; Hussain, A.; Shakeel, F.; Elzayat, E.M.; Mohsin, K.; Ibrahim, M.; Alanazi, F. Enhanced Dissolution of Luteolin by Solid Dispersion Prepared by Different Methods: Physicochemical Characterization and Antioxidant Activity. ACS Omega 2020, 5, 6461–6471. [Google Scholar] [CrossRef]
- Zawar, L.R.; Bari, S.B. Preparation, Characterization and in Vivo Evaluation of Antihyperglycemic Activity of Microwave Generated Repaglinide Solid Dispersion. Chem. Pharm. Bull. 2012, 60, 482–487. [Google Scholar] [CrossRef]
- Sami, F.; Philip, B.; Pathak, K. Effect of auxiliary substances on complexation efficiency and intrinsic dissolution rate of gemfibrozil-beta-CD complexes. AAPS PharmSciTech 2010, 11, 27–35. [Google Scholar] [CrossRef]
- Bera, H.; Chekuri, S.; Sarkar, S.; Kumar, S.; Muvva, N.B.; Mothe, S.; Nadimpalli, J. Novel pimozide-β-cyclodextrin-polyvinylpyrrolidone inclusion complexes for Tourette syndrome treatment. J. Mol. Liq. 2016, 215, 135–143. [Google Scholar] [CrossRef]
- Ren, Z.; Xu, Y.; Lu, Z.; Wang, Z.; Chen, C.; Guo, Y.; Shi, X.; Li, F.; Yang, J.; Zheng, Y. Construction of a water-soluble and photostable rubropunctatin/β-cyclodextrin drug carrier. RSC Adv. 2019, 9, 11396–11405. [Google Scholar] [CrossRef]
- Shirwaikar, A.; Shirwaikar, A.; Rajendran, K.; Punitha, I.S.R. In Vitro Antioxidant Studies on the Benzyl Tetra Isoquinoline Alkaloid Berberine. Biol. Pharm. Bull. 2006, 29, 1906–1910. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, H.; Jarraya, R.; Lassoued, I.; Masmoudi, O.; Damak, M.; Nasri, M. GC/MS and LC/MS analysis, and antioxidant and antimicrobial activities of various solvent extracts from Mirabilis jalapa tubers. Process. Biochem. 2010, 45, 1486–1493. [Google Scholar] [CrossRef]
- Soni, K.; Rizwanullah; Kohli, K. Development and optimization of sulforaphane-loaded nanostructured lipid carriers by the Box-Behnken design for improved oral efficacy against cancer: In vitro, ex vivo and in vivo assessments. Artif. Cells Nanomed. Biotechnol. 2018, 46, 15–31. [Google Scholar] [CrossRef]
- Aleem, O.; Kuchekar, B.; Pore, Y.; Late, S. Effect of β-cyclodextrin and hydroxypropyl β-cyclodextrin complexation on physicochemical properties and antimicrobial activity of cefdinir. J. Pharm. Biomed. Anal. 2008, 47, 535–540. [Google Scholar] [CrossRef]




| Piperine Binary Complex | Piperine Ternary Complex | ||||
|---|---|---|---|---|---|
| Physical Mixture | Solvent Evaporation | Microwave Irradiation | Physical Mixture | Solvent Evaporation | Microwave Irradiation |
| PPR:β CD | PPR:β CD | PPR:β CD | PPR:β CD: HPMC * | PPR:β CD: HPMC * | PPR:β CD: HPMC * |
| 1:1 | 1:1 | 1:1 | 1:1 | 1:1 | 1:1 |
| Samples | Release Study (%) | Antioxidant Study | Antimicrobial Activity (in mm) (ZOI Noted after Subtracting the Value of Well 6 mm) | |||
|---|---|---|---|---|---|---|
| (60 min) | (%) | S. aureus | B. subtilis | E. coli | E. faecalis | |
| Pure PPR | 22.04 | 38.92 ± 2.38 | 11.4 ± 0.45 | 9.3 ± 0.38 | 11.8 ± 0.65 | 6.9 ± 0.53 |
| PPR TPM | 62.42 | 59.42 ± 4.1 | - | - | - | - |
| PPR TCS | 95.78 | 72.37 ± 5.35 | 15.5 ± 0.89 | 13.1 ± 0.72 | 16.5 ± 0.66 | 11.3 ± 0.85 |
| PPR TCM | 97.61 | 79.19 ± 4.34 | 15.7 ± 0.73 | 12.9 ± 0.79 | 16.2 ± 0.82 | 11.9 ± 0.53 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alshehri, S.; Imam, S.S.; Hussain, A.; Altamimi, M.A. Formulation of Piperine Ternary Inclusion Complex Using β CD and HPMC: Physicochemical Characterization, Molecular Docking, and Antimicrobial Testing. Processes 2020, 8, 1450. https://doi.org/10.3390/pr8111450
Alshehri S, Imam SS, Hussain A, Altamimi MA. Formulation of Piperine Ternary Inclusion Complex Using β CD and HPMC: Physicochemical Characterization, Molecular Docking, and Antimicrobial Testing. Processes. 2020; 8(11):1450. https://doi.org/10.3390/pr8111450
Chicago/Turabian StyleAlshehri, Sultan, Syed Sarim Imam, Afzal Hussain, and Mohammad A. Altamimi. 2020. "Formulation of Piperine Ternary Inclusion Complex Using β CD and HPMC: Physicochemical Characterization, Molecular Docking, and Antimicrobial Testing" Processes 8, no. 11: 1450. https://doi.org/10.3390/pr8111450
APA StyleAlshehri, S., Imam, S. S., Hussain, A., & Altamimi, M. A. (2020). Formulation of Piperine Ternary Inclusion Complex Using β CD and HPMC: Physicochemical Characterization, Molecular Docking, and Antimicrobial Testing. Processes, 8(11), 1450. https://doi.org/10.3390/pr8111450








