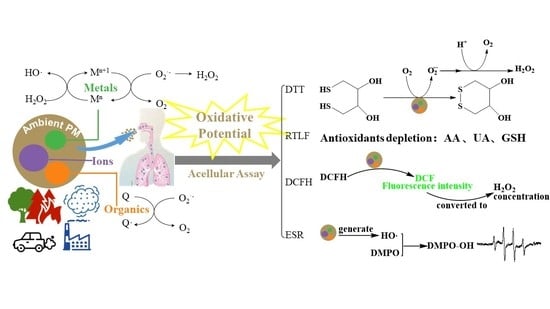
Oxidative Potential Induced by Ambient Particulate Matters with Acellular Assays: A Review
Abstract
1. Introduction
2. Oxidative Potential Measurement Methods
2.1. Respiratory Tract Lining Fluid Assay
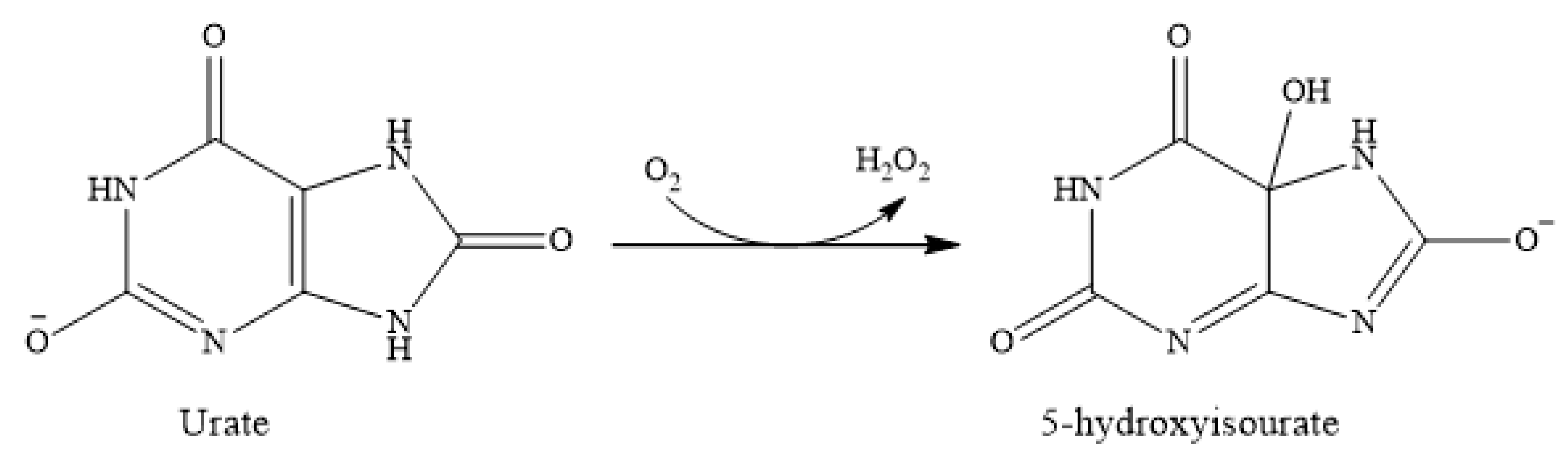
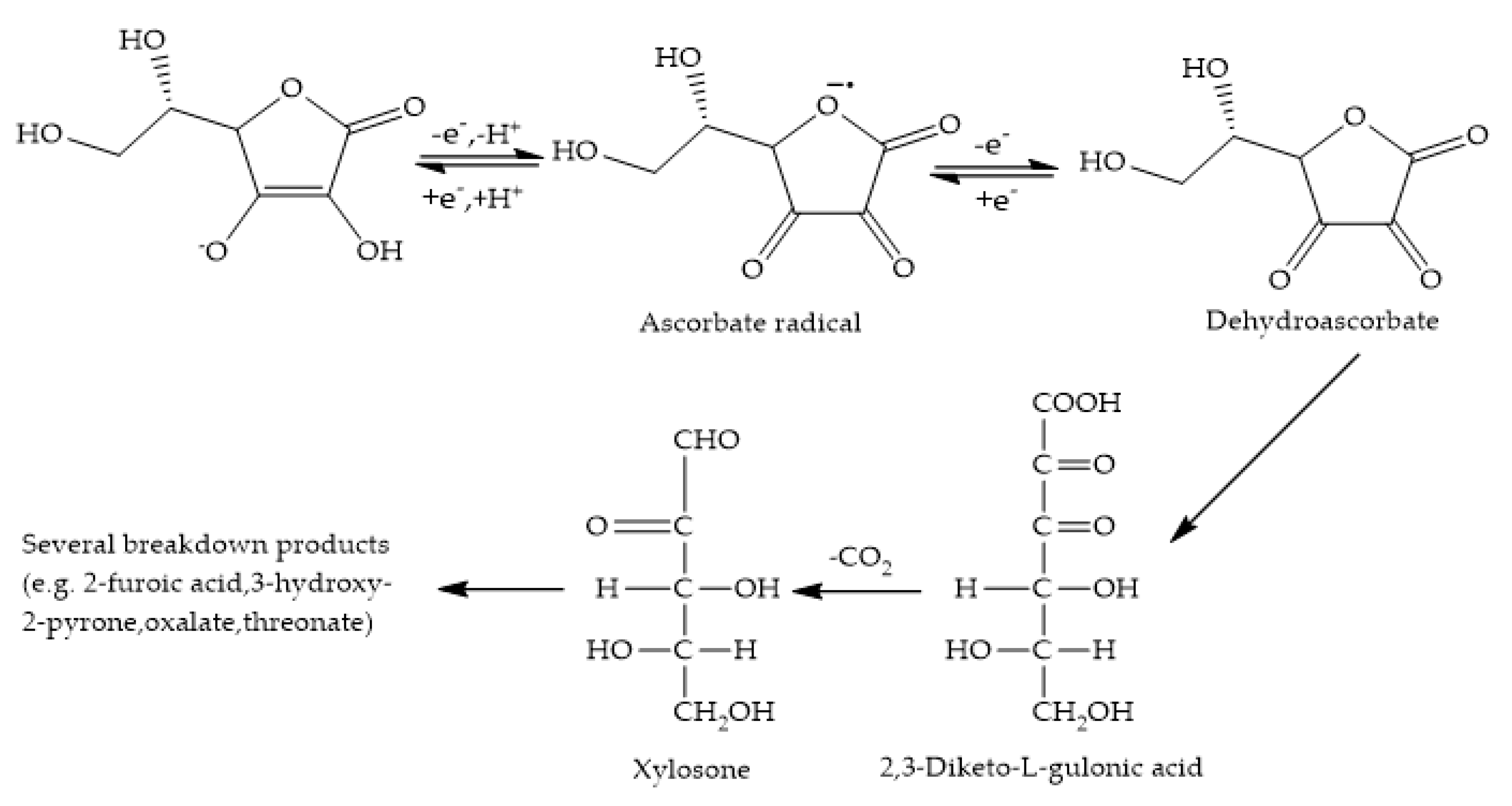
The Ascorbate Depletion Assay
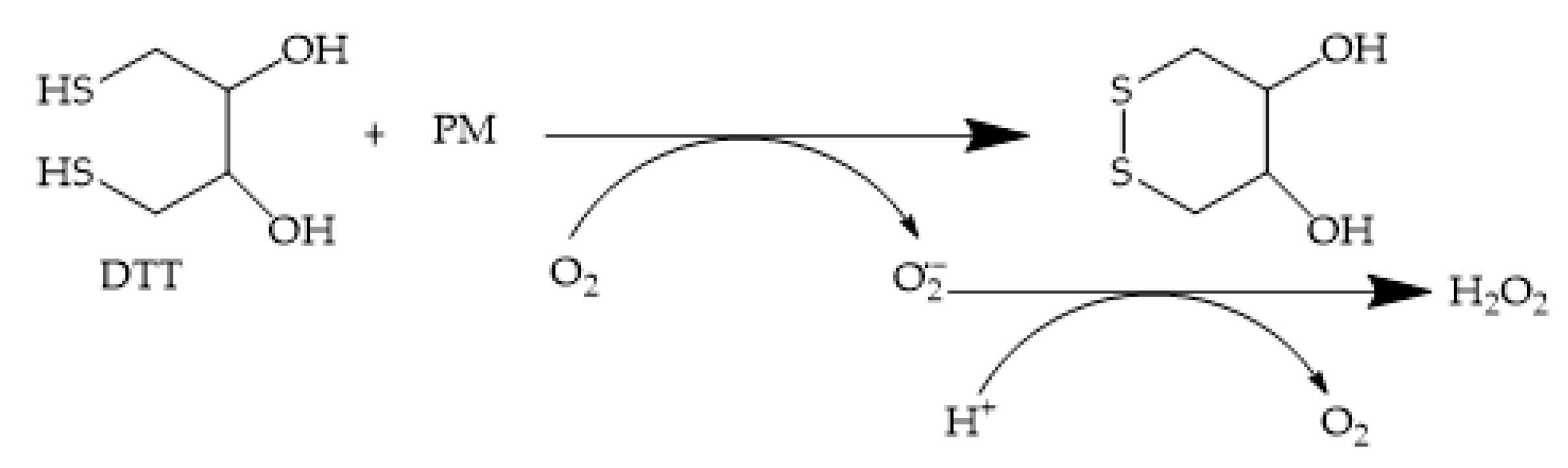
2.2. Dithiothreitol Assay
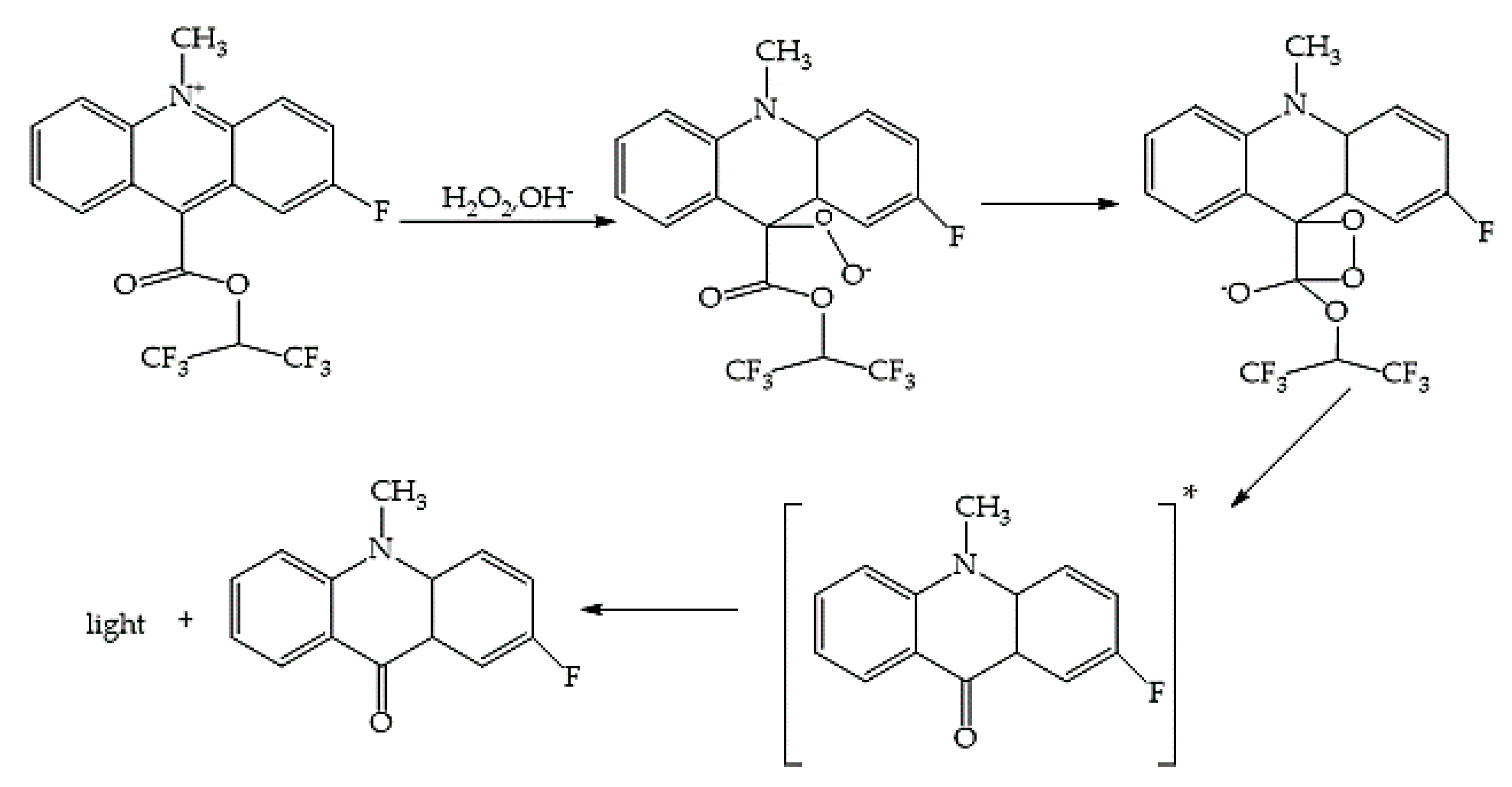
2.3. Chemiluminescent Reductive Acridinium Triggering Assay
2.4. Dichlorofluorescin Assay
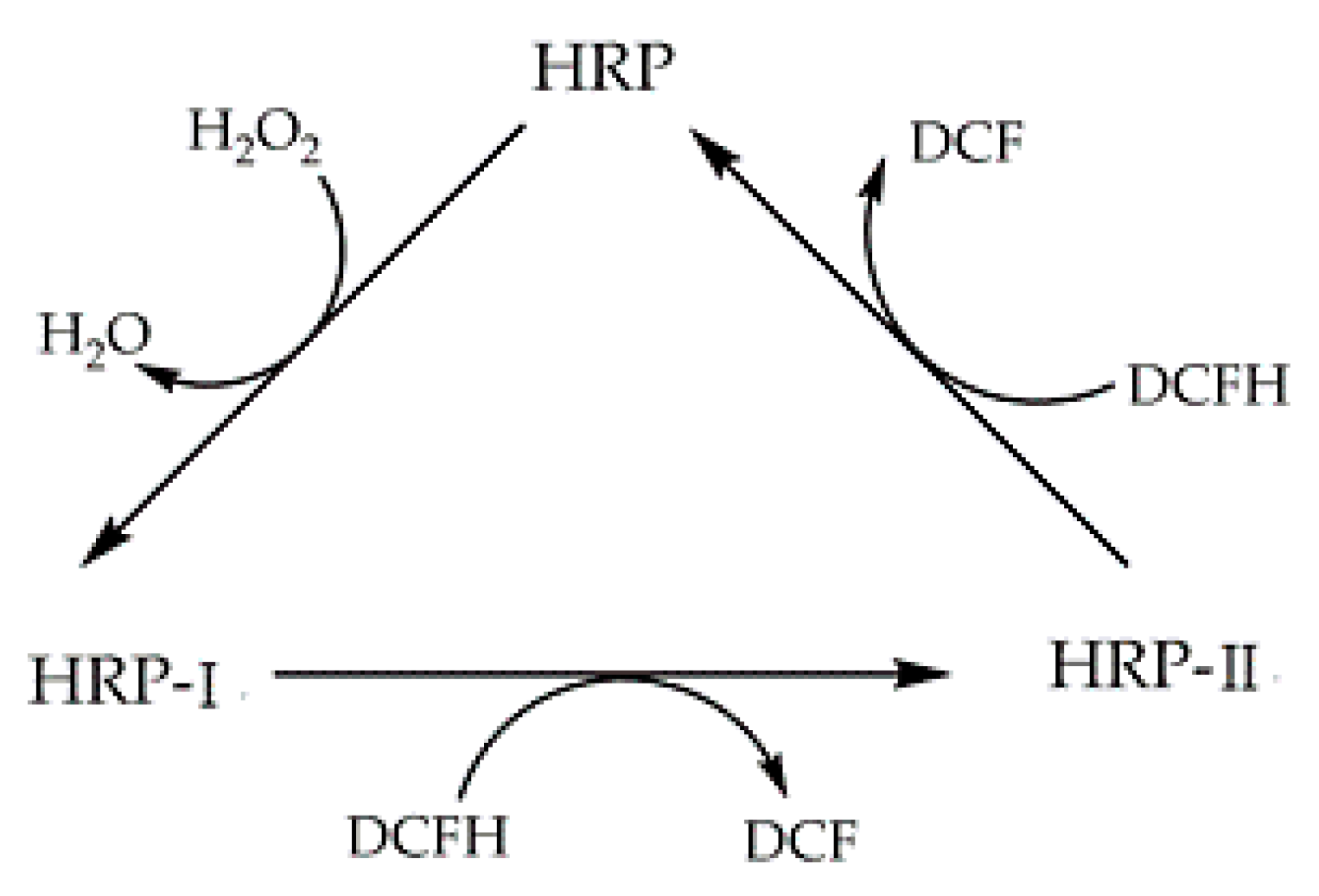
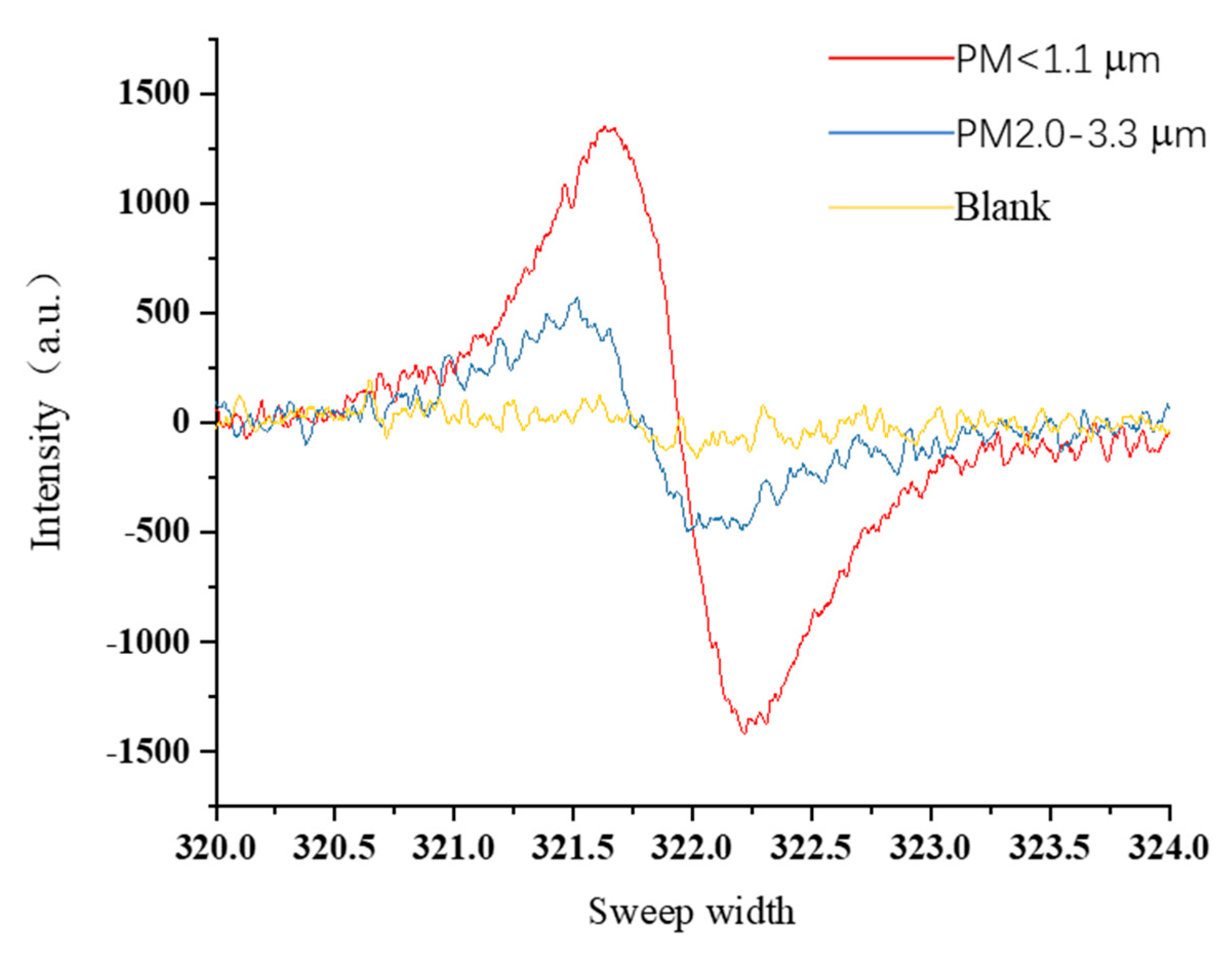
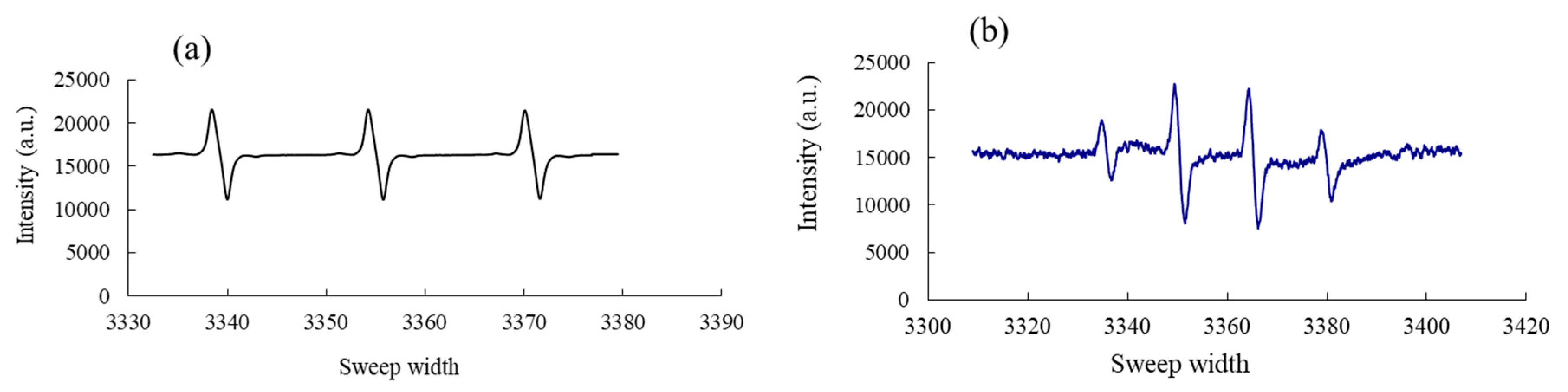
2.5. Electron Paramagnetic/Spin Resonance Assay
3. Comparison of Acellular Assays
3.1. Sensitivity of Different Acellular Assays
3.2. PMs Size Distribution
3.3. OP Related with the Chemical Composition of PMs Collected in Different Seasons
3.4. Correlation with Health Impacts
4. Drivers of Oxidative Potential
4.1. Trace Metals
4.2. Carbonaceous Species
4.3. Ionic Species
4.4. Water-Insoluble Components
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hamanaka, R.B.; Mutlu, G.M. Particulate Matter Air Pollution: Effects on the Cardiovascular System. Front. Endocrinol. 2018, 9, 680. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.C.; Thurston, G.D. Air Pollution, Oxidative Stress, and Diabetes: A Life Course Epidemiologic Perspective. Curr. Diabetes Rep. 2019, 19, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Rao, X.; Zhong, J.; Brook, R.D.; Rajagopalan, S. Effect of Particulate Matter Air Pollution on Cardiovascular Oxidative Stress Pathways. Antioxid. Redox Signal. 2018, 28, 797–818. [Google Scholar] [CrossRef] [PubMed]
- Rajper, S.A.; Ullah, S.; Wang, J.-C. Exposure to air pollution and self-reported effects on Chinese students: A case study of 13 megacities. PLoS ONE 2018, 13, e0194364. [Google Scholar] [CrossRef]
- Delfino, R.J.; Staimer, N.; Tjoa, T.; Gillen, D.L.; Schauer, J.J.; Shafer, M.M. Airway inflammation and oxidative potential of air pollutant particles in a pediatric asthma panel. J. Expo. Sci. Environ. Epidemiol. 2013, 23, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Romano, S.; Perrone, M.; Becagli, S.; Pietrogrande, M.; Russo, M.; Caricato, R.; Lionetto, M. Ecotoxicity, genotoxicity, and oxidative potential tests of atmospheric PM10 particles. Atmos. Environ. 2020, 221, 117085. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, Y.; Li, R.; Chen, W.; Chung, C.K.A.; Cai, Z. The cellular effects of PM2.5 collected in Chinese Taiyuan and Guangzhou and their associations with polycyclic aromatic hydrocarbons (PAHs), nitro-PAHs and hydroxy-PAHs. Ecotoxicol. Environ. Saf. 2020, 191, 110225. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, M.; Wang, Y.; Zhang, L.; Li, Y.; Han, Y. Oxidative Potential of Water-Soluble Matter Associated with Chromophoric Substances in PM2.5 over Xi’an, China. Environ. Sci. Technol. 2019, 53, 8574–8584. [Google Scholar] [CrossRef] [PubMed]
- Bates, J.T.; Fang, T.; Verma, V.; Zeng, L.; Weber, R.J.; Tolbert, P.E.; Abrams, J.Y.; Sarnat, S.E.; Klein, M.; Mulholland, J.A.; et al. Review of Acellular Assays of Ambient Particulate Matter Oxidative Potential: Methods and Relationships with Composition, Sources, and Health Effects. Environ. Sci. Technol. 2019, 53, 4003–4019. [Google Scholar] [CrossRef]
- Leni, Z.; Künzi, L.; Geiser, M. Air pollution causing oxidative stress. Curr. Opin. Toxicol. 2020, 1–8. [Google Scholar] [CrossRef]
- Øvrevik, J. Oxidative Potential Versus Biological Effects: A Review on the Relevance of Cell-Free/Abiotic Assays as Predictors of Toxicity from Airborne Particulate Matter. Int. J. Mol. Sci. 2019, 20, 4772. [Google Scholar] [CrossRef]
- Weber, S.; Uzu, G.; Calas, A.; Chevrier, F.; Besombes, J.-L.; Charron, A.; Salameh, D.; Ježek, I.; Močnik, G.; Jaffrezo, J.-L. An apportionment method for the oxidative potential of atmospheric particulate matter sources: Application to a one-year study in Chamonix, France. Atmos. Chem. Phys. Discuss. 2018, 18, 9617–9629. [Google Scholar] [CrossRef]
- Gao, D.; Ripley, S.; Weichenthal, S.; Pollitt, K.J.G. Ambient particulate matter oxidative potential: Chemical determinants, associated health effects, and strategies for risk management. Free. Radic. Biol. Med. 2020, 151, 7–25. [Google Scholar] [CrossRef]
- Mudway, I.S.; Stenfors, N.; Duggan, S.T.; Roxborough, H.; Zielinski, H.; Marklund, S.L.; Blomberg, A.; Frew, A.J.; Sandström, T.; Kelly, F.J. An in vitro and in vivo investigation of the effects of diesel exhaust on human airway lining fluid antioxidants. Arch. Biochem. Biophys. 2004, 423, 200–212. [Google Scholar] [CrossRef]
- Ayres, J.G.; Borm, P.; Cassee, F.R.; Castranova, V.; Donaldson, K.; Ghio, A.; Harrison, R.M.; Hider, R.; Kelly, F.; Kooter, I.M.; et al. Evaluating the Toxicity of Airborne Particulate Matter and Nanoparticles by Measuring Oxidative Stress Potential—A Workshop Report and Consensus Statement. Inhal. Toxicol. 2008, 20, 75–99. [Google Scholar] [CrossRef]
- Godri, K.J.; Duggan, S.T.; Fuller, G.W.; Baker, T.; Green, D.; Kelly, F.J.; Mudway, I.S. Particulate Matter Oxidative Potential from Waste Transfer Station Activity. Environ. Health Perspect. 2009, 118, 493–498. [Google Scholar] [CrossRef]
- Crobeddu, B.; Aragao-Santiago, L.; Bui, L.-C.; Boland, S.; Squiban, A.B. Oxidative potential of particulate matter 2.5 as predictive indicator of cellular stress. Environ. Pollut. 2017, 230, 125–133. [Google Scholar] [CrossRef]
- Zielinski, H.; Mudway, I.S.; Bérubé, K.; Murphy, S.; Richards, R.; Kelly, F.J. Modeling the interactions of particulates with epithelial lining fluid antioxidants. Am. J. Physiol. Content 1999, 277, L719–L726. [Google Scholar] [CrossRef]
- Gao, D.; Pollitt, K.J.G.; Mulholland, J.A.; Russell, A.G.; Weber, R.J. Characterization and comparison of PM2.5 oxidative potential assessed by two acellular assays. Atmos. Chem. Phys. Discuss. 2020, 20, 5197–5210. [Google Scholar] [CrossRef]
- Pipolo, S.; Percudani, R.; Cammi, R. Absolute stereochemistry and preferred conformations of urate degradation intermediates from computed and experimental circular dichroism spectra. Org. Biomol. Chem. 2011, 9, 5149–5155. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.; Jedynska, A.; Hellack, B.; Kooter, I.; Hoek, G.; Brunekreef, B.; Kuhlbusch, T.A.; Cassee, F.R.; Janssen, N.A. Measurement of the oxidative potential of PM2.5 and its constituents: The effect of extraction solvent and filter type. Atmos. Environ. 2014, 83, 35–42. [Google Scholar] [CrossRef]
- Visentin, M.; Pagnoni, A.; Sarti, E.; Pietrogrande, M.C. Urban PM2.5 oxidative potential: Importance of chemical species and comparison of two spectrophotometric cell-free assays. Environ. Pollut. 2016, 219, 72–79. [Google Scholar] [CrossRef]
- Bradshaw, M.P.; Barril, C.; Clark, A.; Prenzler, P.D.; Scollary, G.R. Ascorbic Acid: A Review of its Chemistry and Reactivity in Relation to a Wine Environment. Crit. Rev. Food Sci. Nutr. 2011, 51, 479–498. [Google Scholar] [CrossRef]
- Lyu, Y.; Guo, H.; Cheng, T.; Li, X. Particle Size Distributions of Oxidative Potential of Lung-Deposited Particles: Assessing Contributions from Quinones and Water-Soluble Metals. Environ. Sci. Technol. 2018, 52, 6592–6600. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Verma, V.; Schauer, J.J.; Cassee, F.R.; Cho, A.K.; Sioutas, C. Oxidative Potential of Semi-Volatile and Non Volatile Particulate Matter (PM) from Heavy-Duty Vehicles Retrofitted with Emission Control Technologies. Environ. Sci. Technol. 2009, 43, 3905–3912. [Google Scholar] [CrossRef]
- Cho, A.K.; Sioutas, C.; Miguel, A.H.; Kumagai, Y.; Schmitz, D.A.; Singh, M.; Eiguren-Fernandez, A.; Froines, J.R. Redox activity of airborne particulate matter at different sites in the Los Angeles Basin. Environ. Res. 2005, 99, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Q.; Yu, H.; Wang, R.; Wei, J.; Verma, V. Rethinking Dithiothreitol-Based Particulate Matter Oxidative Potential: Measuring Dithiothreitol Consumption versus Reactive Oxygen Species Generation. Environ. Sci. Technol. 2017, 51, 6507–6514. [Google Scholar] [CrossRef]
- Zomer, B.; Collé, L.; Jedyńska, A.; Pasterkamp, G.; Kooter, I.; Bloemen, H. Chemiluminescent reductive acridinium triggering (CRAT)—Mechanism and applications. Anal. Bioanal. Chem. 2011, 401, 2945–2954. [Google Scholar] [CrossRef]
- Venkatachari, P.; Hopke, P.K.; Grover, B.D.; Eatough, D.J. Measurement of Particle-Bound Reactive Oxygen Species in Rubidoux Aerosols. J. Atmos. Chem. 2005, 50, 49–58. [Google Scholar] [CrossRef]
- Perrone, M.G.; Zhou, J.; Malandrino, M.; Sangiorgi, G.; Rizzi, C.; Ferrero, L.; Dommen, J.; Bolzacchini, E. PM chemical composition and oxidative potential of the soluble fraction of particles at two sites in the urban area of Milan, Northern Italy. Atmos. Environ. 2016, 128, 104–113. [Google Scholar] [CrossRef]
- Fuller, S.; Wragg, F.; Nutter, J.; Kalberer, M. Comparison of on-line and off-line methods to quantify reactive oxygen species (ROS) in atmospheric aerosols. Atmos. Environ. 2014, 92, 97–103. [Google Scholar] [CrossRef]
- Venkatachari, P.; Hopke, P.K.; Brune, W.H.; Ren, X.; Lesher, R.; Mao, J.; Mitchell, M. Characterization of Wintertime Reactive Oxygen Species Concentrations in Flushing, New York. Aerosol Sci. Technol. 2007, 41, 97–111. [Google Scholar] [CrossRef][Green Version]
- Pietrogrande, M.C.; Russo, M.; Zagatti, E. Review of PM Oxidative Potential Measured with Acellular Assays in Urban and Rural Sites across Italy. Atmosphere 2019, 10, 626. [Google Scholar] [CrossRef]
- Lu, S.; Duffin, R.; Poland, C.; Daly, P.; Murphy, F.; Drost, E.; MacNee, W.; Stone, V.; Donaldson, K. Efficacy of Simple Short-Term in Vitro Assays for Predicting the Potential of Metal Oxide Nanoparticles to Cause Pulmonary Inflammation. Environ. Health Perspect. 2009, 117, 241–247. [Google Scholar] [CrossRef]
- Senlin, L.; Zhang, W.; Zhang, R.; Liu, P.; Wang, Q.; Shang, Y.; Wu, M.; Donaldson, K.; Wang, Q. Comparison of cellular toxicity caused by ambient ultrafine particles and engineered metal oxide nanoparticles. Part. Fibre Toxicol. 2015, 12, 5. [Google Scholar] [CrossRef]
- Shi, T.; Schins, R.P.F.; Knaapen, A.M.; Kuhlbusch, T.; Pitz, M.; Heinrich, J.; Borm, P.J.A. Hydroxyl radical generation by electron paramagnetic resonance as a new method to monitor ambient particulate matter composition. J. Environ. Monit. 2003, 5, 550–556. [Google Scholar] [CrossRef]
- Shi, T.; Knaapen, A.; Begerow, J.; Birmili, W.; Borm, P.; Schins, R.P.F. Temporal variation of hydroxyl radical generation and 8-hydroxy-2′-deoxyguanosine formation by coarse and fine particulate matter. Occup. Environ. Med. 2003, 60, 315–321. [Google Scholar] [CrossRef]
- Boogaard, H.; Janssen, N.A.; Fischer, P.H.; Kos, G.P.; Weijers, E.P.; Cassee, F.R.; Van Der Zee, S.C.; De Hartog, J.J.; Brunekreef, B.; Hoek, G. Contrasts in Oxidative Potential and Other Particulate Matter Characteristics Collected Near Major Streets and Background Locations. Environ. Health Perspect. 2012, 120, 185–191. [Google Scholar] [CrossRef]
- Yang, A.; Hellack, B.; Leseman, D.; Brunekreef, B.; Kuhlbusch, T.A.; Cassee, F.; Hoek, G.; Janssen, N.A. Temporal and spatial variation of the metal-related oxidative potential of PM 2.5 and its relation to PM 2.5 mass and elemental composition. Atmos. Environ. 2015, 102, 62–69. [Google Scholar] [CrossRef]
- Tong, H.; Lakey, P.S.J.; Arangio, A.M.; Socorro, J.; Shen, F.; Lucas, K.; Brune, W.H.; Pöschl, U.; Shiraiwa, M. Reactive Oxygen Species Formed by Secondary Organic Aerosols in Water and Surrogate Lung Fluid. Environ. Sci. Technol. 2018, 52, 11642–11651. [Google Scholar] [CrossRef]
- Dikalov, S.; Skatchkov, M.; Bassenge, E. Quantification of Peroxynitrite, Superoxide, and Peroxyl Radicals by a New Spin Trap Hydroxylamine 1-Hydroxy-2,2,6,6-tetramethyl-4-oxo-piperidine. Biochem. Biophys. Res. Commun. 1997, 230, 54–57. [Google Scholar] [CrossRef] [PubMed]
- Janssen, N.A.; Yang, A.; Strak, M.; Steenhof, M.; Hellack, B.; Gerlofs-Nijland, M.E.; Kuhlbusch, T.; Kelly, F.; Harrison, R.; Brunekreef, B.; et al. Oxidative potential of particulate matter collected at sites with different source characteristics. Sci. Total. Environ. 2014, 472, 572–581. [Google Scholar] [CrossRef]
- Weichenthal, S.; Shekarrizfard, M.; Traub, A.; Kulka, R.; Al-Rijleh, K.; Anowar, S.; Evans, G.J.; Hatzopoulou, M. Within-City Spatial Variations in Multiple Measures of PM2.5 Oxidative Potential in Toronto, Canada. Environ. Sci. Technol. 2019, 53, 2799–2810. [Google Scholar] [CrossRef] [PubMed]
- Fang, T.; Verma, V.; Bates, J.T.; Abrams, J.; Klein, M.; Strickland, M.J.; Sarnat, S.E.; Chang, H.H.; Mulholland, J.A.; Tolbert, P.E.; et al. Oxidative potential of ambient water-soluble PM2.5 in the southeastern United States: Contrasts in sources and health associations between ascorbic acid (AA) and dithiothreitol (DTT) assays. Atmos. Chem. Phys. Discuss. 2016, 16, 3865–3879. [Google Scholar] [CrossRef]
- Godri, K.J.; Harrison, R.M.; Evans, T.; Baker, T.; Dunster, C.; Mudway, I.S.; Kelly, F.J. Increased Oxidative Burden Associated with Traffic Component of Ambient Particulate Matter at Roadside and Urban Background Schools Sites in London. PLoS ONE 2011, 6, e21961. [Google Scholar] [CrossRef]
- Molina, C.; Toro, R.; Manzano, C.A.; Canepari, S.; Massimi, L.; Leiva-Guzmán, M.A. Airborne Aerosols and Human Health: Leapfrogging from Mass Concentration to Oxidative Potential. Atmosphere 2020, 11, 917. [Google Scholar] [CrossRef]
- Chirizzi, D.; Cesari, D.; Guascito, M.R.; Dinoi, A.; Giotta, L.; Donateo, A.; Contini, D. Influence of Saharan dust outbreaks and carbon content on oxidative potential of water-soluble fractions of PM2.5 and PM10. Atmos. Environ. 2017, 163, 1–8. [Google Scholar] [CrossRef]
- Perrone, M.R.; Bertoli, I.; Romano, S.; Russo, M.; Rispoli, G.; Pietrogrande, M.C. PM2.5 and PM10 oxidative potential at a Central Mediterranean Site: Contrasts between dithiothreitol- and ascorbic acid-measured values in relation with particle size and chemical composition. Atmos. Environ. 2019, 210, 143–155. [Google Scholar] [CrossRef]
- Simonetti, G.; Conte, E.; Perrino, C.; Canepari, S. Oxidative potential of size-segregated PM in an urban and an industrial area of Italy. Atmos. Environ. 2018, 187, 292–300. [Google Scholar] [CrossRef]
- Fang, T.; Zeng, L.; Gao, D.; Verma, V.; Stefaniak, A.B.; Weber, R.J. Ambient Size Distributions and Lung Deposition of Aerosol Dithiothreitol-Measured Oxidative Potential: Contrast between Soluble and Insoluble Particles. Environ. Sci. Technol. 2017, 51, 6802–6811. [Google Scholar] [CrossRef]
- Gao, D.; Mulholland, J.A.; Russell, A.G.; Weber, R.J. Characterization of water-insoluble oxidative potential of PM2.5 using the dithiothreitol assay. Atmos. Environ. 2020, 224, 117327. [Google Scholar] [CrossRef]
- Liu, W.; Xu, Y.; Liu, W.; Liu, Q.; Yu, S.; Liu, Y.; Wang, X.; Tao, S. Oxidative potential of ambient PM2.5 in the coastal cities of the Bohai Sea, northern China: Seasonal variation and source apportionment. Environ. Pollut. 2018, 236, 514–528. [Google Scholar] [CrossRef] [PubMed]
- Cesari, D.; Merico, E.; Grasso, F.M.; Decesari, S.; Belosi, F.; Manarini, F.; De Nuntiis, P.; Rinaldi, M.; Volpi, F.; Gambaro, A.; et al. Source Apportionment of PM2.5 and of its Oxidative Potential in an Industrial Suburban Site in South Italy. Atmosphere 2019, 10, 758. [Google Scholar] [CrossRef]
- Calas, A.; Uzu, G.; Kelly, F.J.; Houdier, S.; Martins, J.M.F.; Thomas, F.; Molton, F.; Charron, A.; Dunster, C.; Oliete, A.; et al. Comparison between five acellular oxidative potential measurement assays performed with detailed chemistry on PM10 samples from the city of Chamonix (France). Atmos. Chem. Phys. Discuss. 2018, 18, 7863–7875. [Google Scholar] [CrossRef]
- Taghvaee, S.; Sowlat, M.H.; Diapouli, E.; Manousakas, M.I.; Vasilatou, V.; Eleftheriadis, K.; Sioutas, C. Source apportionment of the oxidative potential of fine ambient particulate matter (PM2.5) in Athens, Greece. Sci. Total. Environ. 2019, 653, 1407–1416. [Google Scholar] [CrossRef]
- Yu, S.; Liu, W.; Xu, Y.; Yi, K.; Zhou, M.; Tao, S.; Liu, W. Characteristics and oxidative potential of atmospheric PM2.5 in Beijing: Source apportionment and seasonal variation. Sci. Total. Environ. 2019, 650, 277–287. [Google Scholar] [CrossRef]
- Janssen, N.A.; Strak, M.; Yang, A.; Hellack, B.; Kelly, F.J.; Kuhlbusch, T.A.J.; Harrison, R.M.; Brunekreef, B.; Cassee, F.R.; Steenhof, M.; et al. Associations between three specific a-cellular measures of the oxidative potential of particulate matter and markers of acute airway and nasal inflammation in healthy volunteers. Occup. Environ. Med. 2014, 72, 49–56. [Google Scholar] [CrossRef]
- Wang, Y.; Plewa, M.J.; Mukherjee, U.K.; Verma, V. Assessing the cytotoxicity of ambient particulate matter (PM) using Chinese hamster ovary (CHO) cells and its relationship with the PM chemical composition and oxidative potential. Atmos. Environ. 2018, 179, 132–141. [Google Scholar] [CrossRef]
- Bates, J.T.; Weber, R.J.; Abrams, J.; Verma, V.; Fang, T.; Klein, M.; Strickland, M.J.; Sarnat, S.E.; Chang, H.H.; Mulholland, J.A.; et al. Reactive Oxygen Species Generation Linked to Sources of Atmospheric Particulate Matter and Cardiorespiratory Effects. Environ. Sci. Technol. 2015, 49, 13605–13612. [Google Scholar] [CrossRef]
- Yang, A.; Janssen, N.A.H.; Brunekreef, B.; Cassee, F.R.; Hoek, G.; Gehring, U. Children’s respiratory health and oxidative potential of PM2.5: The PIAMA birth cohort study. Occup. Environ. Med. 2016, 73, 154–160. [Google Scholar] [CrossRef]
- Maikawa, C.L.; Weichenthal, S.; Wheeler, A.J.; Dobbin, N.A.; Smargiassi, A.; Evans, G.; Liu, L.; Goldberg, M.S.; Pollitt, K.J.G. Particulate Oxidative Burden as a Predictor of Exhaled Nitric Oxide in Children with Asthma. Environ. Health Perspect. 2016, 124, 1616–1622. [Google Scholar] [CrossRef]
- Fang, T.; Guo, H.; Verma, V.; Peltier, R.E.; Weber, R.J. PM2.5 water-soluble elements in the southeastern United States: Automated analytical method development, spatiotemporal distributions, source apportionment, and implications for heath studies. Atmos. Chem. Phys. Discuss. 2015, 15, 17189–17227. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, M.; Li, S.; Sun, H.; Mu, Z.; Zhang, L.; Li, Y.; Chen, Q. Study on the oxidation potential of the water-soluble components of ambient PM2.5 over Xi’an, China: Pollution levels, source apportionment and transport pathways. Environ. Int. 2020, 136, 105515. [Google Scholar] [CrossRef]
- Patel, A.; Rastogi, N. Oxidative potential of ambient fine aerosol over a semi-urban site in the Indo-Gangetic Plain. Atmos. Environ. 2018, 175, 127–134. [Google Scholar] [CrossRef]
- Forman, H.J.; Finch, C.E. A critical review of assays for hazardous components of air pollution. Free. Radic. Biol. Med. 2018, 117, 202–217. [Google Scholar] [CrossRef]
- Valko, M.; Jomova, K.; Rhodes, C.J.; Kuča, K.; Musílek, K. Redox- and non-redox-metal-induced formation of free radicals and their role in human disease. Arch. Toxicol. 2016, 90, 1–37. [Google Scholar] [CrossRef]
- Yu, H.; Wei, J.; Cheng, Y.; Subedi, K.; Verma, V. Synergistic and Antagonistic Interactions among the Particulate Matter Components in Generating Reactive Oxygen Species Based on the Dithiothreitol Assay. Environ. Sci. Technol. 2018, 52, 2261–2270. [Google Scholar] [CrossRef]
- Pietrogrande, M.; Perrone, M.R.; Manarini, F.; Romano, S.; Udisti, R.; Becagli, S. PM10 oxidative potential at a Central Mediterranean Site: Association with chemical composition and meteorological parameters. Atmos. Environ. 2018, 188, 97–111. [Google Scholar] [CrossRef]
- Ma, Y.; Cheng, Y.; Qiu, X.; Cao, G.; Fang, Y.; Wang, J.; Zhu, T.; Yu, J.; Xinghua, Q. Sources and oxidative potential of water-soluble humic-like substances (HULISWS) in fine particulate matter (PM2.5) in Beijing. Atmos. Chem. Phys. Discuss. 2018, 18, 5607–5617. [Google Scholar] [CrossRef]
- Pietrogrande, M.; Dalpiaz, C.; Dell’Anna, R.; Lazzeri, P.; Manarini, F.; Visentin, M.; Tonidandel, G. Chemical composition and oxidative potential of atmospheric coarse particles at an industrial and urban background site in the alpine region of northern Italy. Atmos. Environ. 2018, 191, 340–350. [Google Scholar] [CrossRef]
- Shirmohammadi, F.; Wang, D.; Hasheminassab, S.; Verma, V.; Schauer, J.J.; Shafer, M.M.; Sioutas, C. Oxidative potential of on-road fine particulate matter (PM 2.5 ) measured on major freeways of Los Angeles, CA, and a 10-year comparison with earlier roadside studies. Atmos. Environ. 2017, 148, 102–114. [Google Scholar] [CrossRef]
- Wei, J.; Yu, H.; Wang, Y.; Verma, V. Complexation of Iron and Copper in Ambient Particulate Matter and Its Effect on the Oxidative Potential Measured in a Surrogate Lung Fluid. Environ. Sci. Technol. 2018, 53, 1661–1671. [Google Scholar] [CrossRef]
- Charrier, J.G.; Anastasio, C. On dithiothreitol (DTT) as a measure of oxidative potential for ambient particles: Evidence for the importance of soluble transition metals. Atmos. Chem. Phys. Discuss. 2012, 12, 9321–9333. [Google Scholar] [CrossRef]
- Senlin, L.; Win, M.S.; Zeng, J.; Yao, C.; Zhao, M.; Xiu, G.; Lin, Y.; Xie, T.; Dai, Y.; Rao, L.; et al. A characterization of HULIS-C and the oxidative potential of HULIS and HULIS-Fe(II) mixture in PM2.5 during hazy and non-hazy days in Shanghai. Atmos. Environ. 2019, 219, 117058. [Google Scholar] [CrossRef]
- Tapparo, A.; Di Marco, V.; Badocco, D.; D’Aronco, S.; Soldà, L.; Pastore, P.; Mahon, B.M.; Kalberer, M.; Giorio, C. Formation of metal-organic ligand complexes affects solubility of metals in airborne particles at an urban site in the Po valley. Chemosphere 2019, 241, 125025. [Google Scholar] [CrossRef] [PubMed]
- Xie, T.; Senlin, L.; Zeng, J.; Rao, L.; Wang, X.; Win, M.S.; Zhang, D.; Lu, H.; Liu, X.; Wang, Q. Soluble Fe release from iron-bearing clay mineral particles in acid environment and their oxidative potential. Sci. Total. Environ. 2020, 726, 138650. [Google Scholar] [CrossRef]
- Verma, V.; Fang, T.; Guo, H.; King, L.; Bates, J.T.; Peltier, R.E.; Edgerton, E.S.; Russell, A.G.; Weber, R.J. Reactive oxygen species associated with water-soluble PM2.5 in the southeastern United States: Spatiotemporal trends and source apportionment. Atmos. Chem. Phys. Discuss. 2014, 14, 12915–12930. [Google Scholar] [CrossRef]
- Samet, J.M.; Chen, H.; Pennington, E.R.; Bromberg, P.A. Non-redox cycling mechanisms of oxidative stress induced by PM metals. Free. Radic. Biol. Med. 2019, 151, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Nenes, A.; Weber, R.J. The underappreciated role of nonvolatile cations in aerosol ammonium-sulfate molar ratios. Atmos. Chem. Phys. Discuss. 2018, 18, 17307–17323. [Google Scholar] [CrossRef]
- Wang, Q.; Kobayashi, K.; Senlin, L.; Nakajima, D.; Wang, W.; Zhang, W.; Sekiguchi, K.; Terasaki, M. Studies on size distribution and health risk of 37 species of polycyclic aromatic hydrocarbons associated with fine particulate matter collected in the atmosphere of a suburban area of Shanghai city, China. Environ. Pollut. 2016, 214, 149–160. [Google Scholar] [CrossRef]
- Kumagai, Y.; Koide, S.; Taguchi, K.; Endo, A.; Nakai, Y.; Yoshikawa, T.; Shimojo, N. Oxidation of Proximal Protein Sulfhydryls by Phenanthraquinone, a Component of Diesel Exhaust Particles. Chem. Res. Toxicol. 2002, 15, 483–489. [Google Scholar] [CrossRef]
- Shang, Y.; Fan, L.; Feng, J.; Lv, S.; Wu, M.; Li, B.; Zang, Y.-S. Genotoxic and inflammatory effects of organic extracts from traffic-related particulate matter in human lung epithelial A549 cells: The role of quinones. Toxicol. In Vitro 2013, 27, 922–931. [Google Scholar] [CrossRef] [PubMed]
- Walgraeve, C.; Demeestere, K.; Dewulf, J.; Zimmermann, R.; Van Langenhove, H. Oxygenated polycyclic aromatic hydrocarbons in atmospheric particulate matter: Molecular characterization and occurrence. Atmos. Environ. 2010, 44, 1831–1846. [Google Scholar] [CrossRef]
- Li, L.J.; Ho, S.S.H.; Feng, B.; Xu, H.; Wang, T.; Wu, R.; Huang, W.; Qu, L.; Wang, Q.; Cao, J. Characterization of particulate-bound polycyclic aromatic compounds (PACs) and their oxidations in heavy polluted atmosphere: A case study in urban Beijing, China during haze events. Sci. Total. Environ. 2019, 660, 1392–1402. [Google Scholar] [CrossRef]
- Vreeland, H.; Weber, R.J.; Bergin, M.; Greenwald, R.; Golan, R.; Russell, A.G.; Verma, V.; Sarnat, J.A. Oxidative potential of PM 2.5 during Atlanta rush hour: Measurements of in-vehicle dithiothreitol (DTT) activity. Atmos. Environ. 2017, 165, 169–178. [Google Scholar] [CrossRef]
- Weinstein, J.R.; Asteria-Peñaloza, R.; Diaz-Artiga, A.; Davila, G.; Hammond, S.K.; Ryde, I.T.; Meyer, J.N.; Benowitz, N.; Thompson, L.M. Exposure to polycyclic aromatic hydrocarbons and volatile organic compounds among recently pregnant rural Guatemalan women cooking and heating with solid fuels. Int. J. Hyg. Environ. Health 2017, 220, 726–735. [Google Scholar] [CrossRef]
- Jiang, H.; Jang, M. Dynamic Oxidative Potential of Atmospheric Organic Aerosol under Ambient Sunlight. Environ. Sci. Technol. 2018, 52, 7496–7504. [Google Scholar] [CrossRef]
- Wang, S.; Ye, J.; Soong, R.; Wu, B.; Yu, L.; Simpson, M.J.; Chan, A.W.H. Relationship between chemical composition and oxidative potential of secondary organic aerosol from polycyclic aromatic hydrocarbons. Atmos. Chem. Phys. Discuss. 2018, 18, 3987–4003. [Google Scholar] [CrossRef]
- Win, M.S.; Tian, Z.; Zhao, H.; Xiao, K.; Peng, J.; Shang, Y.; Wu, M.; Xiu, G.; Lu, S.; Yonemochi, S.; et al. Atmospheric HULIS and its ability to mediate the reactive oxygen species (ROS): A review. J. Environ. Sci. 2018, 71, 13–31. [Google Scholar] [CrossRef] [PubMed]
- Charrier, J.G.; Anastasio, C. Rates of Hydroxyl Radical Production from Transition Metals and Quinones in a Surrogate Lung Fluid. Environ. Sci. Technol. 2015, 49, 9317–9325. [Google Scholar] [CrossRef]
- Kuang, X.M.; Gonzalez, D.H.; Scott, J.A.; Vu, K.; Hasson, A.S.; Charbouillot, T.; Hawkins, L.; Paulson, S.E. Cloud Water Chemistry Associated with Urban Aerosols: Rapid Hydroxyl Radical Formation, Soluble Metals, Fe(II), Fe(III), and Quinones. ACS Earth Space Chem. 2019, 4, 67–76. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, W. Size characteristics and health risks of inorganic species in PM1.1 and PM2.0 of Shanghai, China, in spring, 2017. Environ. Sci. Pollut. Res. 2020, 27, 14690–14701. [Google Scholar] [CrossRef] [PubMed]
- Daher, N.; Ning, Z.; Cho, A.K.; Shafer, M.; Schauer, J.J.; Sioutas, C. Comparison of the Chemical and Oxidative Characteristics of Particulate Matter (PM) Collected by Different Methods: Filters, Impactors, and BioSamplers. Aerosol Sci. Technol. 2011, 45, 1294–1304. [Google Scholar] [CrossRef]
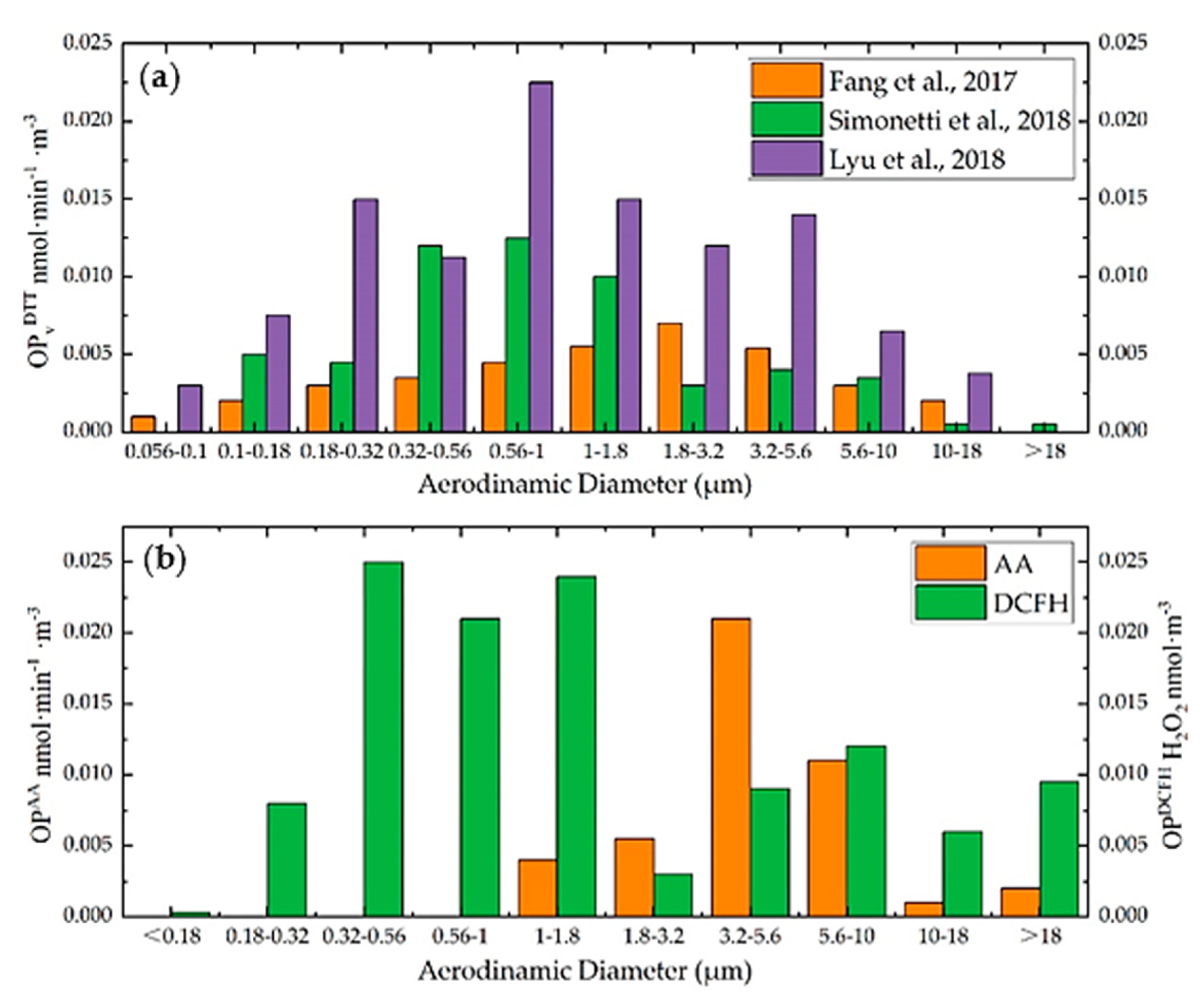
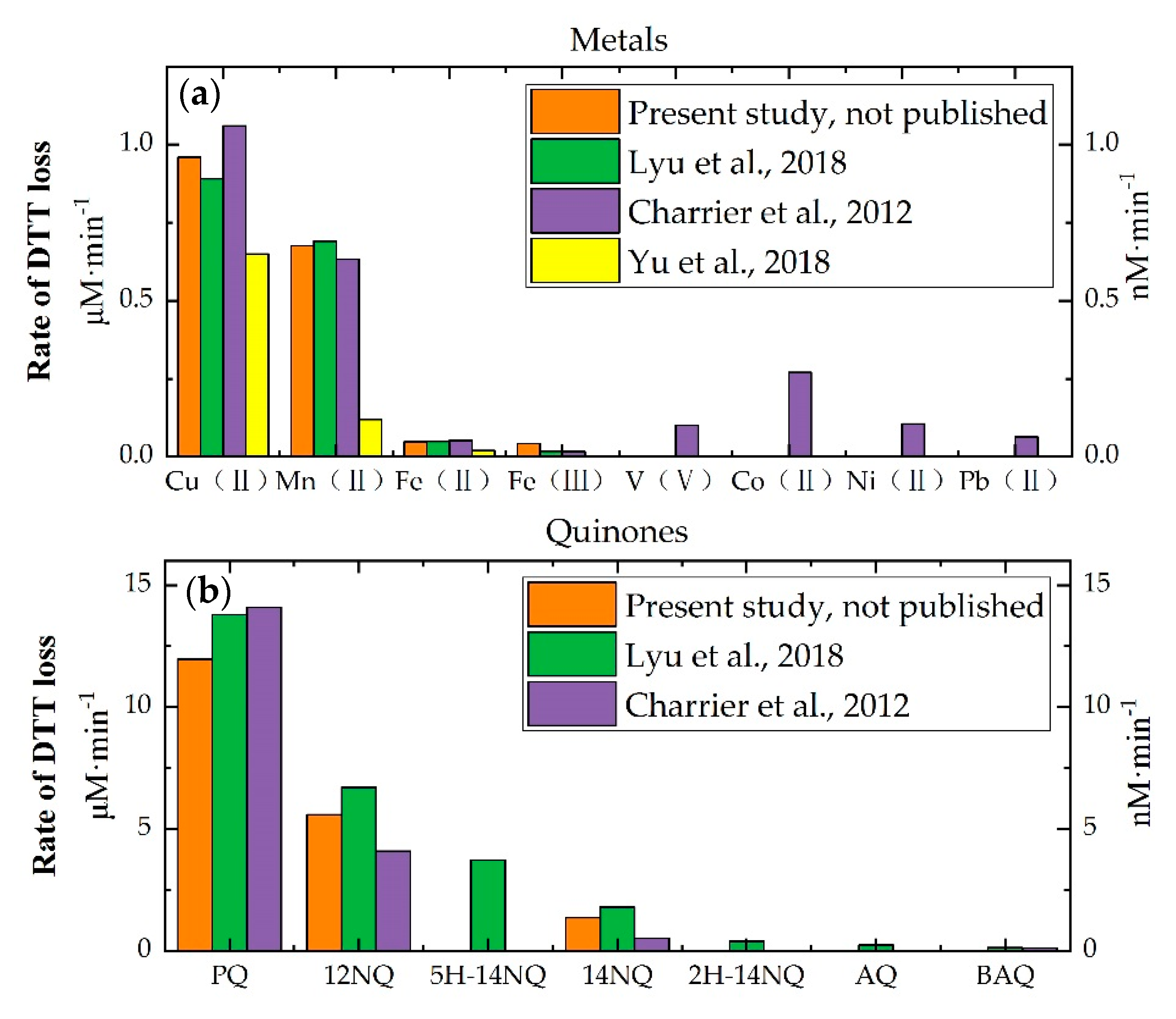
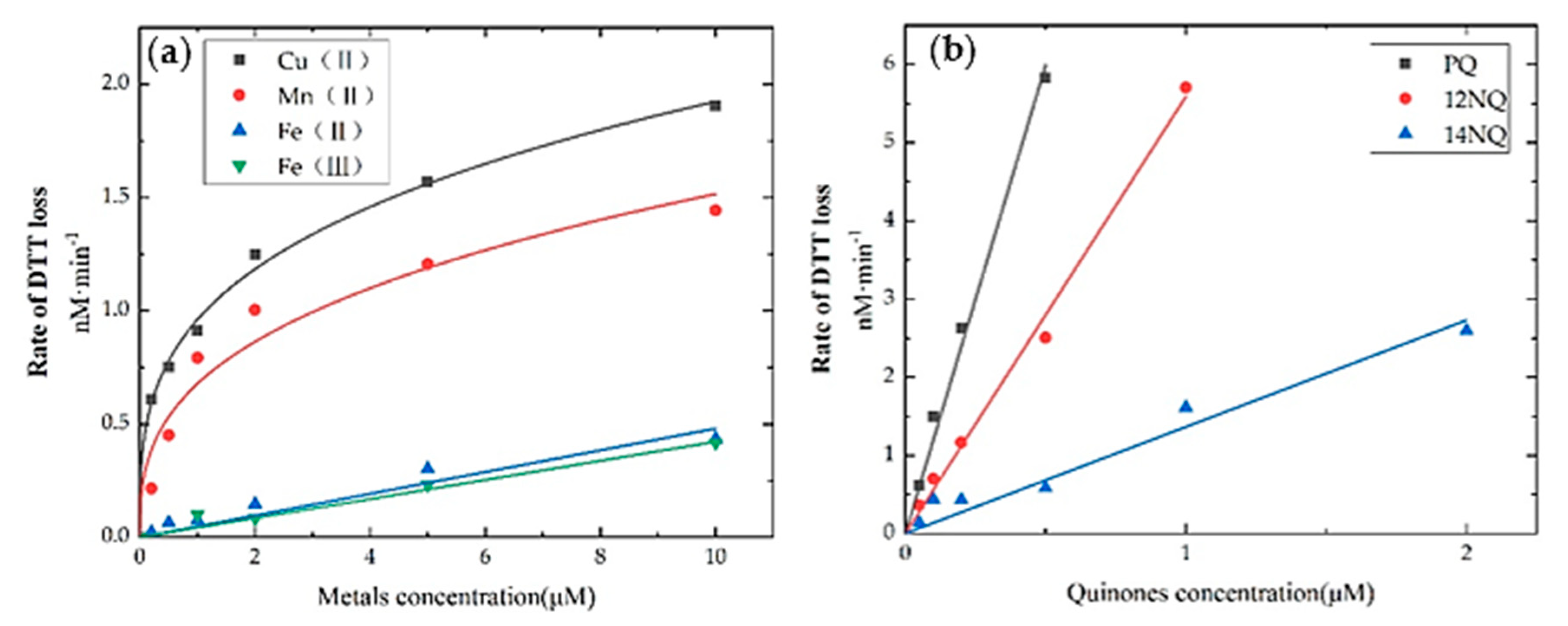

| Assay | Requirements | Estimated Way | Sensitivity | Sources | Characteristic | Reference |
|---|---|---|---|---|---|---|
| DTT | fast, inexpensive, easy to perform and suitable for automation | the depletion rate of chemical proxies for cellular reductants | organic compounds; traffic-related metals; inorganic ions | biomass burning; brake/tire wear; traffic/fossil fuel combustion; photochemical aging | associated with fine fraction | [24,42,47,50] |
| AA | the antioxidant loss rate | metals | non-exhaust traffic emissions | associated with coarse particles | [48,49] | |
| GSH | the antioxidant loss rate | Cu | non-exhaust traffic emissions | not a strong marker for traffic | [43] | |
| DCFH | the increase in fluorescence intensity over time | organic compounds; inorganic ions | anthropic combustion; secondary aerosol | associated with fine fraction | [49] | |
| CRAT | has not been widely used | the chemiluminescence reaction | transition metals, quinones | ambient particles | highly correlated with PM mass concentration | [21] |
| ESR | relatively little material, inexpensive | the ability of PMs to generate •OH | transition metals; organic components | ambient particles | associated with coarse/fine particles | [42] |
| Location | Particles | Seasons | Sampling Period | Assay | Driving Species | Reference |
|---|---|---|---|---|---|---|
| Atlanta | PM2.5 | One year | Jan–Dec, 2017 | DTT | BrC, EC, K, Fe, Cu | [51] |
| Central Mediterranean Sea | PM10, PM2.5 | One year | 2014-2015 | DTT, AA | K+, NO3−, Ba, Cd, Cu, Fe, Mn, P, V, OC, EC | [48] |
| Atlanta | PM2.5 | One year | 2017 | DTT, RTLF | WSOC, OC, EC, Fe, Cu, Mn | [19] |
| the University of Illinois, Urbana−Champaign | Ambient PM2.5 | Spring | Feb–Apr, 2017 | DTT | HULIS, Fe, Cu, Mn | [67] |
| the Central Mediterranean basin | PM10 | One year | Dec 2014–Oct 2015 | DTT AA | Ba, Cd, Ce, Cr, Cu, Fe, EC, OC | [68] |
| Indo-Gangetic Plain | PM2.5 | Winter | 2014 | DTT | OC, EC, WSOC, | [64] |
| Beijing | PM2.5 | One year | 2012 | DTT | HULIS | [69] |
| Italy | PM10 | One year | Feb–Nov 2015 Apr–May 2016 | DTT, AA | SO42−, NH4+, K+, Mg2+, Ca2+, Ca, Mg, K, Mn, Cu, Rb, Zn, WSOC | [70] |
| the littoral zone of the Bohai Sea | PM2.5 | One year | 2016 | DTT | WSOC, EC, Mn, Co, Fe, Cr, Cd, SO42−, NH4+, NO3− | [52] |
| Italy | Size-segregated PMs | spring | Feb–Mar, 2017 | AA, DTT, DCFH | Cu, Fe, Mn, As, B, Cd, Cr, Mo, Se, Ni, Pb, K, Rb | [49] |
| the Los Angeles Basin | PM2.5 | winter | Oct 2014–Jan 2015; Nov 2015–Jan 2016 | DTT | Ba, Cr, Cu, Mn, Ni, Pb, Sb and Zn, EC, OC | [71] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rao, L.; Zhang, L.; Wang, X.; Xie, T.; Zhou, S.; Lu, S.; Liu, X.; Lu, H.; Xiao, K.; Wang, W.; et al. Oxidative Potential Induced by Ambient Particulate Matters with Acellular Assays: A Review. Processes 2020, 8, 1410. https://doi.org/10.3390/pr8111410
Rao L, Zhang L, Wang X, Xie T, Zhou S, Lu S, Liu X, Lu H, Xiao K, Wang W, et al. Oxidative Potential Induced by Ambient Particulate Matters with Acellular Assays: A Review. Processes. 2020; 8(11):1410. https://doi.org/10.3390/pr8111410
Chicago/Turabian StyleRao, Lanfang, Luying Zhang, Xingzi Wang, Tingting Xie, Shumin Zhou, Senlin Lu, Xinchun Liu, Hui Lu, Kai Xiao, Weiqian Wang, and et al. 2020. "Oxidative Potential Induced by Ambient Particulate Matters with Acellular Assays: A Review" Processes 8, no. 11: 1410. https://doi.org/10.3390/pr8111410
APA StyleRao, L., Zhang, L., Wang, X., Xie, T., Zhou, S., Lu, S., Liu, X., Lu, H., Xiao, K., Wang, W., & Wang, Q. (2020). Oxidative Potential Induced by Ambient Particulate Matters with Acellular Assays: A Review. Processes, 8(11), 1410. https://doi.org/10.3390/pr8111410