Thermo-Acoustic Catalytic Effect on Oxidizing Woody Torrefaction
Abstract
1. Introduction
2. Material and Methods
2.1. Feedstock and Sample Analysis
2.2. Thermal Gravimetric (TG) Experimental Analysis
2.3. Thermoacoustic Kinetics Modeling
3. Results and Discussions
3.1. Thermoacoustic Experiments
3.1.1. TG and DTG Curve Assessment
3.1.2. Torrefaction Severity Index (TSI) for the Catalytic Process
3.2. Physical and Chemical Property Analysis
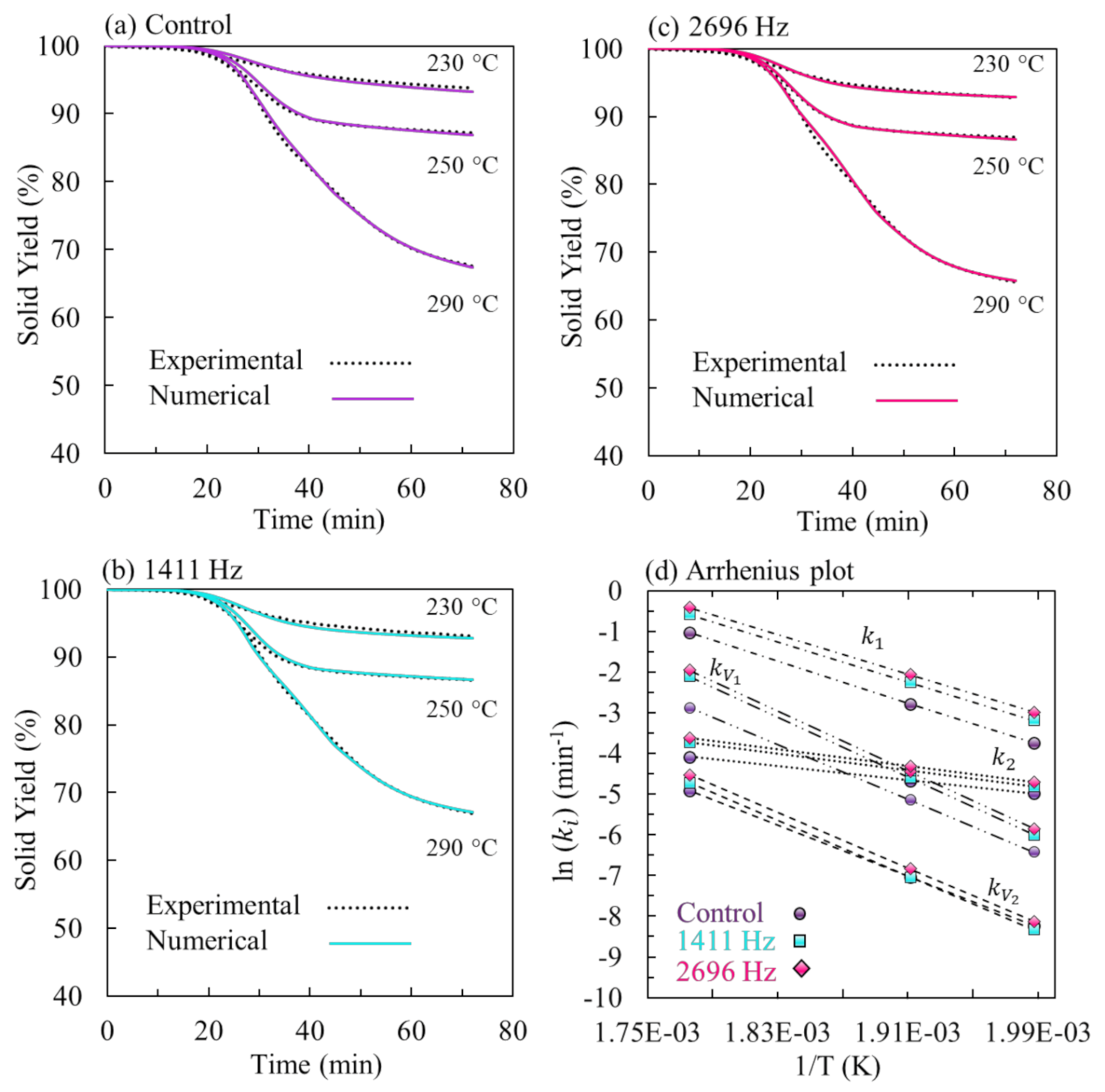
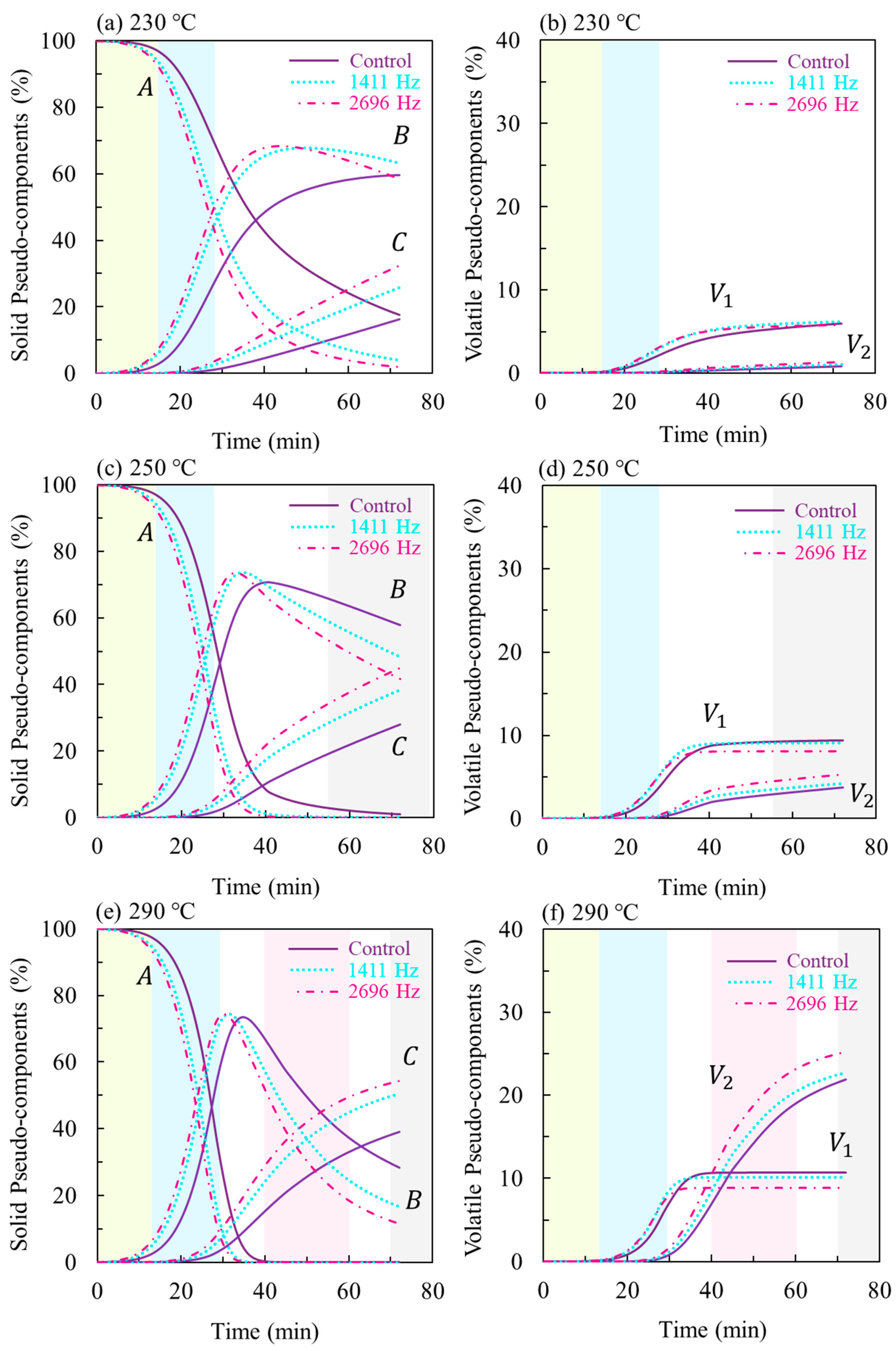
3.3. Thermoacoustic Kinetic Modeling
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- EPE Balanço energético nacional: Ano base 2018. EPE—Empres. Pesqui. Energ. 2019, 67, 23.
- Chen, W.-H.; Peng, J.; Bi, X.T. A state-of-the-art review of biomass torrefaction, densification and applications. Renew. Sustain. Energy Rev. 2015, 44, 847–866. [Google Scholar] [CrossRef]
- Mamvura, T.A.; Danha, G. Biomass torrefaction as an emerging technology to aid in energy production. Heliyon 2020, 6, e03531. [Google Scholar] [CrossRef] [PubMed]
- Cahyanti, M.N.; Doddapaneni, T.R.K.C.; Kikas, T. Biomass torrefaction: An overview on process parameters, economic and environmental aspects and recent advancements. Bioresour. Technol. 2020, 301, 122737. [Google Scholar] [CrossRef] [PubMed]
- Silveira, E.A.; De Morais, M.V.G.; Rousset, P.; Caldeira-Pires, A.; Pétrissans, A.; Galvão, L.G.O. Coupling of an acoustic emissions system to a laboratory torrefaction reactor. J. Anal. Appl. Pyrolysis 2017, 129, 29–36. [Google Scholar] [CrossRef]
- Turner, I.; Rousset, P.; Rémond, R.; Perré, P. An experimental and theoretical investigation of the thermal treatment of wood (Fagus sylvatica L.) in the range 200–260 °C. Int. J. Heat Mass Transf. 2010, 53, 715–725. [Google Scholar] [CrossRef]
- Santos, E.A.; Martins, C.A.; Nascimento, C.L. A new approach to treating pressure oscillations in combustion instability phenomena. Appl. Acoust. 2016, 114, 27–35. [Google Scholar] [CrossRef]
- Cintra, B.F.C.; Fernandes, E.C. Thermoacoustic instabilities of lean disc flames. Fuel 2016, 184, 973–986. [Google Scholar] [CrossRef]
- Matveev, K.I.; Culick, F.E.C. A study of the transition to instability in a Rijke tube with axial temperature gradient. J. Sound Vib. 2003, 264, 689–706. [Google Scholar] [CrossRef]
- Garrett, S.L. TA-1: Thermoacoustic engines and refrigerators. Am. J. Phys. 2004, 72, 11–17. [Google Scholar] [CrossRef]
- Guédra, M.; Bannwart, F.; Penelet, G.; Lotton, P. Parameter estimation for the characterization of thermoacoustic stacks and regenerators. Appl. Therm. Eng. 2015, 80, 229–237. [Google Scholar] [CrossRef]
- Komarov, S.; Hirasawa, M. Enhancement of gas phase heat transfer by acoustic field application. Ultrasonics 2003, 41, 289–293. [Google Scholar] [CrossRef]
- Bennett, G.J.; Mahon, J.; Murray, D.; Persoons, T.; Davis, I. Heat Transfer Enhancement in Ducts Due to Acoustic Excitation. In Proceedings of the 7th World Conference on Experimental Heat Transfer, Fluid Mechanics and Thermodynamics (ExHFT-7), Krakow, Poland, 28 June 2009. [Google Scholar]
- Lin, B.J.; Colin, B.; Chen, W.H.; Pétrissans, A.; Rousset, P.; Pétrissans, M. Thermal degradation and compositional changes of wood treated in a semi-industrial scale reactor in vacuum. J. Anal. Appl. Pyrolysis 2018, 130, 249–255. [Google Scholar] [CrossRef]
- Chen, W.H.; Lin, B.J.; Colin, B.; Chang, J.S.; Pétrissans, A.; Bi, X.; Pétrissans, M. Hygroscopic transformation of woody biomass torrefaction for carbon storage. Appl. Energy 2018, 231, 768–776. [Google Scholar] [CrossRef]
- Carrier, M.; Hardie, A.G.; Uras, Ü.; Görgens, J.; Knoetze, J. Production of char from vacuum pyrolysis of South-African sugar cane bagasse and its characterization as activated carbon and biochar. J. Anal. Appl. Pyrolysis 2012, 96, 24–32. [Google Scholar] [CrossRef]
- Garcìa-Pérez, M.; Chaala, A.; Pakdel, H.; Kretschmer, D.; Roy, C. Vacuum pyrolysis of softwood and hardwood biomass. Comparison between product yields and bio-oil properties. J. Anal. Appl. Pyrolysis 2007, 78, 104–116. [Google Scholar] [CrossRef]
- Huang, Y.F.; Sung, H.T.; Chiueh, P.T.; Lo, S.L. Co-torrefaction of sewage sludge and leucaena by using microwave heating. Energy 2016, 116, 1–7. [Google Scholar] [CrossRef]
- Huang, Y.F.; Cheng, P.H.; Chiueh, P.T.; Lo, S.L. Leucaena biochar produced by microwave torrefaction: Fuel properties and energy efficiency. Appl. Energy 2017, 204, 1018–1025. [Google Scholar] [CrossRef]
- Huang, Y.-F.F.; Sung, H.-T.T.; Chiueh, P.-T.T.; Lo, S.-L.L. Microwave torrefaction of sewage sludge and leucaena. J. Taiwan Inst. Chem. Eng. 2017, 70, 236–243. [Google Scholar] [CrossRef]
- Mohd Fuad, M.A.H.; Hasan, M.F.; Ani, F.N. Microwave torrefaction for viable fuel production: A review on theory, affecting factors, potential and challenges. Fuel 2019, 253, 512–526. [Google Scholar] [CrossRef]
- Bach, Q.V.; Tran, K.Q.; Skreiberg, Ø. Comparative study on the thermal degradation of dry- and wet-torrefied woods. Appl. Energy 2017, 185, 1051–1058. [Google Scholar] [CrossRef]
- Bhutto, A.W.; Qureshi, K.; Harijan, K.; Abro, R.; Abbas, T.; Bazmi, A.A.; Karim, S.; Yu, G. Insight into progress in pre-treatment of lignocellulosic biomass. Energy 2017, 122, 724–745. [Google Scholar] [CrossRef]
- De Macedo, L.A.; Commandré, J.M.; Rousset, P.; Valette, J.; Pétrissans, M. Influence of potassium carbonate addition on the condensable species released during wood torrefaction. Fuel Process. Technol. 2018, 169, 248–257. [Google Scholar] [CrossRef]
- Khazraie Shoulaifar, T.; Demartini, N.; Karlström, O.; Hupa, M. Impact of organically bonded potassium on torrefaction: Part 1. Experimental. Fuel 2016, 165, 544–552. [Google Scholar] [CrossRef]
- Safar, M.; Lin, B.-J.J.; Chen, W.-H.H.; Langauer, D.; Chang, J.-S.S.; Raclavska, H.; Pétrissans, A.; Rousset, P.; Pétrissans, M. Catalytic effects of potassium on biomass pyrolysis, combustion and torrefaction. Appl. Energy 2019, 235, 346–355. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, M.; Zhang, M.; Zhang, K.; Wang, D.; Lei, C. A fundamental research on synchronized torrefaction and pelleting of biomass. Renew. Energy 2019, 142, 668–676. [Google Scholar] [CrossRef]
- Silveira, E.A.; Galvão, L.G.O.; Sá, I.A.; Silva, B.F.; Macedo, L.; Rousset, P.; Caldeira-Pires, A.; Luce, B.F.S.; Caldeira-Pires, A. Effect of torrefaction on thermal behavior and fuel properties of Eucalyptus grandis macro-particulates. J. Therm. Anal. Calorim. 2019, 138, 3645–3652. [Google Scholar] [CrossRef]
- Silveira, E.A.; Lin, B.J.; Colin, B.; Chaouch, M.; Pétrissans, A.; Rousset, P.; Chen, W.H.; Pétrissans, M. Heat treatment kinetics using three-stage approach for sustainable wood material production. Ind. Crops Prod. 2018, 124, 563–571. [Google Scholar] [CrossRef]
- Di Blasi, C.; Lanzetta, M. Intrinsic kinetics of isothermal xylan degradation in inert atmosphere. J. Anal. Appl. Pyrolysis 1997, 40, 287–303. [Google Scholar] [CrossRef]
- Lin, B.J.; Silveira, E.A.; Colin, B.; Chen, W.H.; Pétrissans, A.; Rousset, P.; Pétrissans, M. Prediction of higher heating values (HHVs) and energy yield during torrefaction via kinetics. In Proceedings of the 10th International Conference on Applied Energy (ICAE2018), Hong Kong, China, 23 August 2019; Volume 158, pp. 111–116. [Google Scholar]
- Lin, B.-J.; Silveira, E.A.; Colin, B.; Chen, W.-H.; Lin, Y.-Y.; Leconte, F.; Pétrissans, A.; Rousset, P.; Pétrissans, M. Modeling and prediction of devolatilization and elemental composition of wood during mild pyrolysis in a pilot-scale reactor. Ind. Crops Prod. 2019, 131, 357–370. [Google Scholar] [CrossRef]
- Bates, R.B.; Ghoniem, A.F. Biomass torrefaction: Modeling of volatile and solid product evolution kinetics. Bioresour. Technol. 2012, 124, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Candelier, K.; Thevenon, M.F.; Petrissans, A.; Dumarcay, S.; Gerardin, P.; Petrissans, M. Control of wood thermal treatment and its effects on decay resistance: A review. Ann. For. Sci. 2016, 73, 571–583. [Google Scholar] [CrossRef]
- Rousset, P.; Macedo, L.; Commandré, J.M.; Moreira, A. Biomass torrefaction under different oxygen concentrations and its effect on the composition of the solid by-product. J. Anal. Appl. Pyrolysis 2012, 96, 86–91. [Google Scholar] [CrossRef]
- Rodrigues, T.O.; Rousset, P.L.A. Effects of torrefction on energy properties of Eucalyptus grandis wood. Cerne 2009, 15, 446–452. [Google Scholar] [CrossRef]
- Uemura, Y.; Omar, W.; Othman, N.A.; Yusup, S.; Tsutsui, T. Torrefaction of oil palm EFB in the presence of oxygen. Fuel 2013, 103, 156–160. [Google Scholar] [CrossRef]
- Chen, W.H.; Lu, K.M.; Liu, S.H.; Tsai, C.M.; Lee, W.J.; Lin, T.C. Biomass torrefaction characteristics in inert and oxidative atmospheres at various superficial velocities. Bioresour. Technol. 2013, 146, 152–160. [Google Scholar] [CrossRef]
- Chen, W.H.; Huang, M.Y.; Chang, J.S.; Chen, C.Y. Thermal decomposition dynamics and severity of microalgae residues in torrefaction. Bioresour. Technol. 2014, 169, 258–264. [Google Scholar] [CrossRef]
- Acharya, B.; Sule, I.; Dutta, A. A review on advances of torrefaction technologies for biomass processing. Biomass Convers. Biorefin. 2012, 2, 349–369. [Google Scholar] [CrossRef]
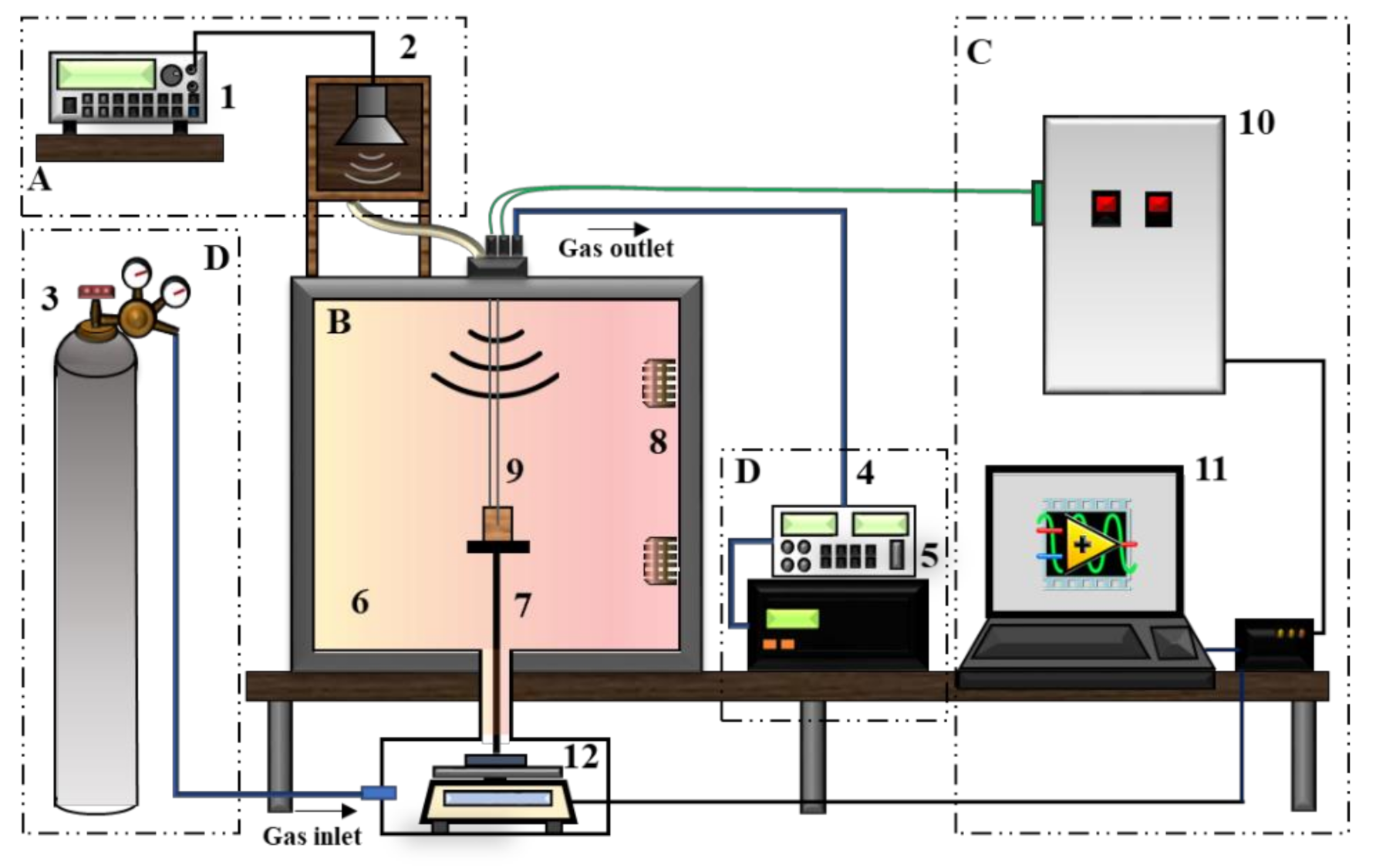
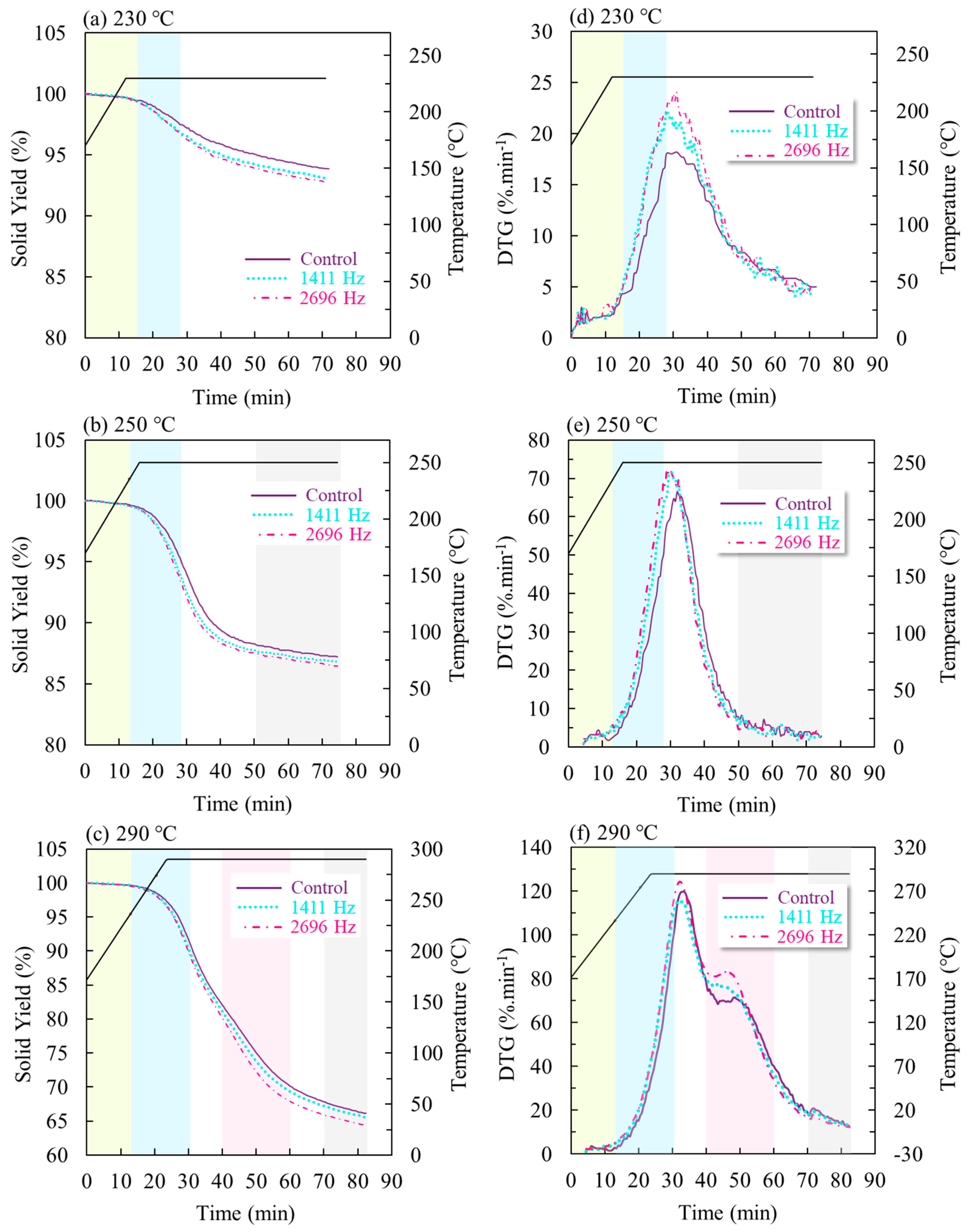
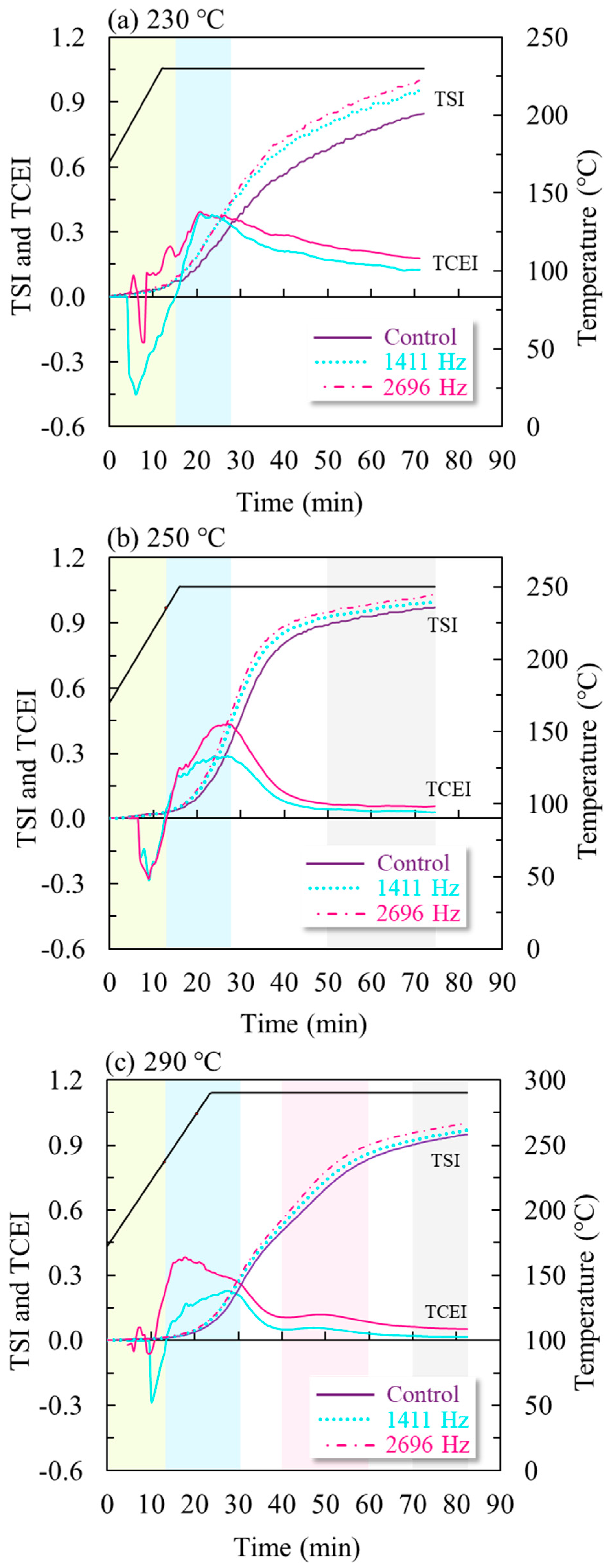
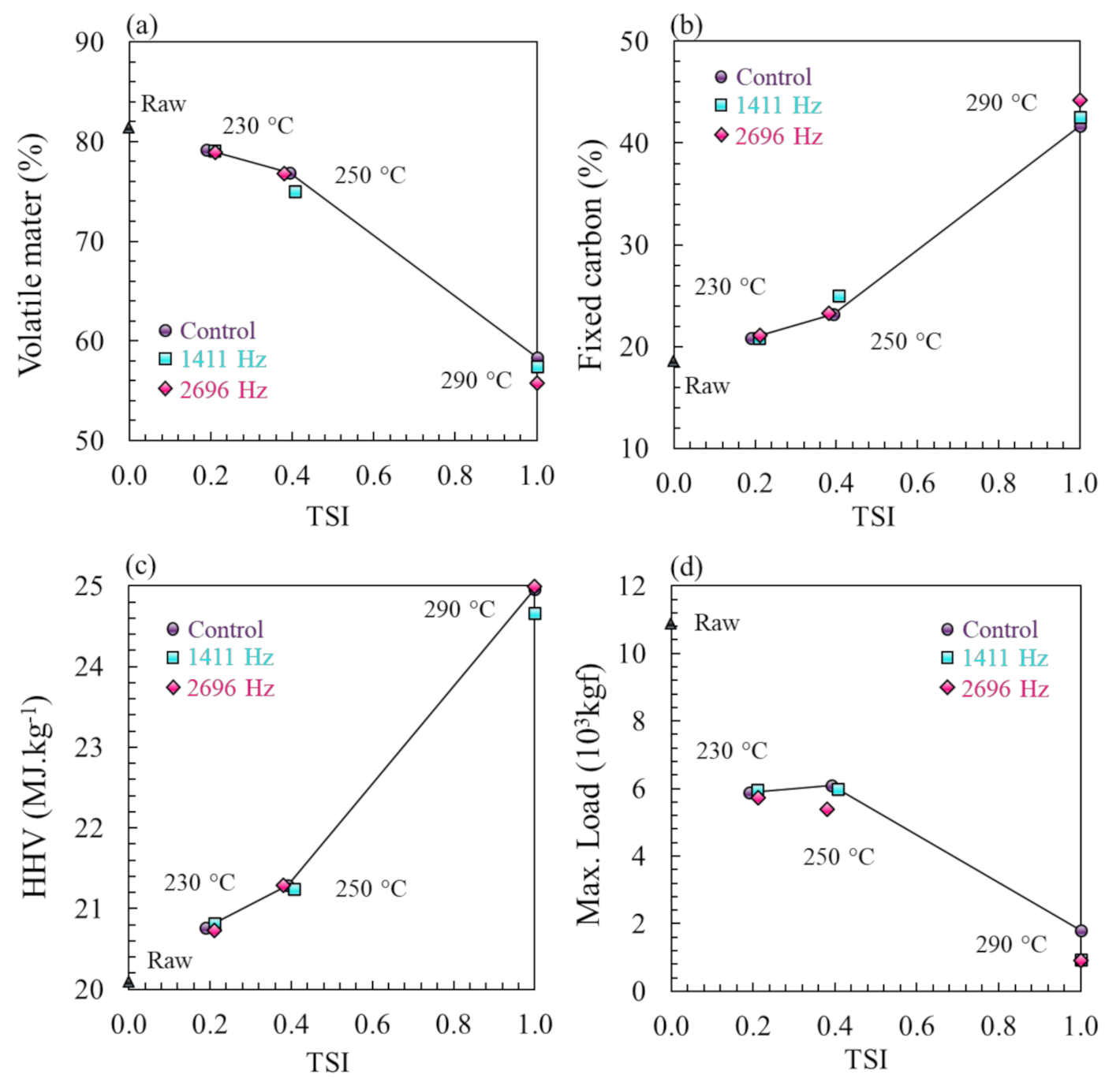
| Raw Material | Eucalyptus grandis |
|---|---|
| Proximate analysis a | |
| Fixed carbon (%) | 18.51 |
| Volatile matter (%) | 81.4 |
| Ash (%) | 0.09 |
| HHV (MJ kg−1) | 20.09 |
| Max. load (kgf) | 10,874.16 |
| Thermoacoustic Torrefaction Conditions | |
|---|---|
| Raw Material | Eucalyptus grandis |
| Duration | 60 min |
| Heating rate | 5 °C min−1 |
| Atmosphere | 10% O2 |
| Final temperature/acoustic frequency | |
| 230 °C a/- | |
| 230 °C/1411 Hz | |
| 230 °C/2696 Hz | |
| 250 °C a/- | |
| 250 °C/1411 Hz | |
| 250 °C/2696 Hz | |
| 290 °C a/- | |
| 290 °C/1411 Hz | |
| 290 °C/2696 Hz | |
| Reaction | |||||
|---|---|---|---|---|---|
| Control | (kJ·kg−1) | 106,757 | 139,152 | 35,434 | 129,916 |
| (min−1) | 2.85 × 109 | 4.55 × 1011 | 32.61 | 8.22 × 109 | |
| 230 °C | 2.35 × 10−2 | 1.63 × 10−3 | 6.84 × 10−3 | 2.68 × 10−4 | |
| 250 °C | 6.24 × 10−2 | 5.81 × 10−3 | 9.45 × 10−3 | 8.78 × 10−4 | |
| 290 °C | 3.57 × 10−1 | 5.64 × 10−2 | 1.69 × 10−2 | 7.33 × 10−3 | |
| 1411 Hz | (kJ·kg−1) | 102,176 | 152,936 | 42,891 | 142,169 |
| (min−1) | 1.66 × 109 | 1.87 × 1013 | 229.56 | 1.37 × 1011 | |
| 230 °C | 4.09 × 10−2 | 2.48 × 10−3 | 8.10 × 10−3 | 2.39 × 10−4 | |
| 250 °C | 1.04 × 10−1 | 1.00 × 10−2 | 1.20 × 10−2 | 8.77 × 10−4 | |
| 290 °C | 5.52 × 10−1 | 1.22 × 10−1 | 2.41 × 10−2 | 8.94 × 10−3 | |
| 2696 Hz | (kJ·kg−1) | 101,122 | 152,864 | 42,558 | 141,767 |
| (min−1) | 1.58 × 109 | 2.14 × 1013 | 238.45 | 1.53 × 1011 | |
| 230 °C | 5.03 × 10−2 | 2.89 × 10−3 | 9.11 × 10−3 | 2.94 × 10−4 | |
| 250 °C | 1.27 × 10−1 | 1.17 × 10−2 | 1.34 × 10−2 | 1.07 × 10−3 | |
| 290 °C | 6.60 × 10−1 | 1.42 × 10−1 | 2.69 × 10−2 | 1.09 × 10−2 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
A. Silveira, E.; Oliveira Galvão, L.G.; Alves de Macedo, L.; A. Sá, I.; S. Chaves, B.; Girão de Morais, M.V.; Rousset, P.; Caldeira-Pires, A. Thermo-Acoustic Catalytic Effect on Oxidizing Woody Torrefaction. Processes 2020, 8, 1361. https://doi.org/10.3390/pr8111361
A. Silveira E, Oliveira Galvão LG, Alves de Macedo L, A. Sá I, S. Chaves B, Girão de Morais MV, Rousset P, Caldeira-Pires A. Thermo-Acoustic Catalytic Effect on Oxidizing Woody Torrefaction. Processes. 2020; 8(11):1361. https://doi.org/10.3390/pr8111361
Chicago/Turabian StyleA. Silveira, Edgar, Luiz Gustavo Oliveira Galvão, Lucélia Alves de Macedo, Isabella A. Sá, Bruno S. Chaves, Marcus Vinícius Girão de Morais, Patrick Rousset, and Armando Caldeira-Pires. 2020. "Thermo-Acoustic Catalytic Effect on Oxidizing Woody Torrefaction" Processes 8, no. 11: 1361. https://doi.org/10.3390/pr8111361
APA StyleA. Silveira, E., Oliveira Galvão, L. G., Alves de Macedo, L., A. Sá, I., S. Chaves, B., Girão de Morais, M. V., Rousset, P., & Caldeira-Pires, A. (2020). Thermo-Acoustic Catalytic Effect on Oxidizing Woody Torrefaction. Processes, 8(11), 1361. https://doi.org/10.3390/pr8111361








