Magnetite and Hematite in Advanced Oxidation Processes Application for Cosmetic Wastewater Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Wastewater
2.2. Treatment Process
2.3. Analytical Methods
2.4. Treatment Processes Kinetics Calculation
3. Results
3.1. Raw Wastewater
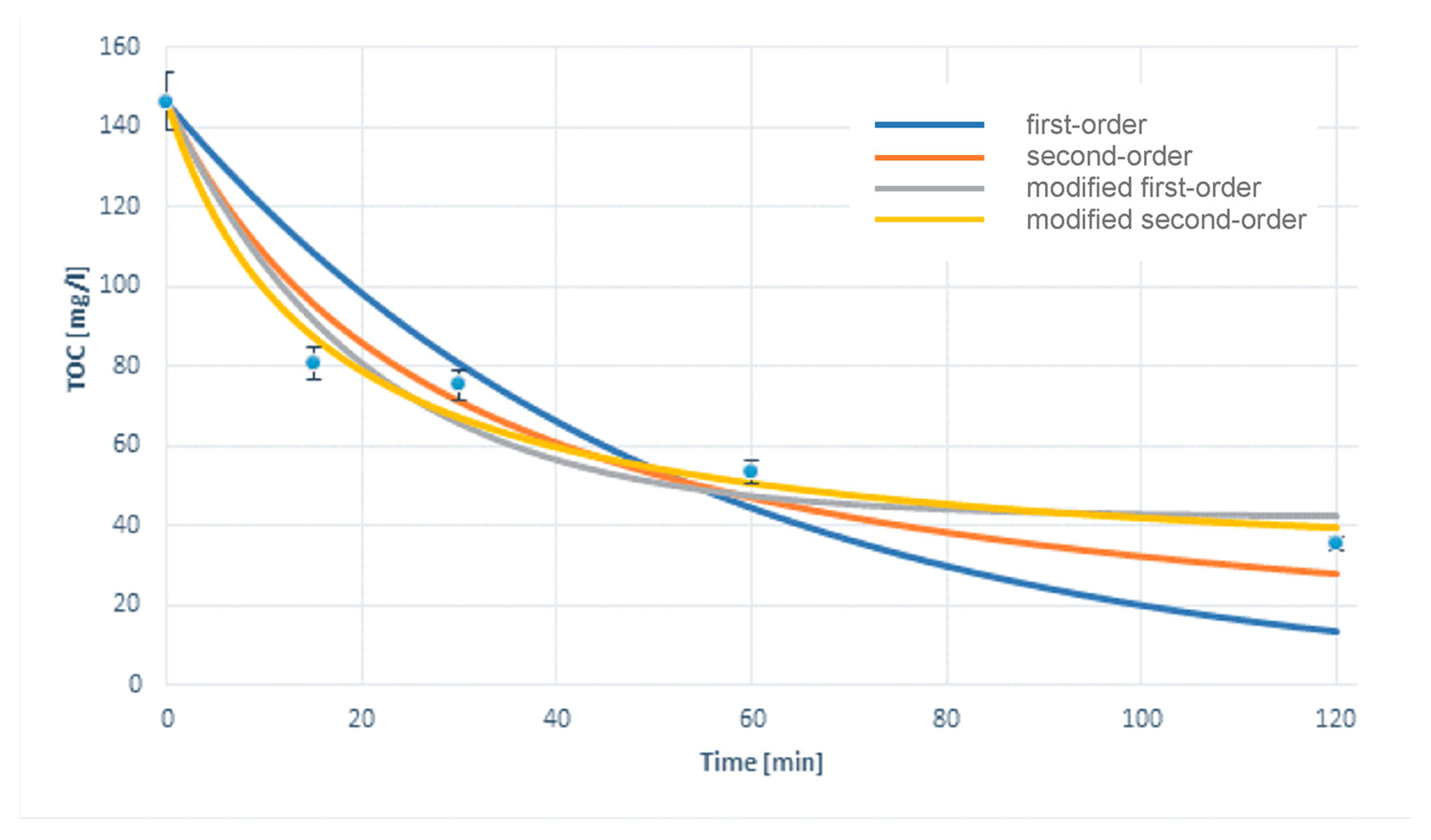
3.2. Results Matching Model
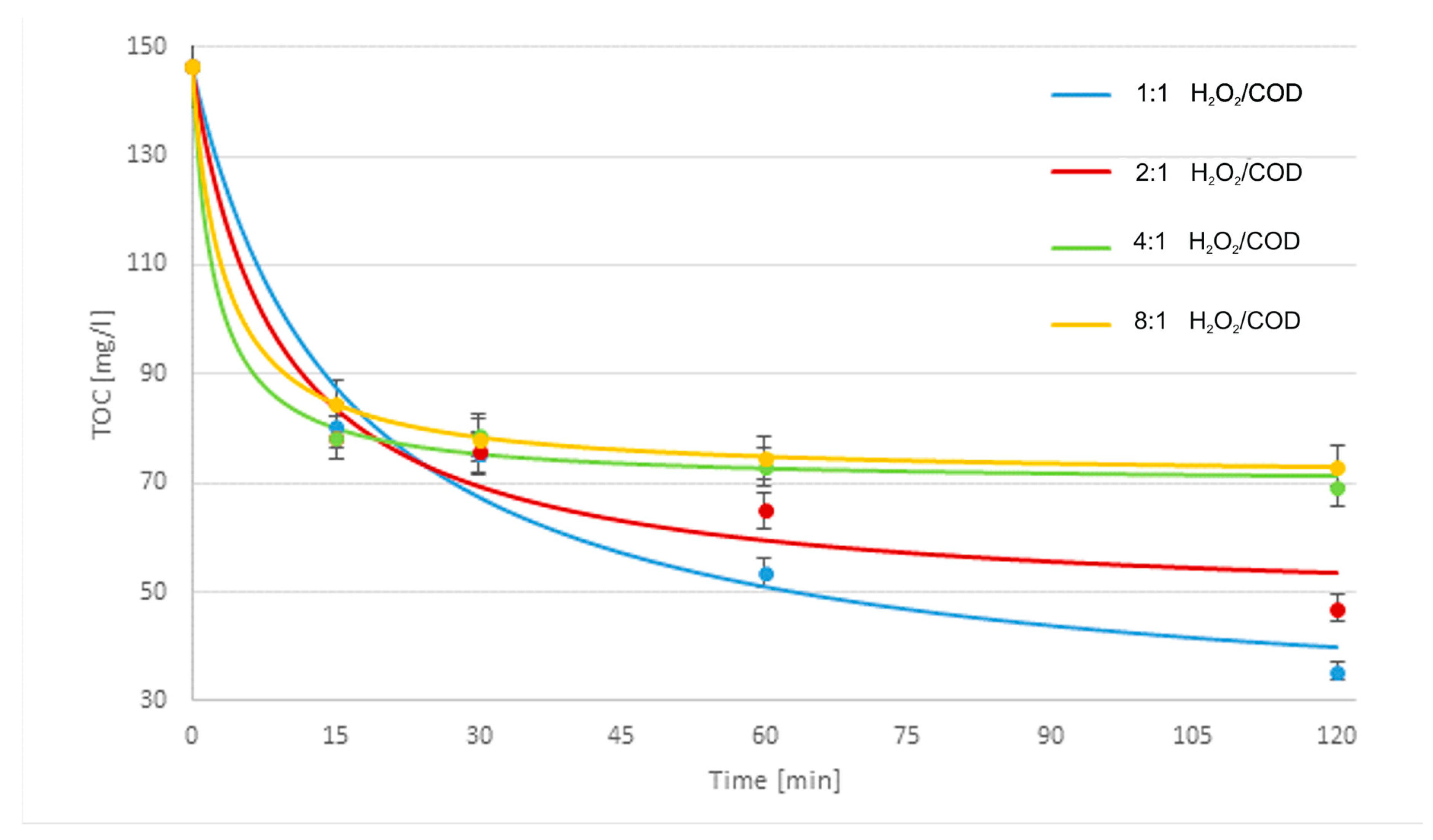
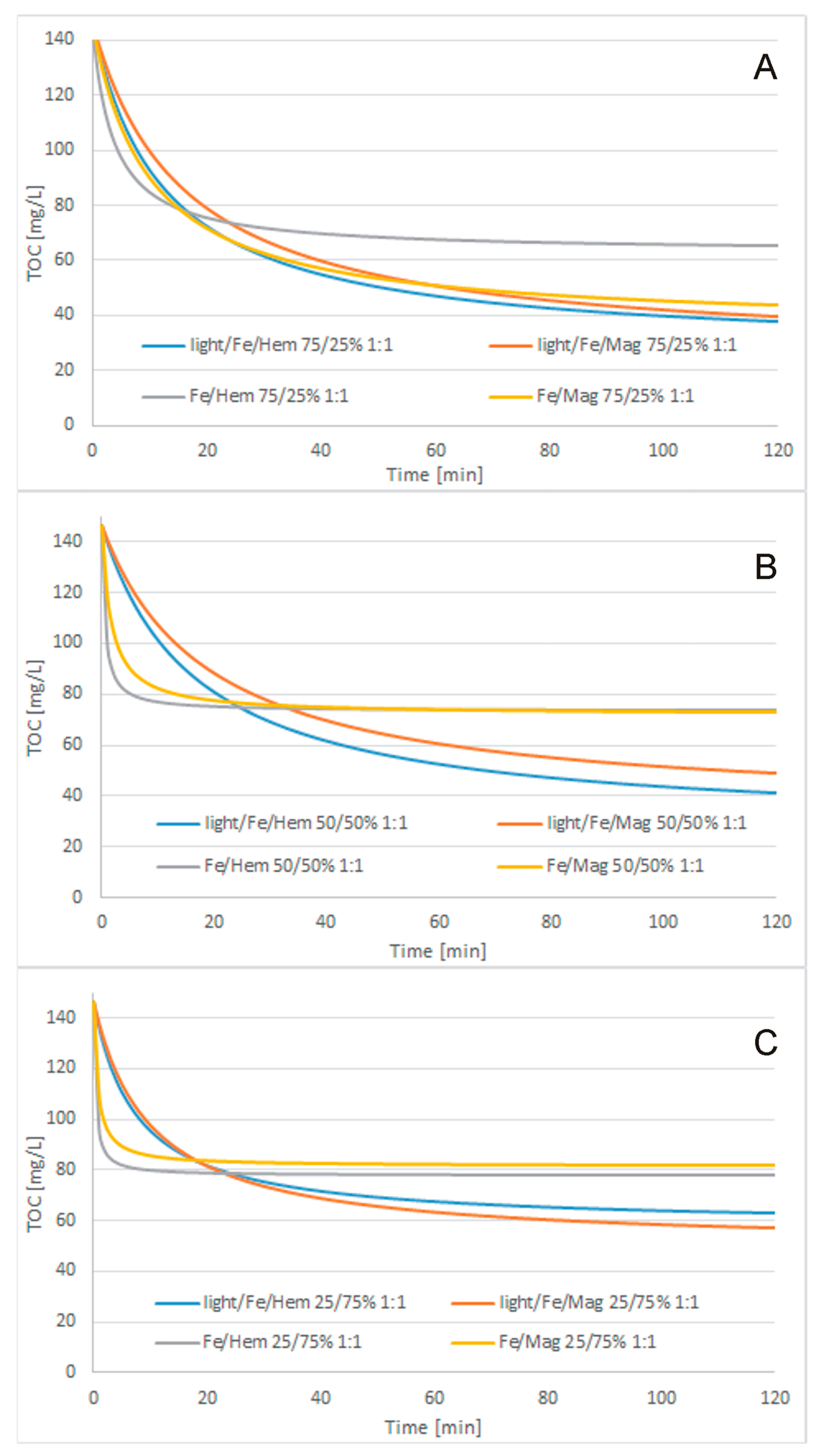
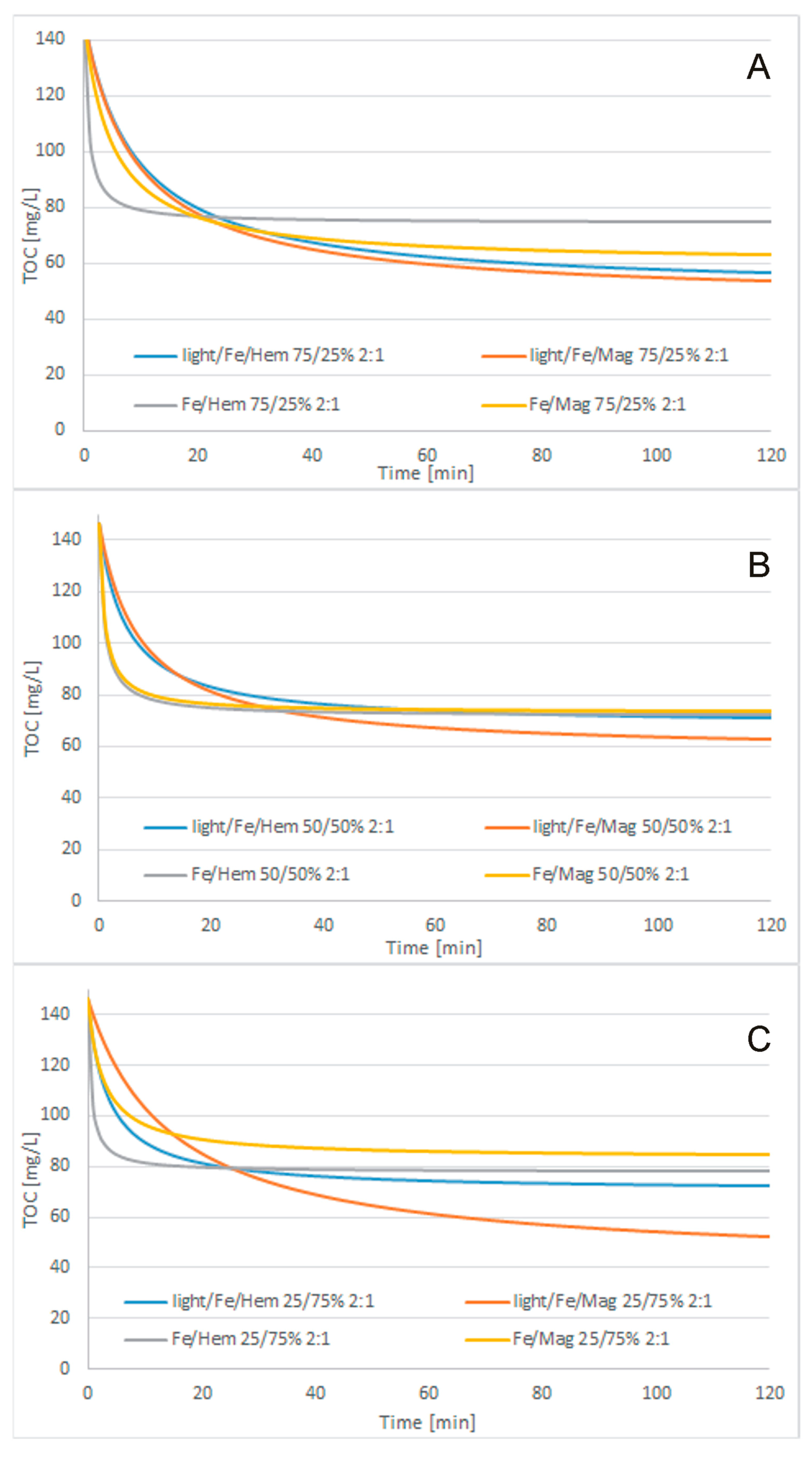
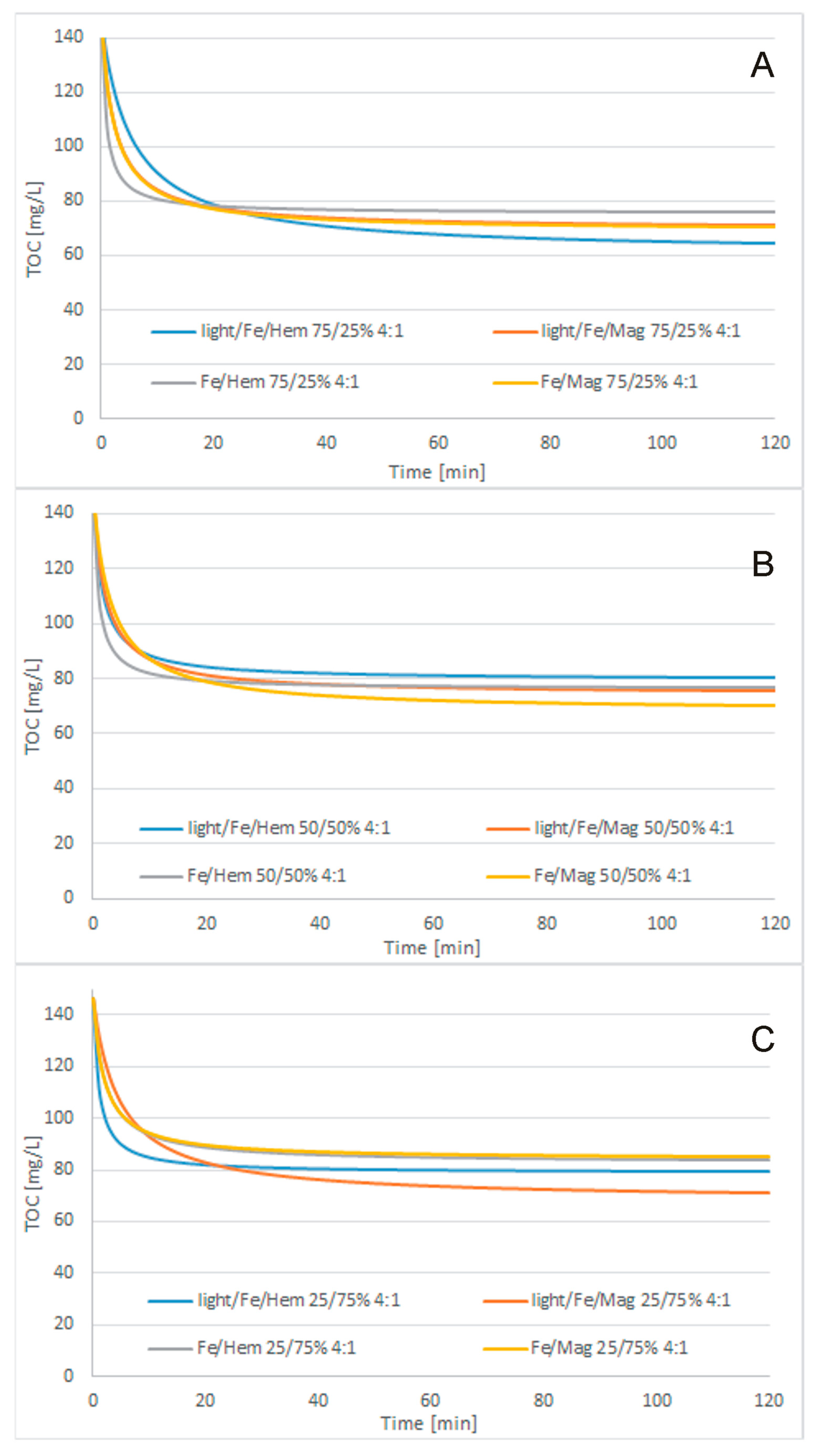
3.3. Treatment Processes
3.4. HS-SPME-GC-MS Analysis
4. Discussion
5. Conclusions
- The wastewater treatment process should be carried out in three stages. The effectiveness of the first stage—coagulation with aluminum-based coagulants—combined with flotation in dissolved air is indicated by relatively low values of the parameters tested (TSS, COD, TOC). With the removal of colloids and suspensions, high TOC/COD remains after the initial process. The second process, i.e. highly effective catalytic oxidation, in which metallic iron, hematite, magnetite, and hydrogen peroxide were used, according to the type of study, allows the removal of potentially toxic organic compounds from wastewater. After eliminating substances that could negatively affect biocenosis and the growth of microorganisms in activated sludge, wastewater can be treated by biological methods in a municipal wastewater treatment plant.
- Cosmetic wastewater can be effectively treated with the use of Fe2O3/Fe0/H2O2, Fe3O4/Fe0/H2O2, light/Fe2O3/Fe0/H2O2, or light/Fe3O4/Fe0/H2O2 processes. Light-supported processes were more effective than lightless processes. The highest efficiency of the catalytic oxidation process was achieved when using a catalyst whose role was played by UV light. If an additional catalyst in the form of UV light is used, the process takes place in the initial stage of purification. Although purification without UV light continues until the end of purification, it is less efficient and effective than the process in which an additional catalyst was used. In most samples, magnetite turned out to be a better catalyst in combination with metallic iron than hematite, but the differences in COD concentration in the purified compounds were not significant. The fastest TOC removal was observed during the first 15 min of the process. The best treatment efficiency was obtained for the light/Fe3O4/Fe0/H2O2 process with 250/750 mg/L Fe3O4/Fe0 reagent doses, 1:1 H2O2/COD mass ratio, and 120 min process time. Under these conditions, 75.7% TOC removal to a final TOC of 35.52 mg/L and 90.5% total nitrogen removal to a final content of 4.9 mg/L were obtained. The BOD5/COD ratio was increased slightly from 0.124 to 0.161.
- The process of catalytic oxidation is an effective method for the treatment of cosmetic wastewater. Numerous studies confirm its effectiveness and encourage scientific teams to expand comprehensive solutions and introduce new innovations to achieve the highest efficiency. Thanks to a properly carried out treatment process, the wastewater flowing into the sewage system will not significantly affect the biological process by significantly interfering with biocenosis and the life processes of microorganisms in the activated sludge.
- Application of HS-SPME-GC-MS analysis allows for the detection and identification of 23 compounds contained in the raw wastewater. The identified compounds were eliminated during the applied process, regardless of process type and reagent doses. No new compounds were detected after the process. The HS-SPME-GC-MS results confirmed the high efficiency of the treatment processes.
- The best fit was obtained for the modified second-order reaction with respect to the TOC value.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ma, G.; Chen, J. Nitrogen and Phosphorus Pollutants in Cosmetics Wastewater and Its Treatment Process of a Certain Brand. IOP Conf. Ser. Earth Environ. Sci. 2018, 113, 012051. [Google Scholar] [CrossRef]
- Bello, L.A.; Omoboye, A.J.; Abiola, T.O.; Oyetade, J.A.; Udorah, D.O.; Ayeola, E.R. Treatment Technologies for Wastewater from Cosmetic Industry—A Review. Int. J. Chem. Biol. Sci. 2018, 4, 69–80. [Google Scholar]
- De Melo, E.D.; Mounteer, A.H.; de Souza Leão, L.H.; Bahia, R.C.B.; Campos, I.M.F. Toxicity identification evaluation of cosmetics industry wastewater. J. Hazard. Mater. 2013, 244–245, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Biel-Maeso, M.; Corada-Fernández, C.; Lara-Martín, P.A. Removal of personal care products (PCPs) in wastewater and sludge treatment and their occurrence in receiving soils. Water Res. 2019, 150, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Juliano, C.; Magrini, G.A. Cosmetic Ingredients as Emerging Pollutants of Environmental and Health Concern. A Mini-Review. Cosmetics 2017, 4, 11. [Google Scholar] [CrossRef]
- Amneklev, J.; Augustsson, A.; Sorme, L.; Bergback, B. Bismuth and Silver in Cosmetic Products A Source of Environmental and Resource Concern? J. Ind. Ecol. 2015, 20, 99–106. [Google Scholar] [CrossRef]
- Guerranti, C.; Martellini, T.; Perra, G.; Scopetani, C.; Cincinelli, A. Microplastics in cosmetics: Environmental issues and needs for global bans. Environ. Toxicol. Pharmacol. 2019, 68, 75–79. [Google Scholar] [CrossRef]
- Kalcíkova, G.; Alic, B.; Skalar, T.; Bundschuh, M.; Zgajnar Gotvajn, A. Wastewater treatment plant effluents as source of cosmetic polyethylene microbeads to freshwater. Chemosphere 2017, 188, 25–31. [Google Scholar] [CrossRef]
- El-Gohary, F.; Tawfik, A.; Mahmoud, U. Comparative study between chemical coagulation/precipitation (C/P) versus coagulation/dissolved air flotation (C/DAF) for pre-treatment of personal care products (PCPs) wastewater. Desalination 2010, 252, 106–112. [Google Scholar] [CrossRef]
- Naumczyk, J.; Marcinowski, P.; Bogacki, J. Highly polluted cosmetic wastewater treatment. Environ. Prot. Eng. 2017, 44, 25–40. [Google Scholar] [CrossRef]
- Michel, M.M.; Tytkowska, M.; Reczek, L.; Trach, Y.; Siwiec, T. Technological Conditions for the Coagulation of Wastewater from Cosmetic Industry. Ecol. Eng. 2019, 20, 78–85. [Google Scholar] [CrossRef]
- Michel, M.M.; Siwiec, T.; Tytkowska, M.; Reczek, L. Analysis of flotation unit operation in coagulation of wastewater from a cosmetic factory. Przem. Chem. 2015, 11, 2000–2005. (In Polish) [Google Scholar]
- Aloui, F.; Kchaou, S.; Sayadi, S. Physicochemical treatments of anionic surfactants wastewater: Effect on aerobic biodegradability. J. Hazard. Mater. 2009, 164, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Martins, R.C.; Nunes, M.; Gando-Ferreira, L.M.; Quinta-Ferreira, R.M. Nanofiltration and Fenton’s process over iron shavings for surfactants removal. Environ. Technol. 2014, 35, 2380–2388. [Google Scholar] [CrossRef]
- Wiliński, P.; Marcinowski, P.; Naumczyk, J.; Bogacki, J. Pretreatment of cosmetic wastewater by dissolved ozone flotation (DOF). Desalin. Water Treat. 2017, 71, 95–106. [Google Scholar] [CrossRef]
- Perdigon-Melon, J.; Carbajo, J.; Petre, A.; Rosal, R.; García-Calvo, E. Coagulation-Fenton coupled treatment for ecotoxicity reduction in highly polluted industrial wastewater. J. Hazard. Mater. 2010, 181, 127–132. [Google Scholar] [CrossRef]
- Bautista, P.; Mohedano, A.F.; Gilarranz, M.A.; Casas, J.A.; Rodriguez, J.J. Application of Fenton oxidation to cosmetic wastewaters treatment. J. Hazard. Mater. 2007, 143, 128–134. [Google Scholar] [CrossRef]
- Bautista, P.; Casas, J.A.; Zazo, J.A.; Rodriguez, J.J.; Mohedano, A.F. Comparison of Fenton and Fenton-like oxidation for the treatment of cosmetic wastewater. Water Sci. Technol. 2014, 70, 472–478. [Google Scholar] [CrossRef]
- Bayhan, Y.K.; Değermenci, G.D. Kozmetik atık sularından fenton prosesiyle organik madde gideriminin ve kinetiğinin incelenmesi. J. Fac. Eng. Archit. Gazi Univ. 2017, 32, 181–188. (In Turkish) [Google Scholar] [CrossRef]
- Bogacki, J.; Marcinowski, P.; Zapałowska, E.; Maksymiec, J.; Naumczyk, J. Cosmetic wastewater treatment by Fe0/H2O2 process. Environ. Technol. 2017, 38, 2589–2600. [Google Scholar] [CrossRef]
- Martins de Andrade, P.; Dufrayer, C.R.; de Brito, N.N. Treatment of Real Cosmetic Effluent Resulting from the Manufacture of Hair Conditioners by Reduction Degradation, Adsorption and the Fenton Reaction. Ozone Sci. Eng. 2019, 41, 221–230. [Google Scholar] [CrossRef]
- Ebrahiem, E.E.; Al-Maghrabi, M.N.; Mobarki, A.R. Removal of organic pollutants from industrial wastewater by applying photo-Fenton oxidation technology. Arab. J. Chem. 2017, 10, S1674–S1679. [Google Scholar] [CrossRef]
- Boroski, M.; Rodrigues, A.C.; Garcia, J.C.; Sampaio, L.C.; Nozaki, J.; Hioka, N. Combined electrocoagulation and TiO2 photoassisted treatment applied to wastewater effluents from pharmaceutical and cosmetic industries. J. Hazard. Mater. 2009, 162, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Maksymiec, J.; Marcinowski, P.; Bogacki, J.; Zapałowska, E.; Dzienio, K. Wstępne wyniki zastosowania magnetytu w oczyszczaniu ścieków z przemysłu kosmetycznego. Gaz Woda Techn. San. 2017, 91, 336–339. (In Polish) [Google Scholar] [CrossRef]
- Friha, I.; Feki, F.; Karray, F.; Sayadi, S. A pilot study for cosmetic wastewater using a submerged flat sheet membrane bioreactor. Procedia Eng. 2012, 44, 819–820. [Google Scholar] [CrossRef]
- Monsalvo, V.M.; Lopez, J.; Mohedano, A.F.; Rodriguez, J.J. Treatment of cosmetic wastewater by a full-scale membrane bioreactor. Environ. Sci. Pollut. 2014, 21, 12662–12670. [Google Scholar] [CrossRef]
- Zhang, C.; Ning, K.; Guo, Y.; Chen, J.; Liang, C.; Zhang, X.; Wang, R.; Guo, L. Cosmetic wastewater treatment by a combined anaerobic/aerobic (ABR + UBAF) biological system. Desal. Water Treat. 2015, 53, 1606–1612. [Google Scholar] [CrossRef]
- Puyol, D.; Monsalvo, V.M.; Mohedano, A.F.; Sanz, J.L.; Rodriguez, J.J. Cosmetic wastewater treatment by upflow anaerobic sludge blanket reactor. J. Hazard. Mater. 2011, 185, 1059–1065. [Google Scholar] [CrossRef]
- Muszyński, A.; Marcinowski, P.; Maksymiec, J.; Beskowska, K.; Kalwarczyk, E.; Bogacki, J. Cosmetic wastewater treatment with combined light/Fe0/H2O2 process coupled with activated sludge. J. Hazard. Mater. 2019, 378, 120732. [Google Scholar] [CrossRef]
- Chávez, A.M.; Gimeno, O.; Rey, A.; Pliego, G.; Oropesa, A.L.; Álvarez, P.M.; Beltrán, F.J. Treatment of highly polluted industrial wastewater by means of sequential aerobic biological oxidation-ozone based AOPs. Chem. Eng. J. 2019, 361, 89–98. [Google Scholar] [CrossRef]
- Banerjee, P.; Dey, T.; Sarkar, S.; Swarnakar, S.; Mukhopadhyay, A.; Ghosh, S. Treatment of cosmetic effluent in different configurations of ceramic UF membrane based bioreactor: Toxicity evaluation of the untreated and treated wastewater using catfish (Heteropneustes fossilis). Chemosphere 2016, 146, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Demichelis, F.; Fiore, S.; Onofrio, M. Pretreatments aimed at increasing the biodegradability of cosmetic industrial waste. Process. Saf. Environ. Prot. 2018, 118, 245–253. [Google Scholar] [CrossRef]
- Minella, M.; Bertinetti, S.; Hanna, K.; Minero, C.; Vione, D. Degradation of ibuprofen and phenol with a Fenton-like process triggered by zero-valent iron (ZVI-Fenton). Environ. Res. 2019, 179, 108750. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, F.; Vione, D. Effect of pH on Zero Valent Iron Performance in Heterogeneous Fenton and Fenton-Like Processes: A Review. Molecules 2018, 23, 3127. [Google Scholar] [CrossRef] [PubMed]
- Jack, R.S.; Ayoko, G.A.; Adebajo, M.O.; Frost, R.L. A review of iron species for visible-light photocatalytic water purification. Environ. Sci. Pollut. Res. 2015, 22, 7439–7449. [Google Scholar] [CrossRef] [PubMed]
- Vorontsov, A.V. Advancing Fenton and photo-Fenton water treatment through the catalyst design. J. Hazard. Mater. 2019, 372, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Bokare, A.D.; Choi, W. Review of iron-free Fenton-like systems for activating H2O2 in advanced oxidation processes. J. Hazard. Mater. 2014, 275, 121–135. [Google Scholar] [CrossRef]
- He, J.; Yang, X.; Men, B.; Wang, D. Interfacial mechanisms of heterogeneous Fenton reactions catalyzed by iron-based materials: A review. Int. J. Environ. Sci. 2016, 39, 97–109. [Google Scholar] [CrossRef]
- Munoz, M.; de Pedro, Z.M.; Casas, J.A.; Rodriguez, J.J. Preparation of magnetite-based catalysts and their application in heterogeneous Fenton oxidation–A review. Appl. Catal. B-Environ. 2015, 176–177, 249–265. [Google Scholar] [CrossRef]
- Demarchis, L.; Minella, M.; Nisticò, R.; Maurino, V.; Minero, C.; Vione, D. Photo–Fenton reaction in the presence of morphologically controlled hematite as iron source. J. Photochem. Photobiol. A 2015, 307, 99–107. [Google Scholar] [CrossRef]
- Babuponnusami, A.; Muthukumar, K. A review on Fenton and improvements to the Fenton process for wastewater treatment. J. Environ. Chem. Eng. 2014, 2, 557–572. [Google Scholar] [CrossRef]
| Parameter | Unit | Value |
|---|---|---|
| TOC | mg/L | 146.4 |
| COD | mg/L | 819 |
| BOD5 | mg/L | 102 |
| DCMEO | mg/L | 7.2 |
| TSS | mg/L | 14 |
| Surfactants | mg/L | 17 |
| pH | - | 5.03 |
| Conductivity | mS/cm | 1.105 |
| Total N | mg/L | 51.45 |
| Total P | mg/L | 0.07 |
| Process, Reagent Proportions [%], H2O2/COD Ratio | COD [mg/L] | BOD5 [mg/L] |
|---|---|---|
| light/Fe0/Fe3O4, 75/25, 1:1 | 74.5 | 12 |
| light/Fe0/Fe2O3, 75/25, 1:1 | 76 | 17 |
| Fe0/Fe3O4, 75/25, 1:1 | 80 | 31 |
| Fe0/Fe2O3, 75/25, 1:1 | 125 | 64 |
| No. | Compound Name |
|---|---|
| 1 | 2,6-dimethyl-7-octen-2-ol |
| 2 | 2-propenoic acid, heptyl ester |
| 3 | 3,7-dimethyl-1,6-octadien-3-ol |
| 4 | decamethylcyclopentasiloxane |
| 5 | 1-(1-oxobutyl)-1,2-dihydropyridine |
| 6 | 4-trimethyl-3-cyclohexene-1-methanol |
| 7 | 1,7,7-trimethylbicyclo [2.2.1]hept-2-yl acetate |
| 8 | 2-(1,1-dimethylethyl)-cyclohexanol |
| 9 | dodecamethylcyclohexasiloxane |
| 10 | propanoate 3-hexen-1-ol |
| 11 | 1-methyl-4-(1-methylethylidene)cyclohexene |
| 12 | 3-propyl-2,4-pentadien-1-ol |
| 13 | 4-(2,6,6-trimethyl-2-cyclohexen-1-yl)-3-penten-2-one |
| 14 | 2,4-bis(1,1-dimethylethyl)-phenol |
| 15 | di-n-octyl ether (1,1’-oxybisoctane) |
| 16 | cyclopentaneacetic acid, 3-oxo-2-pentyl-,methyl ester |
| 17 | cyclotetradecane |
| 18 | 7a-isopropenyl-4,5-dimethy octahydro-1H-inden-4-yl)methanol |
| 19 | 2-(4a,8-dimethyl-6-oxo-1,2,3,4,4a,5,6,8a-octahydro-naphthalen-2-yl)-propionaldehyde |
| 20 | tricyclo[4.3.0.0(7,9)]nonane, 2,2,5,5,8,8-hexamethyl-, (1.alpha.,6.beta.,7.alpha.,9.alpha.)- |
| 21 | 7-acetyl-6-ethyl-1,2,3,4-tetrahydro-1,1,4,4-tetramethylnaphtalene |
| 22 | 1-hexadecanol |
| 23 | dibutyl phthalate |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marcinowski, P.; Bury, D.; Krupa, M.; Ścieżyńska, D.; Prabhu, P.; Bogacki, J. Magnetite and Hematite in Advanced Oxidation Processes Application for Cosmetic Wastewater Treatment. Processes 2020, 8, 1343. https://doi.org/10.3390/pr8111343
Marcinowski P, Bury D, Krupa M, Ścieżyńska D, Prabhu P, Bogacki J. Magnetite and Hematite in Advanced Oxidation Processes Application for Cosmetic Wastewater Treatment. Processes. 2020; 8(11):1343. https://doi.org/10.3390/pr8111343
Chicago/Turabian StyleMarcinowski, Piotr, Dominika Bury, Monika Krupa, Dominika Ścieżyńska, Prasanth Prabhu, and Jan Bogacki. 2020. "Magnetite and Hematite in Advanced Oxidation Processes Application for Cosmetic Wastewater Treatment" Processes 8, no. 11: 1343. https://doi.org/10.3390/pr8111343
APA StyleMarcinowski, P., Bury, D., Krupa, M., Ścieżyńska, D., Prabhu, P., & Bogacki, J. (2020). Magnetite and Hematite in Advanced Oxidation Processes Application for Cosmetic Wastewater Treatment. Processes, 8(11), 1343. https://doi.org/10.3390/pr8111343






