1. Introduction
According to revised Renewable Energy Directive published in 2018, the European Union (EU) set a goal to become a global leader in the field of renewable energy. To gain this target, the directive establishes a new and binding target for renewable energy for the EU for 2030 of at least 32%. In addition, the energy in the transport sector should increase to at least 14% by 2030 and the limit of 7% for conventional first generation biofuels in transport sector will be reduced to 3.5% by 2030 [
1]. As the goal is to shift the transport sector from fossil fuel use to the use of different fuel mixtures with a larger fraction of different biofuels, such as bioethanol and biodiesel. In addition, biofuel target in transport sector is planned to be covered with biomethane produced by anaerobic digestion of local biomass.
Although the production of liquid and sustainable biofuels from lignocellulosic biomass like ethanol, butanol etc. shows potential in laboratory conditions, the large-scale and cost-effective production of cellulosic biofuels is still hindered by several technical barriers. One of the critical issues is the high capital and processing costs of which the most expensive processing step is pretreatment [
2]. Therefore, there is an urgent need for research and development of new and more effective methods and processes for biomass pretreatment to overcome major hindrances in lignocellulosic bioethanol production.
Over the time, various methods for pretreatment of lignocellulosic biomass have been proposed and many papers studying various methods have been published [
2,
3,
4,
5]. According to working principles, the pretreatment methods can be divided into biological, chemical, and physical methods and, additionally, combined methods that apply characteristics of multiple methods [
5,
6,
7,
8,
9]. Amongst them physical or mechanical methods involve size reduction of biomass by means of grinding chipping, or milling [
10,
11]. The biomass chip size is usually reduced to 10–30 mm after chipping and 0.2–2 mm after grinding or milling [
4,
12]. The size reduction pretreatments are very often used in combination with other pretreatment methods as a first step in the whole pretreatment process to make the subsequent processes easier and more effective [
2,
13]. The physical pretreatment methods are environmentally friendly and usually do not produce any toxic substances. On the other hand, they have high energy consumption and the high costs associated with large-scale implementation, and safety hazards.
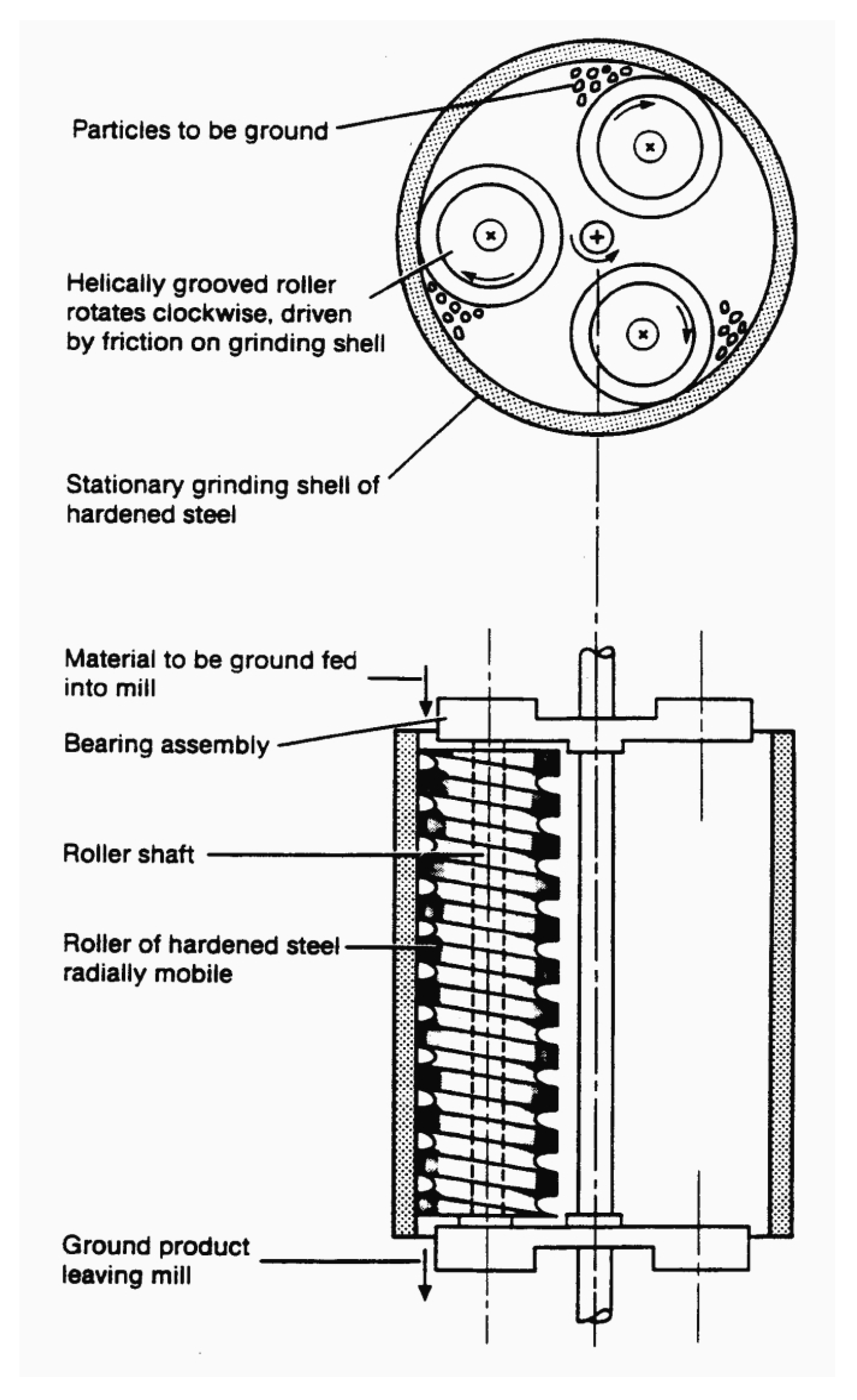
Different types of mills and milling processes like colloid milling, ball milling, hammer milling, vibro-energy milling, and two-roll milling have been proposed [
14,
15]. In this work The Szego Mill™ was used. The Szego Mill™ is a planetary ring-roller mill consisting of a stationary, cylindrical grinding surface inside which a number of helically grooved rollers rotate. The biomass is fed by gravity or pumped into the mill from top, and then discharged at the bottom of the mill. The biomass is crushed and sheared inside the mill between cylindrical surface and rotating rollers. The radial acceleration of the rollers create the crushing force while the high velocity gradients generated in the mill causes the shearing action. The biomass is transported through the mill by roller grooves and exits from the bottom of the mill [
16,
17].
Szego Mills™ have been used to process various woody materials like chips, sawdust, and wood wastes, such as pelletized wood waste and hog fue, as well as minerals and other materials [
18]. However, the effect of this mill has not been tested for biomass milling as a pretreatment method in the second generation lignocellulosic biofuel and biogas production process. In addition, the efficiency of nitrogen gas-assisted milling process by the Szego Mill™ has not been reported for milling any biomass nor material.
Previous research has shown that when using NED (nitrogen explosive decompression pretreatment), a higher efficiencies can be obtained in further biofuel production process when pressurized nitrogen gas was added during pretreatment. The biomass destruction is achieved on sudden pressure release where the gas volume inside the biomass is expanding and thereby breaking the structure of biomass [
19,
20].
The paper proposes that similar effect could be obtained using a Szego Mill™. By adding nitrogen gas to the milling cylinder, high pressure micro-areas arise in the cylinder during milling process. Due to rapid change in pressure, similar effects can occur as in NED pretreatment. During milling, the sudden pressure changes in between high and low pressure areas causes the moisture and gas inside the biomass to increase and decrease volume and this breaks the biomass structure. As a result, the biomass structure is opened for the next processing steps.
The aim of the paper was to investigate the mechanical size reduction of barely straw with a Szego Mill™ as a pretreatment method in biofuels production. The biomass was milled with a Szego Mill™ using three different techniques: dry milling, wet milling and nitrogen assisted wet milling. The milled biomass was used in bioethanol and biogas production processes using different process schemes to evaluate the effect of different pretreatment approaches.
2. Materials and Methods
2.1. Biomass
Barley straw (Hordeum vulgare) used in the experimental procedure was collected in September 2013 from the plant field collection of Estonian University of Life Sciences, near Tartu (Estonia). The samples were dried to gain a moisture content less than 100 g kg−1 and ground with a SM 100 Cutting Mill comfort (Retsch GmbH, Haan, Germany) to a particle-size 4 mm or less (referred to as “original material”). Particle size distribution of milled straw was analysed by the vibratory sieve shaker AS 200 (Retsch GmbH) passing the straw through a series of sieves (2.0, 1.0, 0.5, 0.25, 0.125, 0.05, 0.02 mm) and weighing the amount of material on each sieve.
2.2. Pretreatment with Szego Mill
The Szego Mill™ (SM-220, General Comminution Inc., Toronto, ON, Canada) was used for biomass pretreatment. The details of mill construction and working principles are outlined in published papers [
18] and in
Figure 1. Three modes of milling were used—dry milling, wet milling, and nitrogen assisted wet milling. In all modes, 50 Hz and 1100 RPM milling parameters were used and 3.6 kg of dry biomass was grounded. In case of dry mill, the biomass was fed through the mill twice. After each pass, the biomass was collected and an averaged sample was taken. In case of wet milling, the 3.6 kg of dry biomass was mixed with 18 kg of water (biomass-water ratio 1:5) and fed through the mill once. For the third mode, the biomass was moistened with water similarly to the previous method and additionally, nitrogen was fed through the mill and the collection system with biomass during milling. After milling, samples were used in the enzymatic hydrolysis or stored after drying for later analysis.
2.3. Route Design
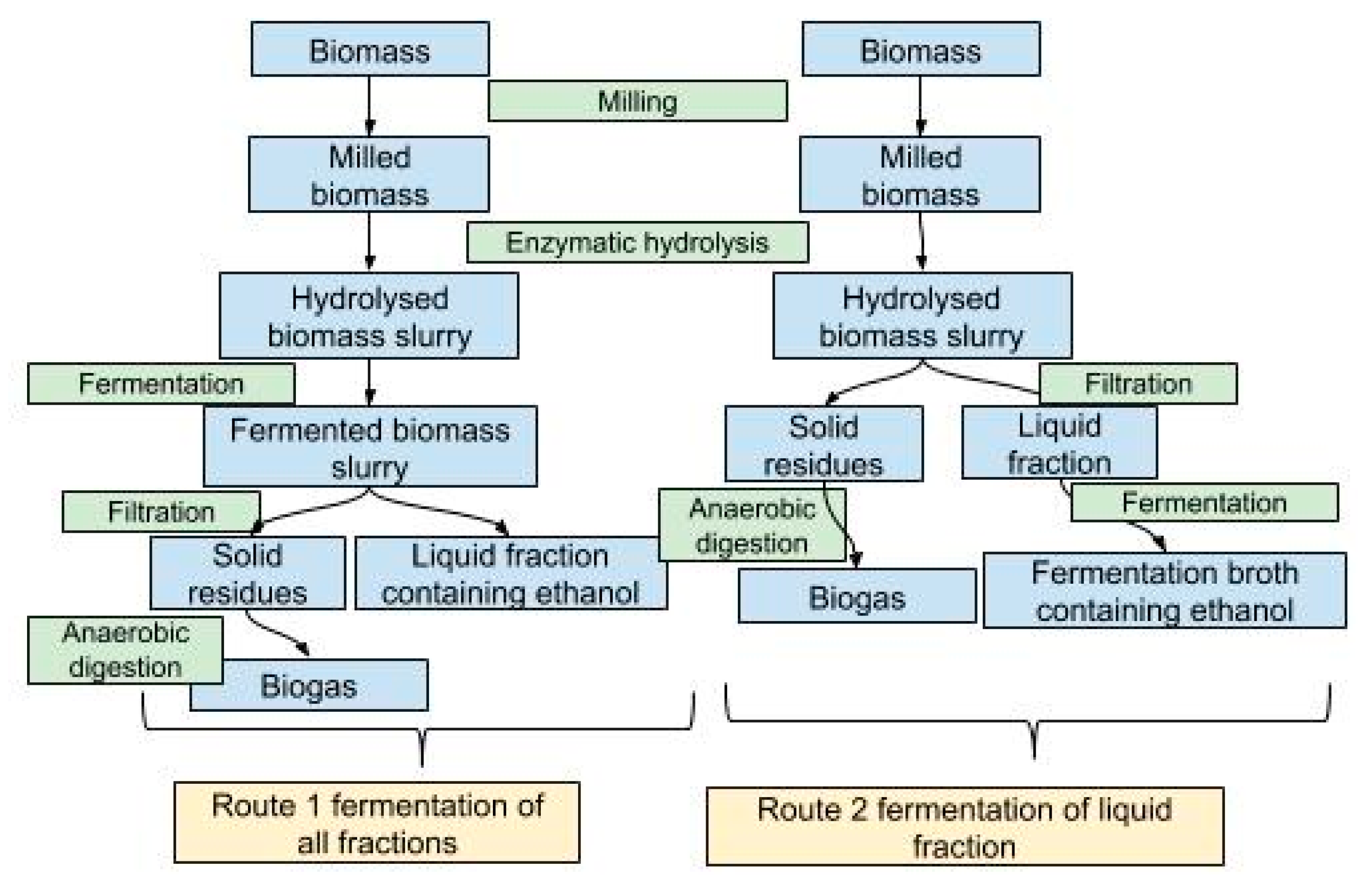
Bioethanol production from biomass was evaluated using two different routes (
Figure 2). In both routes the biomass was used in the traditional three step bioethanol production process. The biomass was pretreated using the Szego Mill™. Enzymatic hydrolysis was applied to convert the cellulose to sugars and finally yeast was used to ferment the sugars to ethanol. In route 2 the solid and liquid fractions were separated after enzymatic hydrolysis and only the liquid part was used in the ethanol production process. In route 1, the hydrolyzed biomass slurry was used in a fermentation process without additional processing and phase separation was applied after the fermentation.
2.4. Enzymatic Hydrolysis and Fermentation
Enzymatic hydrolysis was used to convert cellulose to glucose. Distilled water and enzyme were added to the 100 g of pretreated biomass in order to gain the final volume of the mixture 1000 mL. An enzyme mixture (Accellerase 1500 from DuPont
®, city, USA; 30 FPU g
−1 cellulose) was added to biomass suspension at a ratio of 0.3 mL per g of biomass [
21]. Hydrolysis lasted for 24 h at a temperature of 50 °C under constant stirring in a rotating shaker/incubator (250 min
−1) (IKA 4000 i Control IKA
®-Werke GmbH & Co. KG, Mindelheim, Germany).
After enzymatic hydrolysis, two approaches were used (
Figure 1). In the first option, the biomass slurry was used in fermentation process without separation and 2.5 g of dry yeast
Saccharomyces cerevisiae per 100 g of biomass was added to all samples to start the fermentation process. For second option, centrifugation (Heraeus Megafuge 40, Thermo, USA, 3400 rpm) was used to separate the liquid phase of the suspension from the solid phase and only liquid was used in fermentation. In this case, 1 g of yeast
Saccharomyces cerevisiae was added to 200 mL of liquid to start the fermentation process. In both options, the fermentation process was carried out at room temperature (22 ± 1 °C) for 7 days under low oxygen conditions in 250 or 1000 mL glass bottles sealed with a fermentation tube.
2.5. Biogas Analysis
The BMP (biochemical methane potential) measurements were performed according to the guideline of Angelidaki et al. [
22]. Barley straw, milled straw (dry, wet and N
2 assisted milling) as well as biomass samples from bioethanol production after hydrolysis and fermentation (
Figure 1) were analysed for BMP.
The inoculum was collected from the anaerobic reactor of the municipal wastewater treatment plant in Tartu (Estonia). Before use the inoculum was sieved through 2 mm mesh and preincubated at 36 °C to ensure depletion of degradable organic material.
The experiments were carried out in 575 mL plasma bottles filled with 200 g of inoculum and substrate mixture with the substrate to inoculum VS (volatile solids) ratio of 0.25. The test bottles were flushed with nitrogen to assure anaerobic conditions and incubated at 36 °C in a Mermet isothermal thermo-chamber. All tests were performed in triplicates with each sample. Additional nutrients were not added to the BMP test. The blanks with only inoculum were included to measure the background biogas and methane production from the inoculum, which were later subtracted from that of the tests with substrate.
Biogas production was determined by measuring pressure in the headspace of bottles with pressure meter BMP-Testsystem WAL (WAL Mess- und Regelsysteme GmbH, Oldenburg, Germany). The quantification of methane in the produced biogas was done by gas chromatography (CP-4900 Micro-GC, Varian Inc., Palo Alto, CA, USA) at conditions described by Luna-del Risco et al. [
23].
Biogas and biomethane potential, cumulative yield achieved in the BMP test, were calculated as liters of cumulative gas produced per kg of TS or VS for normal conditions (0 °C, 1 atm). To characterize the methane production rate during anaerobic digestion, the first-order rate constant k was calculated from Equation (1):
where Bt is the cumulative biomethane yield at time t, Bmax is the maximum biomethane yield, k is the first-order rate constant and t is time.
2.6. Biomass Analysis
The solid and liquid parts of the biomass mixture were weighed before and after pretreatment and enzymatic hydrolysis and corresponding samples were taken for analysis. The initial biomass, but also samples of solid biomass gained after pretreatment and enzymatic hydrolysis, were dried for later analysis.
Dry matter content was analyzed with a Kern MLS-D moisture analyzer (KERN & SOHN GmbH, Balingen, Germany) and ash content according to NREL Technical Report NREL/TP-510-42622 [
24].
The biomass was subjected to ADF (acid detergent fiber), NDF (neutral detergent fiber) ADL (acid detergent lignin) tests to determine the contents of cellulose, hemicellulose and lignin using ANKOM 2000 analyzer (ANKOM Technology, Macedon, NY, USA).
The glucose and ethanol concentrations were measured using a Prominence-i LC-2030 3D Plus HPLC system (Shimadzu, Japan) and a Shimadzu RID-20A detector. The analytical separation of glucose was accomplished with the RPM-Monosaccharide Pb+2 and RHM-Monosaccharide H+ columns which were arranged in sequence (RezexTM). The column oven temperature was 85 °C and the detector temperature 60 °C. Autoclaved and degassed distilled water was used as a mobile phase at a flow rate of 0.8 mL/min. For the sugar quantification, a calibration range from 50 mg/L to 2000 mg/L was chosen. The injection volume of the sugar samples was 20 µL.
A RezexTM ROA-Organic Acid H+ column was used for the detection of ethanol. The column oven and detector temperature were set to 50 °C. Autoclaved and degassed 5 mM H2SO4 was used as mobile phase at a flow rate of 0.5 mL/min. The calibration ranged from 200 mg/L to 20 g/L for the acetic acid and ethanol quantification. The sample injection volume was 10 µL. All the measurements were conducted in triplicate and average and standard deviations were calculated.
SEM at the University of Tartu at the Institute of Geology was used to take pictures of the biomass. SEM imaging of uncoated samples was carried out using an EVO MA15 SEM (ZEISS) in variable pressure mode. The images were captured in backscattered electronand variable pressure secondary electron modes.
3. Results
3.1. Effect of Milling
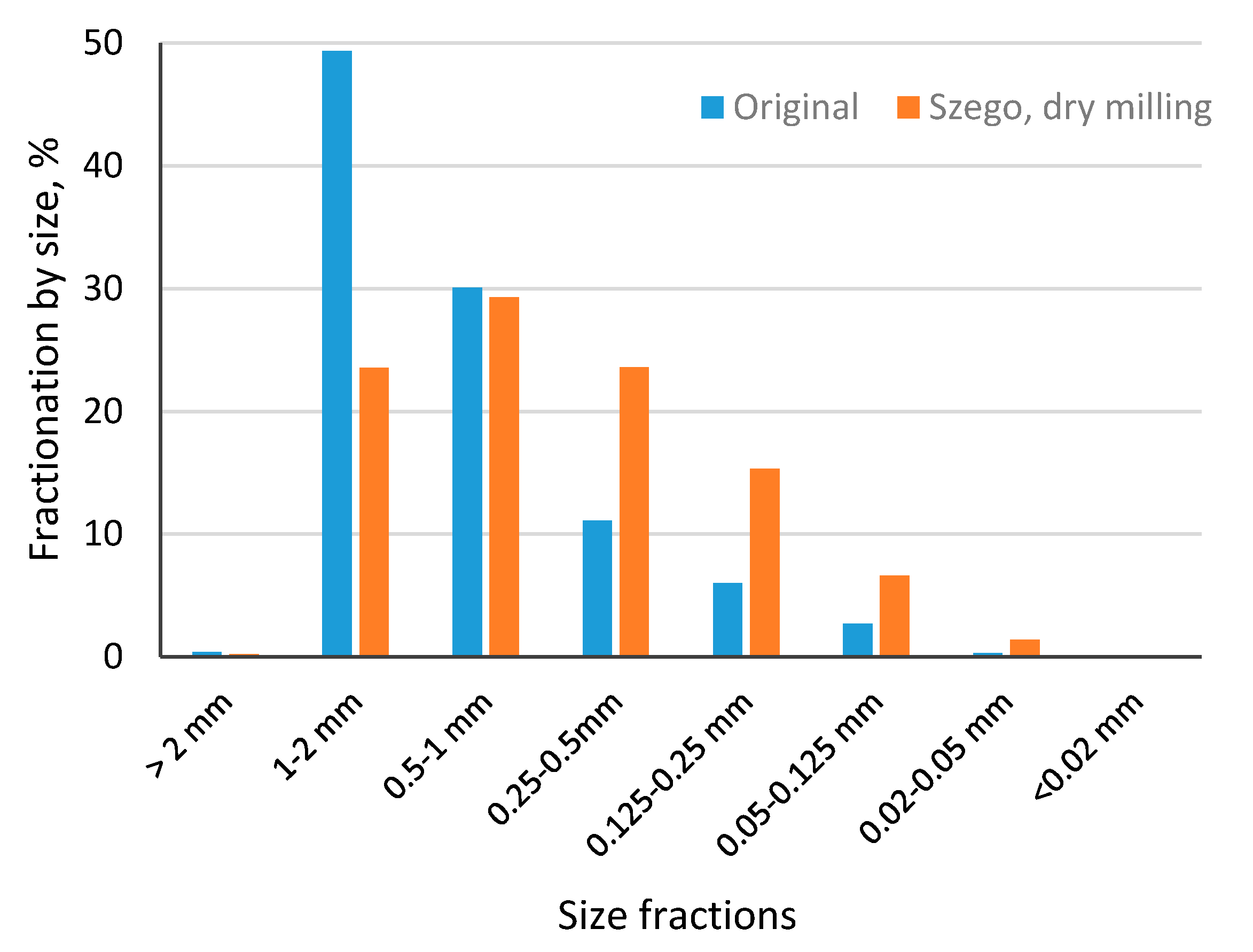
Size distribution of straw samples ground with a cutting mill and the Szego Mill
TM are given in
Figure 2. It can be seen that there is a much higher mass of smaller particles in the sample ground with the Szego Mill
TM. Grinding with the cutting mill provided most of the particles with size 1–2 mm whereas with the Szego Mill
TM size fractions 0.5–1 mm and 0.25–0.5 mm were dominant (
Figure 3). The Szego Mill
TM was more efficient for particle size reduction than the cutting mill, which can be seen also from
Figure 4 where SEM pictures of biomass milled in different ways have been shown. In addition to a decrease in average particle size, the particle structure has also changed due to milling with the Szego Mill
TM (
Figure 5). While particles from the cutting mill have smooth surfaces and straight edges, the surface structure of particles from the Szego Mill
TM have rough surfaces and pieces have sharp edges. Furthermore, the particles from wet milling show a number of loose fibres and ragged ends (
Figure 4B–D). Dry milling (
Figure 4B) has done visibly less damage to the biomass compared to wet milling (
Figure 4C,D). Moreover, the nitrogen assisted wet milling shows even smaller particles and more visible damage to the biomass through fibre separation.
The composition of initial biomass and biomass treated using different modes with the Szego MillTM was characterized by fibre analysis results. The untreated biomass contained 7.8%, 40.0% and 27.1% of lignin, cellulose, and hemicellulose, respectively. The fibre analysis after milling indicated minimal changes in biomass composition after wet milling and no change after dry milling. In case of wet milling methods, the cellulose and lignin contents of biomass decreased 1–2% while the hemicellulose content did not change. This variation in fibre analysis results are in line with measurement variability and standard deviation of parallel measurements. Therefore, it can be concluded that milling did not cause significant chemical changes in biomass composition.
3.2. Bioethanol Production
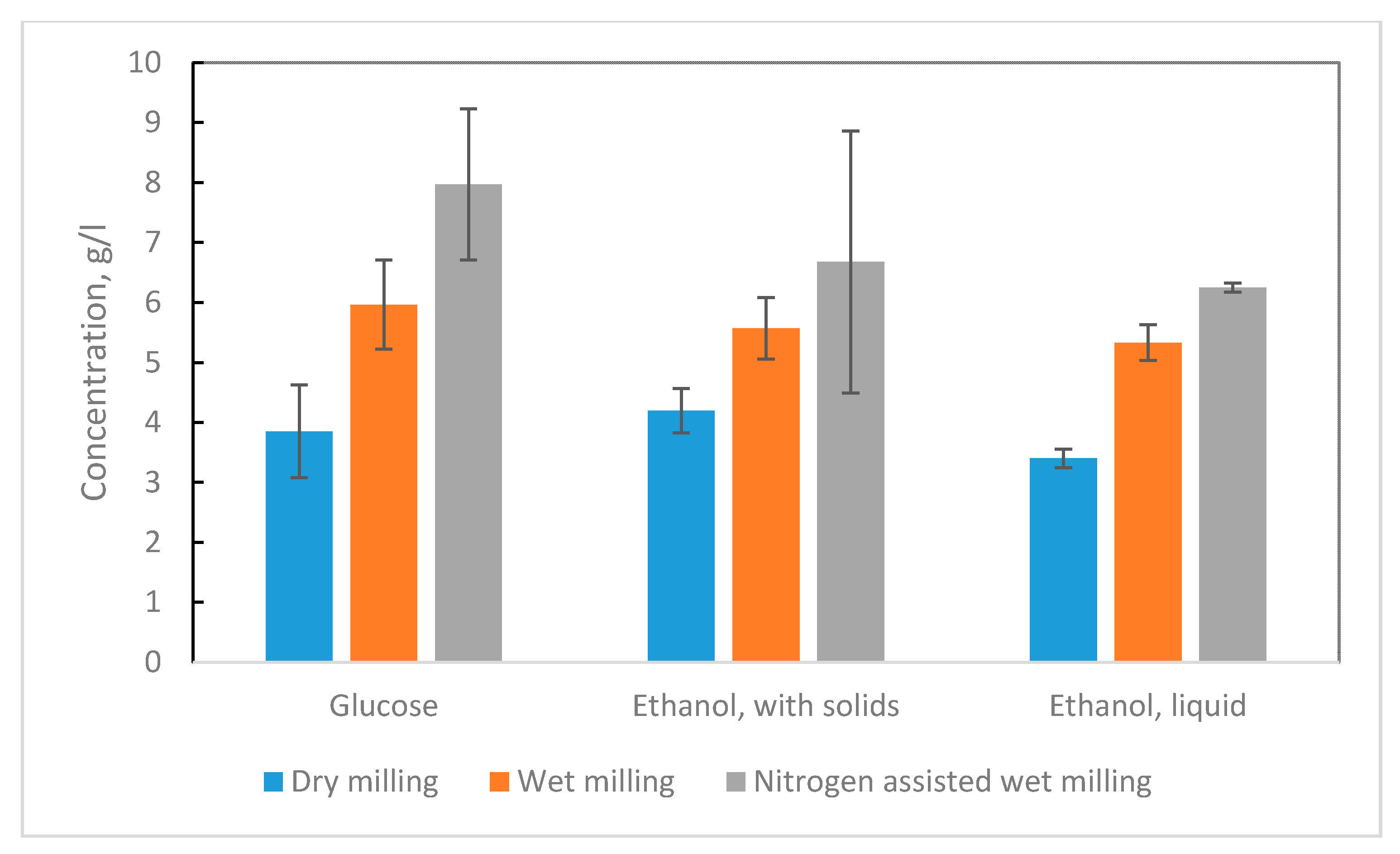
Figure 5 illustrates the glucose and ethanol yields, and hydrolysis and fermentation efficiencies when biomass was milled in different ways using the Szego Mill
TM. The lowest glucose concentration, 3.9 g L
−1 in hydrolysis media, was measured when biomass was dry milled. Higher glucose concentrations, 6.0 and 8.0 g L
−1, were measured when the biomass wet milling and nitrogen assisted wet milling were used, respectively. This can be attributed to the effect of wet milling to biomass. In the SEM pictures, it was also seen that milling gave smaller biomass pieces and wet milling additionally lead to even greater extent of biomass shredding. It has been shown that milling causes the size reduction and rupture of the polysaccharide chains bonds, which increases their exposure at particles surfaces and reduces cellulose crystallinity [
25]. In addition, when wet and dry milling were compared with the
Pennisetum hybrid, then at the same milling conditions, wet milling gave higher pore volume, surface area, and average pore diameter [
26]. This effect helps to increase the accessibility of the substrate to enzymes and, thereby, improve hydrolysis efficiency.
Similarly to glucose, the lowest ethanol concentrations, 3.4–4.2 g L−1, were measured after fermentation when biomass was dry milled and hydrolyzed. In comparison, wet milling methods enabled a gain of 60–100% higher ethanol concentrations. The higher ethanol yields are partially caused by the higher glucose concentrations obtained when milled moist biomass was used in the hydrolysis step. When wet milled and nitrogen assisted wet milled biomass was used in hydrolysis, 54% and 104% higher glucose concentrations were gained compared to glucose yields gained with dry milled straw, respectively. The glucose formed in hydrolysis was directly fermented into ethanol in the fermentation step. Therefore, the proportions that were seen in glucose concentration, when different milling methods were used, remained the same after fermentation in ethanol concentrations.
Fermentation was carried out using two different approaches to find the most effective process in terms of ethanol and biogas yields. In the first approach, the hydrolyzed biomass slurry was used in fermentation, and in the second approach, the biomass and liquid fractions were separated using filtration and only the liquid fraction was used in fermentation (
Figure 1). Applying the phase separation ensured that the sugars available in the fermentation step were only from the hydrolysis step. As expected, the higher ethanol concentrations were measured in samples where biomass slurry was used in fermentation. The measured ethanol concentrations were 5–19% higher compared to the fermentation of only liquid fraction. Without solid and liquid separation, the residual cellulose remaining in the solid fraction is continuously hydrolyzed by enzymes to glucose during the fermentation step as well and then fermented to ethanol. This yielded higher concentrations of ethanol in samples, where biomass slurry was fully used for fermentation compared to samples where only the liquid fraction was used.
Fermentation efficiencies exceeding 100% were caused by sugars available in forms other than glucose. The hydrolysis mixture also contains sugars in oligomeric forms and in short polymeric chains. During fermentation, the enzymes that remained in the fermentation broth continued to hydrolyze these polymers into sugars. These sugars were fermented into ethanol, which increased the final ethanol yield. Similar results have been reported in earlier studies [
19,
21].
Compared to the results obtained with NED pretreatment when the same biomass was used, the glucose and ethanol concentrations gained with Szego milling as a pretreatment were lower. When the same biomass was used with NED pretreatment up to 20 g L
−1 of ethanol was gained with a fermentation efficiency of 46% depending on pretreatment temperature. The ethanol concentrations obtained with NED pretreatment method were up to 12 g L
−1 [
21]. In addition to pressure release, the NED pretreatment also includes use of elevated temperatures which initiate the dissolution of hemicellulose. Therefore, after NED pretreatment the cellulose is more opened to enzymatic treatment and higher efficiencies resulted [
19]. On the other hand, when the Szego Mill was used in different modes, neither the hemicellulose nor lignin were removed from biomass. Due to this, the glucose and ethanol concentrations were lower.
3.3. Biogas
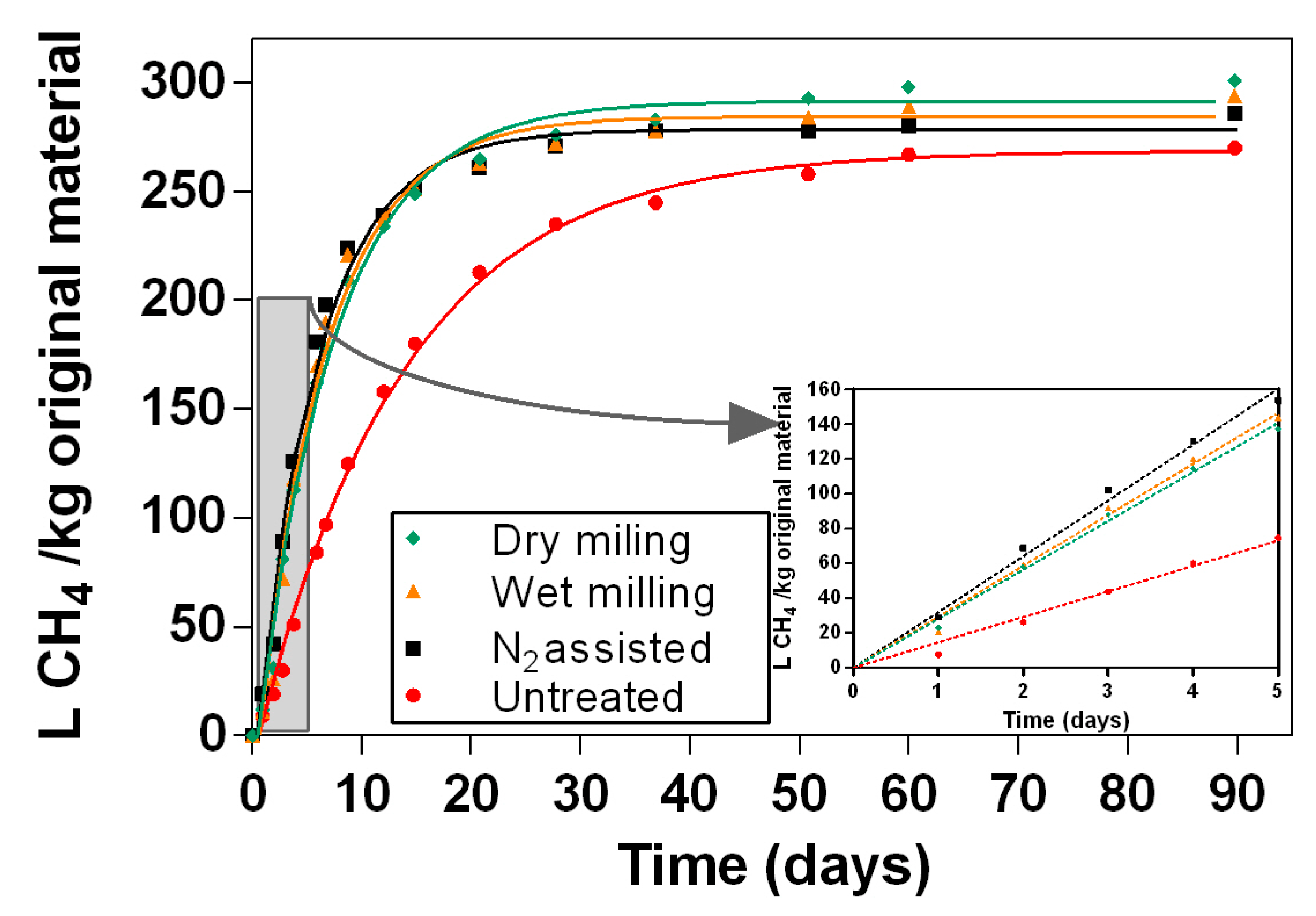
Figure 6 shows the biomethane potential (BMP) of samples that were pretreated with dry milling, wet milling and nitrogen assisted wet milling. Samples that were pretreated using dry milling had the highest methane yields (292 L CH
4 kg
−1 original material), followed by samples that were pretreated with wet milling (285 L CH
4 kg
−1 original material), N
2 assisted milling (278 L CH
4 kg
−1 original material) and untreated barley straw (269 L CH
4 kg
−1 original material). It can be seen from the
Figure 5 that all materials that were Szego milled had higher BMP than the one milled using conventional cutting mill. The differences in BMP results between cutting milling and Szego milling were statistically significant (
p < 0.05). Although the highest BMP was obtained using the dry milling mode, the highest reaction rate was achieved with nitrogen assisted wet milled material (
Figure 5).
Figure 5 illustrates that the Szego milling process allows for better access to the biomass than the regular cutting mill as the kinetic rates (slope of the curve) increased up to 133% (see also
Figure 7). Furthermore, maximum biogas output is also increased with Szego milling, which implies that some crystalline cellulose areas have also been disrupted.
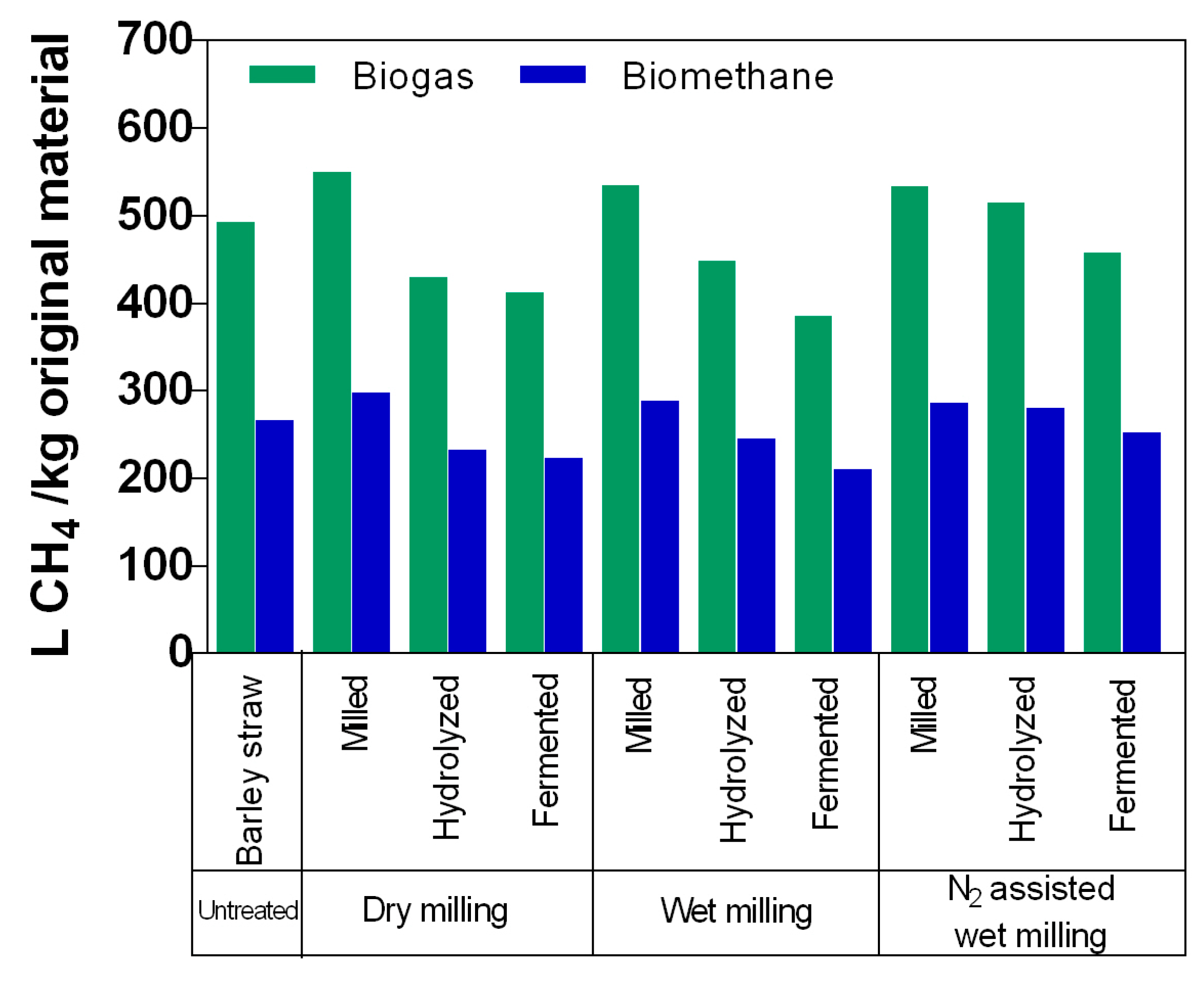
Figure 7 shows the biomethane and biogas potentials for original material and samples that were dry milled, wet milled and nitrogen assisted wet milled and subjected to bioethanol production, calculated per kg of dry matter of the original sample used in bioethanol production. The biomethane yields varied between 210 L CH
4 kg
−1 original material (samples from the fermentation stage that were pretreated with wet milling) and 289 L CH
4 kg
−1 original material (samples from the milling stage that were pretreated with wet milling). A similar trend was obtained for biogas yields. Samples from the fermentation stage that were wet milled had the lowest yields (385 L CH
4 kg
−1 original material), while samples from the milling stage that were dry milled had the highest biogas yields (550 L CH
4 kg
−1 original material). As can be seen from the results, dry milling has higher biogas and biomethane yields. For different milling methods, the highest biogas and methane yields were achieved in trials with samples only milled compared to samples from bioethanol production. However, the fact that even with higher ethanol production, the solid residue from hydrolysis and fermentation steps of the nitrogen assisted wet milled material generated considerably more biogas/biomethane than from other materials implies that the structure of the biomass was more open for the biological processes.
The kinetic rate constants are shown in
Figure 8. As can be seen from the figure, conventionally milled barley straw has the lowest kinetic rate constant (0.074 d
−1). Samples that were N
2 assisted wet milled had the highest kinetic rate (between 0.16 d
−1 and 0.17 d
−1), followed by samples that were wet milled (between 0.12 d
−1 and 0.15 d
−1), and dry milled (being between 0.10 d
−1 and 0.14 d
−1) with the Szego Mill. The results are in accordance with SEM pictures in
Figure 3, where it can be seen that the nitrogen assisted wet milling resulted in the smallest particle size and the most visible damage to the straw. For samples that were N
2 assisted wet milled, the kinetic rate constant was 106% to 133% higher than that for conventionally milled barley straw; for samples that were wet milled, the kinetic rate was 68% to 111% higher than original material; and for samples that were dry milled, the kinetic rate was 39% to 87% higher than that of original biomass. For all the milling methods, the highest kinetic rates were achieved for the samples that were just milled compared to further hydrolyzed and fermented samples. It can be explained by the presence of more easily hydrolyzable substances in the milled samples that were used in the enzymatic hydrolysis step of bioethanol production. Overall, fermented samples had higher kinetic rates than hydrolyzed materials.
3.4. Mass Balances
The liquid and solid phases from all process stages were weighed, fiber analysis results of all biomasses and solid residues, and glucose and ethanol concentrations, as well as methane production, measured after hydrolysis and fermentation stages, respectively, were recorded. Based on these data, mass balances were calculated, and mass flow charts prepared for ethanol and biogas production processes using different milling methods (
Figure 9 and
Figure 10).
The results show that during bioethanol production a small mass loss was recorded after the hydrolysis and fermentation steps. During hydrolysis, the cellulose in the biomass is partially hydrolyzed and dissolved by the enzymes. From an initial 100 g as low as 85.6 g of solid fraction was left after hydrolysis. The hydrolysis process and dissolution of cellulose partially continues during fermentation and causes continuous mass loss in the solid fraction. Therefore 89.9–79.7 g of solid fraction was left after fermentation. After hydrolysis, 272–391 mL of liquid fraction was separated and used for fermentation. When the liquid fraction was separated after fermentation, 339–380 mL of it was obtained. In both fermentation approaches used, the least amount of liquid fraction was obtained after wet milling with nitrogen addition.
Two different strategies were used for fermentation. Depending on the milling method, 1.33–1.70 g of ethanol could be produced when solid and liquid fractions were separated, and only the liquid fraction was used in fermentation. When the biomass broth was fully used in fermentation, 1.45–2.26 g of ethanol could be produced with different milling methods. Depending on the fermentation strategy, wet milling gives 34–45% more ethanol compared to dry milling using the same fermentation strategy. When nitrogen was added to wet milling, the ethanol yield was 27–55% higher than with dry milling using the same fermentation strategy.
Biogas production experiments revealed that the highest amount of biomethane could be produced from milled biomass—28.7–29.8 L methane from 100 g of biomass. After processing the biomass in hydrolysis and fermentation steps the biomethane yield decreases with each step since some of the volatile matter has been removed. If the solid would be removed after hydrolysis and solid residue would be used for biomethane production, then additionally 23.3–28.1 L biomethane could be produced. If the solids would be separated after fermentation, the biomethane yield would be 21.0–25.2 L. Overall, more biomethane was produced in case of nitrogen assisted wet milling in case of combined bioethanol and biomethane production. As the biogas and biomethane potentials were similar in all milling methods the difference in yields was most likely caused by the mass difference of solid residues. When nitrogen assisted wet milling was used as a pretreatment, the larger amount of solid residues after each process step remained and, therefore, more biomethane could be produced.
Although nitrogen assisted wet milling produced almost double the glucose compared to dry milling, all milling methods are too ineffective as a single pretreatment. The same biomass was used with steam explosion pretreatment and explosive decompression pretreatment at similar conditions and up to 9.0 g of ethanol from 100 g of biomass was obtained [
19,
21]. Pretreatment by milling enables a breakdown of the biomass structure but as hemicellulose is not removed with pretreatment, the enzyme accessibility to cellulose is not sufficient for efficient bioethanol production. Compared to earlier studies, less liquid fraction was removed and therefore, the final ethanol yield is smaller although the ethanol concentration in media was comparable.