Cyanobacterial Biomass Produced in the Wastewater of the Dairy Industry and Its Evaluation in Anaerobic Co-Digestion with Cattle Manure for Enhanced Methane Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Dairy Industry Wastewater Characteristics
2.2. Photobioreactors and Culture Conditions
2.3. Anaerobic Co-Digestion
2.4. Analytical Methods
2.5. Statistical Analyses
3. Results
3.1. Growth and Productivity of Arthrospira Platensis in Batch Cultures with Wastewater from Dairy Industries
3.2. Carbohydrates, Protein, and Phycobiliprotein of Arthrospira Platensis in Batch Culture with Wastewater from Dairy Industries
3.3. Nutrients Removal
3.4. Content of VS, TN, TP, OC, and Mesophilic Anaerobic Co-Digestion (CB and CM)
4. Discussion
- −
- The thermal and sonic pre-treatments that break the cell wall of the cyanobacteria and significantly improve its biodegradability;
- −
- The improvement in the carbon-nitrogen ratio of both substrates;
- −
- The thermophilic regime used (35 °C).
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Semyalo, R. The Effects of Cyanobacteria on the Growth, Survival, and Behaviour of a Tropical Fish (Nile Tilapia) and Zooplankton (Daphnia Lumholtzi). Ph.D. Thesis, University of Bergen, Bergen, Norway, 2009. [Google Scholar]
- Cairns, J.; Dickson, K. A simple method for the biological assessment of the effects of waste discharge on aquatic bottom dwelling organisms. J. Water Pollut. Control Fed. 1971, 43, 755–772. [Google Scholar] [PubMed]
- Martins, J.; Peixe, L.; Vasconcelos, V. Unraveling cyanobacteria ecology in wastewater treatment plants (WWTP). Microb. Ecol. 2011, 62, 241–256. [Google Scholar] [CrossRef] [PubMed]
- Tafur, J.; Estrada, L. Tratamiento de aguas residuales in vitro por medio de la microalga Chlorella sp. en el Municipio de Barrancabermeja, Colombia. CITECSA 2015, 6, 5–19. [Google Scholar]
- Sankaran, K.; Premalatha, M. Nutrients uptake from anaerobically digested distillery wastewater by Spirulina sp. under xenon lamp illumination. J. Water Process Eng. 2018, 25, 295–300. [Google Scholar] [CrossRef]
- Yang, X.; Wu, X.; Hao, H.; He, Z. Mechanisms and assessment of water eutrophication. J. Zhejiang Univ. Sci. B 2008, 9, 197–209. [Google Scholar] [CrossRef]
- Pittman, J.; Dean, A.; Osundeko, O. The potential of sustainable algal biofuel production using wastewater resources. Bioresour. Technol. 2011, 102, 17–25. [Google Scholar] [CrossRef]
- Streit, N.; Ramírez-Mérida, L.; Queiroz, L.; Jacob-Lopes, E.; Queiroz, M. Producción de pigmentos por Aphanothece microscopica Nägeli a partir de residuos industriales lácteos. Ingeniare Rev. Chil. Ing. 2017, 25, 350–358. [Google Scholar] [CrossRef]
- Vijayakumar, S. Potential applications of cyanobacteria in industrial effluents—A review. J. Bioremediat. Biodegrad. 2012, 3. [Google Scholar] [CrossRef]
- Rahman, A.; Ellis, J.T.; Miller, C.D. Bioremediation of domestic wastewater and production of bioproducts from microalgae using waste stabilization ponds. J. Bioremediat. Biodegrad. 2012, 3. [Google Scholar] [CrossRef]
- Muylaert, K.; Beuckels, A.; Depraetere, O.; Fourbert, I.; Markou, G.; Vandamme, D. Wastewater as a Source of Nutrients for Microalgae Biomass Production. In Biomass and Biofuels from Microalgae Advances in Engineering and Biology, 1st ed.; Moheimani, N., McHenry, M., de Boer, K., Bahri, P., Eds.; Springer International Publishing: London, UK; New York, NY, USA, 2015; Volume 2, pp. 75–94. [Google Scholar] [CrossRef]
- Oswald, W.J.; Golueke, C.G. Biological transformation of solar energy. Adv. Appl. Microbiol. 1960, 2, 223–262. [Google Scholar] [CrossRef]
- Craggs, R.; Lundquist, T.J.; Benemann, J.R. Wastewater treatment and algal biofuel production. In Algae for Biofuels and Energy, 1st ed.; Borowitzka, M.A., Moheimani, N.R., Eds.; Springer: Berlin, Germany, 2013; Volume 5, pp. 153–163. [Google Scholar] [CrossRef]
- Álvarez, X.; Otero, A. Nutrient removal from the centrate of anaerobic digestion of high ammonium industrial wastewater by a semi-continuous culture of Arthrospira sp. and Nostoc sp. PCC 7413. J. Appl. Phycol. 2020, 32, 2785–2794. [Google Scholar] [CrossRef]
- Arango, O.; Sanches, L. Tratamiento de aguas residuales de la industria láctea en sistemas anaerobios tipo UASB. Biotecnologia en el Sector Agropecuario y Agroindustrial 2009, 7, 24–31. [Google Scholar]
- OECD/FAO. Agricultural Outlook 2019–2028; OECD Publishing: Paris, France, 2019; pp. 180–189. [Google Scholar] [CrossRef]
- Kushwaha, J.; Srivastava, V.; Mall, I. An overview of various technologies for the treatment of dairy wastewaters. Crit. Rev. Food Sci. Nutr. 2011, 51, 442–452. [Google Scholar] [CrossRef] [PubMed]
- Raghunath, B.V.; Punnagaiarasi, A.; Rajarajan, G.; Irshad, A.; Elango, A.; Mahesh-Kumar, A. Impact of dairy effluent on environment—A review. In Integrated Waste Management in India: Status and Future Prospects for Environmental Sustainability, 1st ed.; Prashanthi, M., Sundaram, R., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 229–249. [Google Scholar] [CrossRef]
- Chen, H.; Qiu, T.; Rong, J.; He, C.; Wang, Q. Microalgal biofuel revisited: An informatics-based analysis of developments to date and future prospects. Appl. Energy 2015, 155, 585–598. [Google Scholar] [CrossRef]
- Kerrison, P.D.; Stanley, M.S.; Edwards, M.D.; Black, K.D.; Hughes, A.D. The cultivation of European kelp for bioenergy: Site and species selection. Biomass Bioenergy 2015, 80, 229–242. [Google Scholar] [CrossRef]
- Milledge, J.J.; Heaven, S. Methods of energy extraction from microalgal biomass: A review. Rev. Environ. Sci. Bio 2014, 13, 301–320. [Google Scholar] [CrossRef]
- Milledge, J.J.; Smith, B.; Dyer, P.; Harvey, P. Macroalgae-Derived Biofuel: A Review of Methods of Energy Extraction from Seaweed Biomass. Energies 2014, 7, 7194–7222. [Google Scholar] [CrossRef]
- Sills, D.L.; Paramita, V.; Franke, M.J.; Johnson, M.C.; Akabas, T.M.; Greene, C.H.; Tester, J.W. Quantitative Uncertainty Analysis of Life Cycle Assessment for Algal Biofuel Production. Environ. Sci. Technol. 2012, 47, 687–694. [Google Scholar] [CrossRef]
- Fasaei, F.; Bitter, J.H.; Slegers, P.M.; van Boxtel, A.J.B. Techno-economic evaluation of microalgae harvesting and dewatering systems. Algal Res. 2018, 31, 347–362. [Google Scholar] [CrossRef]
- Horn, S.V. Bioenergy from Brown Seaweeds, 1st ed.; Norwegian University of Science and Technology (NTNU): Trondheim, Norway, 2000. [Google Scholar]
- Murphy, F.; Devlin, G.; Deverell, R.; McDonnell, K. Biofuel Production in Ireland—An Approach to 2020 Targets with a Focus on Algal Biomass. Energies 2013, 6, 6391–6412. [Google Scholar] [CrossRef]
- Milledge, J.J.; Staple, A.; Harvey, P. Slow Pyrolysis as a Method for the Destruction of Japanese Wireweed, Sargassum muticum. Environ. Nat. Resour. Res. 2015, 5, 28–36. [Google Scholar] [CrossRef]
- Barbot, Y.; Al-Ghaili, H.; Benz, R. A Review on the Valorization of Macroalgal Wastes for Biomethane Production. Mar. Drugs 2016, 14, 120. [Google Scholar] [CrossRef] [PubMed]
- Aresta, M.; Dibenedetto, A.; Barberio, G. Utilization of macro-algae for enhanced CO2 fixation and biofuels production: Development of a computing software for an LCA study. Fuel Process. Technol. 2005, 86, 1679–1693. [Google Scholar] [CrossRef]
- Ward, A. The Anaerobic Digestion of Microalgae Feedstock, “Life-Cycle Environmental Impacts of Biofuels and Co-products. In Biomass and Biofuels from Microalgae Advances in Engineering and Biology, 1st ed.; Moheimani, N., McHenry, M., de Boer, K., Bahri, P., Eds.; Springer International Publishing: London, UK; New York, NY, USA, 2015; pp. 331–345. [Google Scholar]
- Maneein, S.; Milledge, J.J.; Nielsen, B.V.; Harvey, P.J. A Review of Seaweed Pre-Treatment Methods for Enhanced Biofuel Production by Anaerobic Digestion or Fermentation. Fermentation 2018, 4, 100. [Google Scholar] [CrossRef]
- Golueke, C.G.; Oswald, W.J.; Gotaas, H.B. Anaerobic Digestion of Algae. Appl. Microbiol. 1957, 5, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Uziel, M. Solar Energy Fixation and Conversion with Algal Bacterial System. Ph.D. Thesis, University of California, Berkeley, CA, USA, 1978. [Google Scholar]
- Matsunaga, T.; Izumida, H. Seawater-based methane production from blue-green algae biomass by marine bacteria coculture. In Biotechnology Bioengineering Symposium; John Wiley & Sons: Gatlinburg, IL, USA, 1984; Volume 14, pp. 407–418. [Google Scholar]
- Chen, P.H. Factors Influencing Methane Fermentation of Micro-Algae. Ph.D. Thesis, University of California, Berkeley, CA, USA, 1987. [Google Scholar]
- Golueke, C.G.; Oswald, W.J. Biological Conversion of Light Energy to the Chemical Energy of Methane. Appl. Environ. Microbiol. 1959, 7, 219–227. [Google Scholar] [CrossRef]
- Golueke, C.G.; Oswald, W.J. Power from solar energy––Via algae-produced methane. Sol. Energy 1963, 7, 86–92. [Google Scholar] [CrossRef]
- Oswald, W.J. Gas production from microalgae. Clean Fuels Biomass Wastes 1976, 311–324. Available online: https://ui.adsabs.harvard.edu/abs/1976cfms.proc..311O/abstract (accessed on 13 September 2020).
- Oswald, W.J.; Green, F.B.; Lundquist, T.J. Performance of methane fermentation pits in advanced integrated wastewater pond systems. Water Sci. Technol. 1994, 30, 287–295. [Google Scholar] [CrossRef]
- Green, F.B.; Lundquist, T.J.; Oswald, W.J. Energetic of advanced integrated wastewater pond systems. Water Sci. Technol. 1995, 31, 9–20. [Google Scholar] [CrossRef]
- Green, F.B.; Bernstone, L.; Lundquist, T.J.; Muir, J.; Tresan, R.B.; Oswald, W.J. Methane fermentation, submerged gas collection, and the fate of carbon un advanced integrated wastewater pond systems. Water Sci. Technol. 1995, 31, 55–65. [Google Scholar] [CrossRef]
- Chen, P.H.; Oswald, W.J. Thermochemical treatment for algal fermentation. Environ. Int. 1998, 24, 889–897. [Google Scholar] [CrossRef]
- Solé-Bundó, M.; Eskicioglu, C.; Garfí, M.; Carrère, H.; Ferrer, I. Anaerobic co-digestion of microalgal biomass and wheat straw with and without thermo-alkaline pre-treatment. Bioresour. Technol. 2017, 237, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Astals, S.; Musenze, R.S.; Bai, X.; Tannock, S.; Tait, S.; Pratt, S.; Jensen, P.D. Anaerobic co-digestion of pig manure and algae: Impact of intracellular algal products recovery on co-digestion performance. Bioresour. Technol. 2015, 181, 97–104. [Google Scholar] [CrossRef]
- Wang, M.; Sahu, A.K.; Rusten, B.; Park, C. Anaerobic co-digestion of microalgae Chlorella sp. and waste activated sludge. Bioresour. Technol. 2013, 142, 585–590. [Google Scholar] [CrossRef]
- Park, S.; Li, Y. Evaluation of methane production and macronutrient degradation in the anaerobic co-digestion of algae biomass residue and lipid waste. Bioresour. Technol. 2012, 111, 42–48. [Google Scholar] [CrossRef]
- Fernández-Rodríguez, M.J.; Rincón, B.; Fermoso, F.G.; Jiménez, A.M.; Borja, R. Assessment of two-phase olive mill solid waste and microalgae co-digestion to improve methane production and process kinetics. Bioresour. Technol. 2014, 157, 263–269. [Google Scholar] [CrossRef]
- Gonzalez-Fernandez, C.; Barreiro-Vescovo, S.; de Godos, I.; Fernandez, M.; Zouhayr, A.; Ballesteros, M. Biochemical methane potential of microalgae biomass using different microbial inocula. Biotechnol. Biofuels 2018, 11, 1–11. [Google Scholar] [CrossRef]
- Zarrouk, C. Contribution à l’étude d’une Cyanophycée. Influence de Divers Facteurs Physiques et Chimiques sur la Croissance et Photosynthèse de Spirulina Maxima Geitler. Ph.D. Thesis, University of Paris, Paris, France, 1966. [Google Scholar]
- Markou, G.; Georgakakis, D. Cultivation of filamentous cyanobacteria (blue-green algae) in agro-industrial wastes and wastewater. A review. Appl. Energy 2011, 88, 3389–3401. [Google Scholar] [CrossRef]
- Sialve, B.; Bernet, N.; Bernard, O. Anaerobic digestion of microalgae as a necessary step to make microalgae biodiesel sustainable. Biotechnol. Adv. 2009, 27, 409–416. [Google Scholar] [CrossRef]
- Janczyk, P.; Franke, H.; Souffrant, W.B. Nutritional value of Chlorella vulgaris: Effects of ultrasonication and electroporation on digestibility in rats. Anim. Feed Sci. Technol. 2007, 132, 163–169. [Google Scholar] [CrossRef]
- Talling, J.F.; Driver, D. Some problems in the estimation of Chlorophyll-a in phytoplankton. In Proceedings of the Primary Productivity Measurement, Marine and Fresh-water, University of Hawaii, Honolulu, HI, USA, 21 August–6 September 1961; Doty, M.S., Ed.; US Atomic Energy Commission: Washington, DC, USA, 1961; pp. 142–146. [Google Scholar]
- Álvarez, X. Modulación de la Producción y Caracterización Estructural de los Exopolisacáridos en Cianobacterias Diazotróficas, y Estudio de su Utilización Para el Tratamiento del Digestato Líquido de la Digestión Anaeróbica de Efluente Efluentes de una Procesadora de Pescado. Ph.D. Thesis, Universidade de Santiago de Compostela, Santiago de Compostela, Spain, 2016. [Google Scholar]
- D´Elia, C.; Steudler, P.; Corwin, N. Determination of total nitrogen in aqueous samples using persulfate digestion. Limnol. Oceanogr. 1977, 22, 760–764. [Google Scholar] [CrossRef]
- Tandon, H.; Cescas, M.; Tyner, E. An acid-free vanadate-molybdate reagent for the determination of total phosphorus in soils. Soil. Sci. Soc. Am. J. 1968, 32, 48–51. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [PubMed]
- Herbert, D.; Phipps, P.J.; Strange, R.E. Chemical Analysis of Microbial Cells. In Methods in Microbiology; Norris, J.R., Ribbons, D.W., Eds.; Academic Press: London, UK; New York, NY, USA, 1971; Volume 5B, pp. 242–264. [Google Scholar] [CrossRef]
- Bennett, A.; Bogorad, L. Complementary chromatic adaptation in a filamentous blue-green alga. J. Cell. Biol. 1973, 58, 419–435. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.J.; Lee, Y.K. Determination of biomass dry weight of marine microalgae. J. Appl. Phycol. 1997, 9, 189–194. [Google Scholar] [CrossRef]
- Degtjaree, W.T.H. Determining soil organic matter by means of hydrogen peroxide and chromic acid. Soil Sci. 1930, 29, 239–246. [Google Scholar] [CrossRef]
- Walkley, A.; Black, I.A. An examination of Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association: Washington, DC, USA, 2017. [Google Scholar]
- Muñoz-Rojas, M.; Chilton, A.; Liyanage, G.S.; Erickson, T.E.; Merritt, D.J.; Neilan, B.A.; Ooi, M.K.J. Effects of indigenous soil cyanobacteria on seed germination and seedling growth of arid species used in restoration. Plant Soil 2018, 429, 91–100. [Google Scholar] [CrossRef]
- Plaza, B.M.; Gómez-Serrano, C.; Acién-Fernández, F.G.; Jimenez-Becker, S. Effect of microalgae hydrolysate foliar application (Arthrospira platensis and Scenedesmus sp.) on Petunia x hybrida growth. J. Appl. Phycol. 2018, 30, 2359–2365. [Google Scholar] [CrossRef]
- Godlewska, K.; Michalak, I.; Pacyga, P.; Baśladyńska, S.; Chojnacka, K. Potential application of cyanobacteria: Spirulina platensis filtrates and homogenates in agriculture. World J. MMicrobiol. Biotechnol. 2019, 35, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Raouf, N.; Al-Homaidan, A.A.; Ibraheem, I.B.M. Microalgae and wastewater treatment. Saudi J. Biol. Sci. 2012, 19, 257–275. [Google Scholar] [CrossRef] [PubMed]
- Batista, A.P.; Ambrosano, L.; Graca, S.; Sousa, C.; Marques, P.A.S.S.; Ribeiro, B.; Botrel, E.P.; Castro-Neto, P.; Gouveia, L. Combining urban wastewater treatment with biohydrogen production—An integrated microalgae-based approach. Bioresour. Technol. 2015, 184, 230–235. [Google Scholar] [CrossRef]
- Cai, T.; Park, S.Y.; Li, Y. Nutrient recovery from wastewater streams by microalgae: Status and prospects. Renew. Sustain. Energy Rev. 2013, 19, 360–369. [Google Scholar] [CrossRef]
- Phasey, J.; Vandamme, D.; Fallowfield, H.J. Harvesting of algae in municipal wastewater treatment by calcium phosphate precipitation mediated by photosynthesis, sodium hydroxide and lime. Algal Res. 2017, 27, 115–120. [Google Scholar] [CrossRef]
- Renuka, N.; Sood, A.; Prasanna, R.; Ahluwalia, A.S. Phycoremediation of wastewater: A synergistic approach using microalgae for bioremediation and biomass generation. Int. J. Environ. Sci. Technol. 2015, 12, 1443–1460. [Google Scholar] [CrossRef]
- Ouhsassi, M.; Khay, E.O.; Bouyahya, A.; El Ouahrani, A.; El Harsal, A.; Abrini, J. Evaluation of self-purifying power of cyanobacteria Pseudanabaena galeata: Case of dairy factory effluents. Appl. Water Sci. 2020, 10, 181. [Google Scholar] [CrossRef]
- Marazzi, F.; Belluci, M.; Fantasia, T.; Ficara, E.; Mezzanotte, V. Interactions between Microalgae and Bacteria in the Treatment of Wastewater from Milk Whey Processing. Water 2020, 12, 197. [Google Scholar] [CrossRef]
- Daneshvar, E.; Zarrinmehr, M.J.; Hashtjin, A.M.; Farhadian, O.; Bhatnagar, A. Versatile applications of freshwater and marine water microalgae in dairy wastewater treatment, lipid extraction and tetracycline biosorption. Bioresour. Technol. 2018, 268, 523–530. [Google Scholar] [CrossRef]
- Markou, G.; Chatzipavlidis, I.; Georgakakis, D. Cultivation of Arthrospira (Spirulina) platensis in olive-oil mill wastewater treated with sodium hypochlorite. Bioresour. Technol. 2012, 112, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Olguín, E.J.; Hernández, B.; Araus, A.; Camacho, R.; González, R.; Ramírez, M.E.; Galicia, S.; Mercado, G. Simultaneous high-biomass protein production and nutrient removal using Spirulina maxima in sea water supplemented with anaerobic effluents. World J. Microbiol. Biotechnol. 1994, 10, 576–578. [Google Scholar] [CrossRef] [PubMed]
- McConnell, M.D.; Koop, R.; Vasil´ev, S.; Bruce, D. Regulation of the distribution of chlorophyll and phycobilin-absorbed excitation energy in cyanobacteria. A structure-based model for the light state transition. Plant Physiol. 2002, 130, 1201–1212. [Google Scholar] [CrossRef] [PubMed]
- Olguín, E.; Galicia, S.; Mercado, G.; Pérez, T. Annual productivity of Spirulina (Arthrospira) and nutrient removal in a pig wastewater recycling process under tropical conditions. J. Appl. Phycol. 2003, 15, 249–257. [Google Scholar] [CrossRef]
- Rizzo, R.F.; dos Santos, B.N.C.; de Castro, G.F.P.S.; Passos, T.S.; Nascimento, M.A.; Guerra, H.D.; da Silva, C.G.; Dias, D.S.; Domingues, J.R.; Lima-Araújo, K.G.; et al. Production of phycobiliprotein by Arthrospira platensis under different lightconditions for application in food products. Food Sci. Technol. 2015, 35, 247–252. [Google Scholar] [CrossRef]
- Kim, T.; Choi, W.S.; Ye, B.R.; Heo, S.J.; Oh, D.; Kim, S.; Choi, K.S.; Kang, D.H. Cultivating Spirulina maxima: Innovative Approaches. In Cyanobacteria, 1st ed.; Tiwari, A., Ed.; IntechOpen: New York, NY, USA, 2018; pp. 61–83. [Google Scholar] [CrossRef][Green Version]
- Michael, A.; Kyewalyanga, M.S.; Lugomela, C.V. Biomass and nutritive value of Spirulina (Arthrospira fusiformis) cultivated in a cost-effective medium. Ann. Microbiol. 2019, 69, 1387–1395. [Google Scholar] [CrossRef]
- Pereira, M.I.B.; Chagas, B.M.E.; Sassi, R.; Medeiros, G.F.; Aguiar, E.M.; Borba, L.H.F.; Silva, E.P.E.; Neto, J.C.A.; Rangel, A.H.N. Mixotrophic cultivation of Spirulina platensis in dairy wastewater: Effects on the production of biomass, biochemical composition and antioxidant capacity. PLoS ONE 2019, 14, e0224294. [Google Scholar] [CrossRef]
- Olguín, E.J.; Galicia, S.; Angulo-Guerrero, O.; Hernández, E. The effect of low light flux and nitrogen deficiency on the chemical composition of Spirulina sp. (Arthrospira) grown on digested pig waste. Bioresour. Technol. 2001, 77, 19–24. [Google Scholar] [CrossRef]
- Chaiklahan, R.; Chirasuwan, N.; Siangdung, W.; Paithoonrangsarid, K.; Bunnag, B. Cultivation of Spirulina platensis Using Pig Wastewater in a Semi-Continuous Process. J. Microbiol. Biotechnol. 2010, 20, 609–614. [Google Scholar] [CrossRef]
- Mahdy, A.; Mendez, L.; Ballesteros, M.; González-Fernández, C. Algaculture integration in conventional wastewater treatments plants: Anaerobic digestion comparison of primary and secondary sludge with microalgae biomass. Bioresour. Technol. 2014, 184, 236–244. [Google Scholar] [CrossRef]
- Biller, P.; Ross, A.B.; Skill, S.C.; Lea-Langton, A.; Balasundaram, B.; Hall, C.; Riley, R.; Llewellyn, C.A. Nutrient recycling of aqueous phase for microalgae cultivation from the hydrothermal liquefaction process. Algal Res. 2012, 1, 70–76. [Google Scholar] [CrossRef]
- Buxy, S.; Diltz, R.; Pullammanappallil, P. Biogasification of Marine Algae Nannochloropsis oculata. In Materials Challenges in Alternative and Renewable Energy II: Ceramic Transactions; Wicks, G., Simon, J., Zidan, R., Brigmon, F.G., Arepalli, S., Norris, A., McCluer, M., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2012; Volume 239, pp. 59–67. [Google Scholar]
- Ras, M.; Lardon, L.; Bruno, S.; Bernet, N.; Steyer, J.P. Experimental study on a coupled process of production and anaerobic digestion of Chlorella vulgaris. Bioresour. Technol. 2011, 102, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Samson, R.; LeDuy, A. Influence of mechanical and thermochemical pre-treatments on anaerobic digestion of Spirulina maxima algal biomass. Biotechnol. Lett. 1983, 5, 671–676. [Google Scholar] [CrossRef]
- Samson, R.; LeDuy, A. Detailed study of anaerobic digestion of Spirulina maxima algal biomass. Biotechnol. Bioeng. 1986, 28, 1014–1023. [Google Scholar] [CrossRef]
- Inglesby, A.E.; Fisher, A.C. Enhanced methane yields from anaerobic digestion of Arthrospira maxima biomass in an advanced flow-through reactor with an integrated recirculation loop microbial fuel cell. Energy Environ. Sci. 2012, 5, 7996–8006. [Google Scholar] [CrossRef]
- Polakovičová, G.; Kušnír, P.; Nagyová, S.; Mikulec, J. Process integration of algae production and anaerobic digestion. Chem. Eng. Trans. 2012, 29, 1129–1134. [Google Scholar] [CrossRef]
- Lakaniemi, A.M.; Hulatt, C.J.; Thomas, D.N.; Tuovinen, O.H.; Puhakka, J.A. Biogenic hydrogen and methane production from Chlorella vulgaris and Dunaliella tertiolecta biomass. Biotechnol. Biofuels 2011, 4, 34. [Google Scholar] [CrossRef]
- Lü, F.; Ji, J.; Shao, L.; He, P. Bacterial bioaugmentation for improving methane and hydrogen production from microalgae. Biotechnol. Biofuels 2013, 6, 92. [Google Scholar] [CrossRef]
- González-Fernández, C.; Sialve, B.; Bernet, N.; Steyer, J.P. Thermal pretreatment to improve methane production of Scenedesmus biomass. Biomass Bioenergy 2012, 40, 105–111. [Google Scholar] [CrossRef]
- Mussnug, J.H.; Klassen, V.; Schlüter, A.; Kruse, O. Microalgae as substrates for fermentative biogas production in a combined biorefinery concept. J. Biotechnol. 2010, 150, 51–56. [Google Scholar] [CrossRef]
- Yang, Z.; Guo, R.; Xu, X.; Fan, X.; Luo, S. Hydrogen and methane production form lipid-extracted microalgal biomass residues. Int. J. Hydrog. Energy 2011, 36, 3465–3470. [Google Scholar] [CrossRef]
- Ward, A.J.; Lewis, D.M.; Green, F.B. Anaerobic digestion of algae biomass: A review. Algal Res. 2014, 5, 204–2014. [Google Scholar] [CrossRef]

 ), and wastewater from the dairy industry (
), and wastewater from the dairy industry ( ). Values are average ±SD (n = 3).
). Values are average ±SD (n = 3).
 ), and wastewater from the dairy industry (
), and wastewater from the dairy industry ( ). Values are average ±SD (n = 3).
). Values are average ±SD (n = 3).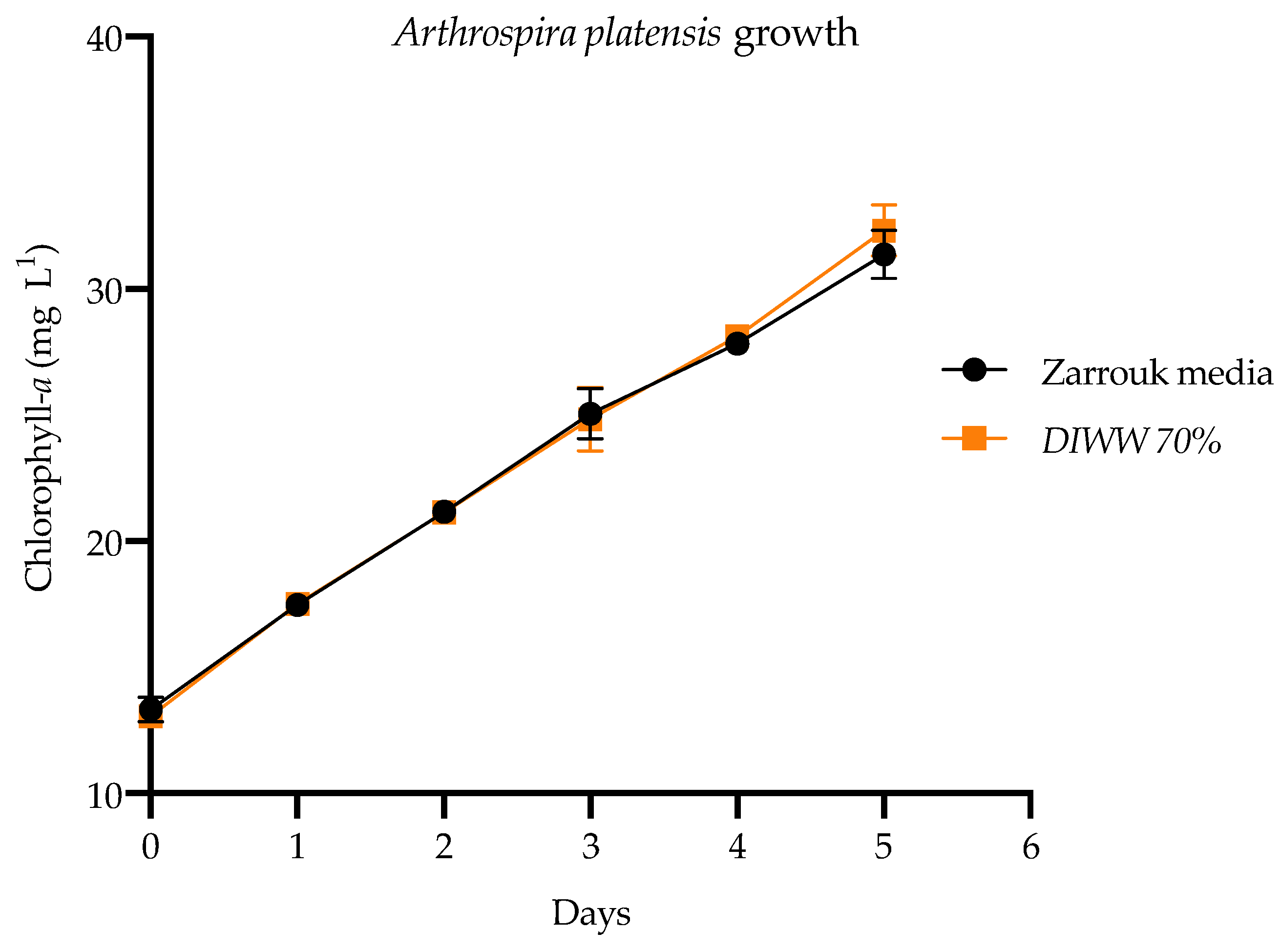
 ), and with wastewater from a dairy industry (
), and with wastewater from a dairy industry ( ). Values are average ± SD (n = 3).
). Values are average ± SD (n = 3).
 ), and with wastewater from a dairy industry (
), and with wastewater from a dairy industry ( ). Values are average ± SD (n = 3).
). Values are average ± SD (n = 3).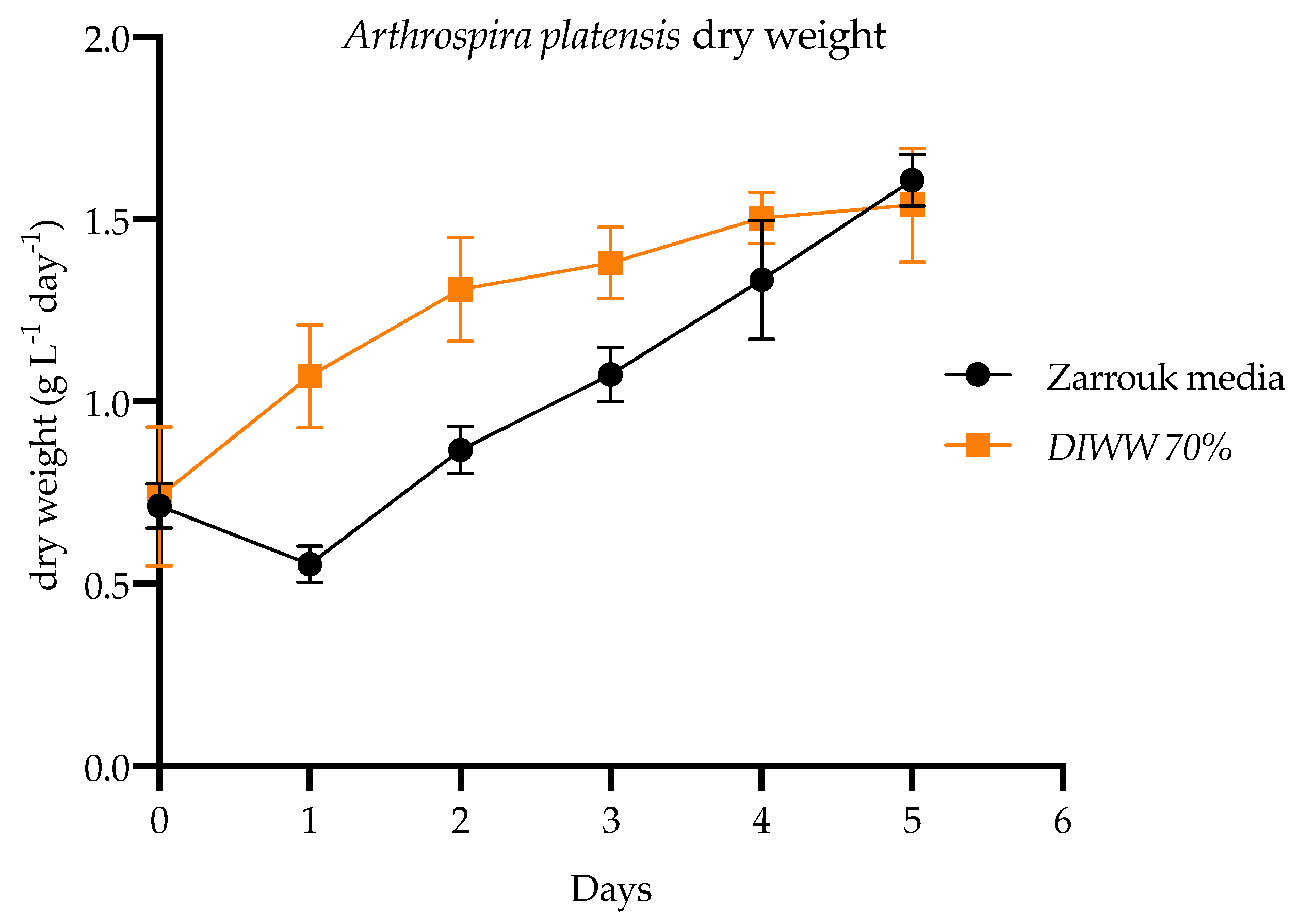
 ); anaerobic digestion of pre-treated Arthrospira platensis biomass (
); anaerobic digestion of pre-treated Arthrospira platensis biomass ( ); and anaerobic co-digestion of bovine manure + of pretreated Arthrospira platensis biomass (
); and anaerobic co-digestion of bovine manure + of pretreated Arthrospira platensis biomass ( ). Values are average ± SD (n = 3).
). Values are average ± SD (n = 3).
 ); anaerobic digestion of pre-treated Arthrospira platensis biomass (
); anaerobic digestion of pre-treated Arthrospira platensis biomass ( ); and anaerobic co-digestion of bovine manure + of pretreated Arthrospira platensis biomass (
); and anaerobic co-digestion of bovine manure + of pretreated Arthrospira platensis biomass ( ). Values are average ± SD (n = 3).
). Values are average ± SD (n = 3).
| Biochemical Composition | Zarrouk Medium | Dairy Industry Wastewater (DIWW) |
|---|---|---|
| Biomass carbohydrate | 261.22 ± 0.01 mg g−1 | 253.35 ± 0.01 mg g−1 |
| Protein | 400.45 ± 32.01 mg g−1 | 487.50 ± 31.14 mg g−1 |
| Phycobiliprotein | ||
| Allophycocyanin | 105.09 ± 9.44 mg g−1 | 94.27 ± 5.23 mg g−1 |
| Phycocyanin | 82.12 ± 7.16 mg g−1 | 77.40 ± 4.69 mg g−1 |
| Substrate | VS % (Volatile Solid) | TN mg g−1 (Total Nitrogen) | TP mg g−1 (Total Phosphorus) | OC mg g−1 (Organic Carbon) |
|---|---|---|---|---|
| Cyanobacterium biomass | 85.03 ± 10.76 | 105.70 ± 7.60 | 3.00 ± 0.25 | 566.90 ± 76.20 |
| Cattle manure | 70.70 ± 17.87 | 5.65 ± 1.18 | 1.75 ± 0.45 | 270.65 ± 10.13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Álvarez, X.; Arévalo, O.; Salvador, M.; Mercado, I.; Velázquez-Martí, B. Cyanobacterial Biomass Produced in the Wastewater of the Dairy Industry and Its Evaluation in Anaerobic Co-Digestion with Cattle Manure for Enhanced Methane Production. Processes 2020, 8, 1290. https://doi.org/10.3390/pr8101290
Álvarez X, Arévalo O, Salvador M, Mercado I, Velázquez-Martí B. Cyanobacterial Biomass Produced in the Wastewater of the Dairy Industry and Its Evaluation in Anaerobic Co-Digestion with Cattle Manure for Enhanced Methane Production. Processes. 2020; 8(10):1290. https://doi.org/10.3390/pr8101290
Chicago/Turabian StyleÁlvarez, Xavier, Olga Arévalo, Miriam Salvador, Ingrid Mercado, and Borja Velázquez-Martí. 2020. "Cyanobacterial Biomass Produced in the Wastewater of the Dairy Industry and Its Evaluation in Anaerobic Co-Digestion with Cattle Manure for Enhanced Methane Production" Processes 8, no. 10: 1290. https://doi.org/10.3390/pr8101290
APA StyleÁlvarez, X., Arévalo, O., Salvador, M., Mercado, I., & Velázquez-Martí, B. (2020). Cyanobacterial Biomass Produced in the Wastewater of the Dairy Industry and Its Evaluation in Anaerobic Co-Digestion with Cattle Manure for Enhanced Methane Production. Processes, 8(10), 1290. https://doi.org/10.3390/pr8101290







