Photochemical Oxidation Process of Copper from Electroplating Wastewater: Process Performance and Kinetic Study
Abstract
1. Introduction
2. Materials and Method
2.1. Materials
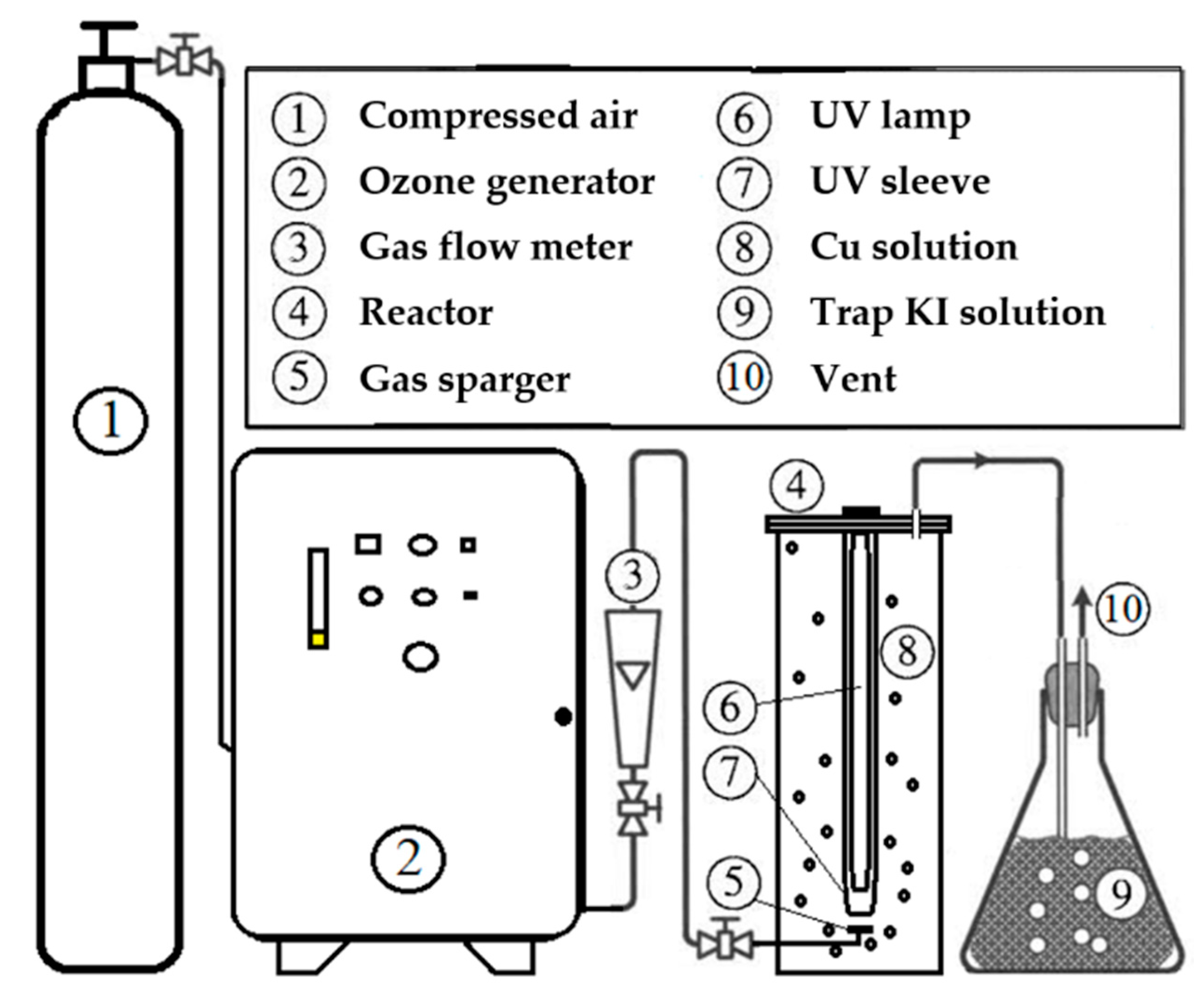
2.2. Experimental System Setup
2.3. Analytical Methods
3. Results and Discussions
3.1. Comparation of UV, Ozone. and Combination UV-Ozone
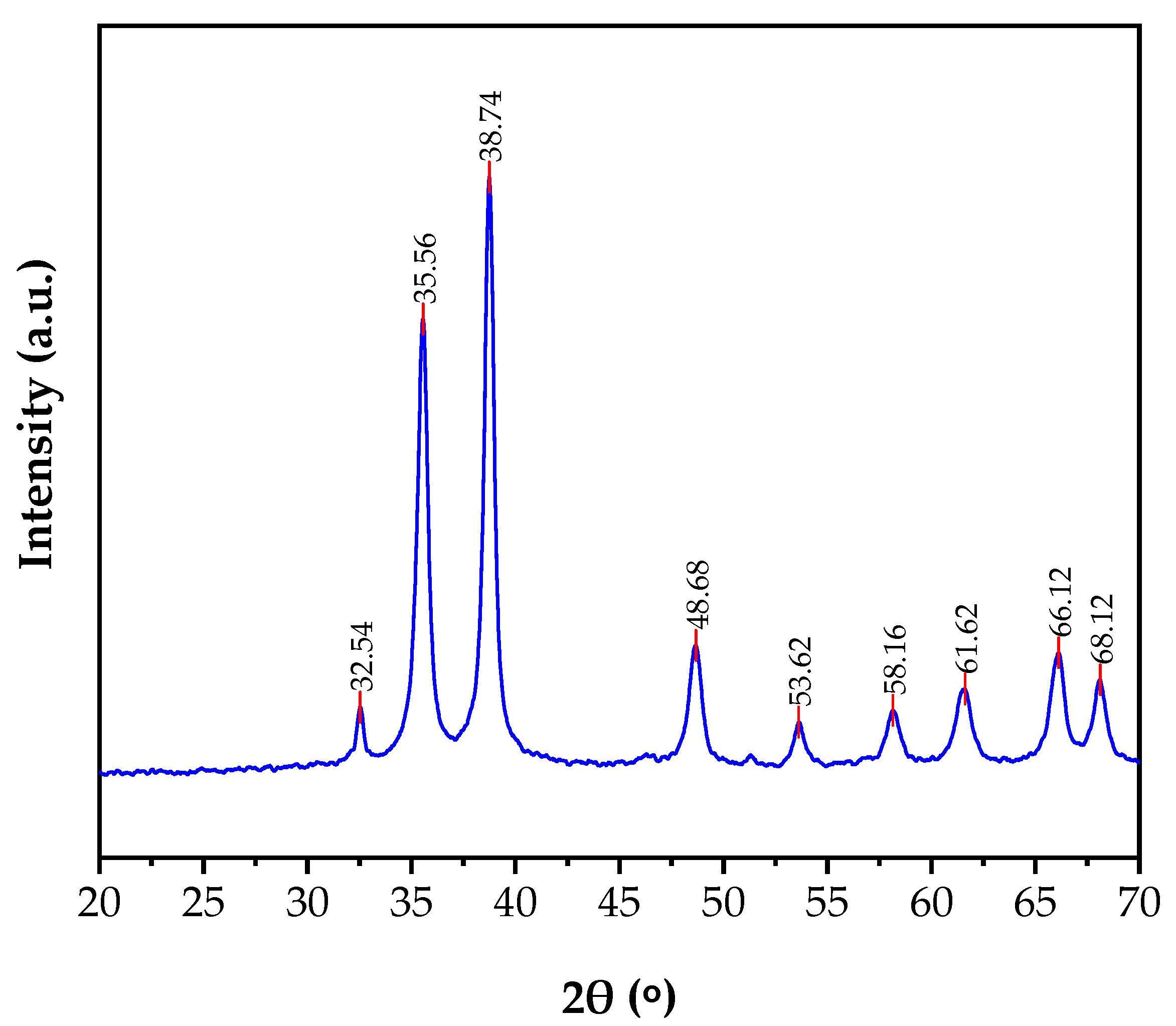
3.2. Characterization of the Solid Product
3.3. The Effect of Operating Parameters
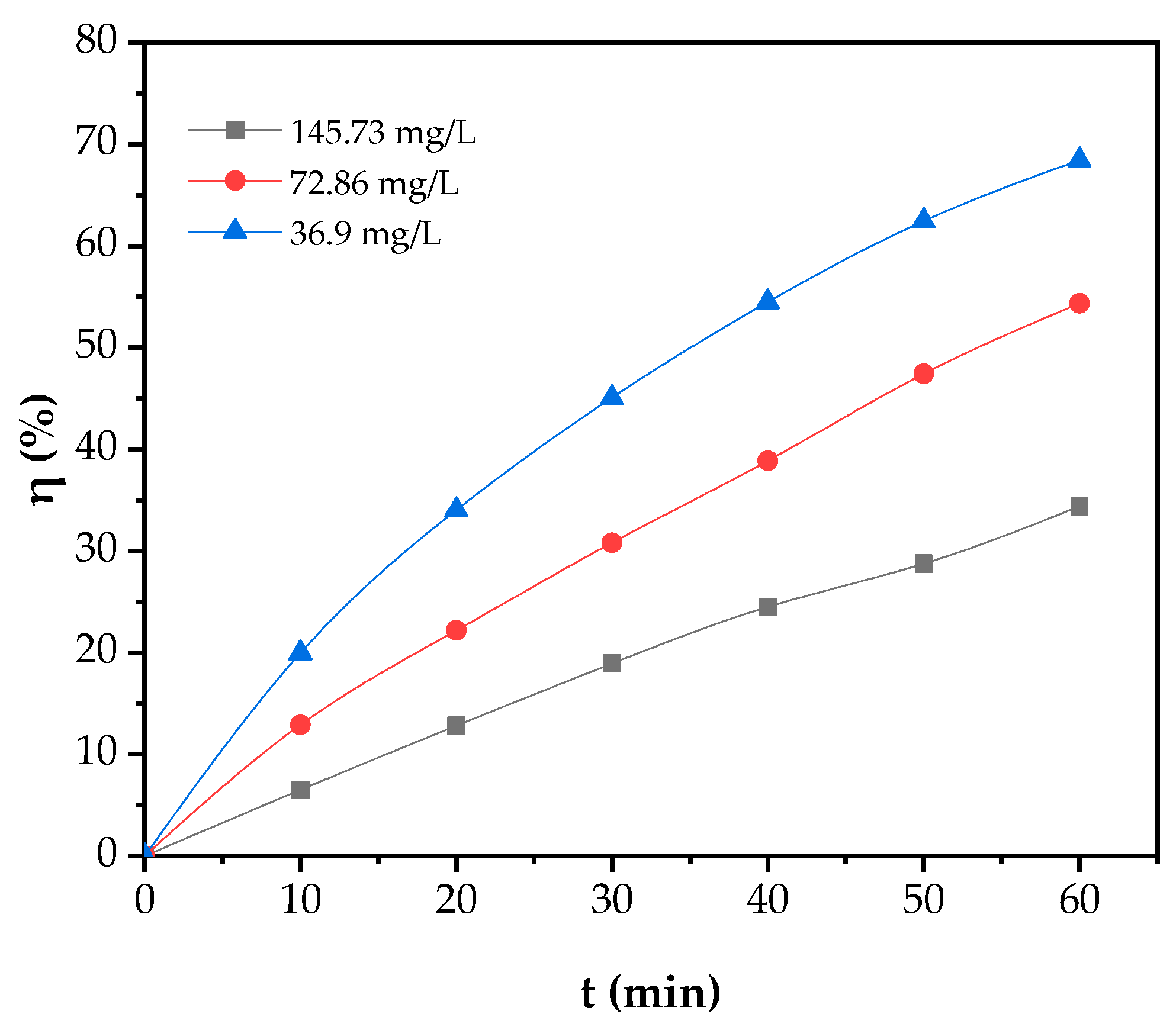
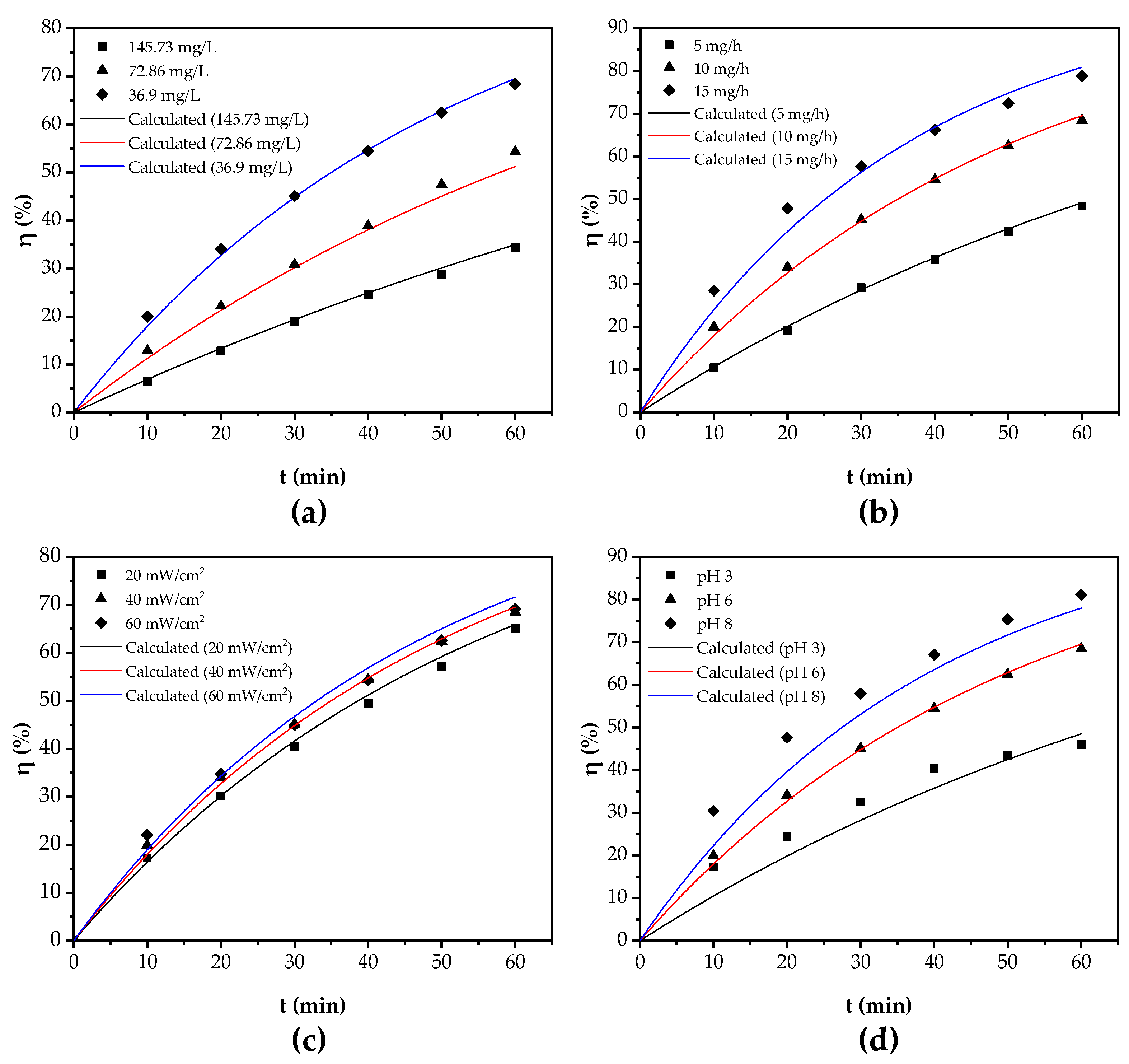
3.3.1. Effect of Initial Concentration
3.3.2. Effect of Ozone Dosage
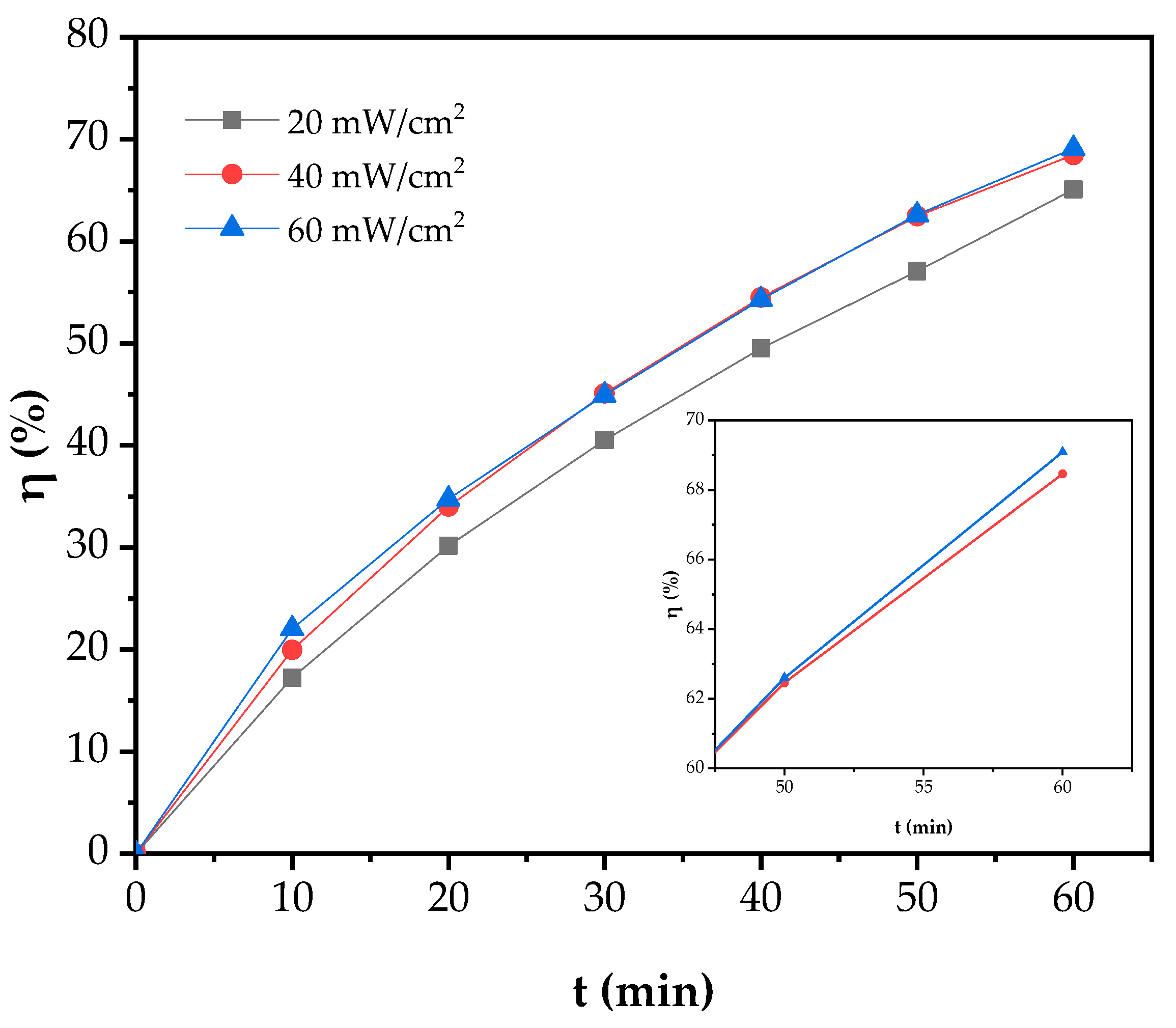
3.3.3. Effect of UV Irradiation Intensity
3.3.4. Effect of pH
3.4. Kinetic Study
3.4.1. Determination of the Kinetic Rate Order
3.4.2. The Effect of Operating Parameters on Kinetic Rate Constant
3.5. Proposed Mechanism
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rahman, M.S.; Sathasivam, K.V. Heavy Metal Adsorption onto Kappaphycus sp. from Aqueous Solutions: The Use of Error Functions for Validation of Isotherm and Kinetics Models. Biomed Res. Int. 2015, 2015, 126298. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.M.; Ali, M.L.; Islam, M.S.; Rahman, M.Z. Preliminary assessment of heavy metals in water and sediment of Karnaphuli River, Bangladesh. Environ. Nanotechnol. Monit. Manag. 2016, 5, 27–35. [Google Scholar] [CrossRef]
- Islam, M.S.; Ahmed, M.K.; Habibullah-Al-Mamun, M.; Islam, K.N.; Ibrahim, M.; Masunaga, S. Arsenic and lead in foods: A potential threat to human health in Bangladesh. Food Addit. Contam. Part A 2014, 31, 1982–1992. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Cao, Q.; Zheng, Y.M.; Huang, Y.Z.; Zhu, Y.G. Health risks of heavy metals in contaminated soils and food crops irrigated with wastewater in Beijing, China. Environ. Pollut. 2008, 152, 686–692. [Google Scholar] [CrossRef] [PubMed]
- Gautam, R.K.; Sharma, S.K.; Mahiya, S.; Chattopadhyaya, M.C. Contamination of Heavy Metals in Aquatic Media: Transport, Toxicity and Technologies for Remediation. In Heavy Metals in Water; Royal Society of Chemistry: Cambridge, UK, 2014; pp. 1–24. [Google Scholar]
- Majumder, S.; Gangadhar, G.; Raghuvanshi, S.; Gupta, S. A comprehensive study on the behavior of a novel bacterial strain Acinetobacter guillouiae for bioremediation of divalent copper. Bioprocess Biosyst. Eng. 2015, 38, 1749–1760. [Google Scholar] [CrossRef]
- Oturan, M.A.; Aaron, J.J. Advanced oxidation processes in water/wastewater treatment: Principles and applications. A review. Crit. Rev. Environ. Sci. Technol. 2014, 44, 2577–2641. [Google Scholar] [CrossRef]
- Krishnan, S.; Rawindran, H.; Sinnathambi, C.M.; Lim, J.W. Comparison of various advanced oxidation processes used in remediation of industrial wastewater laden with recalcitrant pollutants. IOP Conf. Ser. Mater. Sci. Eng. 2017, 206, 012089. [Google Scholar] [CrossRef]
- Al-Kdasi, A.; Idris, A.; Saed, K.; Guan, C.T. Treatment of textile wastewater by advanced oxidation processes—A review. Glob. Nest Int. J. 2004, 6, 222–230. [Google Scholar]
- Hanela, S.; Durán, J.; Jacobo, S. Removal of iron-cyanide complexes from wastewaters by combined UV-ozone and modified zeolite treatment. J. Environ. Chem. Eng. 2015, 3, 1794–1801. [Google Scholar] [CrossRef]
- Bes-Piá, A.; Mendoza-Roca, J.A.; Roig-Alcover, L.; Iborra-Clar, A.; Iborra-Clar, M.I.; Alcaina-Miranda, M.I. Comparison between nanofiltration and ozonation of biologically treated textile wastewater for its reuse in the industry. Desalination 2003, 157, 81–86. [Google Scholar] [CrossRef]
- Deng, Y.; Zhao, R. Advanced Oxidation Processes (AOPs) in Wastewater Treatment. Curr. Pollut. Rep. 2015, 1, 167–176. [Google Scholar] [CrossRef]
- Perkowski, J.; Kos, L. Decolouration of model dyehouse wastewater with advanced oxidation processes. Fibres Text. East. Eur. 2003, 11, 67–71. [Google Scholar]
- Sharrer, M.J.; Summerfelt, S.T. Ozonation followed by ultraviolet irradiation provides effective bacteria inactivation in a freshwater recirculating system. Aquac. Eng. 2007, 37, 180–191. [Google Scholar] [CrossRef]
- Chen, Z.; Fang, J.; Fan, C.; Shang, C. Oxidative degradation of N-Nitrosopyrrolidine by the ozone/UV process: Kinetics and pathways. Chemosphere 2016, 150, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Dai, Q.; Chen, L.; Chen, W.; Chen, J. Degradation and kinetics of phenoxyacetic acid in aqueous solution by ozonation. Sep. Purif. Technol. 2015, 142, 287–292. [Google Scholar] [CrossRef]
- Litter, M.I.; Quici, N. Photochemical Advanced Oxidation Processes for Water and Wastewater Treatment. Recent Patents Eng. 2011, 4, 217–241. [Google Scholar] [CrossRef]
- Badmus, M.A.O.; Audu, T.O.K.; Anyata, B.U. Removal of heavy metal from industrial wastewater using hydrogen peroxide. Afr. J. Biotechnol. 2007, 6, 238–242. [Google Scholar]
- Aravinda, C.L.; Mayanna, S.M.; Muralidharan, V.S. Electrochemical behaviour of alkaline copper complexes. J. Chem. Sci. 2000, 112, 543–550. [Google Scholar] [CrossRef]
- Chand Mali, S.; Raj, S.; Trivedi, R. Biosynthesis of copper oxide nanoparticles using Enicostemma axillare (Lam.) leaf extract. Biochem. Biophys. Reports 2019, 20, 100699. [Google Scholar] [CrossRef]
- Martí, I.; Ferrer, A.; Escorihuela, J.; Burguete, M.I.; Luis, S.V. Copper(II) complexes of bis(amino amide) ligands: Effect of changes in the amino acid residue. Dalt. Trans. 2012, 41, 6764–6776. [Google Scholar] [CrossRef]
- Jin, F.; Wei, M.; Liu, C.; Ma, Y. The mechanism for the formation of OH radicals in condensed-phase water under ultraviolet irradiation. Phys. Chem. Chem. Phys. 2017, 19, 21453–21460. [Google Scholar] [CrossRef] [PubMed]
- Attri, P.; Kim, Y.H.; Park, D.H.; Park, J.H.; Hong, Y.J.; Uhm, H.S.; Kim, K.N.; Fridman, A.; Choi, E.H. Generation mechanism of hydroxyl radical species and its lifetime prediction during the plasma-initiated ultraviolet (UV) photolysis. Sci. Rep. 2015, 5, 9332. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Sun, D.; Chung, J.S. Kinetics study on photochemical oxidation of polyacrylamide by ozone combined with hydrogen peroxide and ultraviolet radiation. J. Environ. Sci. 2006, 18, 660–664. [Google Scholar]
- Shi, L.B.; Tang, P.F.; Zhang, W.; Zhao, Y.P.; Zhang, L.C.; Zhang, H. Green synthesis of CuO nanoparticles using Cassia auriculata leaf extract and in vitro evaluation of their biocompatibility with rheumatoid arthritis macrophages (RAW 264.7). Trop. J. Pharm. Res. 2017, 16, 185–192. [Google Scholar] [CrossRef]
- Etefagh, R.; Azhir, E.; Shahtahmasebi, N. Synthesis of CuO nanoparticles and fabrication of nanostructural layer biosensors for detecting Aspergillus niger fungi. Sci. Iran. 2013, 20, 1055–1058. [Google Scholar]
- Lanje, A.S.; Sharma, S.J.; Pode, R.B.; Ningthoujam, R.S. Synthesis and optical characterization of copper oxide nanoparticles. Adv. Appl. Sci. Res. 2010, 1, 36–40. [Google Scholar]
- Manyasree, D.; Peddi, K.M.; Ravikumar, R. CuO nanoparticles: Synthesis, characterization and their bactericidal efficacy. Int. J. Appl. Pharm. 2017, 9, 71–74. [Google Scholar]
- Zhu, G.; Sun, Q.; Wang, C.; Yang, Z.; Xue, Q. Removal of Sulfamethoxazole, Sulfathiazole and Sulfamethazine in their Mixed Solution by UV/H2O2 Process. Int. J. Environ. Res. Public Health 2019, 16, 1797. [Google Scholar] [CrossRef]
- Hassaan, M.A.; El Nemr, A.; Madkour, F.F. Application of Ozonation and UV assisted Ozonation for Decolorization of Direct Yellow 50 in Sea water. The Pharmaceutical and Chemical Journal. 2016, 3, 131–138. [Google Scholar]
- Dai, C.; Zhou, X.; Zhang, Y.; Duan, Y.; Qiang, Z.; Zhang, T.C. Comparative study of the degradation of carbamazepine in water by advanced oxidation processes. Environ. Technol. 2012, 33, 1101–1109. [Google Scholar] [CrossRef]
- Jing, Z.; Cao, S.; Yu, T.; Hu, J. Degradation Characteristics of Aniline with Ozonation and Subsequent Treatment Analysis. J. Chem. 2015, 2015, 1–6. [Google Scholar] [CrossRef]
- Wang, Z.; Li, J.; Song, W.; Zhang, X.; Song, J. Decomplexation of electroplating wastewater by ozone-based advanced oxidation process. Water Sci. Technol. 2019, 79, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Yang, Z.; Du, J.; Yin, R.; Zhou, X.; Jin, S.; Ren, N. Degradation of sulfadiazine in water by a UV/O3 process: Performance and degradation pathway. RSC Adv. 2016, 6, 57138–57143. [Google Scholar] [CrossRef]
- Barakat, M.A. New trends in removing heavy metals from industrial wastewater. Arab. J. Chem. 2011, 4, 361–377. [Google Scholar] [CrossRef]
- Summerfelt, S.T. Ozonation and UV irradiation—An introduction and examples of current applications. Aquac. Eng. 2003, 28, 21–36. [Google Scholar] [CrossRef]
- Khan, S.; Sayed, M.; Sohail, M.; Shah, L.A.; Raja, M.A. Advanced Oxidation and Reduction Processes. In Advances in Water Purification Techniques; Ahuja, S., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 135–164. ISBN 9780128147917. [Google Scholar]
- Hassaan, M.A.; El Nemr, A.; Madkour, F.F. Advanced oxidation processes of Mordant Violet 40 dye in freshwater and seawater. Egypt. J. Aquat. Res. 2017, 43, 1–9. [Google Scholar] [CrossRef]
- Muniyasamy, A.; Sivaporul, G.; Gopinath, A.; Lakshmanan, R.; Altaee, A.; Achary, A.; Velayudhaperumal Chellam, P. Process development for the degradation of textile azo dyes (mono-, di-, poly-) by advanced oxidation process - Ozonation: Experimental & partial derivative modelling approach. J. Environ. Manag. 2020, 265, 110397. [Google Scholar]
- Colindres, P.; Yee-Madeira, H.; Reguera, E. Removal of Reactive Black 5 from aqueous solution by ozone for water reuse in textile dyeing processes. Desalination 2010, 258, 154–158. [Google Scholar] [CrossRef]
- Zhang, F.; Yediler, A.; Liang, X.; Kettrup, A. Effects of dye additives on the ozonation process and oxidation by-products: A comparative study using hydrolyzed C.I. Reactive Red 120. Dye. Pigment. 2004, 60, 1–7. [Google Scholar] [CrossRef]
- Hassaan, M.A.; El Nemr, A.; Madkour, F.F. Testing the advanced oxidation processes on the degradation of Direct Blue 86 dye in wastewater. Egypt. J. Aquat. Res. 2017, 43, 11–19. [Google Scholar] [CrossRef]
- Munter, R. Advanced Oxidation Processes—Current Status and Prospects. In Proceeding of the Estonian Academy Sciences, Tartu, Estonia, 1 January 2001; pp. 59–80. [Google Scholar]
- Khan, R.; Inam, M.A.; Zam Zam, S.; Akram, M.; Shin, S.; Yeom, I.T. Coagulation and Dissolution of CuO Nanoparticles in the Presence of Dissolved Organic Matter under Different pH Values. Sustainability 2019, 11, 2825. [Google Scholar] [CrossRef]
- Al-Shannag, M.; Al-Qodah, Z.; Bani-Melhem, K.; Qtaishat, M.R.; Alkasrawi, M. Heavy metal ions removal from metal plating wastewater using electrocoagulation: Kinetic study and process performance. Chem. Eng. J. 2015, 260, 749–756. [Google Scholar] [CrossRef]
- Chen, X.; Ren, P.; Li, T.; Trembly, J.P.; Liu, X. Zinc removal from model wastewater by electrocoagulation: Processing, kinetics and mechanism. Chem. Eng. J. 2018, 349, 358–367. [Google Scholar] [CrossRef]
- Vasudevan, S.; Lakshmi, J. Process conditions and kinetics for the removal of copper from water by electrocoagulation. Environ. Eng. Sci. 2012, 29, 563–572. [Google Scholar] [CrossRef]
- Khattab, I.A.; Shaffei, M.F.; Shaaban, N.A.; Hussein, H.S.; Abd El-Rehim, S.S. Study the kinetics of electrochemical removal of copper from dilute solutions using packed bed electrode. Egypt. J. Pet. 2014, 23, 93–103. [Google Scholar] [CrossRef][Green Version]
- Prasetyaningrum, A.; Widayat, W.; Jos, B.; Dharmawan, Y.; Ratnawati, R. UV Irradiation and Ozone Treatment of κ-Carrageenan: Kinetics and Products Characteristics. Bull. Chem. React. Eng. Catal. 2020, 15, 319–330. [Google Scholar] [CrossRef]
- Muruganandham, M.; Suri, R.P.S.; Jafari, S.; Sillanpää, M.; Lee, G.-J.; Wu, J.J.; Swaminathan, M. Recent Developments in Homogeneous Advanced Oxidation Processes for Water and Wastewater Treatment. Int. J. Photoenergy 2014, 2014, 821674. [Google Scholar] [CrossRef]
- Asghar, A.; Raman, A.A.A.; Daud, W.M.A.W. Advanced oxidation processes for in-situ production of hydrogen peroxide/hydroxyl radical for textile wastewater treatment: A review. J. Clean. Prod. 2015, 87, 826–838. [Google Scholar] [CrossRef]






| C0 (mg/L) | CO3 (mg/h) | IUV (mW/cm2) | pH | Kinetic Parameters | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| First-Order | Second-Order | Pseudo-First-Order | ||||||||
| k1 (1/min) | R2 | k2 (L/mg·min) | R2 | kp (1/min) | Ce (mg/L) | R2 | ||||
| 145.73 | 10 | 40 | 6 | 0.0069 | 0.9997 | 5.673 × 10−5 | 0.9921 | 0.0069 | 0.000 | 0.9994 |
| 72.86 | 10 | 40 | 6 | 0.0128 | 0.9993 | 2.451 × 10−4 | 0.9678 | 0.0127 | 0.000 | 0.9983 |
| 36.9 | 10 | 40 | 6 | 0.0196 | 0.9995 | 8.898 × 10−4 | 0.9707 | 0.0237 | 3.769 | 0.9995 |
| 36.9 | 5 | 40 | 6 | 0.0111 | 0.9998 | 3.972 × 10−4 | 0.9875 | 0.0116 | 1.223 | 0.9993 |
| 36.9 | 15 | 40 | 6 | 0.0267 | 0.9959 | 1.480 × 10−3 | 0.9624 | 0.0395 | 5.528 | 0.9979 |
| 36.9 | 10 | 20 | 6 | 0.0173 | 0.9996 | 7.407 × 10−4 | 0.9609 | 0.0188 | 1.850 | 0.9990 |
| 36.9 | 10 | 60 | 6 | 0.0198 | 0.9989 | 9.031 × 10−4 | 0.9654 | 0.0252 | 4.663 | 0.9969 |
| 36.9 | 10 | 40 | 3 | 0.0116 | 0.9847 | 4.160 × 10−4 | 0.9790 | 0.0330 | 12.126 | 0.9940 |
| 36.9 | 10 | 40 | 8 | 0.0282 | 0.9980 | 1.650 × 10−3 | 0.9359 | 0.0376 | 4.170 | 0.9962 |
| 36.9 | 10 | 40 | 10 | 0.0226 | 0.9904 | 1.100 × 10−3 | 0.9909 | 0.0381 | 7.725 | 0.9970 |
| Parameters | Coefficients | Standard Error | p-value |
|---|---|---|---|
| Intercept | −5.1661 | 0.3951 | 3.5663 × 10−6 |
| ln C0 | −0.7401 | 0.0441 | 6.4919 × 10−7 |
| ln CO3 | 0.8154 | 0.0778 | 1.5626 × 10−5 |
| ln IUV | 0.1441 | 0.0778 | 0.1064 |
| ln pH | 0.8407 | 0.0839 | 2.1105 × 10−5 |
| df | SS | MS | F | Significance F | |
|---|---|---|---|---|---|
| Regression | 4 | 1.7467 | 0.4367 | 113.4928 | 1.9188 × 10−6 |
| Residual | 7 | 0.0269 | 0.0038 | ||
| Total | 11 | 1.7736 | |||
| Multiple R | 0.9924 | ||||
| R2 | 0.9848 | ||||
| Adjusted R2 | 0.9761 | ||||
| Standard error | 0.0620 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prasetyaningrum, A.; Riyanto, T.; Djaeni, M.; Widayat, W. Photochemical Oxidation Process of Copper from Electroplating Wastewater: Process Performance and Kinetic Study. Processes 2020, 8, 1276. https://doi.org/10.3390/pr8101276
Prasetyaningrum A, Riyanto T, Djaeni M, Widayat W. Photochemical Oxidation Process of Copper from Electroplating Wastewater: Process Performance and Kinetic Study. Processes. 2020; 8(10):1276. https://doi.org/10.3390/pr8101276
Chicago/Turabian StylePrasetyaningrum, Aji, Teguh Riyanto, Mohamad Djaeni, and Widayat Widayat. 2020. "Photochemical Oxidation Process of Copper from Electroplating Wastewater: Process Performance and Kinetic Study" Processes 8, no. 10: 1276. https://doi.org/10.3390/pr8101276
APA StylePrasetyaningrum, A., Riyanto, T., Djaeni, M., & Widayat, W. (2020). Photochemical Oxidation Process of Copper from Electroplating Wastewater: Process Performance and Kinetic Study. Processes, 8(10), 1276. https://doi.org/10.3390/pr8101276








