Design and Development of Novel Continuous Flow Stirred Multiphase Reactor: Liquid–Liquid–Liquid Phase Transfer Catalysed Synthesis of Guaiacol Glycidyl Ether
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
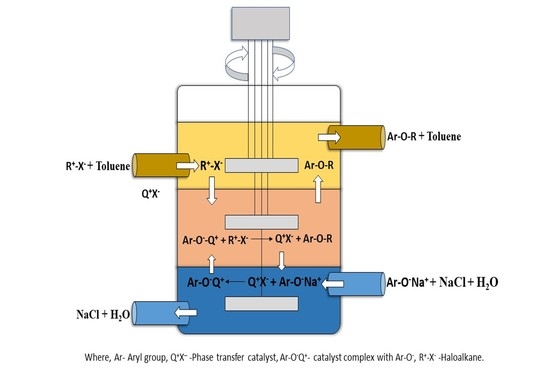
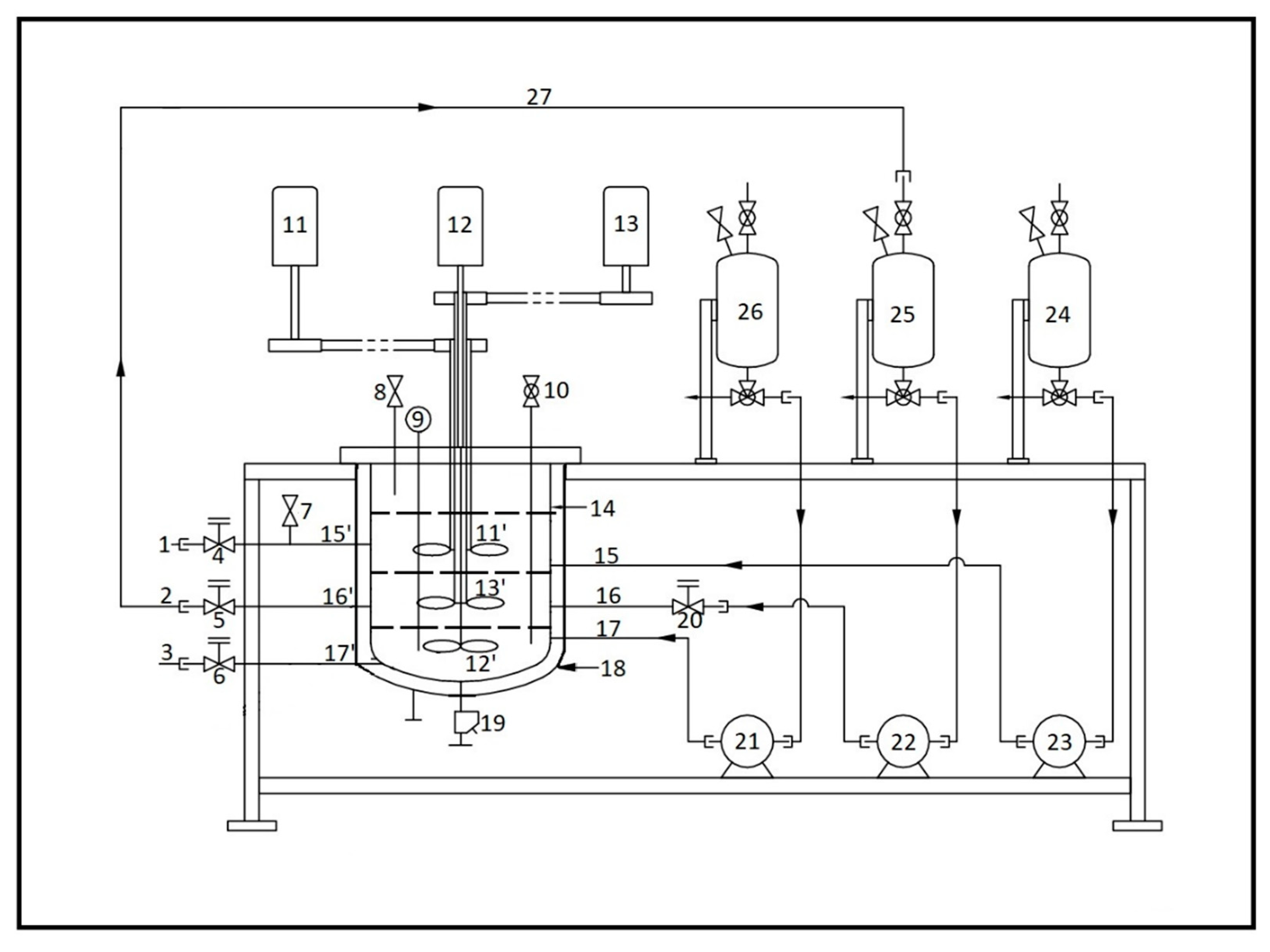
2.2. Experimental Setup
2.3. Reaction Procedure
2.4. Method of Analysis
3. Results and Discussion
3.1. Batch Stirred Multiphase Reactor
3.1.1. Effect of Speed of Agitation in a Batch Stirred Multiphase Reactor
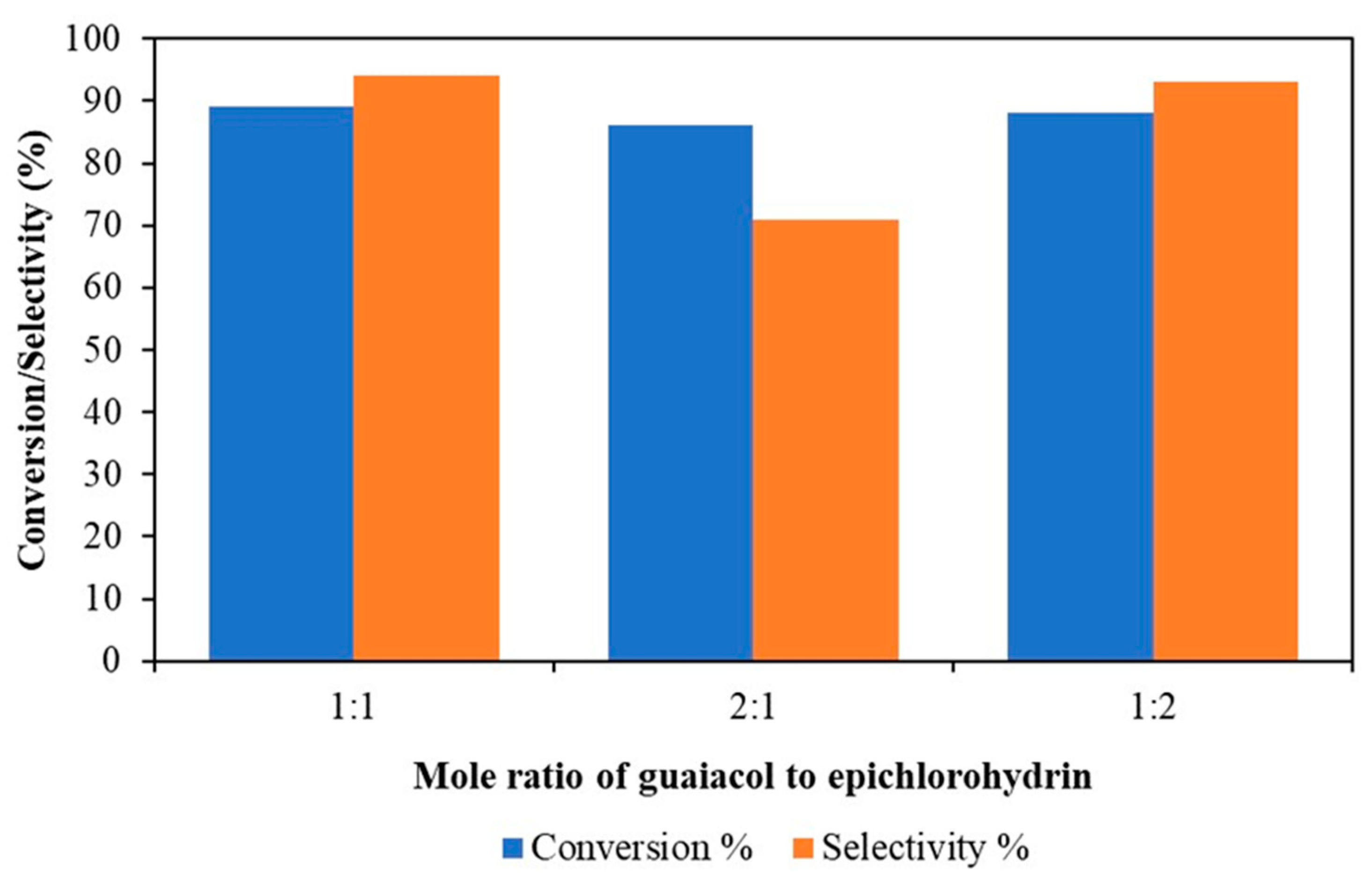
3.1.2. Effect of Mole Ratio of Guaiacol to Epichlorohydrin
3.1.3. Effect of Mole Ratio of Guaiacol to Sodium Hydroxide
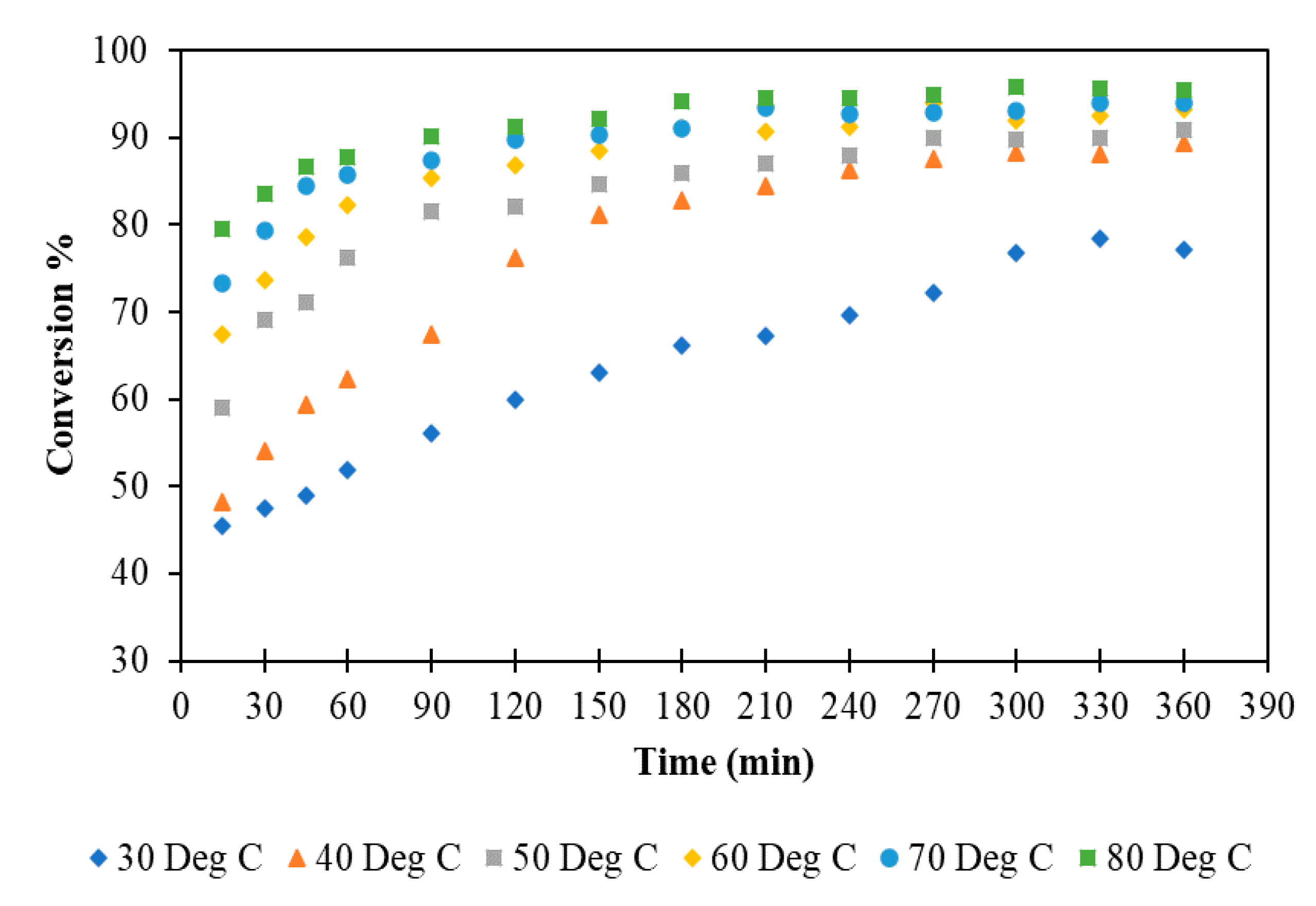
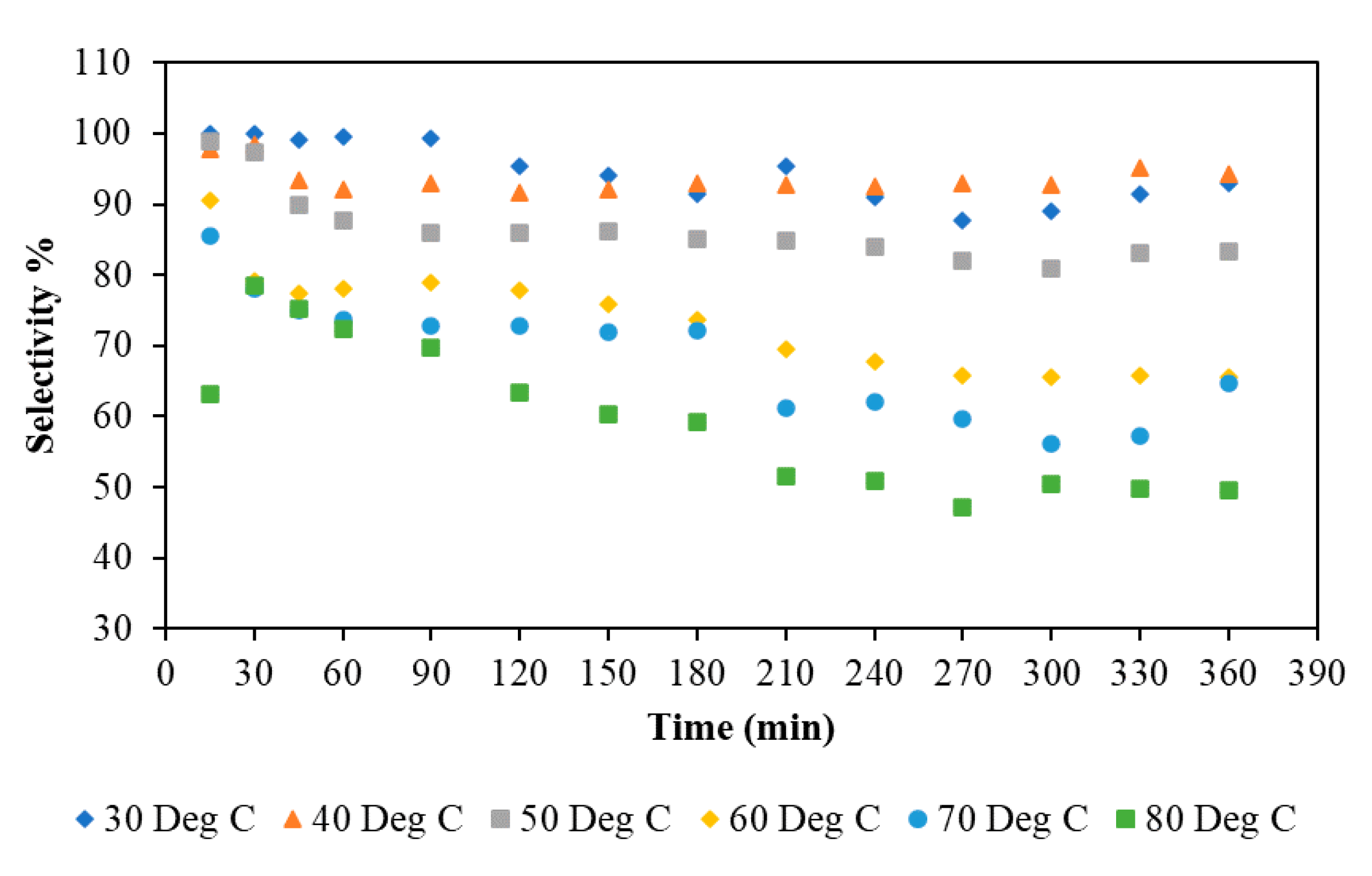
3.1.4. Effect of Temperature
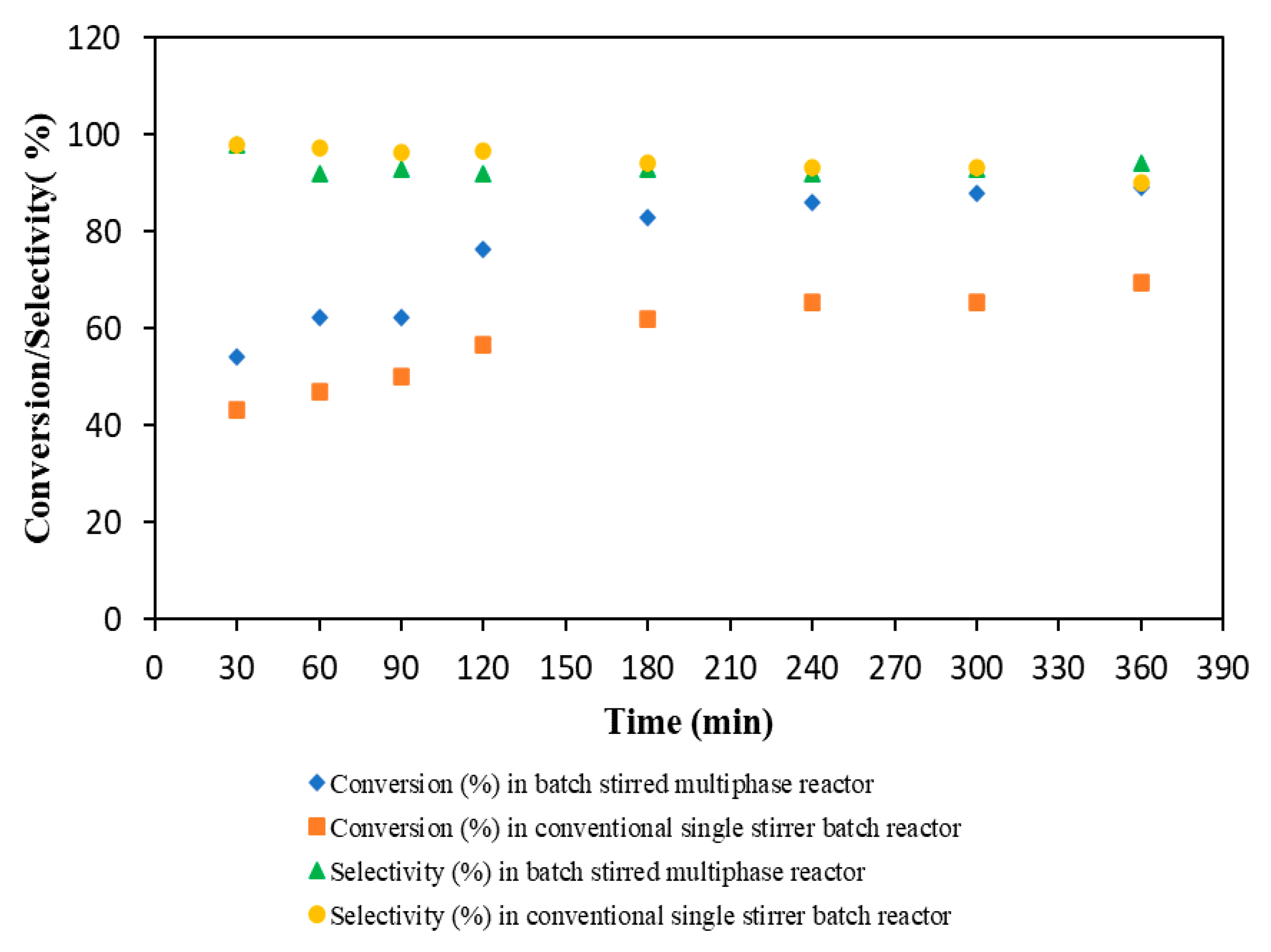
3.1.5. Comparison of Batch Stirred Multiphase Reactor with Conventional Single Stirrer PTC Batch Reactor
3.2. Continuous Flow Stirred Multiphase Reactor
3.2.1. The Effect of Speed of Agitation in a Continuous Flow Stirred Multiphase Reactor
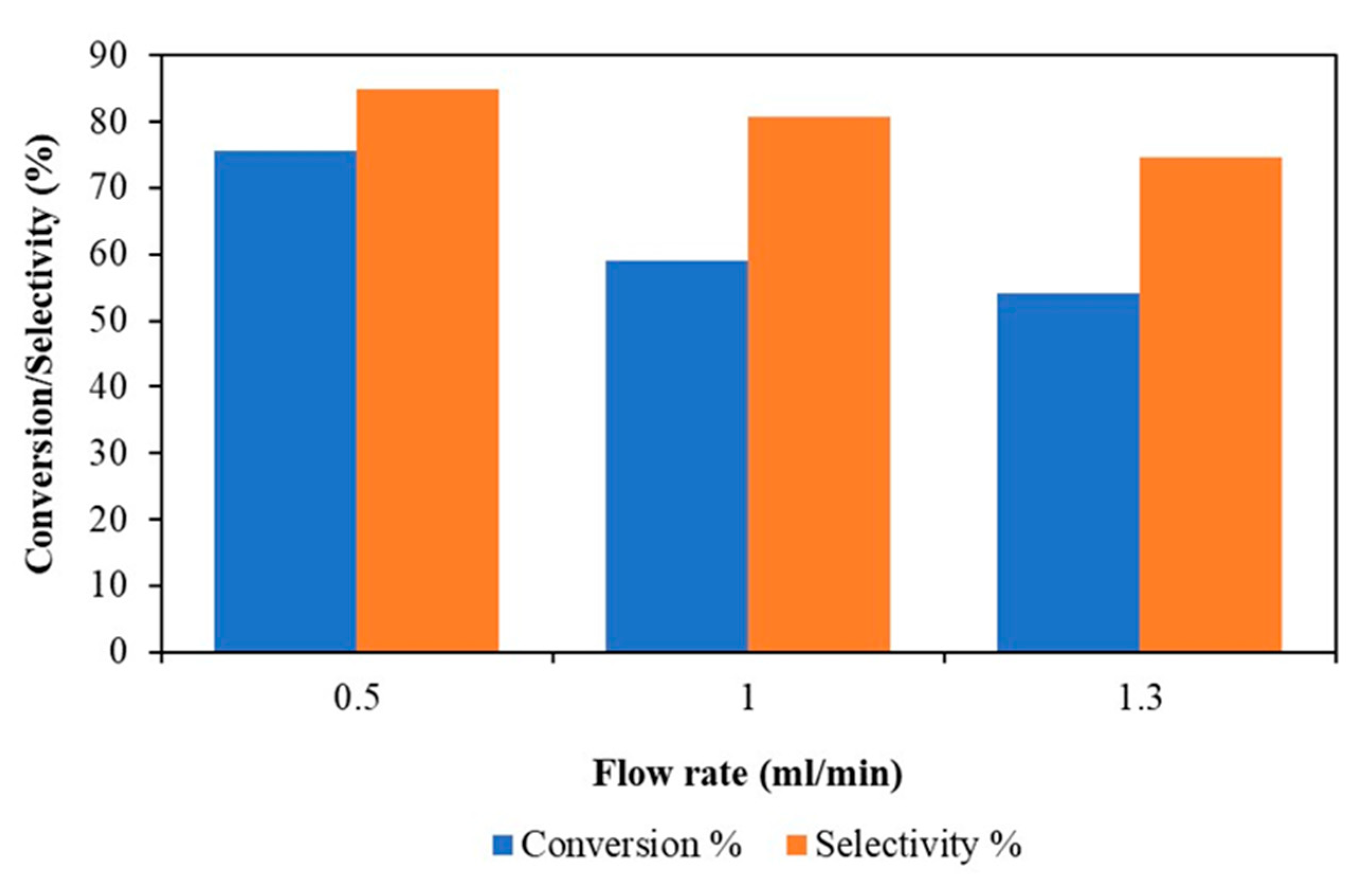
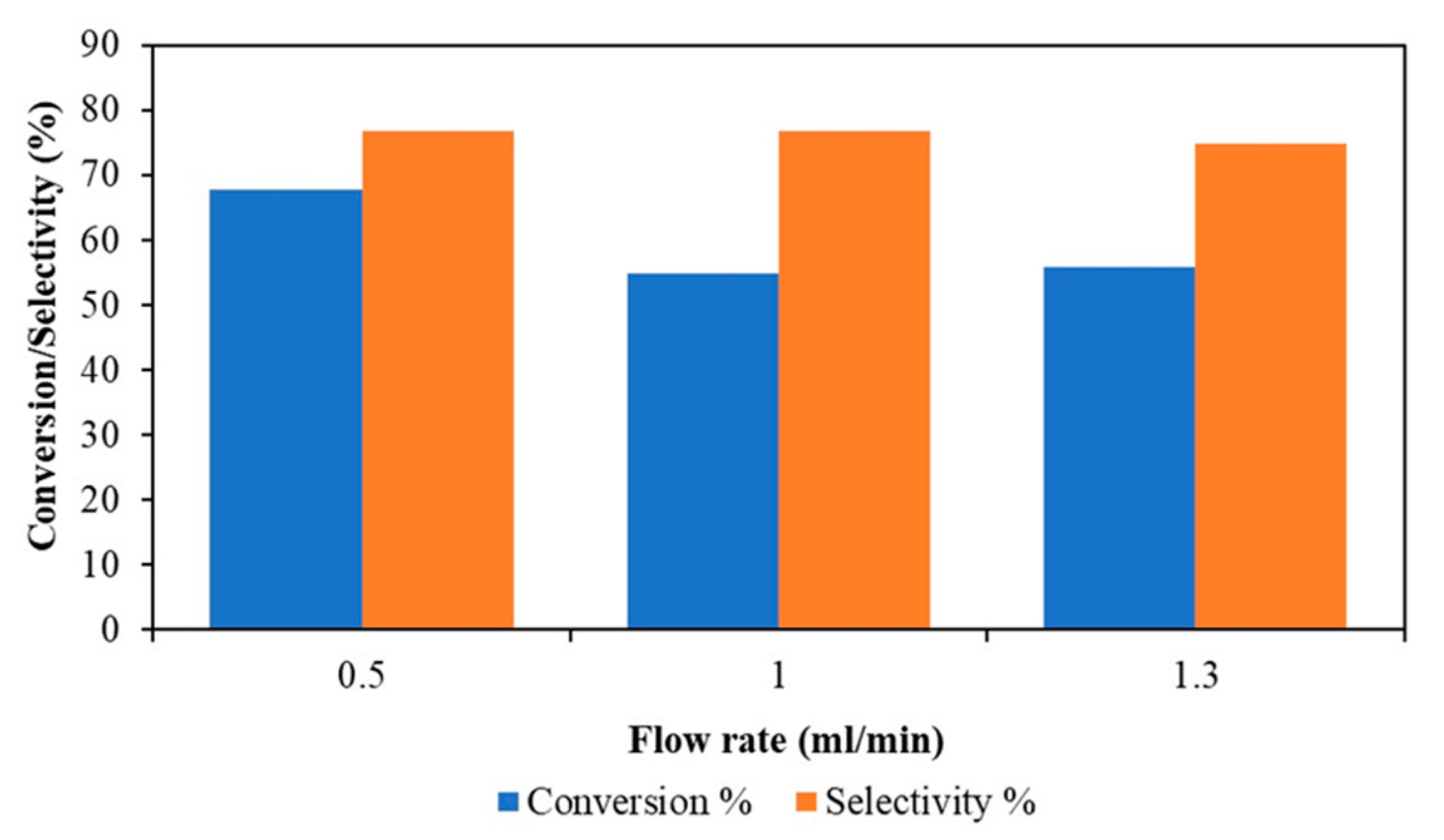
3.2.2. Effect of Flow Rate in a Continuous Flow Stirred Multiphase Reactor
3.2.3. Effect of Middle Catalyst Phase Recirculation in a Continuous Flow Stirred Multiphase Reactor
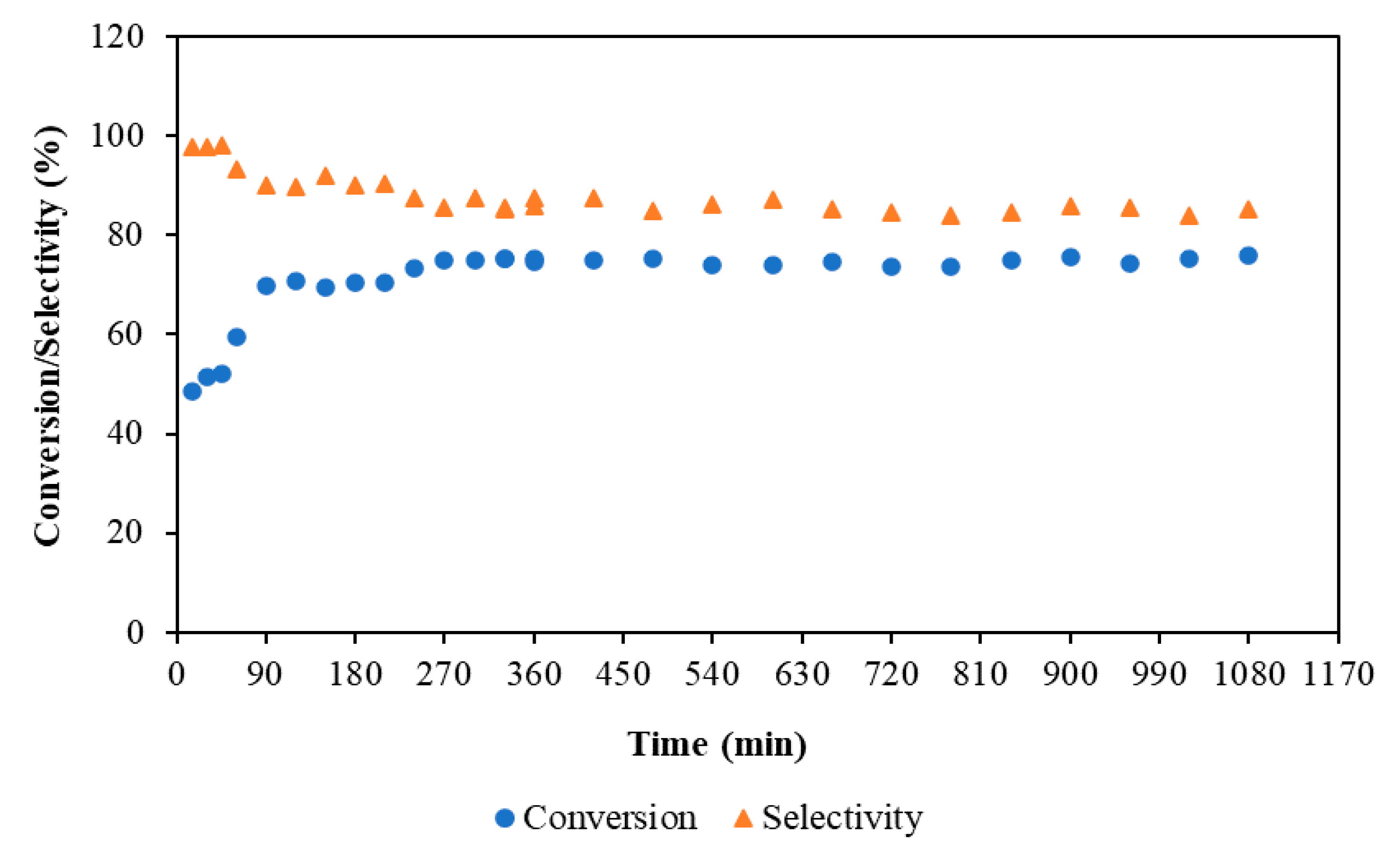
3.2.4. Time on Stream Study in a Continuous Flow Stirred Multiphase Reactor
4. Conclusions
5. Patent
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Starks, C.M.; Liotta, C. Phase Transfer Catalysis Principles and Techniques; Academic Press: New York, NY, USA, 1978. [Google Scholar]
- Makosza, M. Phase-transfer catalysis. A general green methodology in organic synthesis. Pure Appl. Chem. 2000, 72, 1399–1403. [Google Scholar] [CrossRef]
- Naik, S.D.; Doraiswamy, L.K. Phase transfer catalysis: Chemistry and engineering. AIChE J. 1998, 44, 612–646. [Google Scholar] [CrossRef]
- Yadav, G.D.; Sowbna, P.R. Process intensification and waste minimization in liquid–liquid–liquid phase transfer catalyzed selective synthesis of mandelic acid. Chem. Eng. Res. Des. 2012, 90, 1281–1291. [Google Scholar] [CrossRef]
- Yadav, G.D. Insight into Green Phase Transfer Catalysis. Top. Catal. 2004, 29, 145–161. [Google Scholar] [CrossRef]
- Wang, D.H.; Weng, H.S. Preliminary study on the role played by third liquid phase in phase transfer catalysis. Chem. Eng. Sci. 1988, 43, 2019–2024. [Google Scholar] [CrossRef]
- Mason, D.; Magdassi, S.; Sasson, Y. Role of a third liquid phase in phase-transfer catalysis. J. Org. Chem. 1991, 56, 7229–7232. [Google Scholar] [CrossRef]
- Wang, D.H.; Weng, H.S. Solvent and salt effects on the formation of third liquid phase and the reaction mechanisms in the phase transfer catalysis system—Reaction between N-butyl bromide and sodium phenolate. Chem. Eng. Sci. 1995, 50, 3477–3486. [Google Scholar] [CrossRef]
- Hsiao, H.C.; Kao, S.M.; Weng, H.S. Synthesis of n-Butyl Phenyl Ether by Tri-Liquid-Phase Catalysis Using Poly(ethylene glycol)-600 as a Catalyst. 1. Analysis of Factors Affecting the Formation of a Third Liquid Phase. Ind. Eng. Chem. Res. 2000, 39, 2772–2778. [Google Scholar] [CrossRef]
- Ohtani, N.; Ohta, T.; Hosoda, Y.; Yamashita, T. Phase Behavior and Phase-Transfer Catalysis of Tetrabutylammonium Salts. Interface-Mediated Catalysis. Langmuir 2004, 20, 409–415. [Google Scholar] [CrossRef]
- Yadav, G.D.; Jadhav, Y.B.; Sengupta, S. Selectivity engineered phase transfer catalysis in the synthesis of fine chemicals: Reactions of p-chloronitrobenzene with sodium sulphide. J. Mol. Catal. A Chem. 2003, 200, 117–129. [Google Scholar] [CrossRef]
- Yadav, G.D.; Badure, O.V. Role of Third Phase in Intensification of Reaction Rates and Selectivity: Phase-Transfer Catalyzed Synthesis of Benzyl Phenyl Ether. Ind. Eng. Chem. Res. 2007, 46, 8448–8458. [Google Scholar] [CrossRef]
- Yadav, G.D.; Sowbna, P.R. Selectivity Engineering in Synthesis of 4-Benzyloxy Propiophenone Using Liquid–Liquid–Liquid Phase-Transfer Catalysis. Ind. Eng. Chem. Res. 2012, 51, 3256–3264. [Google Scholar] [CrossRef]
- Yadav, G.D.; Motirale, B.G. Selective oxidation of methyl mandelate to methyl phenyl glyoxylate using liquid–liquid–liquid phase transfer catalysis. Chem. Eng. J. 2010, 156, 328–336. [Google Scholar] [CrossRef]
- Katole, D.O.; Yadav, G.D. Process intensification and waste minimization using liquid-liquid-liquid tri-phase transfer catalysis for the synthesis of 2-((benzyloxy)methyl)furan. Mol. Catal. 2019, 466, 112–121. [Google Scholar] [CrossRef]
- Yang, H.; Chen, Y. Ultrasound-assisted third-liquid phase-transfer catalyzed esterification of potassium 4-methoxyphenylacetate by dual-site phase-transfer catalyst. J. Taiwan Inst. Chem. Eng. 2012, 43, 897–903. [Google Scholar] [CrossRef]
- Yang, H.M.; Lin, D.W. Third-liquid phase-transfer catalyzed esterification of sodium benzoate with novel dual-site phase-transfer catalyst under ultrasonic irradiation. Catal. Commun. 2011, 14, 101–106. [Google Scholar] [CrossRef]
- Yang, H.M.; Chen, C.H. Catalytic esterification of sodium salicylate in third-liquid phase under ultrasound-assisted tri-liquid phase-transfer catalysis. J. Mol. Catal. A Chem. 2009, 312, 107–113. [Google Scholar] [CrossRef]
- Huang, C.C.; Yang, H.M. Kinetics for benzoylation of sodium 4-acetylphenoxide via third-liquid phase in the phase-transfer catalysis. Appl. Catal. A Gen. 2005, 290, 65–72. [Google Scholar] [CrossRef]
- Yang, H.-M.; Huang, C.-C. Catalytic Benzoylation of 4-Chloro-3-methylphenol Sodium Salt in Third-Liquid Phase under Conditions of Phase-Transfer Catalysis. Ind. Eng. Chem. Res. 2007, 46, 7915–7920. [Google Scholar] [CrossRef]
- Yang, H.; Hung, Y.; Tu, C. Synthesis of butyl salicylate by phase-transfer catalysis with dual-site phase-transfer catalyst and ionic liquid in tri-liquid system. J. Taiwan Inst. Chem. Eng. 2014, 45, 1421–1427. [Google Scholar] [CrossRef]
- Yang, H.M.; Li, C.C. Kinetics for synthesizing benzyl salicylate by third-liquid phase-transfer catalysis. J. Mol. Catal. A Chem. 2006, 246, 255–262. [Google Scholar] [CrossRef]
- Kopeć, D.; Baj, S.; Siewniak, A. Ultrasound-Assisted Green Synthesis of Dialkyl Peroxides under Phase-Transfer Catalysis Conditions. Molecules 2020, 25, 118. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Sun, J.; Liu, B.; He, J. Novel kinetics model for third-liquid phase-transfer catalysis system of the “complex” carbanion: Competitive role between catalytic cycles. Chem. Eng. J. 2015, 280, 782–795. [Google Scholar] [CrossRef]
- Zhao, Q.; Yang, L.; Shen, Y. Third-Liquid Phase Transfer Catalysis for Horner–Wadsworth–Emmons Reactions of “Moderately Acidic” and “Weakly Acidic” Phosphonates. Ind. Eng. Chem. Res. 2016, 55, 7604–7611. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhao, X.; Sun, J.; Yang, L.; Shen, Y. Catalytic Process for the Hydroxide-Initiated Reaction of the “Weakly Acidic” Substrate in the Third-Liquid Phase-Transfer Catalytic System. Ind. Eng. Chem. Res. 2018, 57, 13318–13326. [Google Scholar] [CrossRef]
- Yadav, G.D.; Sowbna, P.R. Modeling of microwave irradiated liquid–liquid–liquid (MILLL) phase transfer catalyzed green synthesis of benzyl thiocyanate. Chem. Eng. J. 2012, 179, 221–230. [Google Scholar] [CrossRef]
- Yadav, G.D.; Reddy, C.A. Kinetics of the n-Butoxylation of p-Chloronitrobenzene under Liquid−Liquid−Liquid Phase Transfer Catalysis. Ind. Eng. Chem. Res. 1999, 38, 2245–2253. [Google Scholar] [CrossRef]
- Weng, H.S.; Wang, C.M.; Wang, D.H. A Preliminary Study on a Continuous Flow Stirred Vessel Reactor for Tri-Liquid-Phase Phase Transfer Catalysis. Ind. Eng. Chem. Res. 1997, 36, 3613–3618. [Google Scholar] [CrossRef]
- Yang, H.M.; Peng, G.Y. Ultrasound-assisted third-liquid phase-transfer catalyzed esterification of sodium salicylate in a continuous two-phase-flow reactor. Ultrason. Sonochem. 2010, 17, 239–245. [Google Scholar] [CrossRef]
- Yang, H.M.; Huang, Y.S. Green benzylation of sodium salicylate by phase-transfer catalysis with third-liquid phase in a continuous two-phase-flow reactor. J. Taiwan Inst. Chem. Eng. 2011, 42, 265–270. [Google Scholar] [CrossRef]
- Yadav, G.D. An Improved Continuous Flow Stirred Multiphase Reactor. Patent PCT/IN2018/050535, 21 February 2019. [Google Scholar]
- The Engineering ToolBox. Available online: https://www.engineeringtoolbox.com/paraffinic-benzoic-hydroxy-dioic-acids-structure-pka-carboxylic-dissociation-constant-alcohol-phenol-d_1948.html (accessed on 19 September 2020).







© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Margi, N.H.; Yadav, G.D. Design and Development of Novel Continuous Flow Stirred Multiphase Reactor: Liquid–Liquid–Liquid Phase Transfer Catalysed Synthesis of Guaiacol Glycidyl Ether. Processes 2020, 8, 1271. https://doi.org/10.3390/pr8101271
Margi NH, Yadav GD. Design and Development of Novel Continuous Flow Stirred Multiphase Reactor: Liquid–Liquid–Liquid Phase Transfer Catalysed Synthesis of Guaiacol Glycidyl Ether. Processes. 2020; 8(10):1271. https://doi.org/10.3390/pr8101271
Chicago/Turabian StyleMargi, Nikhil H., and Ganapati D. Yadav. 2020. "Design and Development of Novel Continuous Flow Stirred Multiphase Reactor: Liquid–Liquid–Liquid Phase Transfer Catalysed Synthesis of Guaiacol Glycidyl Ether" Processes 8, no. 10: 1271. https://doi.org/10.3390/pr8101271
APA StyleMargi, N. H., & Yadav, G. D. (2020). Design and Development of Novel Continuous Flow Stirred Multiphase Reactor: Liquid–Liquid–Liquid Phase Transfer Catalysed Synthesis of Guaiacol Glycidyl Ether. Processes, 8(10), 1271. https://doi.org/10.3390/pr8101271