Enhanced Degradation of Phenolic Compounds in Coal Gasification Wastewater by Methods of Microelectrolysis Fe-C and Anaerobic-Anoxic—Oxic Moving Bed Biofilm Reactor (A2O-MBBR)
Abstract
1. Introduction
- The effect of electric fields: The electrical field generated microcells in wastewater could trigger the movement of pollutants to move to opposite electrode, where redox reaction would occur against charged pollutants. As a result, chemical structure of the pollutants would be transformed or degraded.
- The reducing effect of hydrogen:In the acidic medium, the following reaction produces atoms [H].Fe + 2H+ → Fe2+ + 2 [H]Then, atoms [H] will reduce pollutants. For example, group pollutants will be reduced and converted to amino group (NH2) compounds.
- The effect of metallic iron: The metallic elements that have weaker reactivity than iron could exchange electrons on the metallic iron surface. Then metal ions with strong toxicity or organic substances will be reduced by iron to metal ions in a less toxic state. For example, Cr(VI) having E0 (/Cr3+) = 1.36 V and strong oxidizing properties would react with metal iron:Then, Cr(VI) with strong oxidizing properties will be converted to Cr(III) with weak reducing properties.In acidic conditions, iron also reduce organic substances containing group to NH2 group.C6H5NO2 + 3Fe + 6H+ → C6H5NH2 + 3Fe2+ + 2H2O
- Reducing effect of Fe2+ ions: Iron oxidized to iron Fe2+, Fe2+ has high reducing properties. For Cr (VI), the reduction reaction occurs as follows.6Fe2+ + 14H+ → 6Fe3+ + 2Cr3+ + 7 H2O
- The flocculation effect of iron ions: In acidic wastewater, metallic iron will corrode, thus expediting the production of Fe2+ and Fe3+ ions. In the presence of O2, following reactions will occur in alkaline environments.Fe2+ + 2OH− → Fe(OH)24Fe2+ + 8OH− + O2 + 2H2O → 4Fe(OH)3The generated Fe (OH)2 and Fe(OH)3 could adsorb organic substances, thus contributing to the treatment process.
- The effect of chemical precipitation: Fe2+ and Fe3+ ions, when being in contact with anions, precipitate into compounds such as FeS, Fe3[Fe(CN)6]2, and Fe4[Fe(CN)6]3. All of which could settle quickly and are easily removed from the wastewater.
2. Materials and Methods
2.1. Fabrication of Materials
2.2. Establishment of the A2O-MBBR System
2.2.1. Culturing Aerobic Activated Sludge
2.2.2. Culturing Anoxic Activated Sludge
2.2.3. Culturing Oxic Activated Sludge
2.2.4. Activated Sludge Culture
3. Results and Discussion
3.1. Characterization of As-Synthesized Fe-C Material
3.1.1. SEM-EDS Analysis of Fe-C
3.1.2. XRD Analysis of Fe-C
3.2. Wastewater Treatment Using Fe-C Internal Electrolysis Material
3.3. Wastewater Treatment Using A2O-MBBR System
3.3.1. pH Change in the A2O-MBBR System
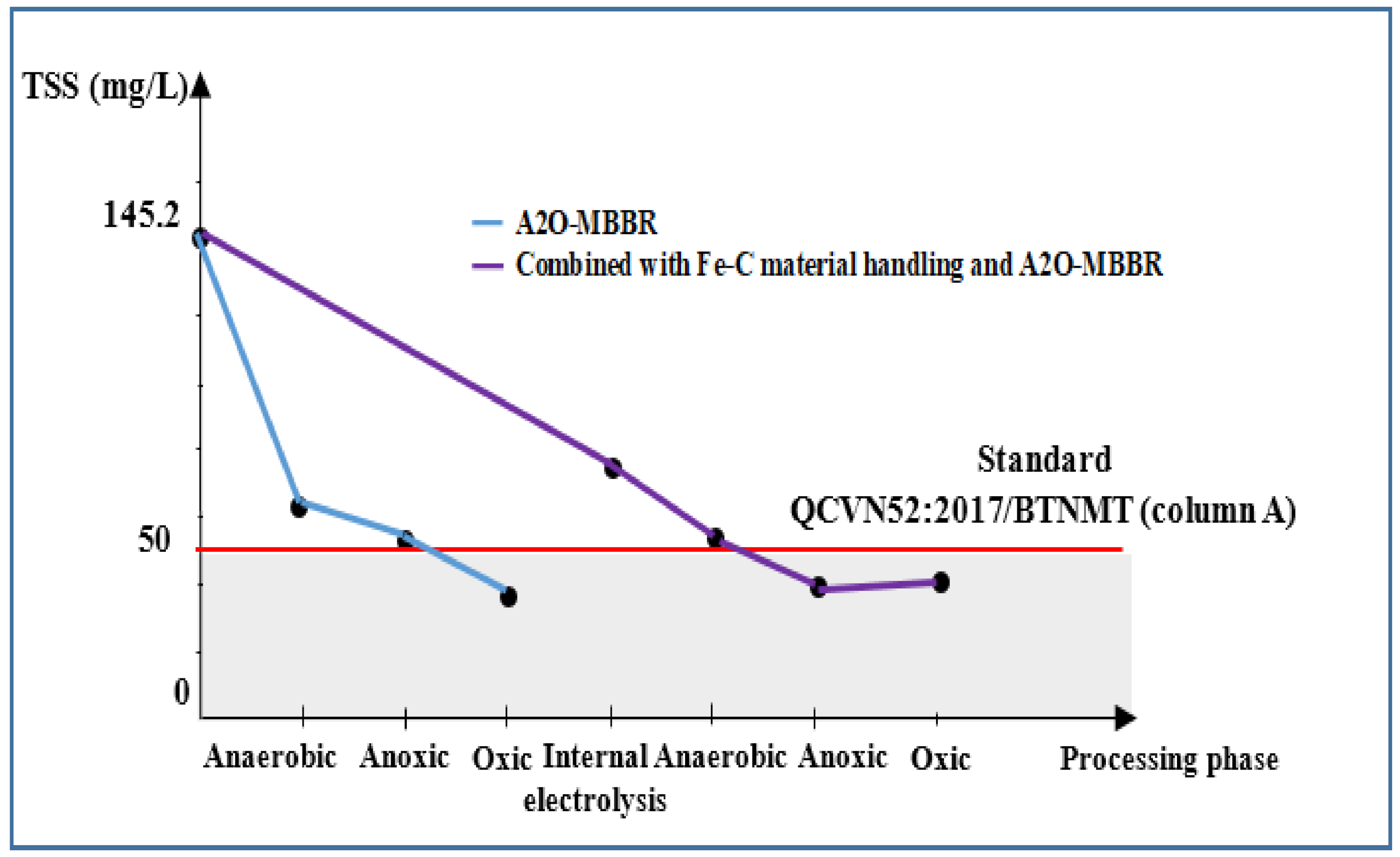
3.3.2. TSS Change in A2O-MBBR System
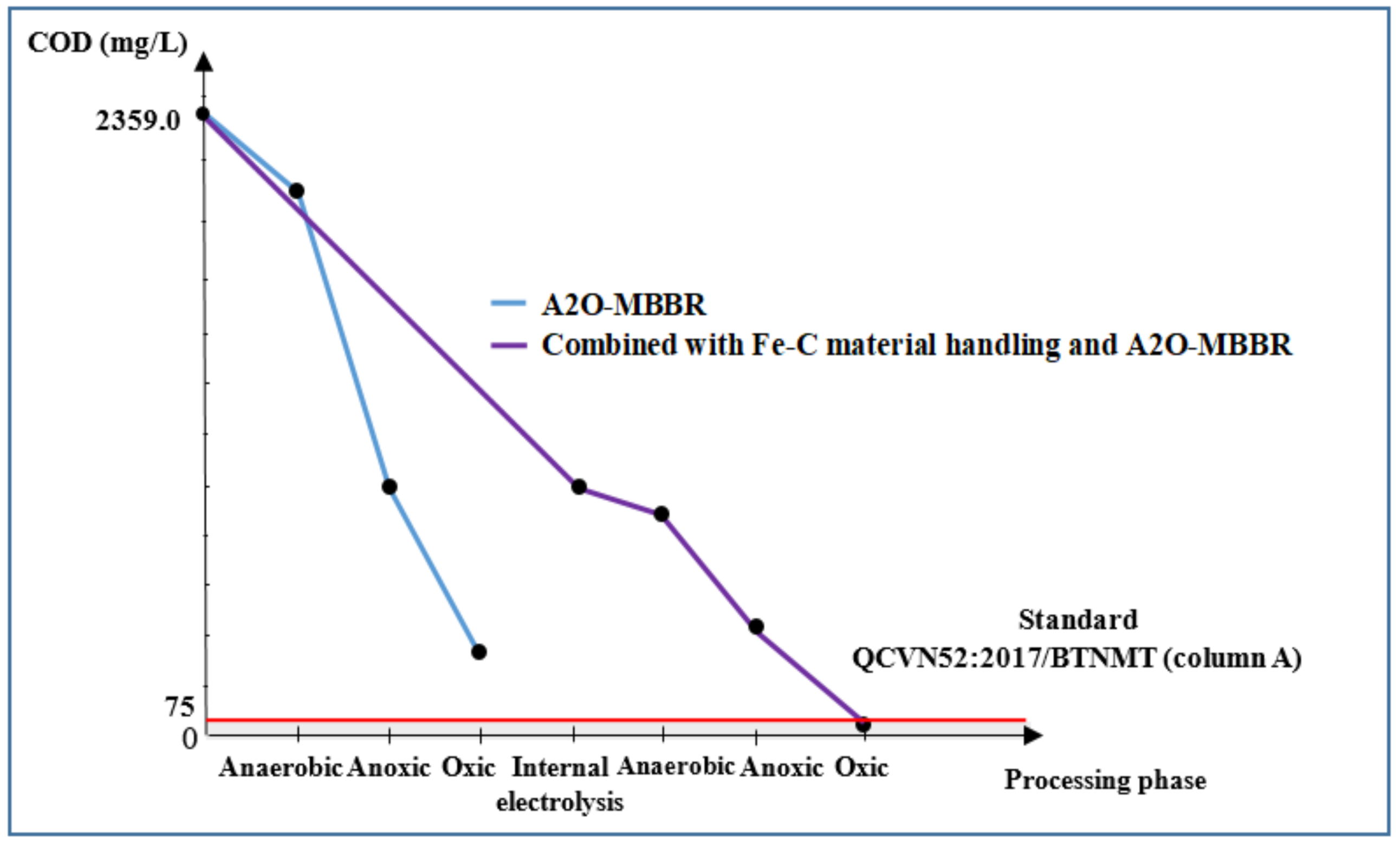
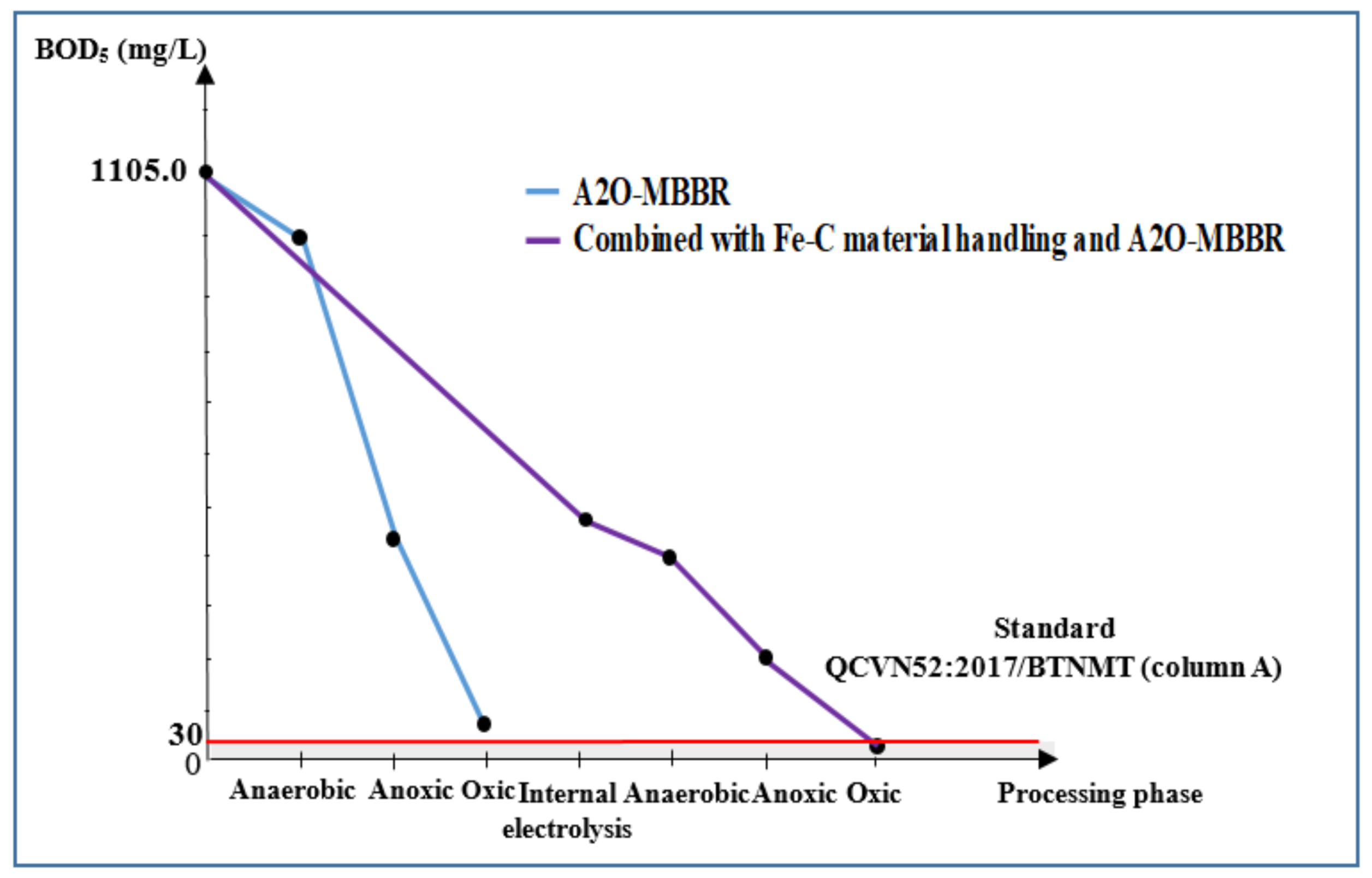
3.3.3. COD and BOD5 of Wastewater Treated Using Internal Electrolysis and A2O-MBBR System
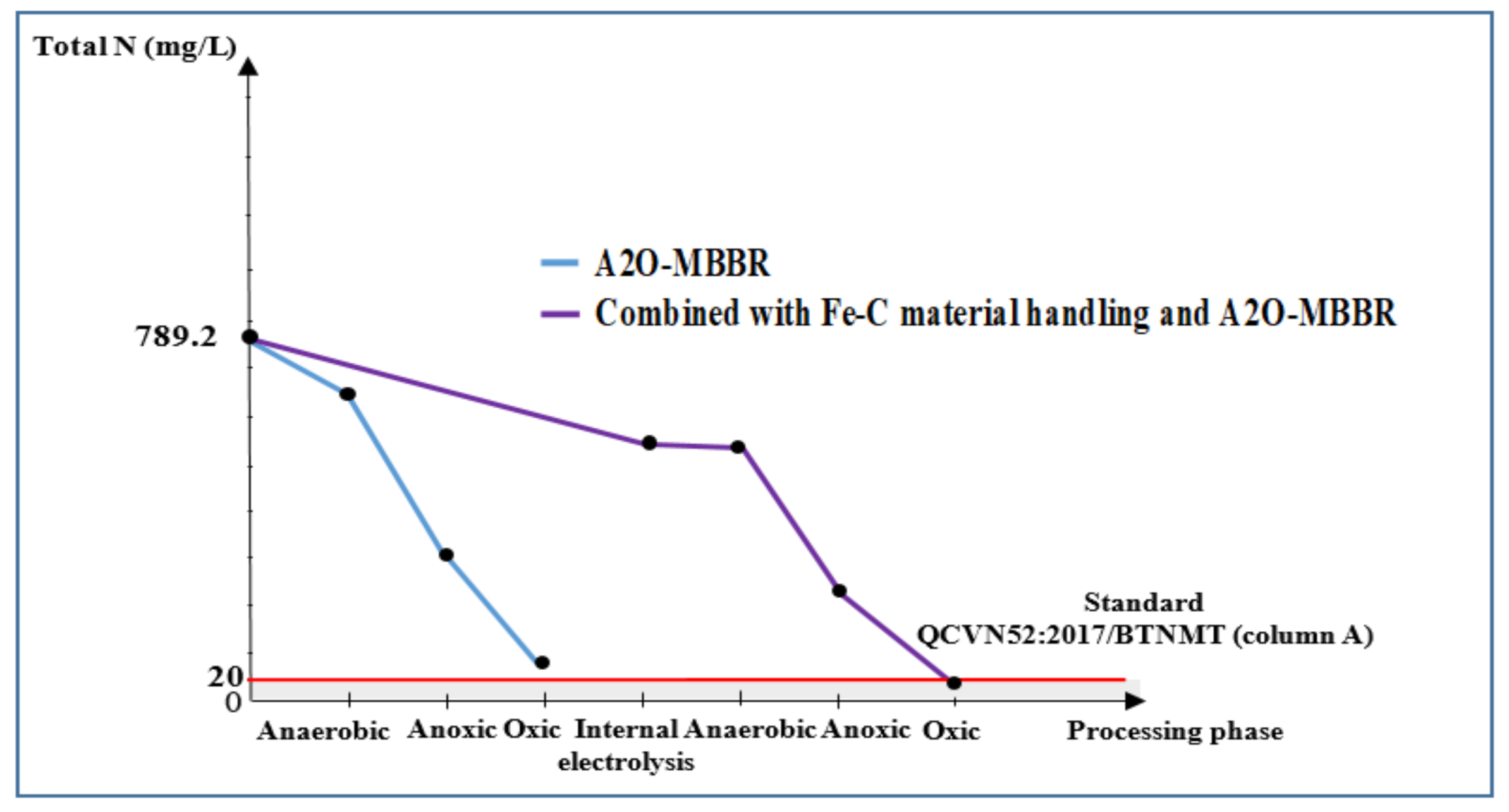
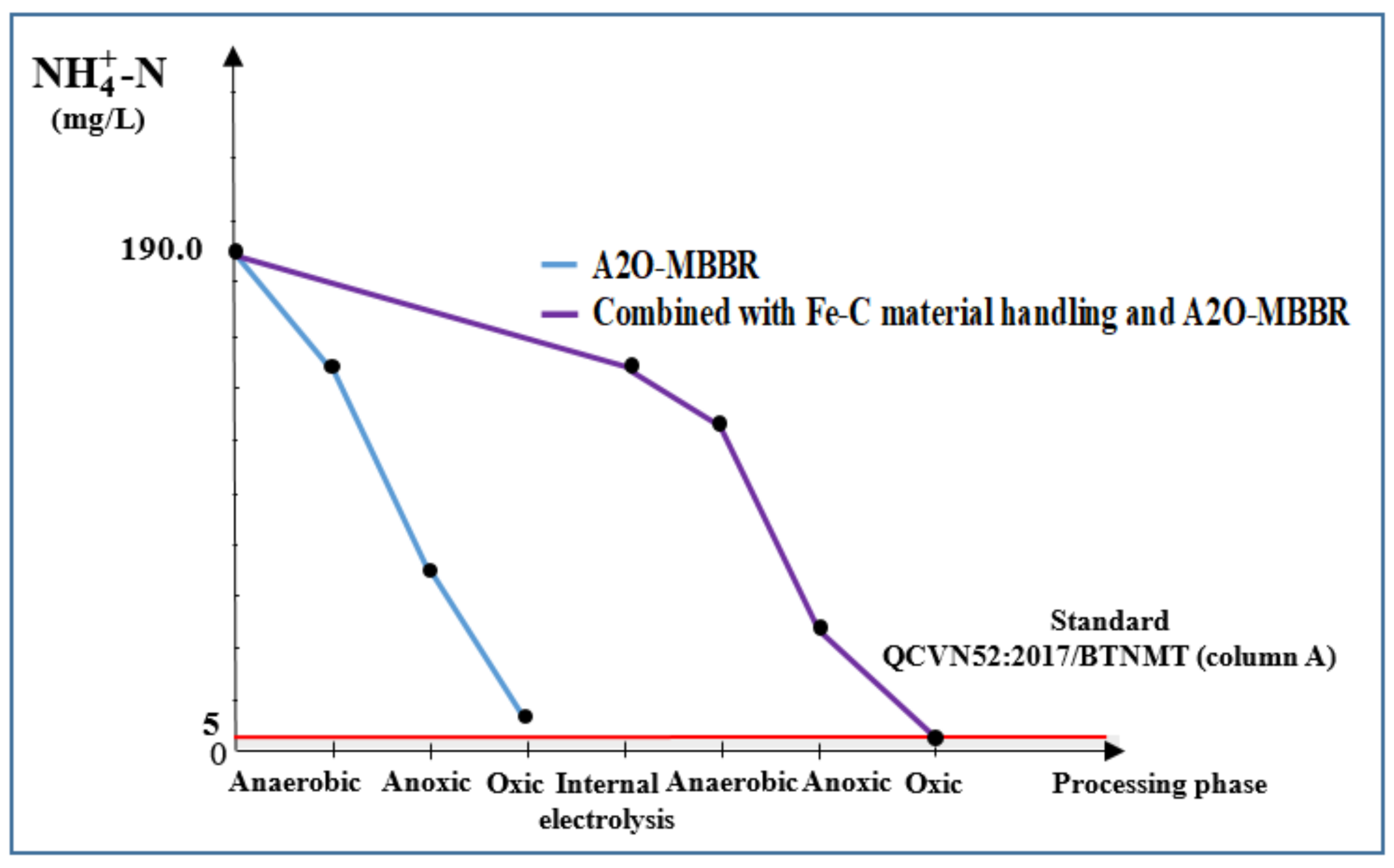
3.3.4. Total N and -N of Wastewater Treated Using Internal Electrolysis and A2O-MBBR System
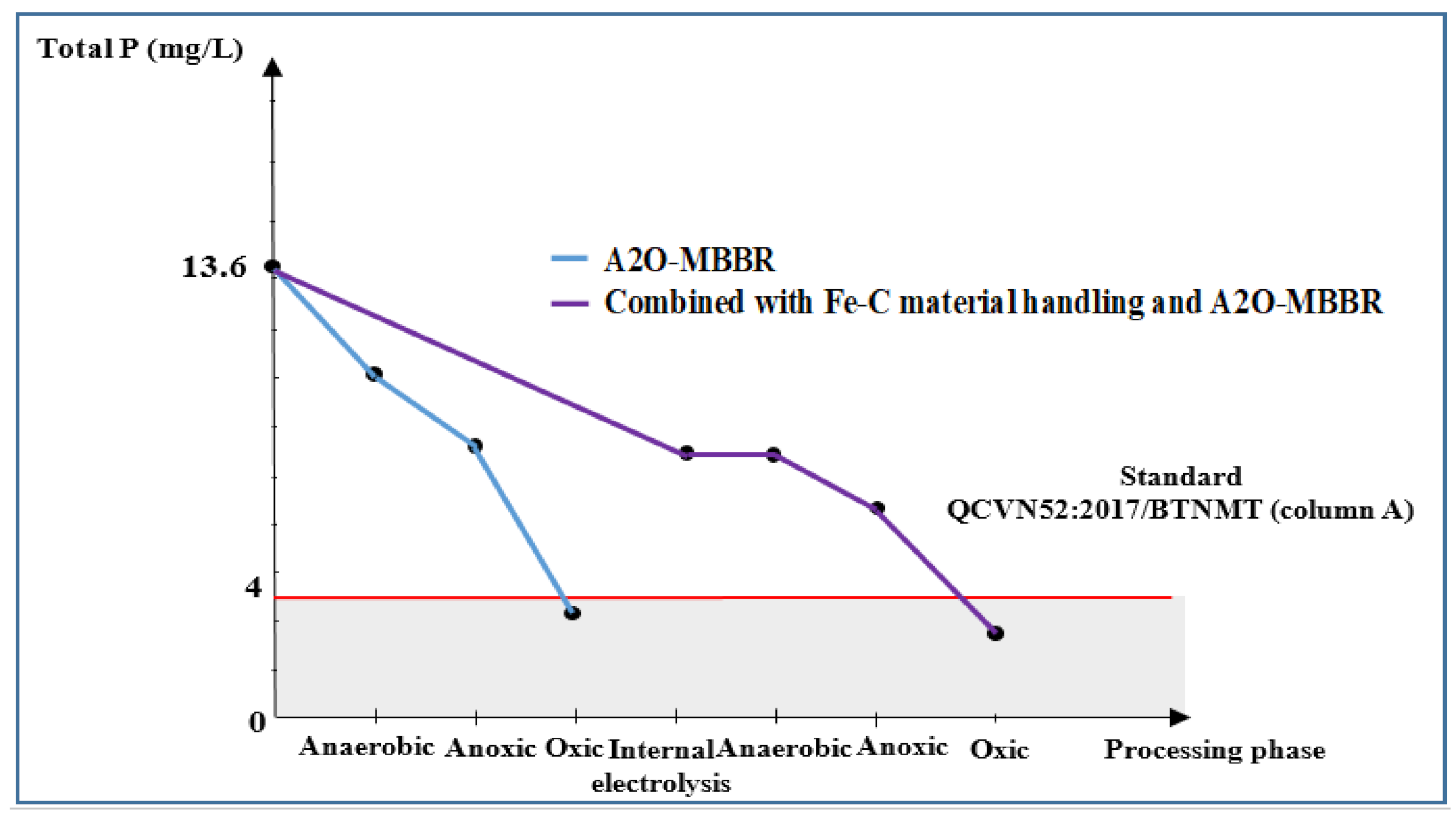
3.3.5. Total P of Wastewater Treated Using Internal Electrolysis and A2O-MBBR System
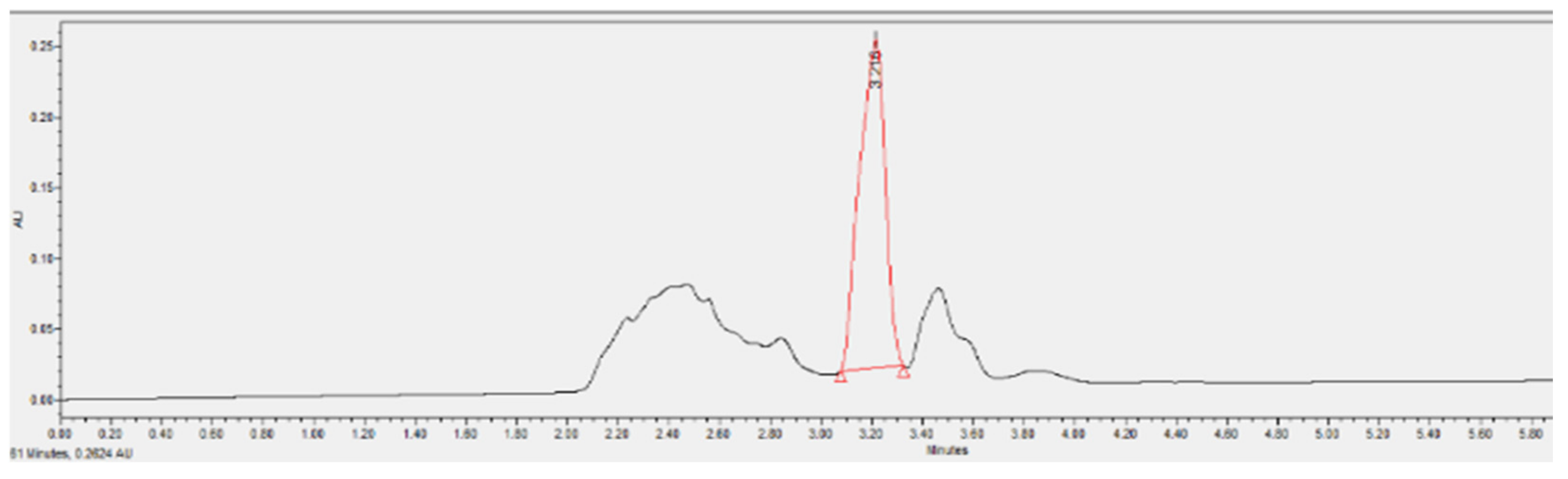
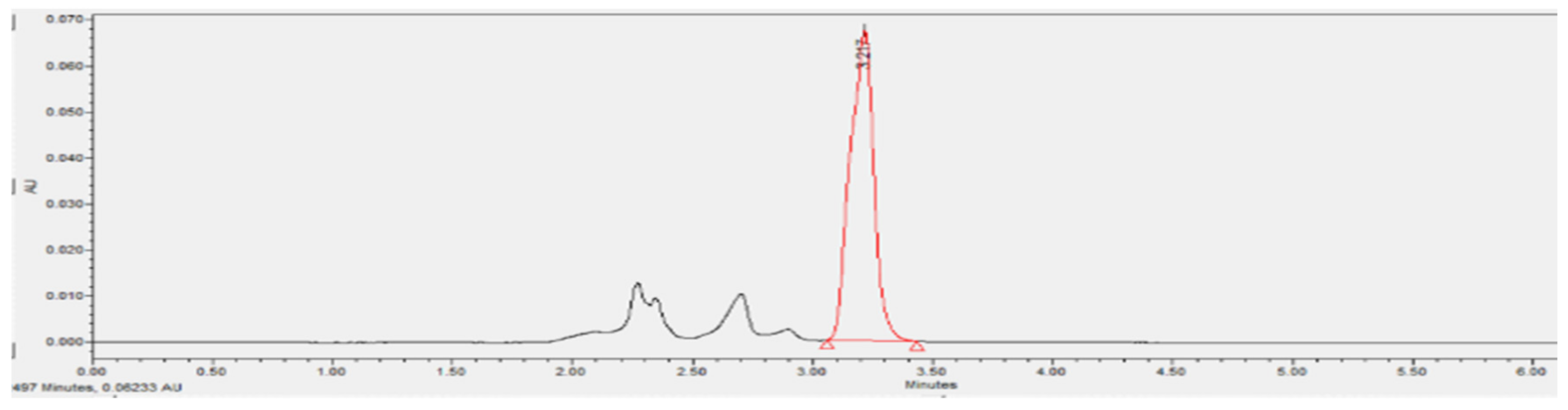
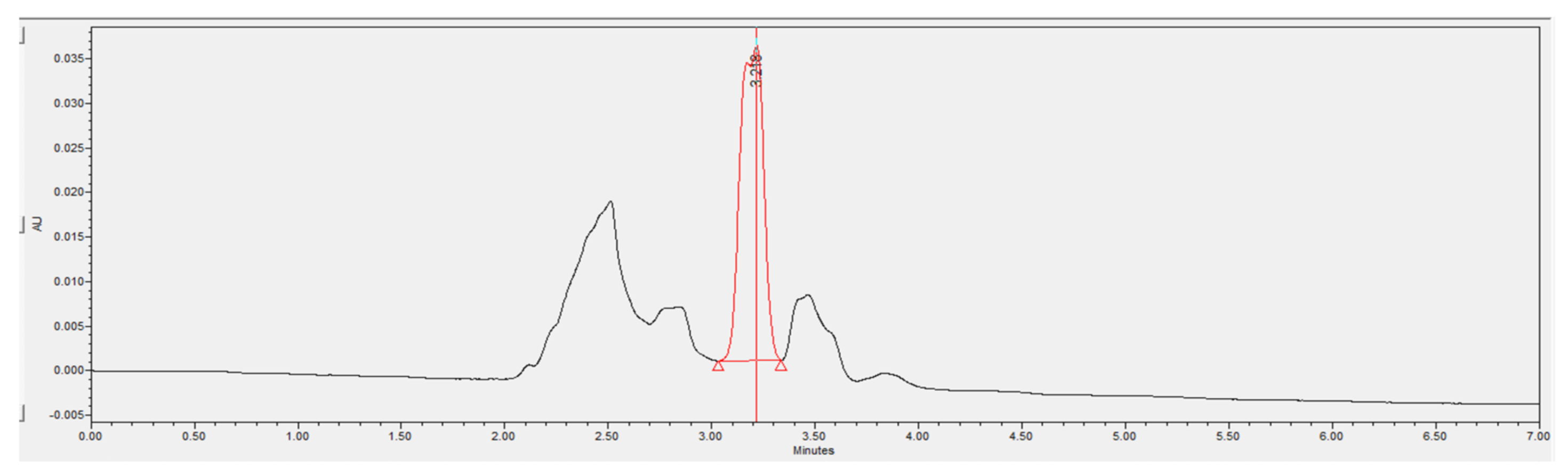
3.3.6. Phenol Removal Efficiency of A2O-MBBR System
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kang, M.; Chen, Q.; Li, J.; Liu, M.; Weng, Y. Preparation and study of a new type of Fe–C microelectrolysis filler in oil-bearing ballast water treatment. Environ. Sci. Pollut. Res. 2019, 26, 10673–10684. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.-Y.; Jin, M.; Zhou, X.; Chen, W.; Lu, D.; Zhang, Y.; Shao, X. Enhanced removal mechanism of iron carbon micro-electrolysis constructed wetland on C, N, and P in salty permitted effluent of wastewater treatment plant. Sci. Total Environ. 2019, 649, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Sun, G.; Yang, T.; Zhang, B.; He, Y.; Wang, X.-H. A preliminary study of anaerobic treatment coupled with micro-electrolysis for anthraquinone dye wastewater. Desalination 2013, 309, 91–96. [Google Scholar] [CrossRef]
- Zhang, L.; Yue, Q.; Yang, K.; Zhao, P.; Gao, B. Analysis of extracellular polymeric substances (EPS) and ciprofloxacin-degrading microbial community in the combined Fe-C micro-electrolysis-UBAF process for the elimination of high-level ciprofloxacin. Chemosphere 2018, 193, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, X.; Yi, J.; Chen, L.; Lan, T.; Dai, J. Pretreatment of printing and dyeing wastewater by Fe/C micro-electrolysis combined with H2O2 process. Water Sci. Technol. 2018, 2017, 707–717. [Google Scholar] [CrossRef]
- Ma, W.; Han, Y.; Xu, C.; Han, H.; Ma, W.; Zhu, H.; Li, K.; Wang, D. Enhanced degradation of phenolic compounds in coal gasification wastewater by a novel integration of micro-electrolysis with biological reactor (MEBR) under the micro-oxygen condition. Bioresour. Technol. 2018, 251, 303–310. [Google Scholar] [CrossRef]
- Liu, M.; Wang, L.; Xiao, X.; He, Z. Fe/C micro electrolysis and Fenton oxidation process for the removal of recalcitrant colored pollutants from mid-stage pulping effluent. J. Bioresour. Bioprod. 2018, 3, 118–122. [Google Scholar] [CrossRef]
- Ji, Q.; Tabassum, S.; Hena, S.; Silva, C.G.; Yu, G.; Zhang, X. A review on the coal gasification wastewater treatment technologies: Past, present and future outlook. J. Clean. Prod. 2016, 126, 38–55. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, Y. State of the art of biological processes for coal gasification wastewater treatment. Biotechnol. Adv. 2016, 34, 1064–1072. [Google Scholar] [CrossRef]
- Xu, L.; Wang, J.; Zhang, X.; Hou, D.; Yü, Y. Development of a novel integrated membrane system incorporated with an activated coke adsorption unit for advanced coal gasification wastewater treatment. Colloids Surfaces A Physicochem. Eng. Asp. 2015, 484, 99–107. [Google Scholar] [CrossRef]
- Banu, J.R.; Uan, D.K.; Yeom, I.T. Nutrient removal in an A2O-MBR reactor with sludge reduction. Bioresour. Technol. 2009, 100, 3820–3824. [Google Scholar] [CrossRef] [PubMed]
- Pimple, S.; Karikkat, S.; Devanna, M.; Yanamadni, V.; Sah, R.; Prasad, S. Comparison of MBR/RO and UF/RO hybrid systems for the treatment of coke-oven effluents. Desalin. Water Treat. 2014, 57, 1–9. [Google Scholar] [CrossRef]
- Zeng, W.; Li, L.; Yang, Y.; Wang, S.; Peng, Y. Nitritation and denitritation of domestic wastewater using a continuous anaerobic–anoxic–aerobic (A2O) process at ambient temperatures. Bioresour. Technol. 2010, 101, 8074–8082. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xu, X.; Gong, Z.; Yang, F. Removal of COD, phenols and ammonium from Lurgi coal gasification wastewater using A2O-MBR system. J. Hazard. Mater. 2012, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Li, L.; Yang, Y.-Y.; Wang, X.-D.; Peng, Y. Denitrifying phosphorus removal and impact of nitrite accumulation on phosphorus removal in a continuous anaerobic–anoxic–aerobic (A2O) process treating domestic wastewater. Enzym. Microb. Technol. 2011, 48, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Li, M.; Zhuang, L.-L.; Zhang, J.; Shen, Y.; Li, X. The Improvement of Pollutant Removal in the Ferric-Carbon Micro-Electrolysis Constructed Wetland by Partial Aeration. Water 2020, 12, 389. [Google Scholar] [CrossRef]
- Zhang, M.; Tay, J.H.; Qian, Y.; Gu, X.S. Coke plant wastewater treatment by fixed biofilm system for COD and NH3-N removal. Water Res. 1998, 32, 519–527. [Google Scholar] [CrossRef]
- Peng, Y.; Wang, X.-L.; Li, B.-K. Anoxic biological phosphorus uptake and the effect of excessive aeration on biological phosphorus removal in the A2O process. Desalination 2006, 189, 155–164. [Google Scholar] [CrossRef]
- You, S.; Hsu, C.; Chuang, S.; Ouyang, C. Nitrification efficiency and nitrifying bacteria abundance in combined AS-RBC and A2O systems. Water Res. 2003, 37, 2281–2290. [Google Scholar] [CrossRef]
- Yang, Z.; Ma, Y.; Liu, Y.; Li, Q.; Zhou, Z.; Ren, Z. Degradation of organic pollutants in near-neutral pH solution by Fe-C micro-electrolysis system. Chem. Eng. J. 2017, 315, 403–414. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.; Quan, X. Electricity assisted anaerobic treatment of salinity wastewater and its effects on microbial communities. Water Res. 2012, 46, 3535–3543. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Qi, Y.; Fan, C.; Dai, B.; Huang, J.; Zhou, W.; He, S.; Gao, L. Improvement of anaerobic biological treatment effect by catalytic micro-electrolysis for monensin production wastewater. Chem. Eng. J. 2016, 296, 260–267. [Google Scholar] [CrossRef]
- Ministry of Natural Resources and Environment of Vietnam. National Technical Regulation on Wastewater of Steel Industry; QCVN 52:2017/BTNMT; Hanoi, Vietnam, 2017. Available online: http://vea.gov.vn/Quy%20chun%20Vit%20Nam/QCVN%2052-2017-BTNMT.pdf (accessed on 18 September 2020).
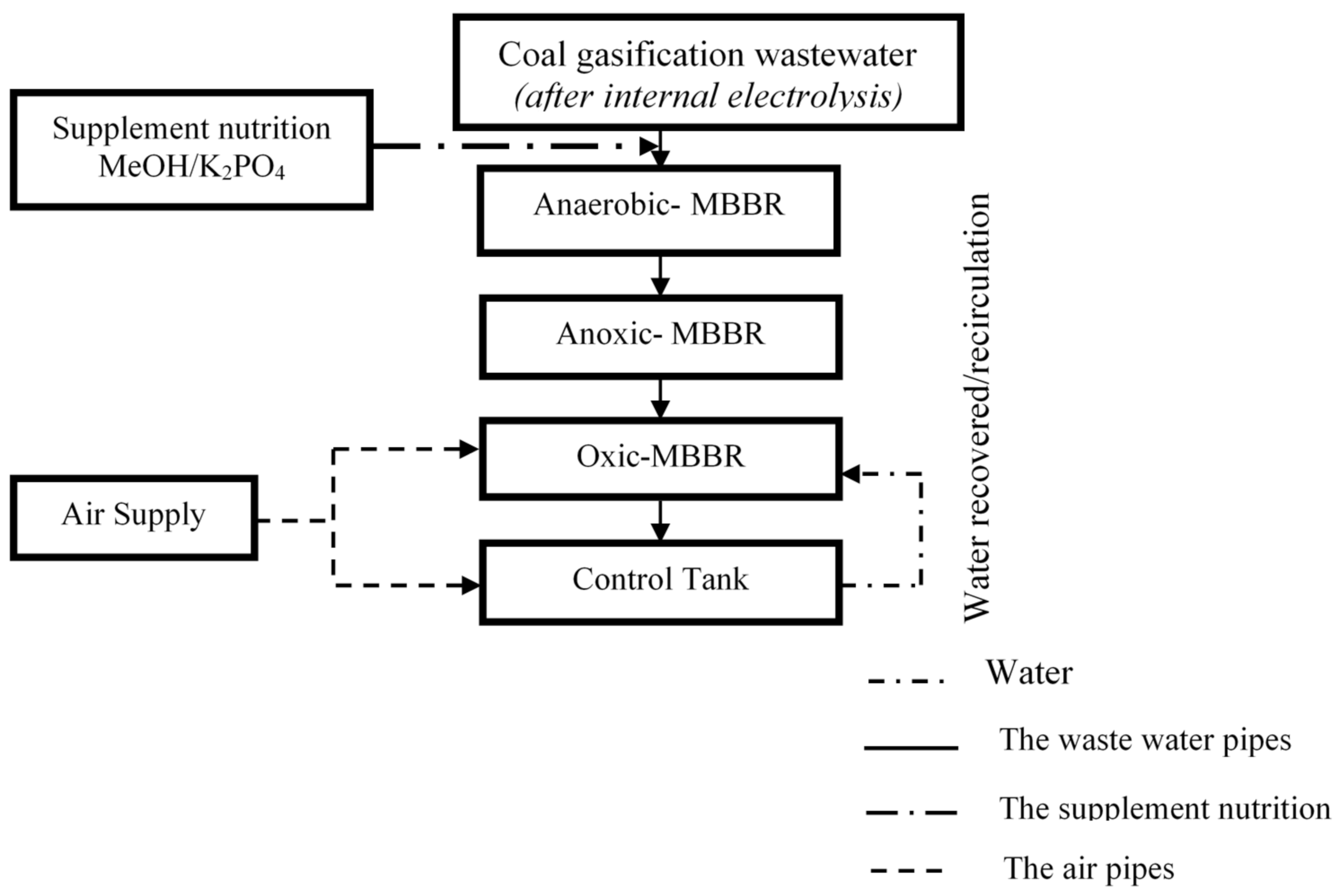

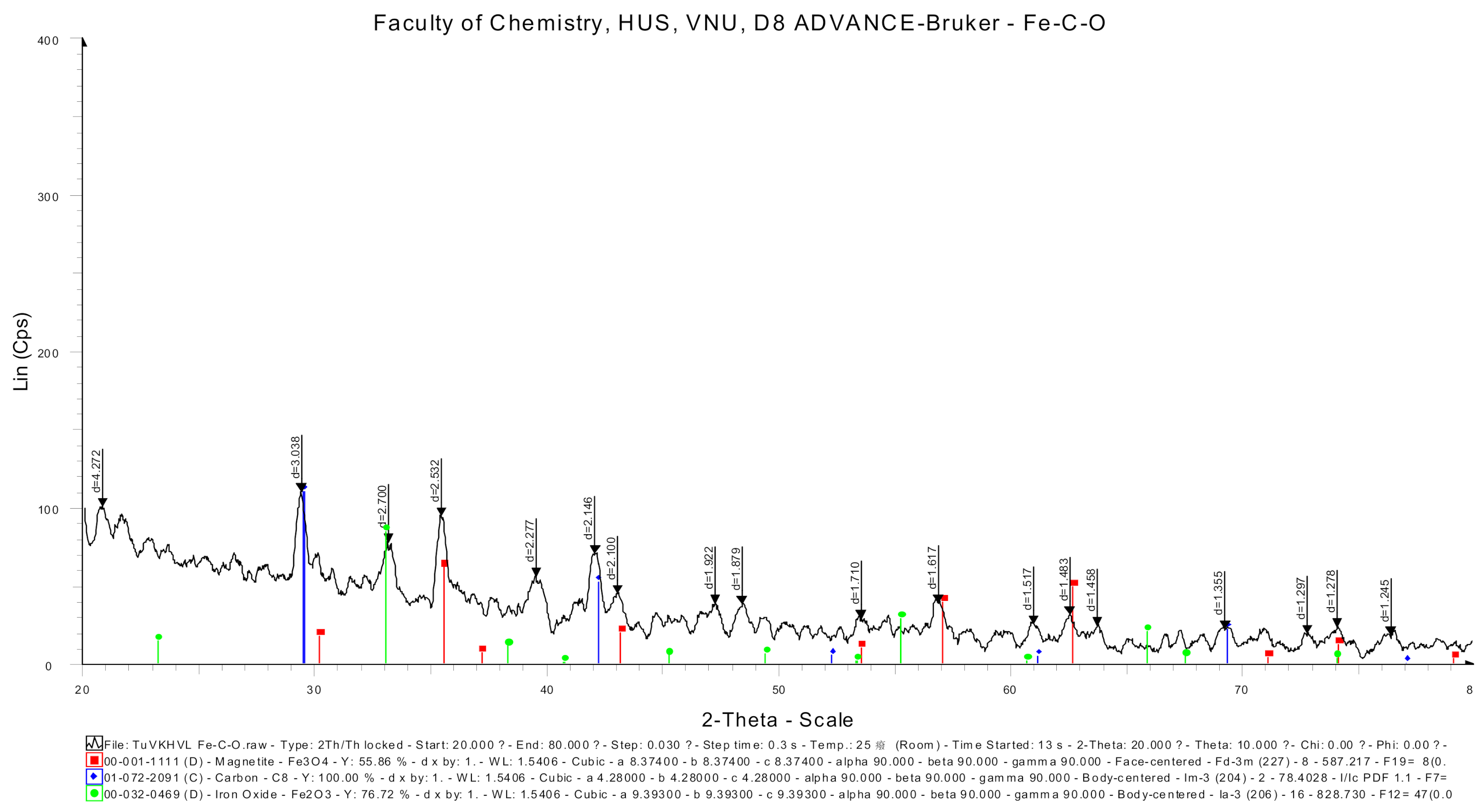

| Reaction Tank | COD (mg/L) | MLSS (mg/L) | pH | DO (mg/L) | Retention Time (h) |
|---|---|---|---|---|---|
| Anaerobic | 2359 | 1800−2000 | 7.5–8 | – | 14 |
| Anoxic tank | 2000−2300 | 1−2 | 6 | ||
| Aerobic | 4000−4500 | 5–8 | 4 |
| Element | Weigh (%) | Atomics (%) |
|---|---|---|
| C | 14.59 | 23.57 |
| O | 50.16 | 60.86 |
| Al | 1.89 | 1.36 |
| Si | 5.47 | 3.78 |
| Ca | 5.40 | 2.61 |
| Fe | 22.48 | 7.81 |
| Total | 100.00 | 100.00 |
| Parameters | Unit | Method of Analysis | Result (mg/L) | Efficiency H (%) | |
|---|---|---|---|---|---|
| Before | After | ||||
| pH | – | TCVN 6492:2011 | 8.3 | 6.7 | – |
| DO | mg/L | TCVN 7325:2004 | 0.6 | 2.8 | - |
| TSS | mg/L | SMEWW 2540 D:2012 | 156.2 | 76.5 | 51.02 |
| BOD5 (20 °C) | mg/L | TCVN 6001–1:2008 | 1150.0 | 498.0 | 56.7 |
| COD | mgO2/L | SMEWW 5220C:2012 | 2231.0 | 1032.0 | 53.7 |
| Phenol | mg/L | TCVN 6216:1996 | 172.0 | 45.9 | 73,32 |
| CN− | mg/L | SMEWW4500 CN−B:2012 | 0.05 | <0.01 | – |
| Total N | mg/L | TCVN 6638:2000 | 865.0 | 562.4 | 34.98 |
| -N | mg/L | TCVN 6179–1:1996 | 391.0 | 156.0 | 60.1 |
| Total P | mg/L | TCVN 6202:2008 | 16.1 | 8.5 | 47.2 |
| Indicator | Before Treatment | After Fe-C | After A2O-MBBR | Fe-C & A2O-MBBR | QCVN 52:2017/BTNMT (Column A) | |
|---|---|---|---|---|---|---|
| After Treatment | H% | |||||
| TSS, mg/L | 145.2 | 76.5 | 40.3 | 41.6 | 71.3 | 50.0 |
| BOD5, mg/L | 1105.0 | 498.0 | 68.7 | 25.7 | 97.7 | 30.0 |
| COD, mg/L | 2359.0 | 1032.0 | 387.7 | 67.3 | 97.1 | 75.0 |
| Total N, mg/L | 789.2 | 562.4 | 81.9 | 19.1 | 97.6 | 20.0 |
| Total P, mg/L | 13.6 | 8.5 | 3.0 | 2.5 | 81.6 | 4.0 |
| -N, mg/L | 190 | 156.0 | 15.3 | 4.7 | 97.5 | 5.0 |
| Phenol, mg/L | 172.0 | 45.9 | 25.8 | 0.0 | 100 | 0.1 |
| pH | 8.0 | 4.0 | 6.5–7.2 | 6.5–7.2 | – | 6.0–9.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huong, D.T.; Nguyen, V.T.; Ha, X.L.; Nguyen Thi, H.L.; Duong, T.T.; Nguyen, D.C.; Nguyen Thi, H.-T. Enhanced Degradation of Phenolic Compounds in Coal Gasification Wastewater by Methods of Microelectrolysis Fe-C and Anaerobic-Anoxic—Oxic Moving Bed Biofilm Reactor (A2O-MBBR). Processes 2020, 8, 1258. https://doi.org/10.3390/pr8101258
Huong DT, Nguyen VT, Ha XL, Nguyen Thi HL, Duong TT, Nguyen DC, Nguyen Thi H-T. Enhanced Degradation of Phenolic Compounds in Coal Gasification Wastewater by Methods of Microelectrolysis Fe-C and Anaerobic-Anoxic—Oxic Moving Bed Biofilm Reactor (A2O-MBBR). Processes. 2020; 8(10):1258. https://doi.org/10.3390/pr8101258
Chicago/Turabian StyleHuong, Do Tra, Van Tu Nguyen, Xuan Linh Ha, Hien Lan Nguyen Thi, Thi Thoa Duong, Duy Chinh Nguyen, and Hong-Tham Nguyen Thi. 2020. "Enhanced Degradation of Phenolic Compounds in Coal Gasification Wastewater by Methods of Microelectrolysis Fe-C and Anaerobic-Anoxic—Oxic Moving Bed Biofilm Reactor (A2O-MBBR)" Processes 8, no. 10: 1258. https://doi.org/10.3390/pr8101258
APA StyleHuong, D. T., Nguyen, V. T., Ha, X. L., Nguyen Thi, H. L., Duong, T. T., Nguyen, D. C., & Nguyen Thi, H.-T. (2020). Enhanced Degradation of Phenolic Compounds in Coal Gasification Wastewater by Methods of Microelectrolysis Fe-C and Anaerobic-Anoxic—Oxic Moving Bed Biofilm Reactor (A2O-MBBR). Processes, 8(10), 1258. https://doi.org/10.3390/pr8101258





