Effects of Alginate and Chitosan on Activated Carbon as Immobilisation Beads in Biohydrogen Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganism, GAC Carrier, Alginate, and Chitosan Carriers
2.2. Biofilm Formation on Activated Carbon
2.3. Development of GAC—Attached Biofilm Entrapped in Alginate Beads (GAC–Alg)
2.4. Entrapment of GAC–Alg Beads with Chitosan (GAC–AlgC)
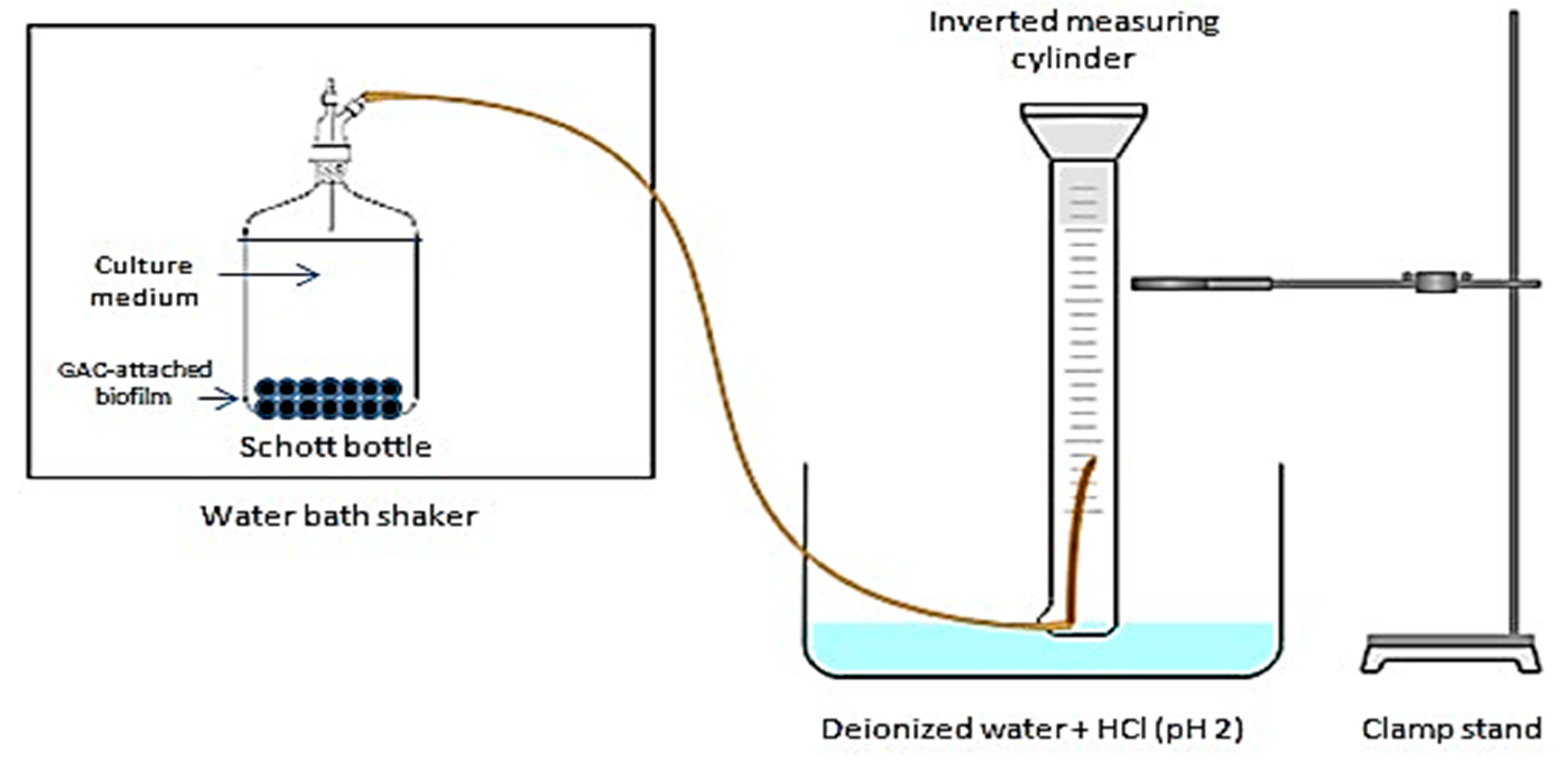
2.5. Batch Fermentation of Biohydrogen Production
2.6. Analysis of Gaseous, Hydrogen Yield, and Productivity
2.7. Analysis of Volatile Fatty Acid and Sugar
2.8. Scanning Electron Microscope (SEM)
3. Results and Discussion
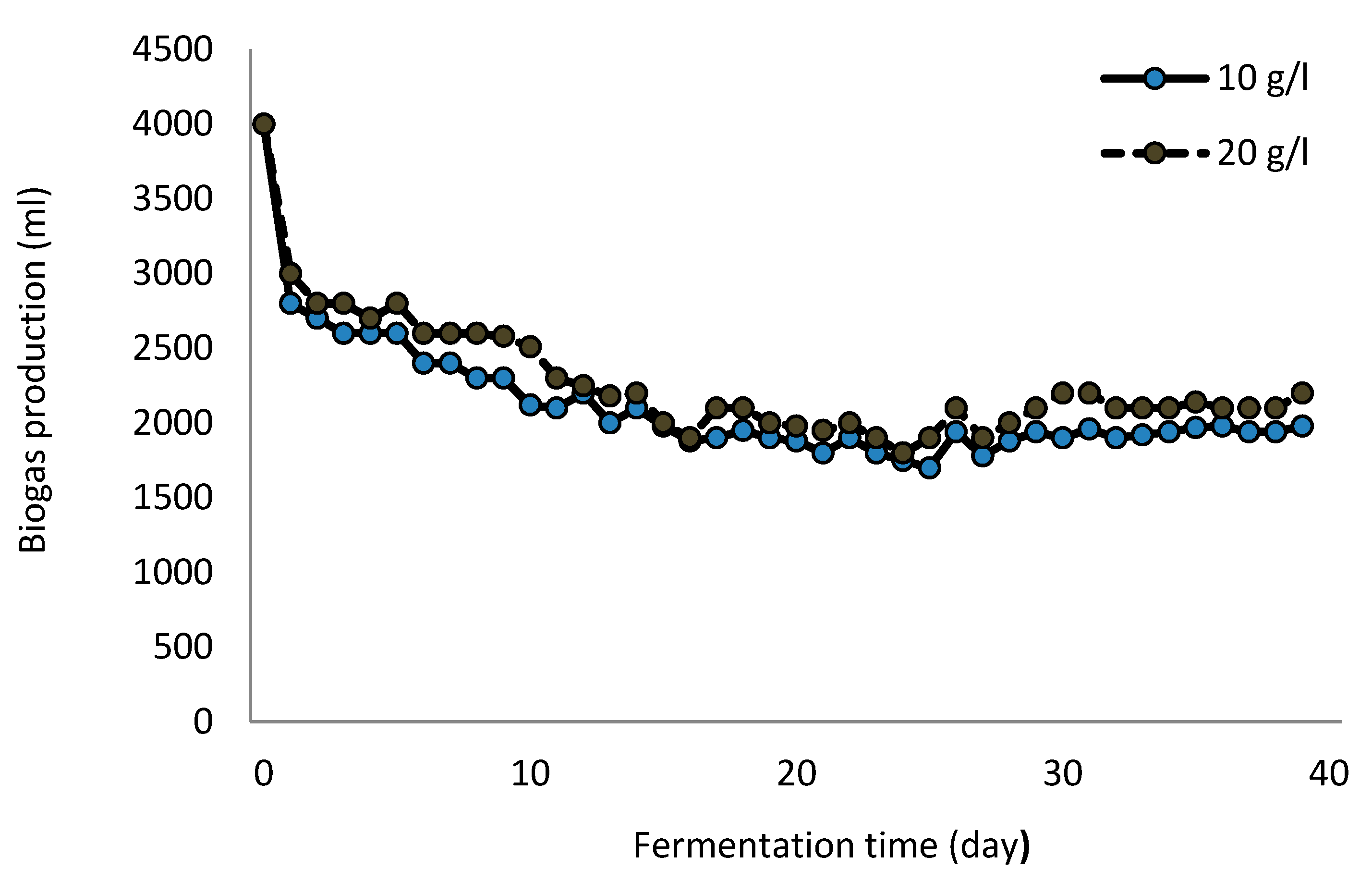
3.1. Microbial Cells Self-Attached to GAC for Hydrogen Production
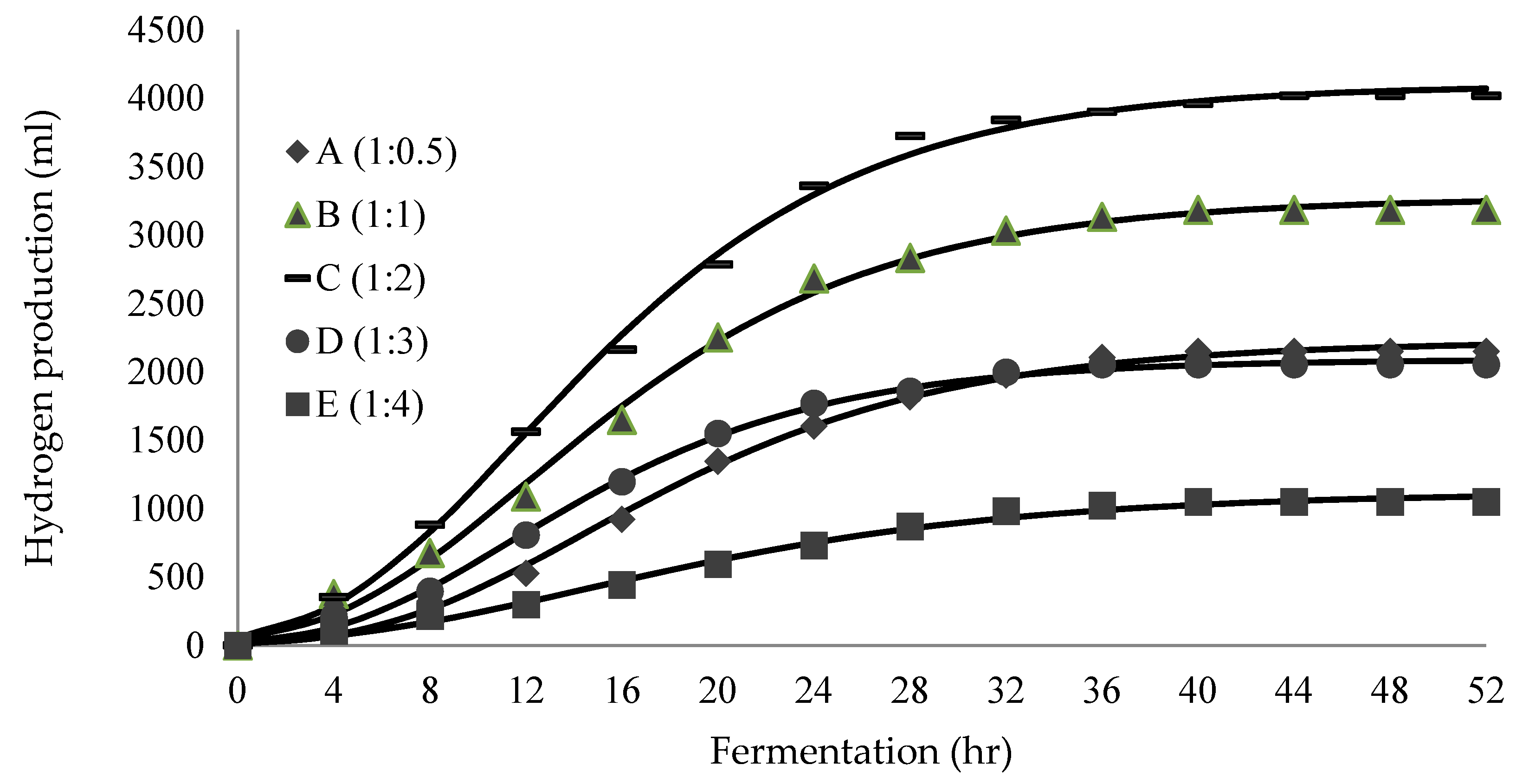
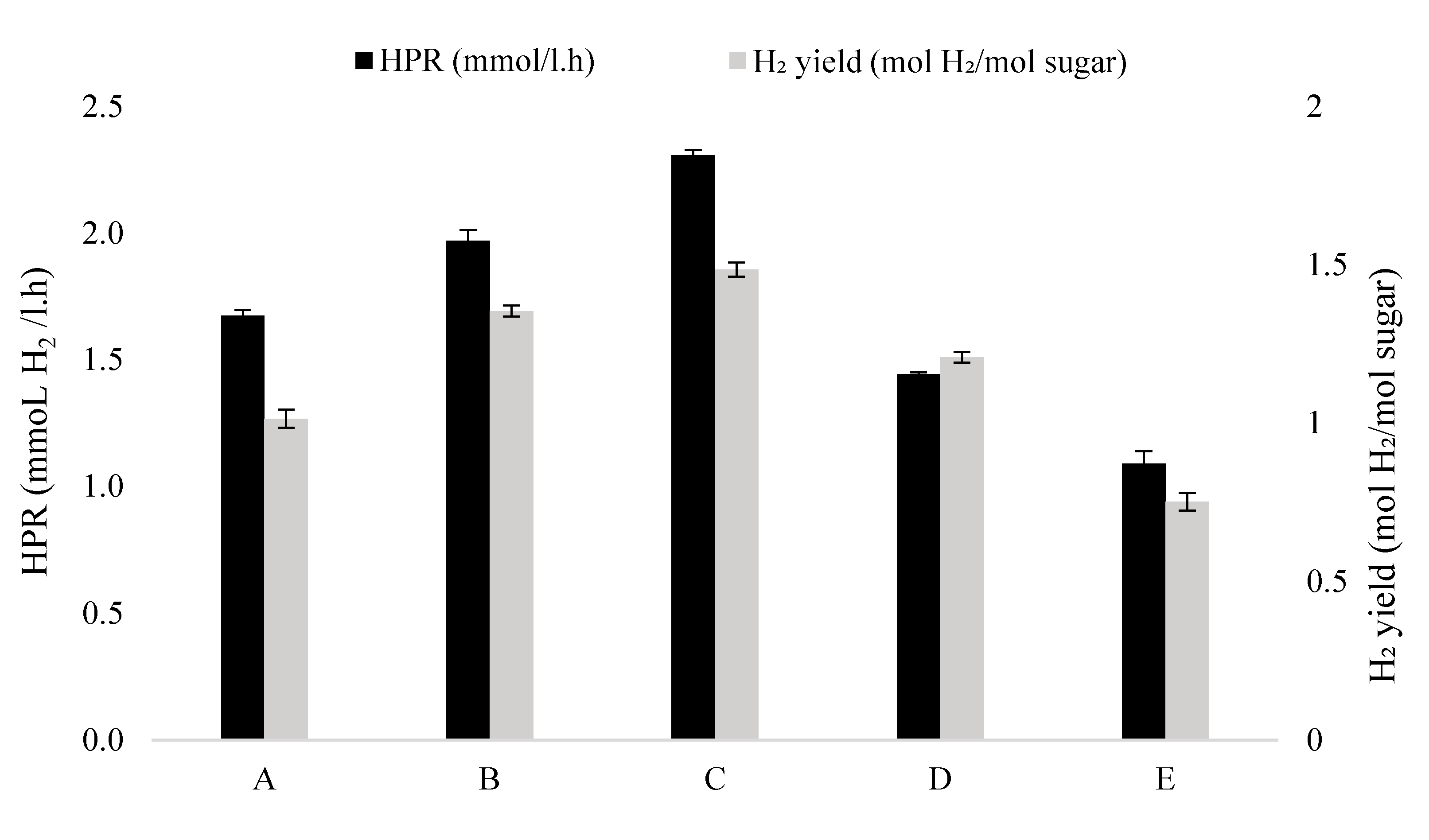
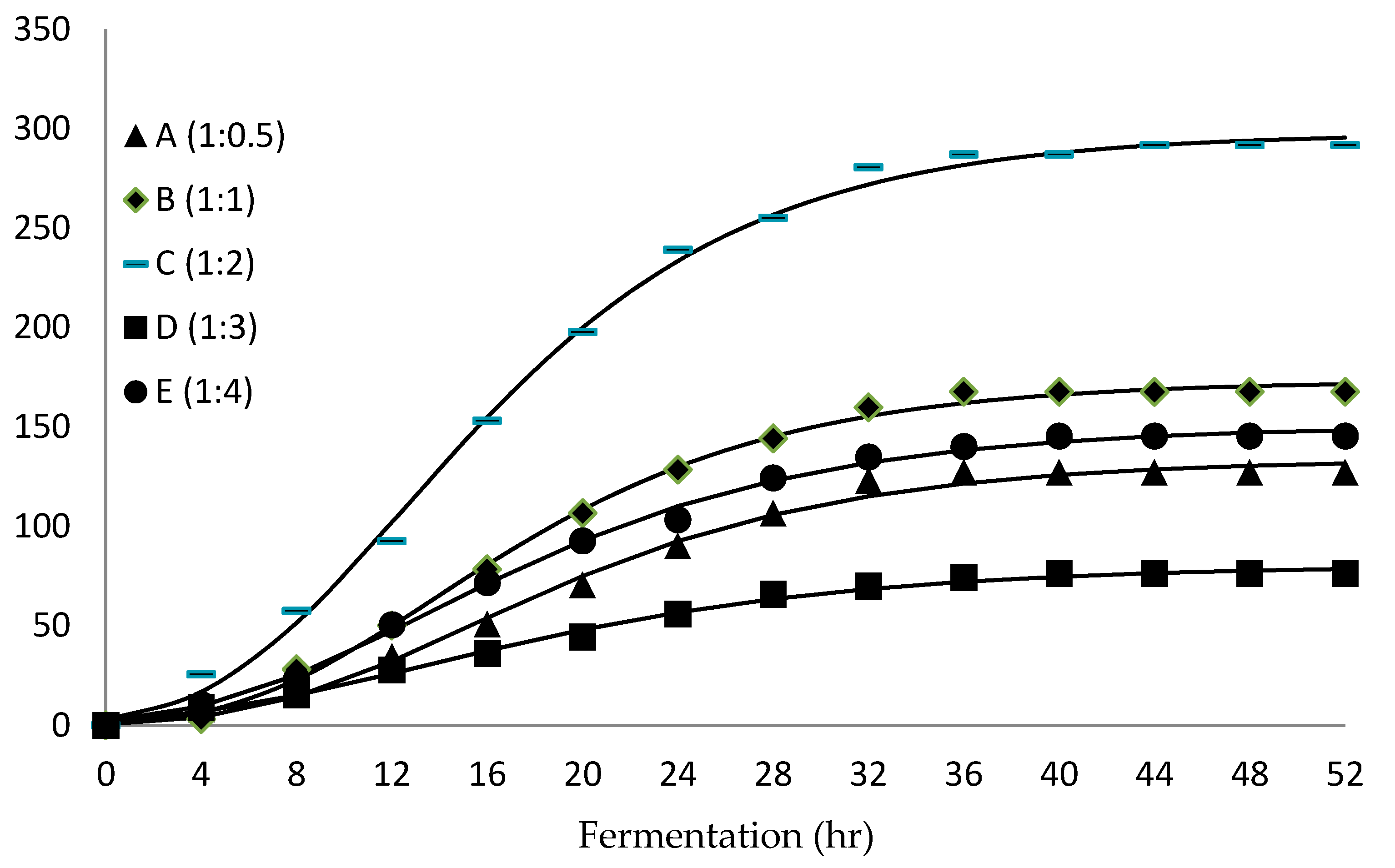
3.2. Biohydrogen Production of Immobilised Beads GAC–Alg
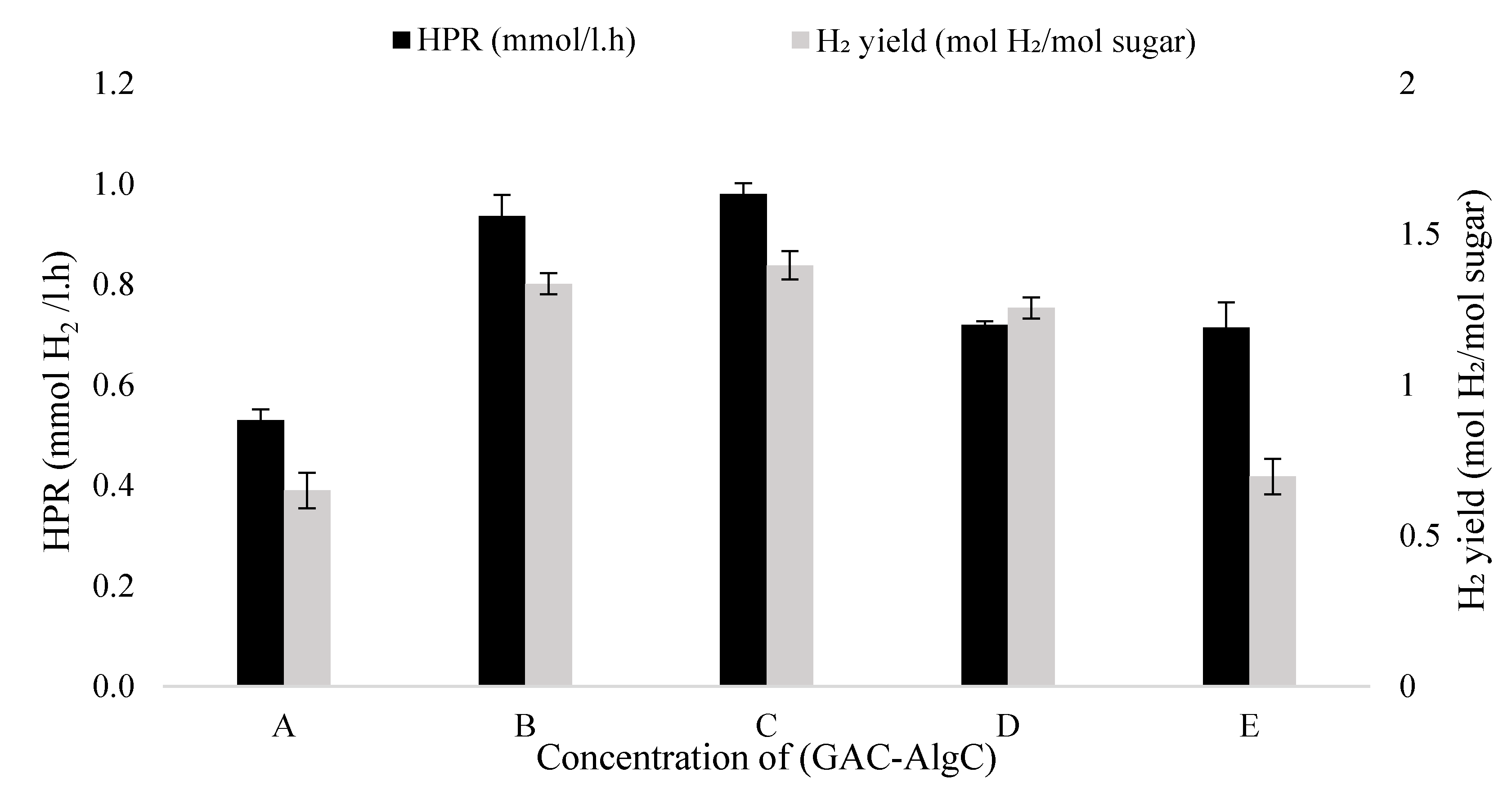
3.3. Bacteria Immobilisation in GAC–Alg Entrapped with Chitosan on Hydrogen Production
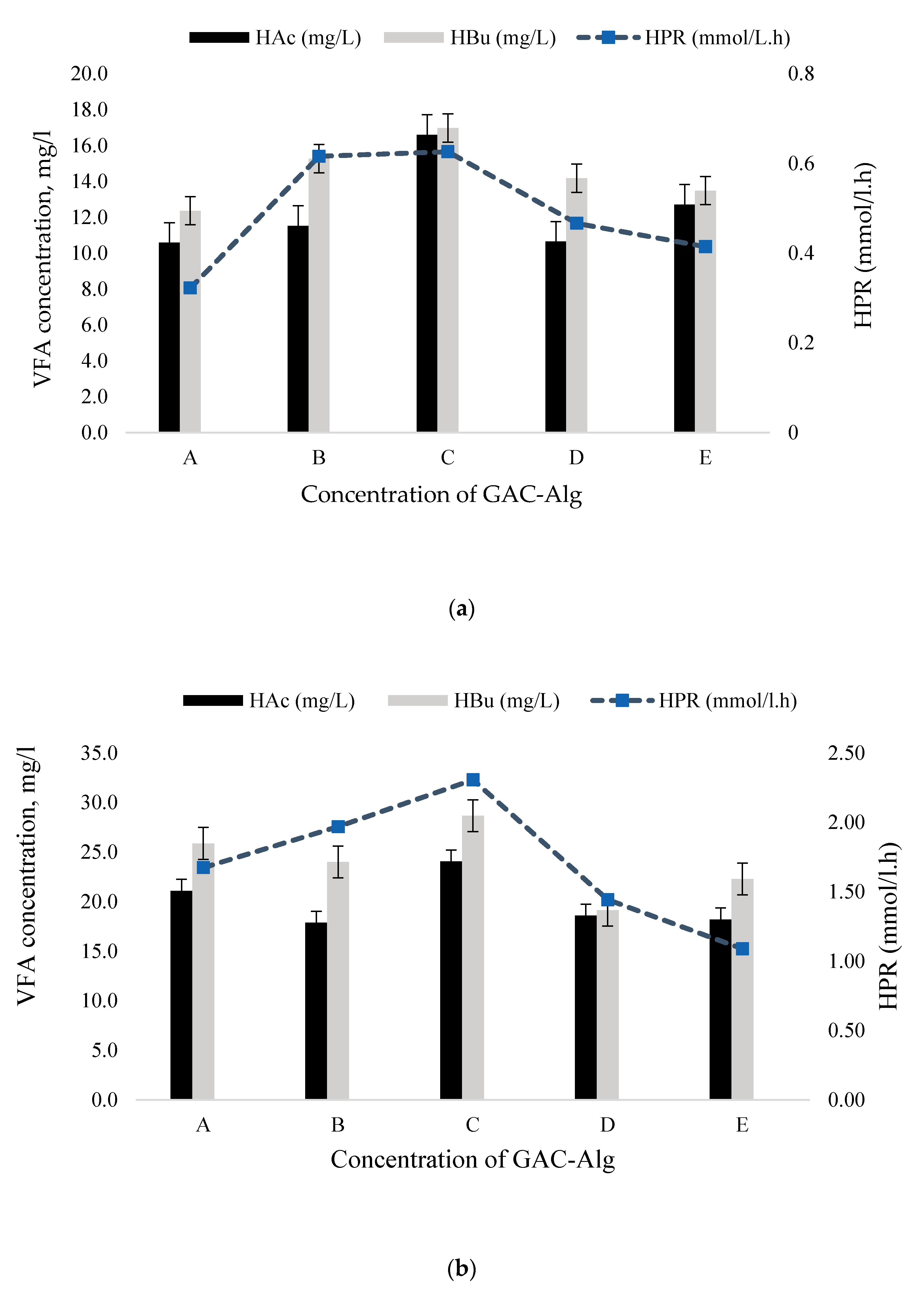
3.4. Effect of Alginate and Chitosan Concentration on Volatile Fatty Acid Production
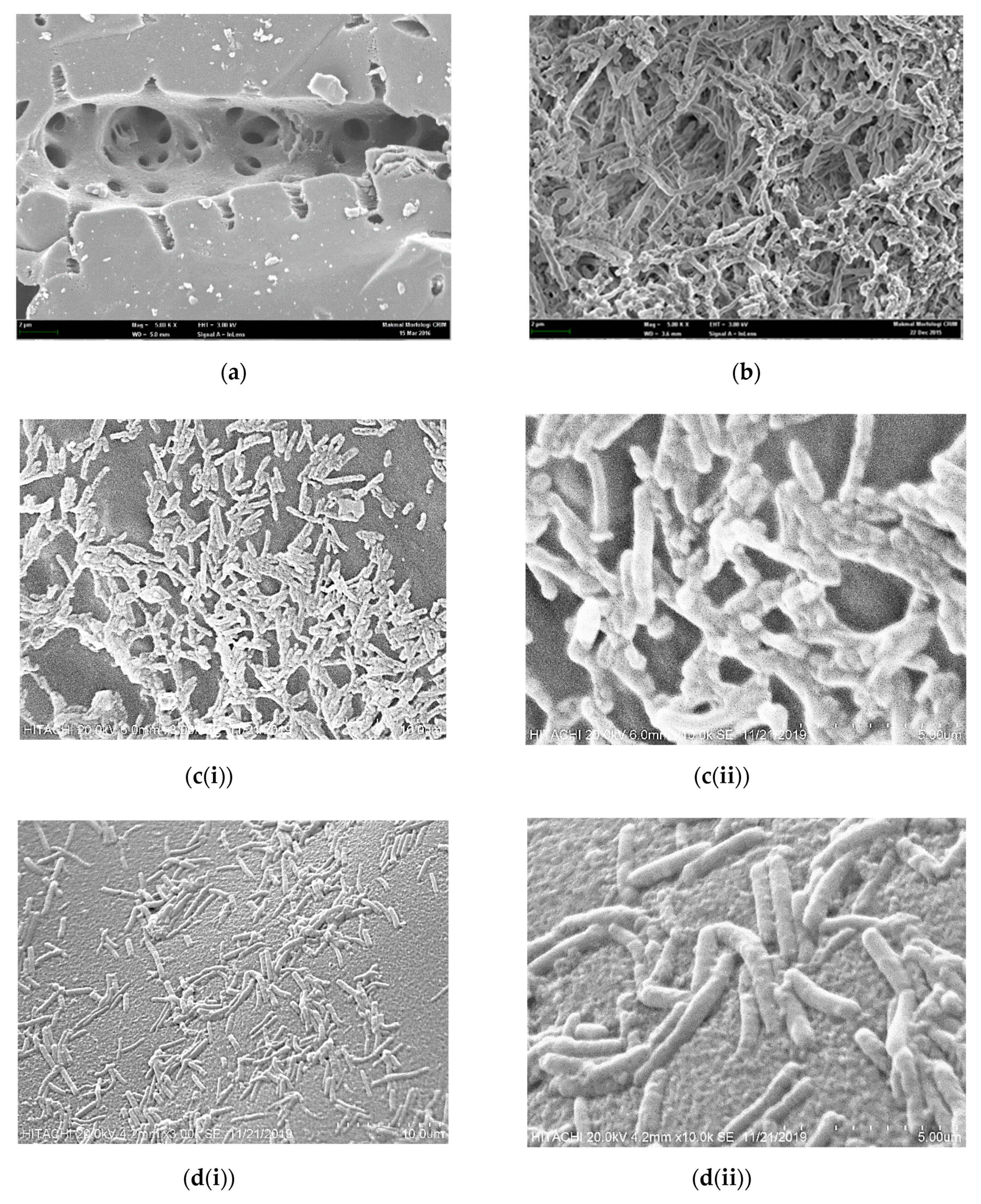
3.5. SEM of Immobilized Beads
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kim, J.K.; Nhat, L.; Chun, Y.N.; Kim, S.W. Hydrogen production conditions from food waste by dark fermentation with Clostridium beijerinckii KCTC 1785. Biotechnol. Bioprocess Eng. 2008, 13, 499–504. [Google Scholar] [CrossRef]
- Hu, B.; Liu, Y.; Chi, Z.; Chen, S. Biological hydrogen production via bacteria immobilized in calcium alginate gel beads. Biol. Eng. Trans. 2007, 1, 25–37. [Google Scholar] [CrossRef]
- Liu, X.; Yu, X. Enhancement of Butanol Production: From Biocatalysis to Bioelectrocatalysis. ACS Energy Lett. 2020, 5, 867–878. [Google Scholar] [CrossRef]
- Jamali, N.S.; Jahim, J.; Nor, W.; Wan, R.; Abdul, P.M. Particle size variations of activated carbon on biofilm formation in thermophilic biohydrogen production from palm oil mill effluent. Energy Convers. Manag. 2016, 141, 354–366. [Google Scholar] [CrossRef]
- Mohamad, N.R.; Marzuki, N.H.C.; Buang, N.A.; Huyop, F.; Wahab, R.A. An overview of technologies for immobilization of enzymes and surface analysis techniques for immobilized enzymes. Biotechnol. Biotechnol. Equip. 2015, 29, 205–220. [Google Scholar] [CrossRef]
- Mishra, P.; Krishnan, S.; Rana, S.; Singh, L.; Sakinah, M.; Ab Wahid, Z. Outlook of fermentative hydrogen production techniques: An overview of dark, photo and integrated dark-photo fermentative approach to biomass. Energy Strategy Rev. 2019, 24, 27–37. [Google Scholar] [CrossRef]
- Singh, L.; Wahid, Z.A.; Siddiqui, M.F.; Ahmad, A.; Hasbi, M.; Rahim, A.; Sakinah, M. Biohydrogen production from palm oil mill effluent using immobilized Clostridium butyricum EB6 in polyethylene glycol. Process Biochem. 2013, 48, 294–298. [Google Scholar] [CrossRef][Green Version]
- Wu, S.-Y.; Lin, C.-N.; Chang, J.-S. Hydrogen Production with Immobilized Sewage Sludge in Three-Phase Fluidized-Bed Bioreactors. Biotechnol. Prog. 2003, 19, 828–832. [Google Scholar] [CrossRef]
- Smidsrød, O.; Skjåk-Bræk, G. Alginate as immobilization matrix for cells. Trends Biotechnol. 1990, 8, 71–78. [Google Scholar] [CrossRef]
- Wu, S.-Y.; Lin, C.-N.; Chang, J.-S.; Lee, K.-S.; Lin, P.-J. Microbial Hydrogen Production with Immobilized Sewage Sludge. Biotechnol. Prog. 2002, 18, 921–926. [Google Scholar] [CrossRef]
- Sekoai, P.T.; Awosusi, A.A.; Yoro, K.O.; Oloye, O.; Ayeni, A.O.; Bodunrin, M.; Thabang, P.; Awosusi, A.A.; Yoro, K.O.; Oloye, O.; et al. Critical Reviews in Biotechnology Microbial cell immobilization in biohydrogen production: A short overview. Crit. Rev. Biotechnol. 2017, 38, 157–171. [Google Scholar] [CrossRef]
- Sekoai, P.T.; Yoro, K.O.; Bodunrin, M.O.; Ayeni, A.O.; Daramola, M.O. Integrated system approach to dark fermentative biohydrogen production for enhanced yield, energy efficiency and substrate recovery. Rev. Environ. Sci. Bio Technol. 2018, 17, 501–529. [Google Scholar] [CrossRef]
- Ngah, W.S.W.; Fatinathan, S. Adsorption of Cu (II) ions in aqueous solution using chitosan beads, chitosan-GLA beads and chitosan-alginate beads. Chem. Eng. J. 2008, 143, 62–72. [Google Scholar] [CrossRef]
- Shu, X.; Zhu, K. The release behavior of brilliant blue from calcium-alginate gel beads coated by chitosan: The preparation method effect. Eur. J. Pharm. Biopharm. 2002, 53, 193–201. [Google Scholar] [CrossRef]
- Overgaard, S.; Scharer, J.M.; Moo-Young, M.; Bols, N.C. Immobilization of hybridoma cells in chitosan alginate beads. Can. J. Chem. Eng. 1991, 69, 439–443. [Google Scholar] [CrossRef]
- Žuža, M.G.; Obradović, B.M.; Knežević-Jugović, Z.D. Hydrolysis of Penicillin G by Penicillin G Acylase Immobilized on Chitosan Microbeads in Different Reactor Systems. Chem. Eng. Technol. 2011, 34, 1706–1714. [Google Scholar] [CrossRef]
- Stojkovska, J.; Kostić, D.; Jovanović, Ž.; Vukašinović-Sekulić, M.; Mišković-Stanković, V.; Obradović, B. A comprehensive approach to in vitro functional evaluation of Ag/alginate nanocomposite hydrogels. Carbohydr. Polym. 2014, 111, 305–314. [Google Scholar] [CrossRef]
- Obradović, N.S.; Krunić, T.Ž.; Damnjanović, I.D.; Vukašinović-Sekulić, M.S.; Rakin, M.B.; Rakin, M.P.; Bugarski, B.M. Influence of whey proteins addition on mechanical stability of biopolymer beads with immobilized probiotics. Tehnika 2015, 70, 397–400. [Google Scholar] [CrossRef]
- Mahajan, R.; Gupta, V.K.; Sharma, J. Comparison and suitability of gel matrix for entrapping higher content of enzymes for commercial applications. Indian J. Pharm. Sci. 2010, 72, 223–228. [Google Scholar] [CrossRef]
- Kumar, G.; Mudhoo, A.; Sivagurunathan, P.; Nagarajan, D.; Ghimire, A.; Lay, C.-H.; Lin, C.-Y.; Lee, D.-J.; Chang, J.-S. Recent insights into the cell immobilization technology applied for dark fermentative hydrogen production. Bioresour. Technol. 2016, 219, 725–737. [Google Scholar] [CrossRef]
- Jamali, N.S.; Farahana, N.; Rashidi, D.; Jahim, J.; O-thong, S.; Jehlee, A.; Engliman, N.S. Thermophilic biohydrogen production from palm oil mill effluent: Effect of immobilized cells on granular activated carbon in fluidized bed reactor. Food Bioprod. Process. 2019, 117, 231–240. [Google Scholar] [CrossRef]
- Angelidaki, I.; Sanders, W. Assessment of the anaerobic biodegradability of macropollutants. Rev. Environ. Sci. Biotechnol. 2004, 3, 117–129. [Google Scholar] [CrossRef]
- Duarte, J.C.; Rodrigues, J.A.R.; Moran, P.J.S.; Valença, G.P.; Nunhez, J.R. Effect of immobilized cells in calcium alginate beads in alcoholic fermentation. AMB Express 2013, 3, 1–8. [Google Scholar] [CrossRef]
- Khanna, N.; Das, D. Biohydrogen production by dark fermentation. Wiley Interdiscip. Rev. Energy Environ. 2013, 2, 401–421. [Google Scholar] [CrossRef]
- Wang, C.C.; Chang, C.W.; Chu, C.P.; Lee, D.J.; Chang, B.V.; Liao, C.S. Producing hydrogen from wastewater sludge by Clostridium bifermentans. J. Biotechnol. 2003, 102, 83–92. [Google Scholar] [CrossRef]
- Sekoai, P.T.; Yoro, K.O.; Daramola, M.O. Batch Fermentative Biohydrogen Production Process Using Immobilized Anaerobic Sludge from Organic Solid Waste. Environments 2016, 3, 38. [Google Scholar] [CrossRef]
- Chen, W.-H.; Chen, S.-Y.; Kumar Khanal, S.; Sung, S. Kinetic study of biological hydrogen production by anaerobic fermentation. Int. J. Hydrog. Energy 2006, 31, 2170–2178. [Google Scholar] [CrossRef]
- Syakina, N.; Jahim, J.; O-thong, S. ScienceDirect Hydrodynamic characteristics and model of fluidized bed reactor with immobilised cells on activated carbon for biohydrogen production. Int. J. Hydrog. Energy 2019. [Google Scholar] [CrossRef]
- Chu, Y.; Wei, Y.; Yuan, X.; Shi, X. Bioconversion of wheat stalk to hydrogen by dark fermentation: Effect of different mixed microflora on hydrogen yield and cellulose solubilisation. Bioresour. Technol. 2011, 102, 3805–3809. [Google Scholar] [CrossRef]
- Syakina, N.; Jahim, J. ScienceDirect Biofilm formation on granular activated carbon in xylose and glucose mixture for thermophilic biohydrogen production. Int. J. Hydrog. Energy 2016, 1–11. [Google Scholar] [CrossRef]
- Seifan, M.; Samani, A.K.; Hewitt, S.; Berenjian, A. The Effect of Cell Immobilization by Calcium Alginate on Bacterially Induced Calcium Carbonate Precipitation. Fermentation 2017, 3, 57. [Google Scholar] [CrossRef]
- Lutpi, N.A.; Jahim, J.; Mumtaz, T. RSC Advances Physicochemical characteristics of attached bio fi lm on granular activated carbon for thermophilic biohydrogen production. RSC Adv. 2015, 19382–19392. [Google Scholar] [CrossRef]
- Mesran, M.H.; Mamat, S.; Pang, Y.R.; Tan, Y.H.; Muneera, Z.; Ghazali, N.F.; Ali, M.A.; Mahmood, N.A. Preliminary Studies on Immobilized Cells-Based Microbial Fuel Cell System on Its Power Generation Performance. J. Asian Sci. Res. 2014, 4, 428–435. [Google Scholar]
- Damayanti, A.; Sediawan, W.B.; Syamsiah, S. Performance analysis of immobilized and co-immobilized enriched-mixed culture for hydrogen production. J. Mech. Eng. Sci. 2018, 12, 3515–3528. [Google Scholar] [CrossRef]
- Szymańska, E.; Winnicka, K. Stability of chitosan—A challenge for pharmaceutical and biomedical applications. Mar. Drugs 2015, 13, 1819–1846. [Google Scholar] [CrossRef]
- Talebnia, F.; Taherzadeh, M.J. In situ detoxification and continuous cultivation of dilute-acid hydrolyzate to ethanol by encapsulated S. cerevisiae. J. Biotechnol. 2006, 125, 377–384. [Google Scholar] [CrossRef]
- Wu, K.; Chang, J. Biohydrogen Production Using Suspended and Immobilized Mixed Microflora. J. Chin. Inst. Chem. Eng. 2006, 37, 545–550. [Google Scholar]
- Basile, M.A.; Dipasquale, L.; Gambacorta, A.; Vella, M.F.; Calarco, A.; Cerruti, P.; Malinconico, M.; Gomez d’Ayala, G. The effect of the surface charge of hydrogel supports on thermophilic biohydrogen production. Bioresour. Technol. 2010, 101, 4386–4394. [Google Scholar] [CrossRef]
- Kumar, A.; Jain, S.R.; Sharma, C.B.; Joshi, A.P.; Kalia, V.C. Increased H2 production by immobilized microorganisms. World J. Microbiol. Biotechnol. 1995, 11, 156–159. [Google Scholar] [CrossRef]
- Zhu, K.; Arnold, W.A.; Sakkos, J.; Davis, C.W.; Novak, P.J. Achieving high-rate hydrogen recovery from wastewater using customizable alginate polymer gel matrices encapsulating biomass. Environ. Sci. Water Res. Technol. 2018, 4, 1867–1876. [Google Scholar] [CrossRef]
- Zhang, Z.P.; Show, K.Y.; Tay, J.H.; Liang, D.T.; Lee, D.J. Enhanced Continuous Biohydrogen Production by Immobilized Anaerobic Microflora. Energy Fuels 2008, 22, 87–92. [Google Scholar] [CrossRef]
- Wu, S.Y.; Lin, C.N.; Chang, J.S.; Chang, J.S. Biohydrogen production with anaerobic sludge immobilized by ethylene-vinyl acetate copolymer. Int. J. Hydrog. Energy 2005, 30, 1375–1381. [Google Scholar] [CrossRef]
- Zhao, L.; Cao, G.; Wang, A.; Guo, W.; Liu, B.; Ren, H.; Ren, N.; Ma, F. Enhanced bio-hydrogen production by immobilized Clostridium sp. T2 on a new biological carrier. Int. J. Hydrog. Energy 2011, 37, 162–166. [Google Scholar] [CrossRef]
- Karimi, K. Lignocellulose-Based Bioproducts; Springer: Berlin/Heidelberg, Germany, 2015; ISBN 9783319350349. [Google Scholar]
- Zhang, S.; Kim, T.-H.; Lee, Y.; Hwang, S.-J. Effects of VFAs Concentration on Bio-hydrogen Production with Clostridium Bifermentans 3AT-ma. Energy Procedia 2012, 14, 518–523. [Google Scholar] [CrossRef]
- Jamali, N.S.; Jamaliah, J. Optimization of Thermophilic Biohydrogen Production by Microflora of Palm Oil Mill Effluent: Cell Attachment on Granular Activated Carbon as Support Media Zirconia ceramic membrane as a separator in microbial fuel cell View project. Artic. Malays. J. Anal. Sci. 2016. [Google Scholar] [CrossRef]
- Ueno, Y.; Sasaki, D.; Fukui, H.; Haruta, S.; Ishii, M.; Igarashi, Y. Changes in bacterial community during fermentative hydrogen and acid production from organic waste by thermophilic anaerobic microflora. J. Appl. Microbiol. 2006, 101, 331–343. [Google Scholar] [CrossRef]
- Fu, Y.-L.; Xiong, Y.; Liu, X.-D.; Yu, W.-T.; Wang, Y.-L.; Yu, X.-J.; Ma, X.-J. Study of alginate/chitosan microcapsules for immobilization of Escherichia coli DH5 alpha. Sheng Wu Gong Cheng Xue Bao 2002, 18, 239–241. [Google Scholar]
- Zucca, P.; Sanjust, E. Inorganic Materials as Supports for Covalent Enzyme Immobilization: Methods and Mechanisms. Molecules 2014, 19, 14139–14194. [Google Scholar] [CrossRef]
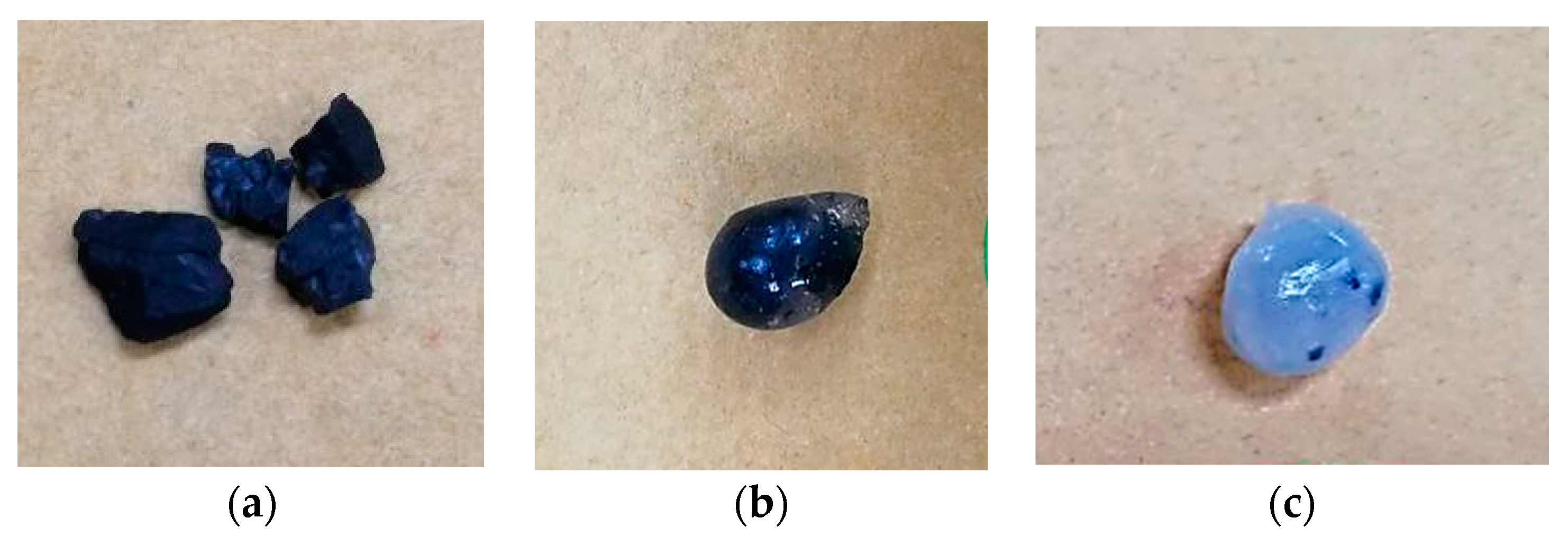
| Sample Labelling | GAC-Alg | GAC-AlgC |
|---|---|---|
| A | 1:0.5 | 1:0.5 |
| B | 1:1 | 1:1 |
| C | 1:2 | 1:2 |
| D | 1:3 | 1:3 |
| E | 1:4 | 1:4 |
| Sugar Loading | Glucose | Xylose | H2 | H2 Productivity Rate (HPR) | H2 Yield | |
|---|---|---|---|---|---|---|
| g/L | g/L | g/L | mL | mmol H2/l.d | mmol H2/l.h | mol H2/mol Total Sugar |
| 10 | 5 | 5 | 1743.42 ± 42.16 | 3.71 ± 0.09 | 2.56 ± 0.17 | 3.27 ± 0.08 |
| 20 | 10 | 10 | 1840.42 ± 97.76 | 3.92 ± 0.21 | 2.70 ± 0.29 | 3.38 ± 0.18 |
| Samples | H2 | Modified Gompertz Equation Parameter Values for H2 Production (Per Working Volume) | |||
|---|---|---|---|---|---|
| Yield | HPR | Hm | Rm | λ | |
| mol H2/mol Sugar Consumed | mmol H2/l.h | mL | mL/h | h | |
| A (1:0.5) | 1.36 ± 0.13 | 1.66 ± 0.05 | 2225.03 | 95.83 | 5.89 |
| B (1:1) | 1.73 ± 0.06 | 1.92 ± 0.05 | 3273.37 | 145.48 | 3.84 |
| C (1:2) | 2.09 ± 0.22 | 2.47 ± 0.47 | 4099.59 | 187.58 | 3.72 |
| D (1:3) | 1.54 ± 0.06 | 1.51 ± 0.45 | 2091.09 | 104.85 | 4.11 |
| E (1:4) | 1.11 ± 0.34 | 1.00 ± 0.17 | 1119.49 | 39.79 | 4.10 |
| Samples | H2 | Modified Gompertz Equation Parameter for H2 Production (Per Working Volume) | |||
|---|---|---|---|---|---|
| Yield | HPR | Hm | Rm | λ | |
| mol H2/mol Sugar Consumed | mmol H2/l.h | mL | mL/h | h | |
| A (1:0.5) | 0.46 ± 0.12 | 0.58 ± 0.20 | 133.77 | 5.55 | 6.26 |
| B (1:1) | 0.97 ± 0.21 | 0.85 ± 0.08 | 173.19 | 7.76 | 5.57 |
| C (1:2) | 1.11 ± 0.35 | 0.93 ± 0.05 | 297.76 | 13.53 | 4.45 |
| D (1:3) | 0.88 ± 0.12 | 0.74 ± 0.15 | 80.37 | 2.90 | 3.01 |
| E (1:4) | 0.67 ± 0.30 | 0.70 ± 0.20 | 150.61 | 6.03 | 4.14 |
| Carbon Source | Type of Carrier | Temp | Fermentation Process | Hydrogen Yield | References |
|---|---|---|---|---|---|
| Glucose | CA-AC | 35 | Batch | 2.6 mol H2/mol sucrose | [10] |
| Sucrose | CA-C-TiO2 | 35 | Batch | 2.60 mol H2/mol sucrose | [37] |
| Glucose | CA | 60 | Batch | 1.90 mol H2/mol glucose | [38] |
| Glucose | CA | 40 | Batch | 17 L/g mol glucose | [39] |
| Brewery wastewater | CA | 37 | Batch | 14 g/L COD | [40] |
| glucose | GAC | 37 | Continuous | 0.4–1.7 mol H2/mol sugar | [41] |
| Glucose | CA-AC | 36 | Batch | 0.029 mol H2/mol glucose | [34] |
| Sucrose | CA | 35 | Batch | 1.7 mol H2/mol sucrose | [42] |
| Xylose | CA | 37 | Continuous | 3.15 mmol H2/mol xylose | [43] |
| Sucrose | CA-AC | 35 | Continuous | 2.67 mol H2/mol sucrose | [8] |
| Glucose/Xylose | GAC-AlgC | 60 | Batch | 1.11 mol H2/mol sugar | This study |
| Glucose/Xylose | GAC-Alg | 60 | Batch | 2.09 mol H2/mol sugar | This study |
| Samples | HPR | Hydrogen | HAc | HBu | TVFAs | |
|---|---|---|---|---|---|---|
| mmol/l.d | mmol/l.h | % | mM | mM | mM | |
| GAC–Alg | ||||||
| A (1:0.5) | 3.63 | 1.66 | 84.60 | 21.08 | 25.88 | 46.95 |
| B (1:1) | 4.27 | 1.92 | 78.97 | 17.87 | 24.01 | 41.87 |
| C (1:2) | 5.00 | 2.47 | 85.87 | 24.05 | 28.66 | 52.71 |
| D (1:3) | 3.13 | 1.51 | 65.74 | 18.58 | 19.14 | 37.72 |
| E (1:4) | 2.36 | 1.00 | 73.18 | 18.21 | 22.29 | 40.50 |
| GAC–AlgC | ||||||
| A (1:0.5) | 1.15 | 0.58 | 52.94 | 10.59 | 12.36 | 22.95 |
| B (1:1) | 2.03 | 0.85 | 84.79 | 11.53 | 15.26 | 26.79 |
| C (1:2) | 2.12 | 0.93 | 86.63 | 16.60 | 16.97 | 33.57 |
| D (1:3) | 1.56 | 0.74 | 64.54 | 10.64 | 14.18 | 24.82 |
| E (1:4) | 1.55 | 0.70 | 76.43 | 12.71 | 13.49 | 26.20 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dzul Rashidi, N.F.; Jamali, N.S.; Mahamad, S.S.; Ibrahim, M.F.; Abdullah, N.; Ismail, S.F.; Siajam, S.I. Effects of Alginate and Chitosan on Activated Carbon as Immobilisation Beads in Biohydrogen Production. Processes 2020, 8, 1254. https://doi.org/10.3390/pr8101254
Dzul Rashidi NF, Jamali NS, Mahamad SS, Ibrahim MF, Abdullah N, Ismail SF, Siajam SI. Effects of Alginate and Chitosan on Activated Carbon as Immobilisation Beads in Biohydrogen Production. Processes. 2020; 8(10):1254. https://doi.org/10.3390/pr8101254
Chicago/Turabian StyleDzul Rashidi, Nur Farahana, Nur Syakina Jamali, Siti Syazwani Mahamad, Mohamad Faizal Ibrahim, Norhafizah Abdullah, Siti Fatimah Ismail, and Shamsul Izhar Siajam. 2020. "Effects of Alginate and Chitosan on Activated Carbon as Immobilisation Beads in Biohydrogen Production" Processes 8, no. 10: 1254. https://doi.org/10.3390/pr8101254
APA StyleDzul Rashidi, N. F., Jamali, N. S., Mahamad, S. S., Ibrahim, M. F., Abdullah, N., Ismail, S. F., & Siajam, S. I. (2020). Effects of Alginate and Chitosan on Activated Carbon as Immobilisation Beads in Biohydrogen Production. Processes, 8(10), 1254. https://doi.org/10.3390/pr8101254






