Modeling of Biofilm Growth on Fine Spherical Particles with the Use of Cellular Automata: The Influence of Cell Death and Lysis on the Biofilm Structure
Abstract
1. Introduction
2. Mathematical Model of the Biofilm Dynamics
- The algorithm of biomass spreading proposed by Picioreanu et al. [7]. Cell death and lysis are neglected. It will be further referred to as CA-1.
- The algorithm of biomass spreading proposed in this study. Cell death is taken into account. It will be further referred to as CA-2.
- An algorithm of biomass spreading that is the same as in CA-2. However, both death and lysis of microbiological cells are taken into account. It will be further referred to as CA-3.
Algorithm for Biomass Spreading Used in CA-2 and CA-3
- Checkif the following condition is fulfilled:If yes, go to step 2. Otherwise, finish the algorithm.
- Calculate the amount of biomass thatexceeds the maximum density:
- Calculate the amount of active and dead bacteria in the value calculated in point 1, as follows:
- Determine the concentrations of active and dead bacteria in time t + Δt:
- Find a grid cell with indices [i2, j2] at the lowest distance from [i, j] for which the following condition is fulfilled:
- Change the concentrations inthe target cells:
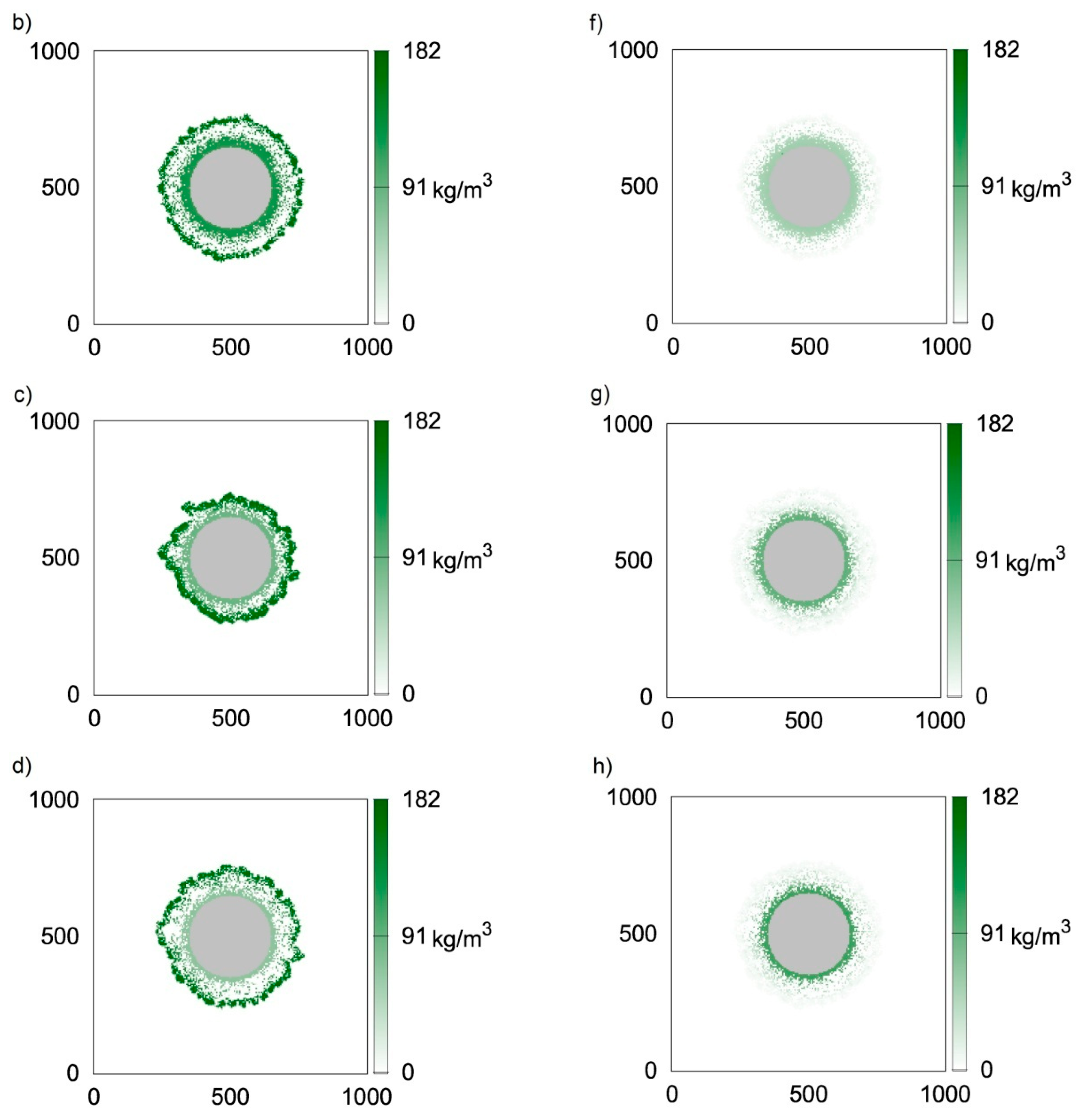
3. Dynamics of the Biofilm Growth
4. Comparison of Model Predictions with Experimental Observations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| cA, cB, cT | mass concentration of carbonaceous substrate, biomass and oxygen (kg∙m−3) |
| De | effective diffusion coefficient in biofilm (m2∙h−1) |
| k | maximum specific growth rate (h−1) |
| Kdet | detachment probability coefficient |
| ko | cell death rate coefficient (h−1) |
| klys | cell lysis rate coefficient (h−1) |
| Lb | total thickness of the biofilm (m) |
| rA, rT | uptake rate of carbonaceous substrate and oxygen, respectively (kg∙m−3∙h−1) |
| pdet | detachment probability |
| rB | growth rate of biomass (kg∙m−3∙h−1) |
| rz | radius of the inert particle (μm) |
| t | time (h) |
| wBA, wBT | growth yield coefficients (kg B∙(kg A)−1), (kg B∙(kg T)−1) |
| x | distance from the carrier surface (m) |
| Δt | time step for diffusion and utilization processes (h) |
| Δtg | time step for biomass growth and detachment (h) |
| ρb | concentration of biomass in the biofilm (kg∙m−3) |
| ρbo | concentration of dead bacteria in the biofilm (kg∙m−3) |
| ρba | concentration of active biomass in biofilm phase (kg∙m−3) |
| maximum biomass concentration in the biofilm (kg∙m−3) | |
| Superscripts | |
| b | biofilm phase |
| c | liquid (continuous) phase |
| Subscripts | |
| A, B, T | refers to carbonaceous substrate, biomass and oxygen, respectively |
| a | active bacteria |
| b | biofilm |
| o | dead bacteria |
Appendix A. Rules Governing the Cellular Automata
Rule 1—Diffusion of the Substrates
Rule 2—Utilization of the Substrates in the Biofilm
Rule 3—Growth of Microorganisms and Biofilm Detachment
References
- Tanyolaç, A.; Beyenal, H. Prediction of average biofilm density and performance of a spherical bioparticle under substrate inhibition. Biotechnol. Bioeng. 1997, 56, 319–329. [Google Scholar] [CrossRef]
- Al-Qodah, Z. Antibiotics production in a fluidized bed reactor utilizing a transverse magnetic field. Bioprocess Eng. 2000, 22, 299–308. [Google Scholar] [CrossRef]
- Nicolella, C.; van Loosdrecht, M.C.M.; Heijnen, S.J. Particle-based biofilm reactor technology. Trends Biotechnol. 2000, 18, 312–320. [Google Scholar] [CrossRef]
- Atkinson, B.; Daoud, I.S.; Williams, D.A. A theory for biological film reactor. Trans. Inst. Chem. Eng. Chem. Eng. 1968, 46, 245–250. [Google Scholar]
- Julian, W.; Werner, M.; Ulrich, S. Heterogeneity in biofilms. FEMS Microbiol. Rev. 2006, 24, 661–671. [Google Scholar]
- Picioreanu, C.; van Loosdrecht, M.C.M.; Heijnen, J.J. Mathematical modeling of biofilm structure with a hybrid differential-discrete cellular automaton approach. Biotechnol. Bioeng. 1998, 58, 101–116. [Google Scholar] [CrossRef]
- Picioreanu, C.; van Loosdrecht, M.C.M.; Heijnen, J.J. A new combined differential-discrete cellular automaton approach for biofilm modeling: Application for growth in gel beads. Biotechnol. Bioeng. 1998, 57, 718–731. [Google Scholar] [CrossRef]
- Xavier, J.B.; Picioreanu, C.; Van Loosdrecht, M.C.M. A modelling study of the activity and structure of biofilms in biological reactors. Biofilms 2004, 1, 377–391. [Google Scholar] [CrossRef][Green Version]
- Wanner, O.; Eberl, H.J.; Morgenroth, E.; Noguera, D.R.; Picioreanu, C.; Rittmann, B.E.; van Loosdrecht, M.C.M. Mathematical Modeling of Biofilms; WA Scientific and Technical Report No. 18; IWA Task Group on Biofilm Modeling: London, UK, 2006. [Google Scholar]
- Graf von der Schulenburg, D.A.; Pintelon, T.R.R.; Picioreanu, C.; van Loosdrecht, M.C.M.; Johns, M.J. Three-dimensional simulations of biofilm growth in porous media. AIChE J. 2008, 55, 494–504. [Google Scholar] [CrossRef]
- Skoneczny, S. Cellular automata-based modelling and simulation of biofilm structure on multi-core computers. Water Sci. Technol. 2015, 72, 2071–2081. [Google Scholar] [CrossRef]
- Skoneczny, S. Cellular automata as an effective tool for modelling of biofilm morphology. Environ. Prot. Eng. 2017, 43, 177–190. [Google Scholar] [CrossRef]
- Bishop, P.L.; Zhang, T.C.; Fu, Y.-C. Effects of biofilm structure, microbial distributions and mass transport on biodegradation processes. Water Sci. Technol. 1995, 31, 143–152. [Google Scholar] [CrossRef]
- Pizarro, G.E.; Garcia, C.; Moreno, R.; Sepulveda, M.E. Two-dimensional cellular automaton model for mixed-culture biofilm. Water Sci. Technol. 2004, 49, 193–198. [Google Scholar] [CrossRef]
- Tang, Y.; Valocchi, A.J. An improved cellular automaton method to model multispecies biofilms. Water Res. 2013, 47, 5729–5742. [Google Scholar] [CrossRef] [PubMed]
- Karel, S.F.; Libicki, S.B.; Robertson, C.R. The immobilization of whole cells: Engineering principles. Chem. Eng. Sci. 1985, 40, 1321–1354. [Google Scholar] [CrossRef]
- Tang, W.-T.; Wisecarver, K.; Fan, L.-S. Dynamics of a draft tube gas-liquid-solid fluidized bed bioreactor for phenol degradation. Chem. Eng. Sci. 1987, 42, 2123–2134. [Google Scholar] [CrossRef]
- Beyenal, H.; Tanyolaç, A. The effects of biofilm characteristics on the external mass transfer coefficient in a differential fluidized bed biofilm reactor. Biochem. Eng. J. 1998, 1, 53–61. [Google Scholar] [CrossRef]
- Seker, S.; Beyenal, H.; Salih, B.; Tanyolac, A. Multi-substrate growth kinetics of Pseudomonas putida for phenol removal. Appl. Microbiol. Biotechnol. 1997, 47, 610–614. [Google Scholar]
- Chambless, J.D.; Stewart, P.S. A three-dimensional computer model analysis of three hypothetical biofilm detachment mechanisms. Biotechnol. Bioeng. 2007, 97, 1573–1584. [Google Scholar] [CrossRef]
- Şeker, Ş.; Beyenal, H.; Tanyolaç, A. The effects of biofilm thickness on biofilm density and substrate consumption rate in a differential fluidizied bed biofilm reactor (DFBBR). J. Biotechnol. 1995, 41, 39–47. [Google Scholar] [CrossRef]
- Horn, H.; Reiff, H.; Morgenroth, E. Simulation of growth and detachment in biofilm systems under defined hydrodynamic conditions. Biotechnol. Bioeng. 2003, 81, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kumar, S.; Kumar, S. Biodegradation kinetics of phenol and catechol using Pseudomonas putida MTCC 1194. Biochem. Eng. J. 2005, 22, 151–159. [Google Scholar] [CrossRef]
- Tabiś, B.; Siudzińska, R. Ocena rozkładów gęstości i współczynników dyfuzji w biofilmie immobilizowanym na materiale drobnoziarnistym. Przem. Chem. 2005, 84, 250–253. [Google Scholar]
- Beyenal, H.; Şeker, Ş.; Tanyolaç, A.; Salih, B. Diffusion coefficients of phenol and oxygen in a biofilm of Pseudomonas putida. AIChE J. 1997, 43, 243–250. [Google Scholar] [CrossRef]







| k (1/h) | KA (kg/m3) | KT (kg/m3) | Kin (kg/m3) | wBA (kg/kg) | wBT (kg/kg) |
|---|---|---|---|---|---|
| 0.569 | 1.8539 × 10−2 | 4.80 × 10−5 | 9.9374 × 10−2 | 0.521 | 0.338 |
| Model | klys (1/h) | R2 |
|---|---|---|
| CA-1 | - | 0.056 |
| CA-2 | 0 | 0.92 |
| CA-3 | 2.8 × 10−3 | 0.88 |
| 5.6 × 10−3 | 0.93 | |
| 1.12 × 10−2 | 0.96 | |
| 2.24 × 10−2 | 0.61 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skoneczny, S.; Cioch-Skoneczny, M. Modeling of Biofilm Growth on Fine Spherical Particles with the Use of Cellular Automata: The Influence of Cell Death and Lysis on the Biofilm Structure. Processes 2020, 8, 1234. https://doi.org/10.3390/pr8101234
Skoneczny S, Cioch-Skoneczny M. Modeling of Biofilm Growth on Fine Spherical Particles with the Use of Cellular Automata: The Influence of Cell Death and Lysis on the Biofilm Structure. Processes. 2020; 8(10):1234. https://doi.org/10.3390/pr8101234
Chicago/Turabian StyleSkoneczny, Szymon, and Monika Cioch-Skoneczny. 2020. "Modeling of Biofilm Growth on Fine Spherical Particles with the Use of Cellular Automata: The Influence of Cell Death and Lysis on the Biofilm Structure" Processes 8, no. 10: 1234. https://doi.org/10.3390/pr8101234
APA StyleSkoneczny, S., & Cioch-Skoneczny, M. (2020). Modeling of Biofilm Growth on Fine Spherical Particles with the Use of Cellular Automata: The Influence of Cell Death and Lysis on the Biofilm Structure. Processes, 8(10), 1234. https://doi.org/10.3390/pr8101234





