Improvement of Catalytic Activity of Platinum Nanoparticles Decorated Carbon Graphene Composite on Oxygen Electroreduction for Fuel Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Material Synthesis
2.2. Electrochemical Characterization
3. Results and Discussions
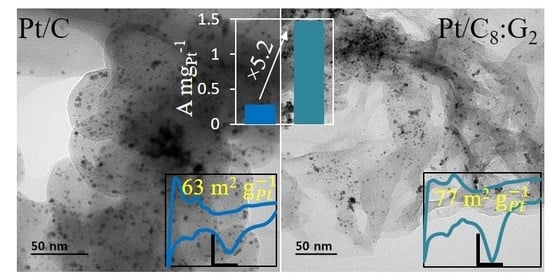
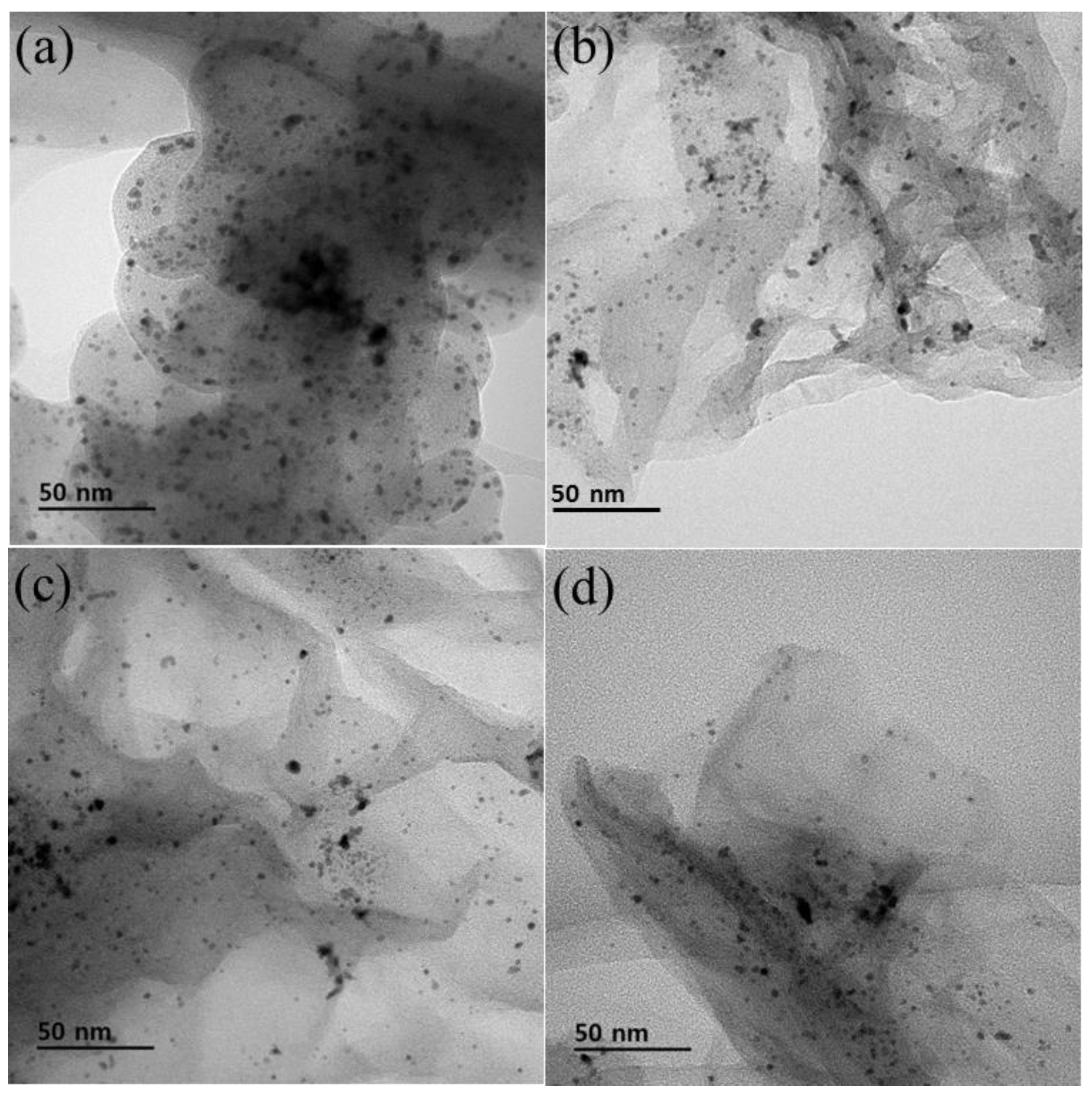
3.1. Surface Analysis of Pt/Cx:G10−x
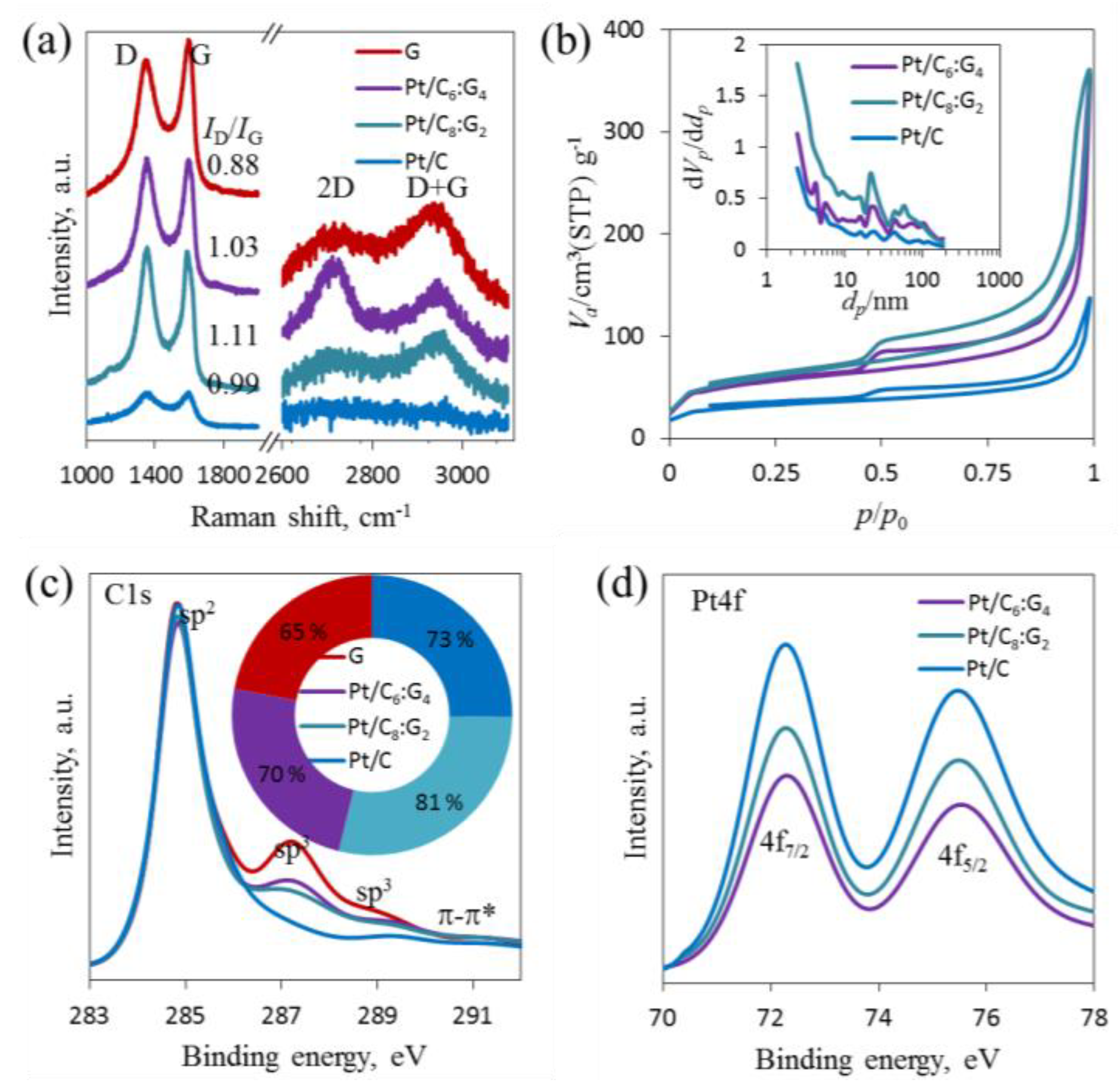
3.2. Raman Analysis of Pt/Cx:G10−x
3.3. SSA Measurement of Pt/Cx:G10−x
3.4. XPS Analysis of Pt/Cx:G10−x
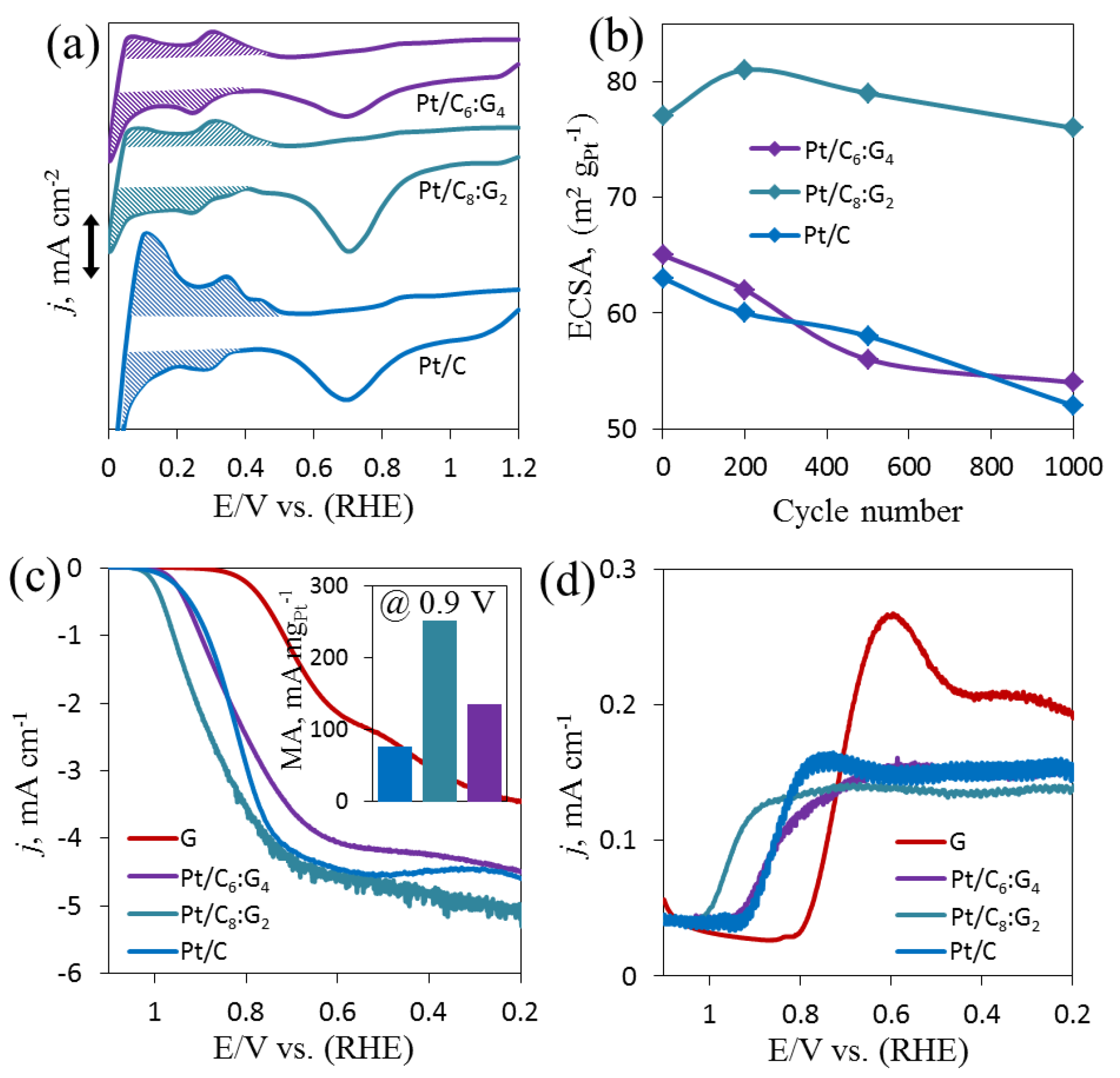
3.5. Electrochemical Analysis of Pt/Cx:G10−x
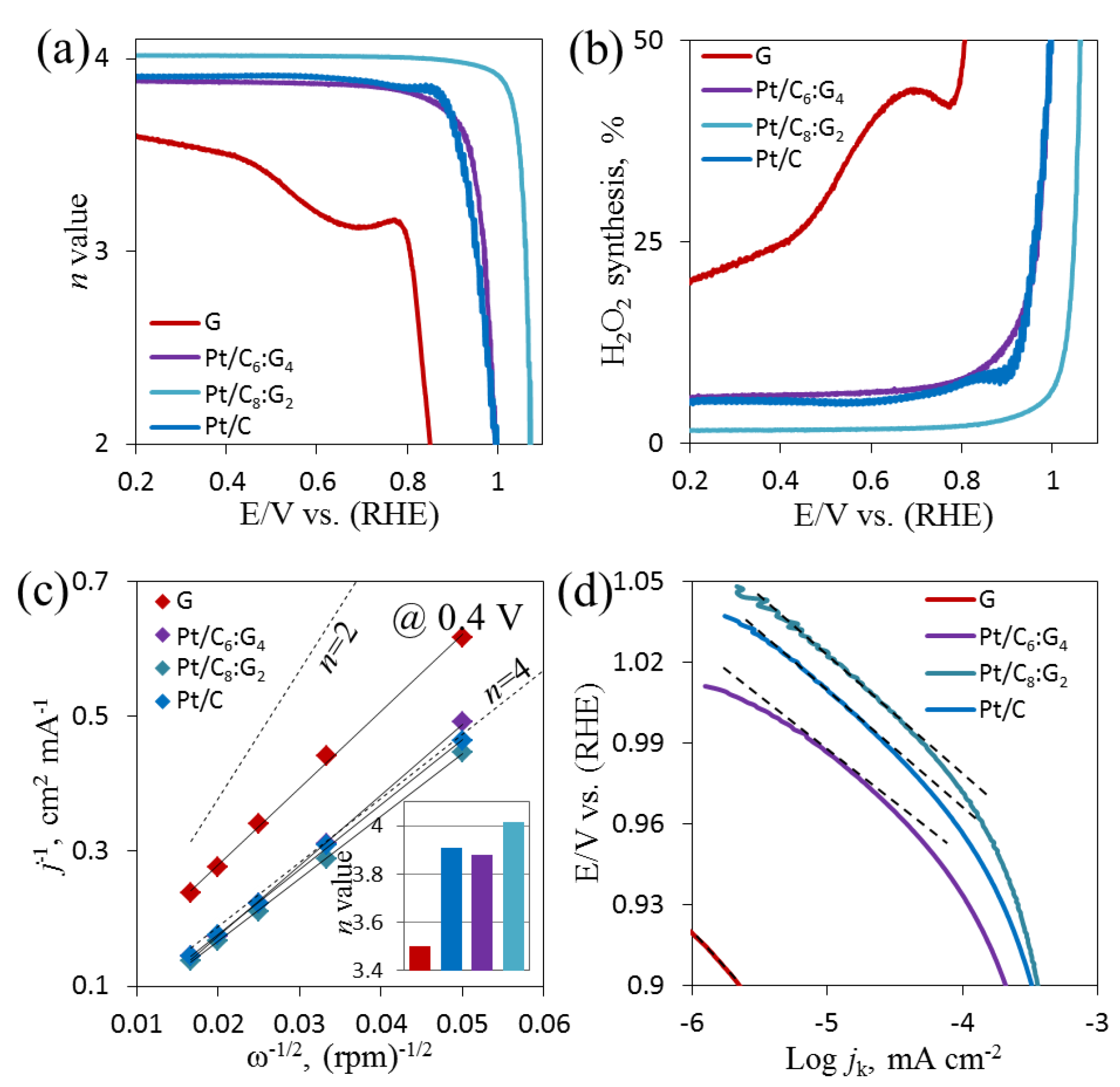
3.6. Electrochemical ORR on Pt/Cx:G10−x
3.7. Kinetics of ORR on Pt/Cx:G10−x
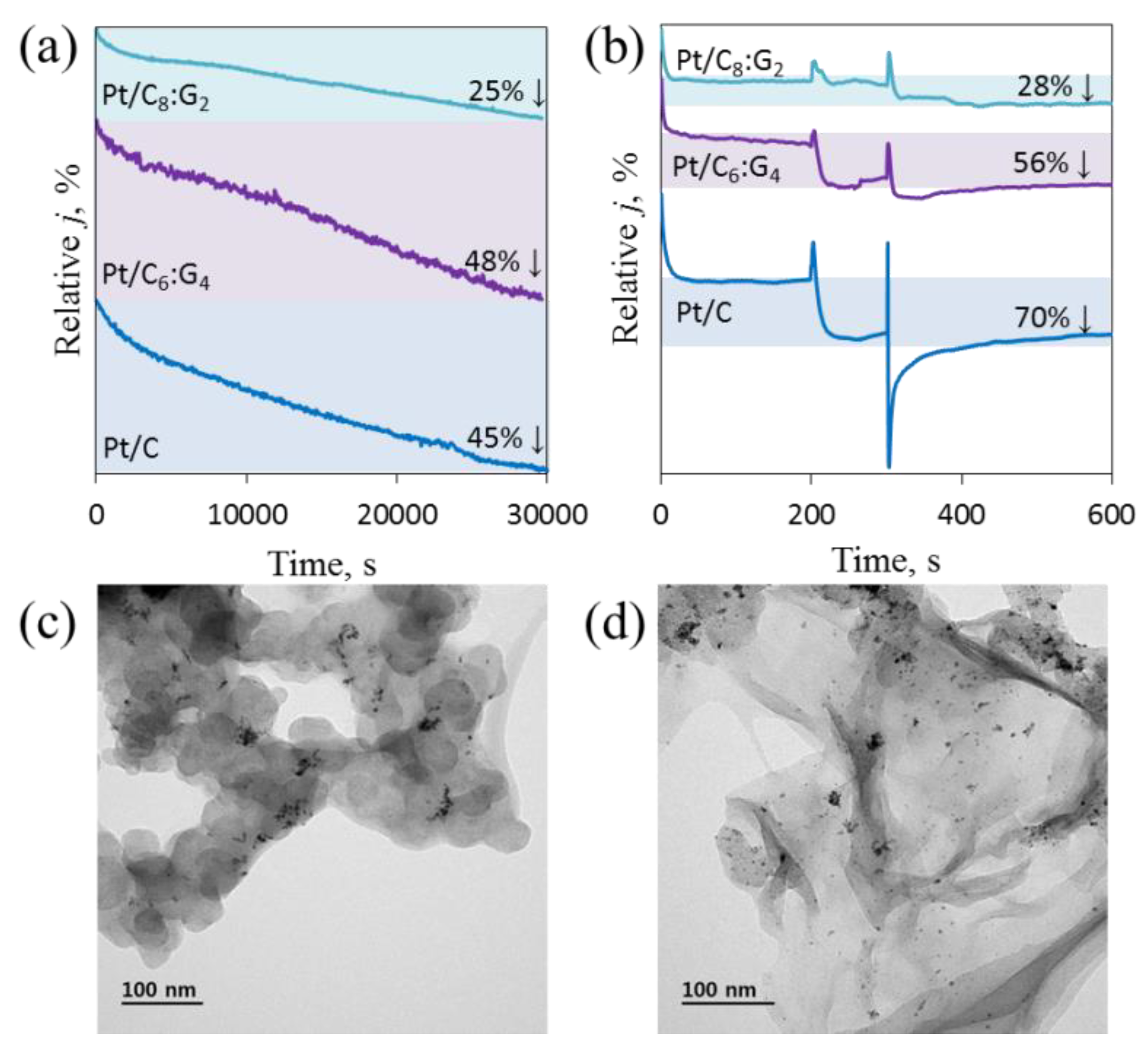
3.8. Stability and Selectivity Towards ORR on Pt/Cx:G10−x
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Debe, M.K. Electrocatalyst approaches and challenges for automotive fuel cells. Nature 2012, 486, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Porosoff, M.D.; Chen, J.G. Review of Pt-based bimetallic catalysis: From model surfaces to supported catalysts. Chem. Rev. 2012, 112, 5780–5817. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Y.; Lim, B.; Lee, E.P.; Xia, Y.N. Shape-controlled synthesis of platinum nanocrystals for catalytic and electrocatalytic applications. Nano Today 2009, 4, 81–95. [Google Scholar] [CrossRef]
- Wu, J.; Yang, H. Platinum-based oxygen reduction electrocatalysts. Acc. Chem. Res. 2013, 46, 1848–1857. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Ahmed, M.S.; Jeon, S. Electrochemical deposition of silver on manganese dioxide coated reduced graphene oxide for enhanced oxygen reduction reaction. J. Power Sources 2015, 288, 261. [Google Scholar] [CrossRef]
- Markovic, N.M.; Grgur, B.N.; Ross, P.N. Temperature-dependent hydrogen electrochemistry on platinum low-index single-crystal surfaces in acid solutions. J. Phys. Chem. B 1997, 101, 5405–5413. [Google Scholar] [CrossRef]
- Paulus, U.A.; Schmidt, T.J.; Gasteiger, H.A.; Behm, R.J. Oxygen reduction on a high-surface area Pt/Vulcan carbon catalyst: A thin-film rotating ring-disk electrode study. J. Electroanal. Chem. 2001, 495, 134–145. [Google Scholar] [CrossRef]
- Ahmed, M.S.; Kim, D.; Jeon, S. Covalently grafted platinum nanoparticles to multi walled carbon nanotubes for enhanced electrocatalytic oxygen reduction. Electrochimca Acta 2013, 92, 168. [Google Scholar] [CrossRef]
- Lim, B.; Jiang, M.; Camargo, P.H.C.; Cho, E.C.; Tao, J.; Lu, X.; Zhu, Y.; Xia, Y. Pd-Pt bimetallic nanodendrites with high activity for oxygen reduction. Science 2009, 324, 1302. [Google Scholar] [CrossRef] [PubMed]
- Choe, J.E.; Ahmed, M.S.; Jeon, S. 3,4-ethylenedioxythiophene functionalized graphene with palladium nanoparticles for enhanced electrocatalytic oxygen reduction reaction. J. Power Sources 2015, 281, 211. [Google Scholar] [CrossRef]
- Dong, Y.; Zhou, Y.W.; Wang, M.Z.; Zheng, S.L.; Jiang, K.; Cai, W.B. Facile aqueous phase synthesis of carbon supported B-doped Pt3Ni nanocatalyst for efficient oxygen reduction reaction. Electrochimca Acta 2017, 246, 242. [Google Scholar] [CrossRef]
- Ahmed, M.S.; Jeon, S. Highly active graphene-supported NixPd100−x binary alloyed catalysts for electro-oxidation of ethanol in an alkaline media. ACS Catal. 2014, 4, 1830. [Google Scholar] [CrossRef]
- Begum, H.; Ahmed, M.S.; Lee, D.W.; Kim, Y.B. Carbon nanotubes-based PdM bimetallic catalysts through N4-system for efficient ethanol oxidation and hydrogen evolution reaction. Sci. Rep. 2019, 9, 11051. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.J.; Liu, L.; Lin, X.X.; Yuan, J.; Feng, J.J. One-pot synthesis of 3D freestanding porous PtAg hollow chain-like networks as efficient electrocatalyst for oxygen reduction reaction. Electrochimca Acta 2017, 245, 883. [Google Scholar] [CrossRef]
- Begum, H.; Ahmed, M.S.; Jeon, S. A novel δ-MnO2 with carbon nanotubes nanocomposite as an enzyme-free sensor for hydrogen peroxide electrosensing. RSC Adv. 2016, 6, 50572. [Google Scholar] [CrossRef]
- Bregoli, L.J. The influence of platinum crystallite size on the electrochemical reduction of oxygen in phosphoric acid. Electrochimca Acta 1978, 23, 489–492. [Google Scholar] [CrossRef]
- Kinoshita, K. Particle size effects for oxygen reduction on highly dispersed platinum in acid electrolytes. J. Electrochem. Soc. 1990, 137, 845–848. [Google Scholar] [CrossRef]
- Wang, Y.J.; Zhao, N.; Fang, B.; Li, H.; Bi, X.T.; Wang, H. Carbon-supported Pt-based alloy electrocatalysts for the oxygen reduction reaction in polymer electrolyte membrane fuel cells: Particle size, shape, and composition manipulation and their impact to activity. Chem. Rev. 2015, 115, 3433–3467. [Google Scholar] [CrossRef]
- Peng, Z.; Yang, H. Designer platinum nanoparticles: Control of shape, composition in alloy, nanostructure and electrocatalytic property. Nano Today 2009, 4, 143–164. [Google Scholar] [CrossRef]
- Darling, R.M.; Meyers, J.P. Kinetic model of platinum dissolution in PEMFCs. J. Electrochem. Soc. 2003, 150, A1523–A1527. [Google Scholar] [CrossRef]
- Van der Vliet, D.F.; Wang, C.; Li, D.; Paulikas, A.P.; Greeley, J.; Rankin, R.B.; Strmcnik, D.; Tripkovic, D.; Markovic, N.M.; Stamenkovic, V.R. Unique electrochemical adsorption properties of Pt-skin surfaces. Angew. Chem. Int. Ed. 2012, 51, 3139–3142. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Gan, L.; Heggen, M.; Rudi, S.; Strasser, P. Compositional segregation in shaped Pt alloy nanoparticles and their structural behavior during electrocatalysis. Nat. Mater. 2013, 12, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhao, Z.; Cheng, T.; Fortunelli, A.; Chen, C.-Y.; Yu, R.; Zhang, Q.; Gu, L.; Merinov, B.V.; Lin, Z.; et al. Ultrafine jagged platinum nanowires enable ultrahigh mass activity for the oxygen reduction reaction. Science 2016, 354, 1414–1419. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Markovic, N.M.; Stamenkovic, V.R. Advanced platinum alloy electrocatalysts for the oxygen reduction reaction. ACS Catal. 2012, 2, 891–898. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, S.; Chang, Q.; Su, D.; Yue, J.; Du, Z.; Shao, M. Palladium−platinum core−shell electrocatalysts for oxygen reduction reaction prepared with the assistance of citric acid. ACS Catal. 2016, 6, 3428–3432. [Google Scholar] [CrossRef]
- Yang, J.; Kim, S.H.; Kwak, S.K.; Song, H.K. Curvature-induced metal-support interaction of an islands-byislands composite of platinum catalyst and carbon nano-onion for durable oxygen reduction. ACS Appl. Mater. Interfaces 2017, 9, 23302–23308. [Google Scholar] [CrossRef] [PubMed]
- Begum, H.; Ahmed, M.S.; Cho, S.; Jeon, S. Simultaneous reduction and nitrogen functionalization of graphene oxide using lemon for metal-free oxygen reduction reaction. J. Power Sources 2017, 372, 116–124. [Google Scholar] [CrossRef]
- Balandin, A.A.; Ghosh, S.; Bao, W.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior thermal conductivity of single-layer graphene. Nano Lett. 2008, 8, 902–907. [Google Scholar] [CrossRef] [PubMed]
- Morozov, S.V.; Novoselov, K.S.; Katsnelson, M.I.; Schedin, F.; Elias, D.C.; Jaszczak, J.A.; Geim, A.K. Giant intrinsic carrier mobilities in graphene and its bilayer. Phys. Rev. Lett. 2008, 100, 016602. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.S.; Han, H.S.; Jeon, S. One-step chemical reduction of graphene oxide with oligothiophene for improved electrocatalytic oxygen reduction reactions. Carbon 2013, 61, 164–172. [Google Scholar] [CrossRef]
- Begum, H.; Ahmed, M.S.; Jeon, S. New Approach for porous chitosan−graphene matrix preparation through enhanced amidation for synergic detection of dopamine and uric acid. ACS Omega 2017, 2, 3043–3054. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.S.; Kim, Y.B. Amide-functionalized graphene with 1,4-diaminobutane as efficient metal-free and porous electrocatalyst for oxygen reduction. Carbon 2017, 111, 577–586. [Google Scholar] [CrossRef]
- Hu, C.; Xue, J.; Dong, L.; Jiang, Y.; Wang, X.; Qu, L.; Dai, L. Scalable preparation of multifunctional fire retardant ultralight graphene foams. ACS Nano 2016, 10, 1325–1332. [Google Scholar] [CrossRef] [PubMed]
- Men, B.; Sun, Y.; Li, M.; Hu, C.; Zhang, M.; Wang, L.; Tang, Y.; Chen, Y.; Wan, P.; Pan, J. Hierarchical Metal-free nitrogen-doped porous graphene/carbon composites as an efficient oxygen reduction reaction catalyst. ACS Appl. Mater. Interfaces 2016, 8, 1415–1423. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Wang, J.; Wang, C.; Zhang, H.; Lu, C.; Wang, J. Hierarchical porous graphene carbon-based supercapacitors. Chem. Mater. 2015, 27, 2107–2113. [Google Scholar] [CrossRef]
- Higgins, D.C.; Hoque, M.A.; Hassan, F.; Choi, J.Y.; Kim, B.; Chen, Z. Oxygen reduction on graphene−carbon nanotube composites doped sequentially with nitrogen and sulfur. ACS Catal. 2014, 4, 2734–2740. [Google Scholar] [CrossRef]
- Allen, M.J.; Tung, V.C.; Kaner, R.B. Honeycomb carbon: A review of graphene. Chem. Rev. 2010, 110, 132–145. [Google Scholar] [CrossRef]
- Probst, N.; Grivei, E. Structure and electrical properties of carbon black. Carbon 2002, 40, 201–205. [Google Scholar] [CrossRef]
- Tao, L.; Wang, Q.; Dou, S.; Ma, Z.; Huo, J.; Wang, S.; Dai, L. Edge-rich and dopant-free graphene as a highly efficient metal-free electrocatalyst for the oxygen reduction reaction. Chem. Commun. 2016, 52, 2764–2767. [Google Scholar] [CrossRef]
- Yang, H.B.; Miao, J.; Hung, S.F.; Chen, J.; Tao, H.B.; Wang, X.; Zhang, L.; Chen, R.; Gao, J.; Chen, H.M.; et al. Identification of catalytic sites for oxygen reduction and oxygen evolution in N-doped graphene materials: Development of highly efficient metal-free bifunctional electrocatalyst. Sci. Adv. 2016, 2, e1501122. [Google Scholar] [CrossRef]
- Sanetuntikul, J.; Hang, T.; Shanmugam, S. Hollow nitrogen-doped carbon spheres as efficient and durable electrocatalysts for oxygen reduction. Chem. Commun. 2014, 50, 9473–9476. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wei, Z.; Gou, X. Nitrogen and phosphorus dual-doped graphene/carbon nanosheets as bifunctional electrocatalysts for oxygen reduction and evolution. ACS Catal. 2015, 5, 4133–4142. [Google Scholar] [CrossRef]
- Ahmed, M.S.; Jeon, S. Electrochemical activity evaluation of chemically damaged carbon nanotube with palladium nanoparticles for ethanol oxidation. J. Power Sources 2015, 282, 479–488. [Google Scholar] [CrossRef]
- You, J.M.; Ahmed, M.S.; Han, H.S.; Choe, J.E.; Üstündag, Z.; Jeon, S. New approach of nitrogen and sulfur-doped graphene synthesis using dipyrrolemethane and their electrocatalytic activity for oxygen reduction in alkaline media. J. Power Sources 2015, 275, 73–79. [Google Scholar] [CrossRef]
- Joo, Y.; Ahmed, M.S.; Han, H.S.; Jeon, S. Preparation of electrochemically reduced graphene oxide-based silver-cobalt alloy nanocatalysts for efficient oxygen reduction reaction. Int. J. Hydrogen Energy 2017, 42, 21751–21761. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved synthesis of graphene oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef]
- Rivera, L.M.; Fajardo, S.; Arévalo, M.C.; García, G.; Pastor, E. S-and N-doped graphene nanomaterials for the oxygen reduction reaction. Catalysts 2017, 7, 278–290. [Google Scholar] [CrossRef]
- Wen, Z.; Wang, X.; Mao, S.; Bo, Z.; Kim, H.; Cui, S.; Lu, G.; Feng, X.; Chen, J. Crumpled nitrogen-doped graphene nanosheets with ultrahigh pore volume for high-performance supercapacitor. Adv. Mater. 2012, 24, 5610–5616. [Google Scholar] [CrossRef]
- Ke, Q.; Wang, J. Graphene-based materials for supercapacitor electrodes—A review. J. Materiomics 2016, 2, 37–54. [Google Scholar] [CrossRef]
- Begum, H.; Ahmed, M.S.; Jeon, S. Highly efficient dual active palladium nanonetwork electrocatalyst for ethanol oxidation and hydrogen evolution. ACS Appl. Mater. Interfaces 2017, 9, 39303–39311. [Google Scholar] [CrossRef]
- Ahmed, M.S.; Kim, Y.B. 3D graphene preparation via covalent amide functionalization for efficient metal-free electrocatalysis in oxygen reduction. Sci. Rep. 2017, 7, 43279. [Google Scholar] [CrossRef] [PubMed]
- Begum, H.; Ahmed, M.S.; Cho, S.; Jeon, S. Freestanding palladium nanonetworks electrocatalyst for oxygen reduction reaction in fuel cells. Int. J. Hydrogen Energy 2018, 43, 229–238. [Google Scholar] [CrossRef]
- Han, H.S.; Ahmed, M.S.; Jeong, H.; Jeon, S. Electrochemical sensing of monohydric alcohols on different linkers imbedded in between graphene and platinum nanoparticles. J. Nanosci. Nanotechnol. 2016, 16, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.S.; Park, D.; Jeon, S. Ultrasmall PdmMn1_mOx binary alloyed nanoparticles on graphene catalysts for ethanol oxidation in alkaline media. J. Power Sources 2016, 308, 180–188. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, J.; Zhao, Y.; Wang, F.; Song, Y. Pt–Co secondary solid solution nanocrystals supported on carbon as next-generation catalysts for the oxygen reduction reaction. J. Mater. Chem. A 2015, 3, 20086–20091. [Google Scholar] [CrossRef]
- Ahmed, M.S.; Lee, D.W.; Kim, Y.B. Graphene supported silver nanocrystals preparation for efficient oxygen reduction in alkaline fuel cells. J. Electrochem. Soc. 2016, 163, F1169–F1176. [Google Scholar] [CrossRef]
- Wang, C.H.; Huang, H.C.; Chang, S.T.; Lin, Y.C.; Huang, M.F. Pyrolysis of melamine-treated vitamin B12 as a non-precious metal catalyst for oxygen reduction reaction. RSC Adv. 2014, 4, 4207–4211. [Google Scholar] [CrossRef]
- Ahmed, M.S.; Jeon, S. New functionalized graphene sheets for enhanced oxygen reduction as metal-free cathode electrocatalysts. J. Power Sources 2012, 218, 168–173. [Google Scholar] [CrossRef]
- Ahmed, M.S.; Jeong, H.; You, J.M.; Jeon, S. Electrocatalytic reduction of dioxygen at a modified glassy carbon electrode based on Nafion-dispersed single-walled carbon nanotubes and cobalt–porphyrin with palladium nanoparticles in acidic media. Electrochimca Acta 2011, 56, 4924–4929. [Google Scholar] [CrossRef]
- Ahmed, M.S.; Jeon, S. The nanostructure of nitrogen atom linked carbon nanotubes with platinum employed to the electrocatalytic oxygen reduction. J. Nanosci. Nanotechnol. 2013, 13, 306. [Google Scholar] [CrossRef]
- Yun, M.; Ahmed, M.S.; Jeon, S. Thiolated graphene oxide-supported palladium cobalt alloyed nanoparticles as high performance electrocatalyst for oxygen reduction reaction. J. Power Sources 2015, 293, 380–387. [Google Scholar] [CrossRef]
- Tang, X.; Ng, H.Y. Cobalt and nitrogen-doped carbon catalysts for enhanced oxygen reduction and power production in microbial fuel cells. Electrochimca Acta 2017, 247, 193. [Google Scholar] [CrossRef]
- Sohn, G.J.; Choi, H.J.; Jeon, I.Y.; Chang, D.W.; Dai, L.; Baek, J.B. Water-dispersible, sulfonated hyperbranched poly(ether-ketone) grafted multiwalled carbon nanotubes as oxygen reduction catalysts. ACS Nano 2012, 6, 6345–6355. [Google Scholar] [CrossRef] [PubMed]
- Park, D.; Ahmed, M.S.; Jeon, S. Covalent functionalization of graphene with 1,5-diaminonaphthalene and ultrasmall palladium nanoparticles for electrocatalytic oxygen reduction. Int. J. Hydrogen Energy 2017, 42, 2061–2070. [Google Scholar] [CrossRef]
- Zhang, J.J.; Sui, X.L.; Huang, G.S.; Gu, D.M.; Wang, Z.B. Hierarchical carbon coated molybdenum dioxide nanotubes as a highly active and durable electrocatalytic support for methanol oxidation. J. Mater. Chem. A 2017, 5, 4067–4074. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Begum, H.; Kim, Y.-B. Improvement of Catalytic Activity of Platinum Nanoparticles Decorated Carbon Graphene Composite on Oxygen Electroreduction for Fuel Cells. Processes 2019, 7, 586. https://doi.org/10.3390/pr7090586
Begum H, Kim Y-B. Improvement of Catalytic Activity of Platinum Nanoparticles Decorated Carbon Graphene Composite on Oxygen Electroreduction for Fuel Cells. Processes. 2019; 7(9):586. https://doi.org/10.3390/pr7090586
Chicago/Turabian StyleBegum, Halima, and Young-Bae Kim. 2019. "Improvement of Catalytic Activity of Platinum Nanoparticles Decorated Carbon Graphene Composite on Oxygen Electroreduction for Fuel Cells" Processes 7, no. 9: 586. https://doi.org/10.3390/pr7090586
APA StyleBegum, H., & Kim, Y.-B. (2019). Improvement of Catalytic Activity of Platinum Nanoparticles Decorated Carbon Graphene Composite on Oxygen Electroreduction for Fuel Cells. Processes, 7(9), 586. https://doi.org/10.3390/pr7090586