1. Introduction
As an important strategic material metal, cobalt plays an important role in national economic development. It is also an important raw material for the production of various high temperature and corrosion resistant alloys, cemented carbides, superhard materials, magnetic materials, catalysts, and other materials [
1]. It is widely used in industries such as aviation, aerospace, electrical appliances, machinery manufacturing, chemical industry and ceramics, etc. Cobalt–sulfur concentrate is the main raw material for extracting cobalt in industry. Pyrite is the main sulfur-bearing mineral in cobalt–sulfur concentrate [
2]. As cobalt–sulfur concentrate mainly contains pyrite, pyrrhotite, and gangue minerals such as talc, quartz, and chlorite, it is sometimes difficult to obtain cobalt concentrate and sulfur concentrate separately.
Cobalt-bearing minerals are mainly composed of arsenide, such as cobaltite (arsenopyrite), arsenopyrite, orthoclase arsenopyrite, and skutterudite, etc.; sulfide, such as sulfur–copper–cobalt ore, and sulfur–nickel–cobalt ore, etc.; oxide, such as cobalt oxide, cobalt earth ore, miscellaneous cobalt ore, and spheroidite; and the smelting slags of cobalt-bearing pyrite, cobalt–nickel ore, and cobalt-bearing copper ore [
3].
When separating cobalt minerals from cobalt ores and cobalt-bearing ores, the flotation method is mostly employed, and in rare cases, the gravity beneficiation method is also used. Gravity beneficiation method is mainly used for arsenide with larger specific gravity (e.g., cobaltite with a specific gravity of 6.2 and arsenic–cobalt with a specific gravity of 6.5). As there is small difference between the specific gravity of cobalt oxide mineral and that of gangue, it is difficult to separate the cobalt oxide mineral (the specific gravity of cobalt wafer is 3, and the specific gravity of cobalt earth ratio is 3–3.5) from gangue effectively by gravity beneficiation method, thus making it unable to obtain an ideal cobalt concentrate product [
4,
5].
If cobalt occurs in pyrite, pyrrhotite, chalcopyrite, and other minerals in the form of isomorphism, cobalt concentrate is usually recovered by flotation carrier minerals rather than directly obtained. If cobalt-bearing minerals are cobalt-rich ores and cobalt sulfide ores with good floatability, it is more economical to recover cobalt concentrate by flotation. However, the problem is that cobalt minerals are closely related to other metal minerals and gangue minerals, and their floatability has little difference. Therefore, the emphasis is to develop new collectors of cobalt minerals and effective depressants of gangue minerals [
6].
At present, cobalt minerals are generally recovered by flotation process. The commonly used collectors for flotation of cobalt-bearing minerals are black catching agent, xanthate, amines, palm oil, fatty acids, and so on. However, there are still no specific collectors for cobalt-bearing minerals so far. A lot of research work is needed to find a kind of collector with high efficiency and good selectivity, which is not highly influenced by other factors in pulp.
There are some SKARN-TYPE deposits, in which pyrite contains a small amount of cobalt. It is of great significance to comprehensively recover drilling metal from concentrators in these mines. However, some factories can only produce sulfur concentrate because they can’t reach the cobalt concentrate target, while others dare not adopt flotation technology for various reasons, which wastes a lot of national resources [
7]. Therefore, it is of practical significance to study the flotation of pyrite and cobalt-bearing pyrite. The pyrite shows good floatability. Nevertheless, as the geological conditions and mineral composition of each deposit are different, the flotation process of pyrite is also different.
Tieshanhe iron ore in Jiyuan occurs in the hydrothermal deposit of dolomite related to diorite metasomatism. Gangue minerals are mainly tremolite, actinolite, calcite, dolomite, and chlorite. It is difficult to separate tremolite and actinolite from pyrite, and it is difficult to obtain high quality sulfur–cobalt concentrate by multistage concentration. In order to obtain high quality sulfur–cobalt concentrate, it is necessary to inhibit these magnesium-containing silicate minerals in the flotation process. At present, there is a shortage of cobalt metal in China, and the cobalt grade of sulfur and cobalt concentrate recovered by some concentrators is about 0.30%. Using low-grade sulfer–cobalt concentrates for smelting has resulted in high cost for smelters. Therefore, it is an urgent task to improve the quality of cobalt sulfide concentrate [
8].
The Wissokogolsk magnetite contains copper and cobalt, and the Ural sulfide magnetite also contains copper and cobalt. Their comprehensive recovery rates of the magnetite are as follows: The former has a cobalt concentrate grade of 0.50% and a recovery of 36.82%, while the latter has a cobalt concentrate grade of 0.49% and a recovery of 66.40%. The cobalt grade of Jiyuan iron ore is 0.023% and the sulfur grade is 1.14%. The cobalt grade in sulfur–cobalt concentrate is 0.62%, sulfur grade is 41.37%, and cobalt recovery rate is 65.43% [
9].
The Panxi Region of China is rich in mineral resources. The proven reserves of vanadium–titanium magnetite are 10 billion tons. Among them, iron resources account for 20% of the domestic iron ore reserves, vanadium resources account for 62% of the national vanadium reserves, and titanium resources account for 90.5% of the national titanium resources reserves. In addition, there are 900,000 tons of cobalt, 700,000 tons of nickel, 250,000 tons of scandium, and 180,000 tons of gallium, as well as a large amount of copper, sulfur, and other resources. However, the comprehensive utilization rate of nonferrous metal resources in the Panxi Region is very low. In the four major mining areas, only the Taihe mining area is recovered, while the other three mining areas are not utilized [
10]. The low-grade and scattered distribution of sulfur and cobalt resources in vanadium–titanium magnetite make it difficult to utilize them directly from the original ore [
11,
12]. Most of the cobalt associated with vanadium–titanium magnetite occurs in the form of pyrite, pyrrhotite, and cobalt–nickel pyrite, while a very small amount of cobalt occurs in the form of pyrite. Due to the small flotability differences among pyrite, pyrrhotite, cobalt–nickel pyrite, and cobalt sulfide ore, which could basically be characterized by the surface properties of pyrite minerals, it is difficult to recover some independent minerals by flotation.
Most cobalt minerals are associated with other metal minerals. At present, valuable cobalt-minerals mainly include cobaltite, skutterudite, chalcopyrite, sulfur–cobalt ore, nickel–cobalt ore, hydrocobalt ore, erythrite, and ferromanganese-bound cobalt ore, etc. The most effective way to recover cobalt minerals is flotation, but manual and repeated screening are also employed. Flotation is the most important method to treat cobalt-bearing minerals [
13,
14]. Cobalt concentrate can be obtained directly by flotation of single cobalt minerals such as cobaltite and sulfur–cobalt ore. Cobalt concentrate occurring in pyrite and chalcopyrite cannot be obtained directly by the carrier flotation.
Therefore, recovered cobalt from vanadium ilmenite tailings is essentially a mixture of minerals of pyrite, pyrrhotite, cobalt pyrite, and sulfur–cobalt ore. In this paper, the experimental study on the recovery of cobalt and sulfur from cobalt-bearing vanadium titanomagnetite tailings in the Panxi Region was carried out by a flotation process, providing an important research basis for the associated cobalt and sulfur resources of vanadium titanomagnetite tailings in the Panxi Region.
2. Materials and Methods
2.1. Sampling
The ore samples used in this study were from low grade cobalt-bearing V–Ti magnetite tailings produced by a concentrator in the Panxi Region after concentrating iron and titanium. The water content of the tailings was less than 1% and the particle size was between 0.038 and 20 mm. It was found that the bulk ore samples were all caused by fine-grained caking. In order to avoid large sampling errors, the agglomerated samples were crushed and then shrunk in advance for reserve. The main chemical composition analysis of the samples is shown in
Table 1, the phase analysis of cobalt is shown in
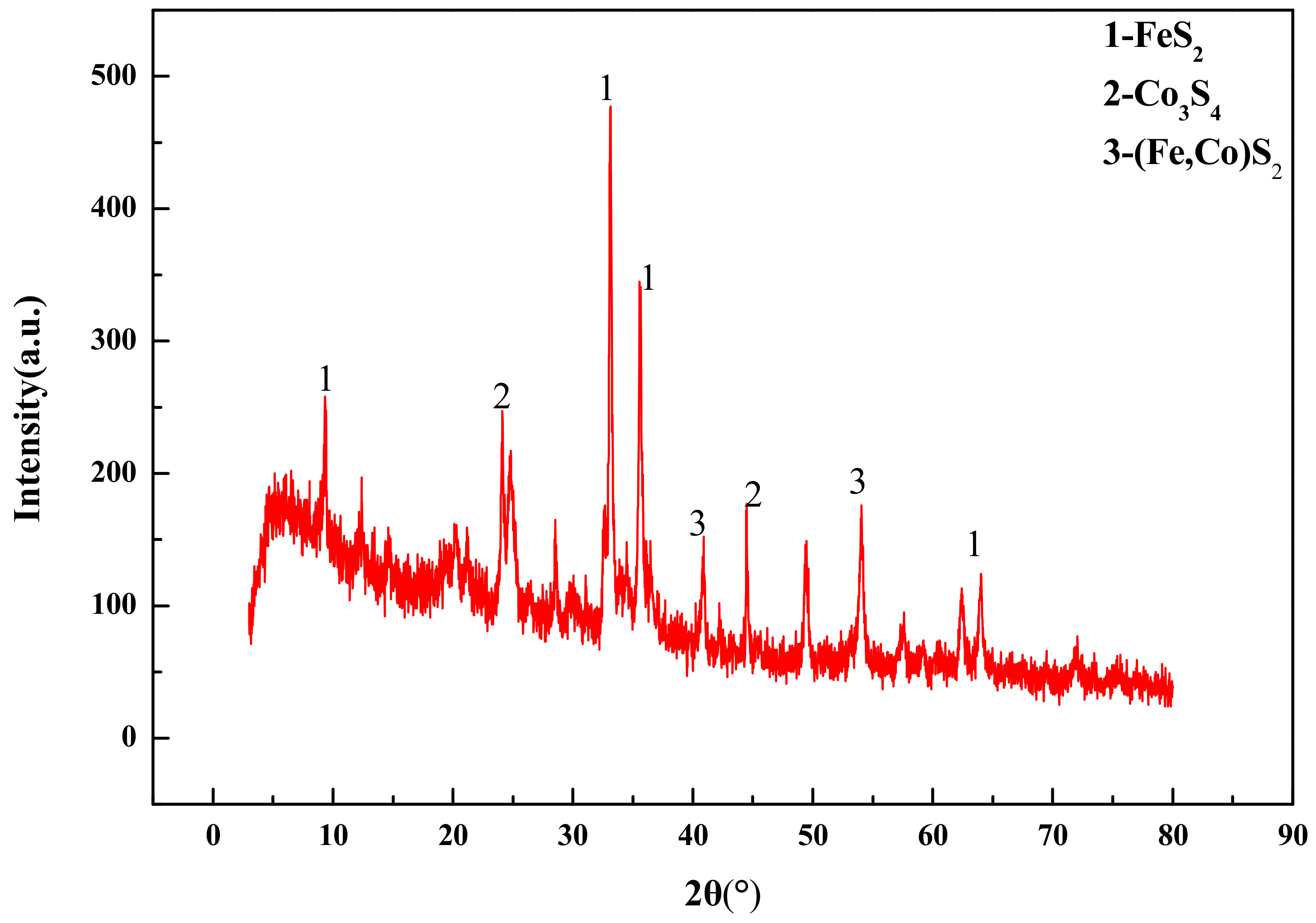
Table 2, and the X-ray diffraction pattern of the samples is shown in
Figure 1. The mineral composition of the ores is complex, with the mineral surface covered with more sludge and the particles bonded to each other. The metal minerals mainly include pyrite, cobalt pyrite, sulfur–cobalt ore, pyrrhotite, and ilmenite. Gangue minerals mainly include titanopyroxene, plagioclase, serpentine, chlorite, mica, amphibole, olivine, and calcite, etc.
2.2. Chemical Reagent
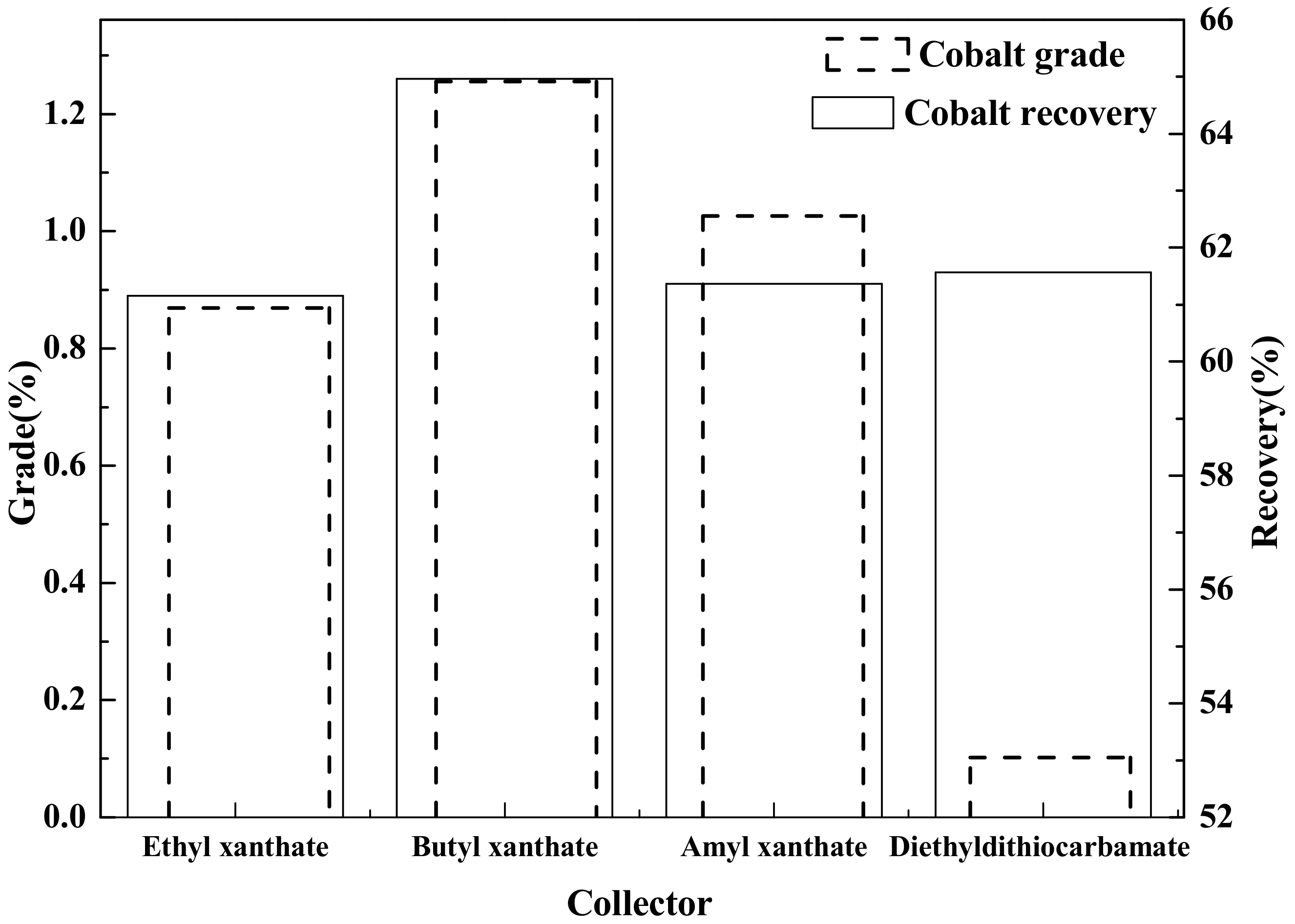
The main chemical reagents used in this test are ethyl xanthate, butyl xanthate, amyl xanthate, black xanthate, ethyl thionitrogen, copper sulfate, sodium carbonate, and sodium silicate, all of which have analytical purity and a producing area in Tianjin Tianli Chemical Regeant, Co., Ltd., Tianjin China.
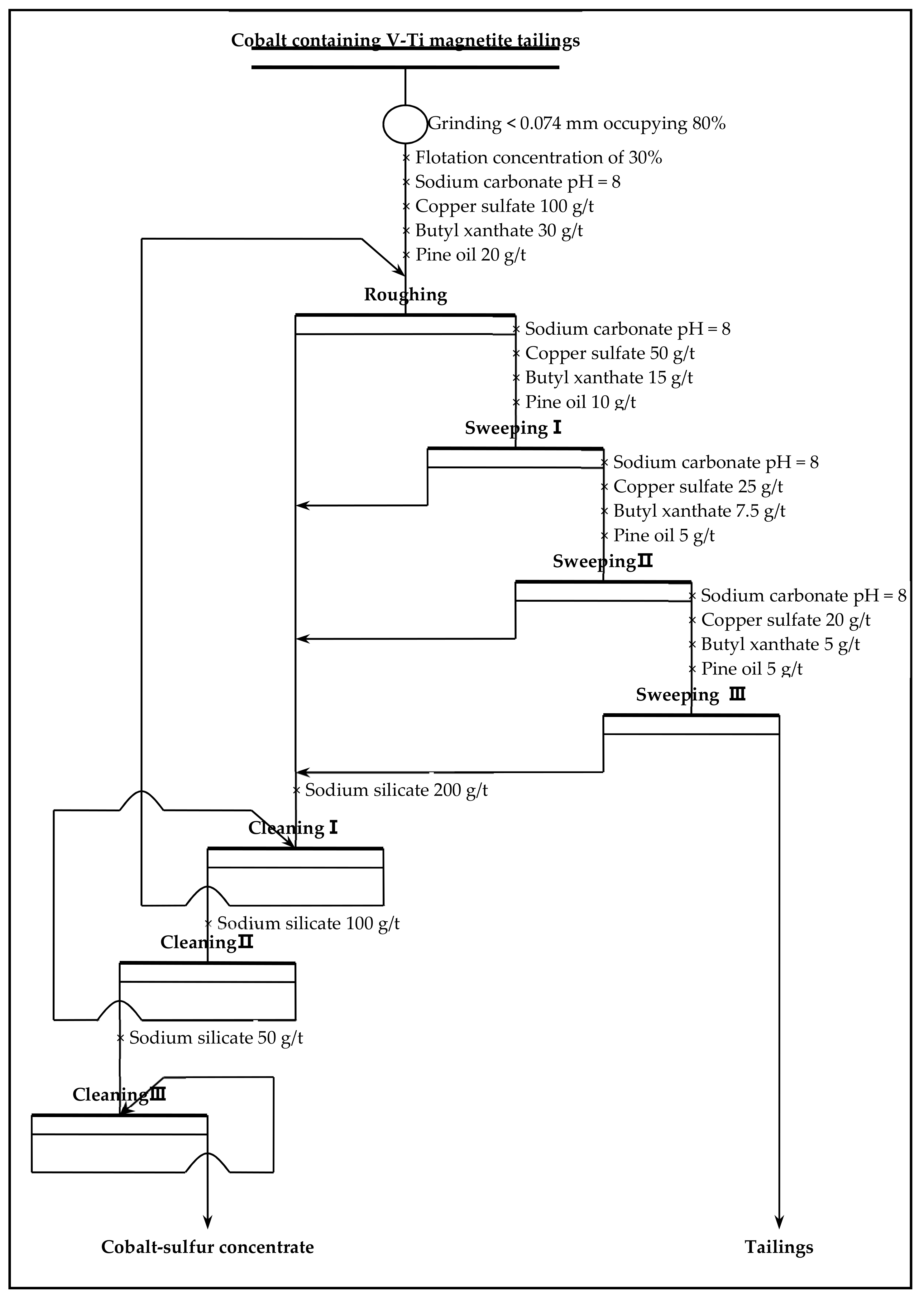
2.3. Flotation
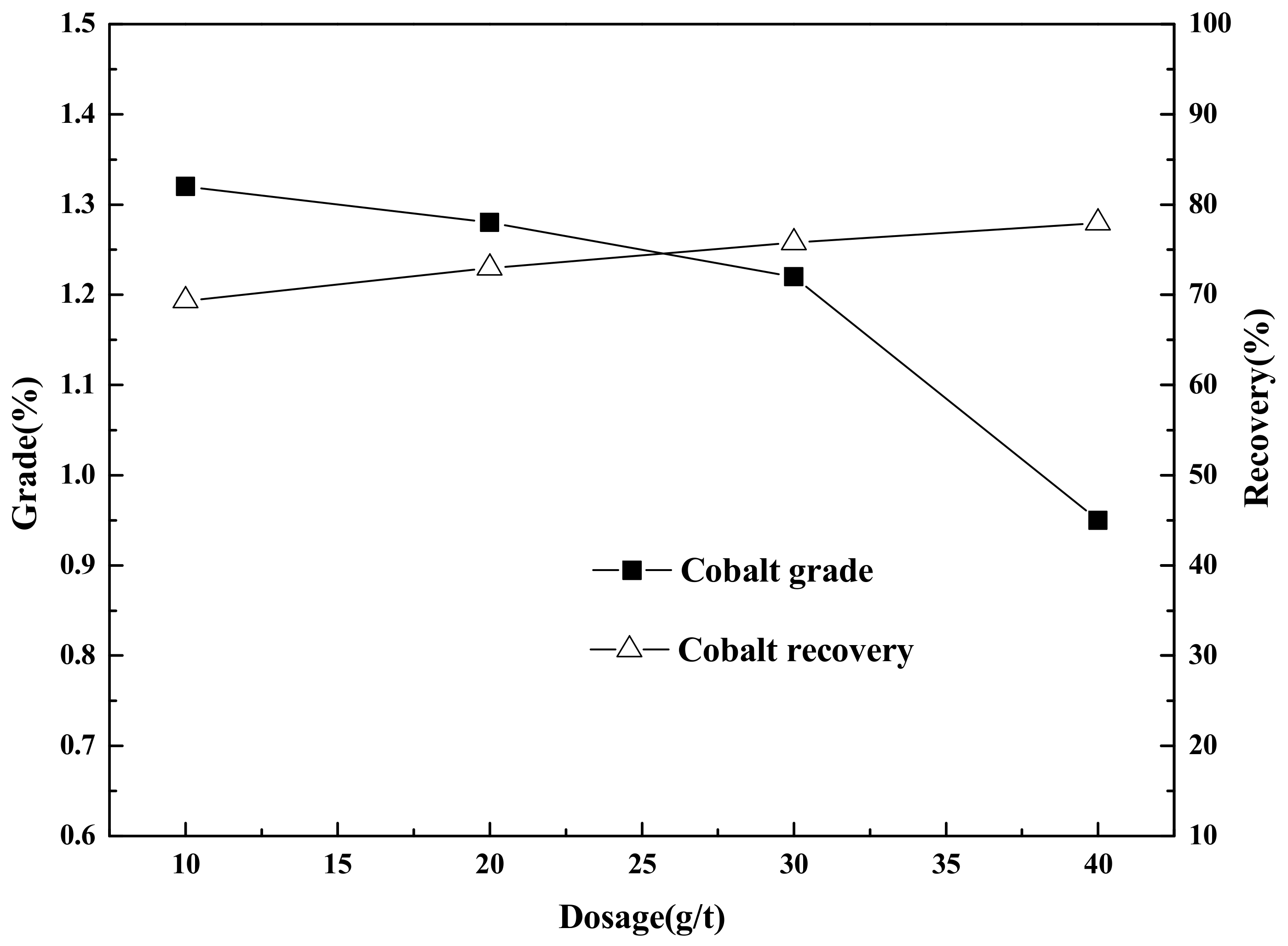
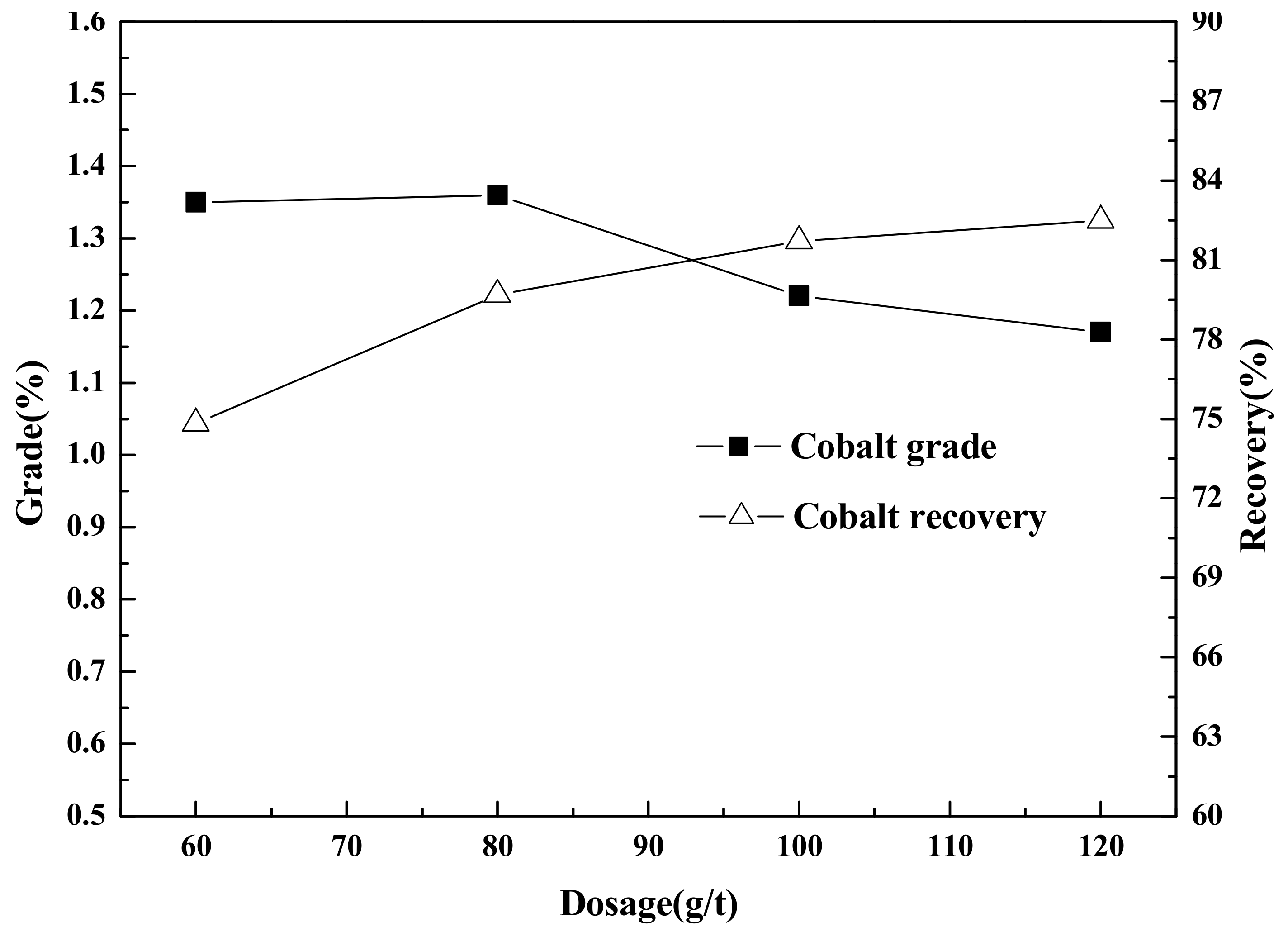
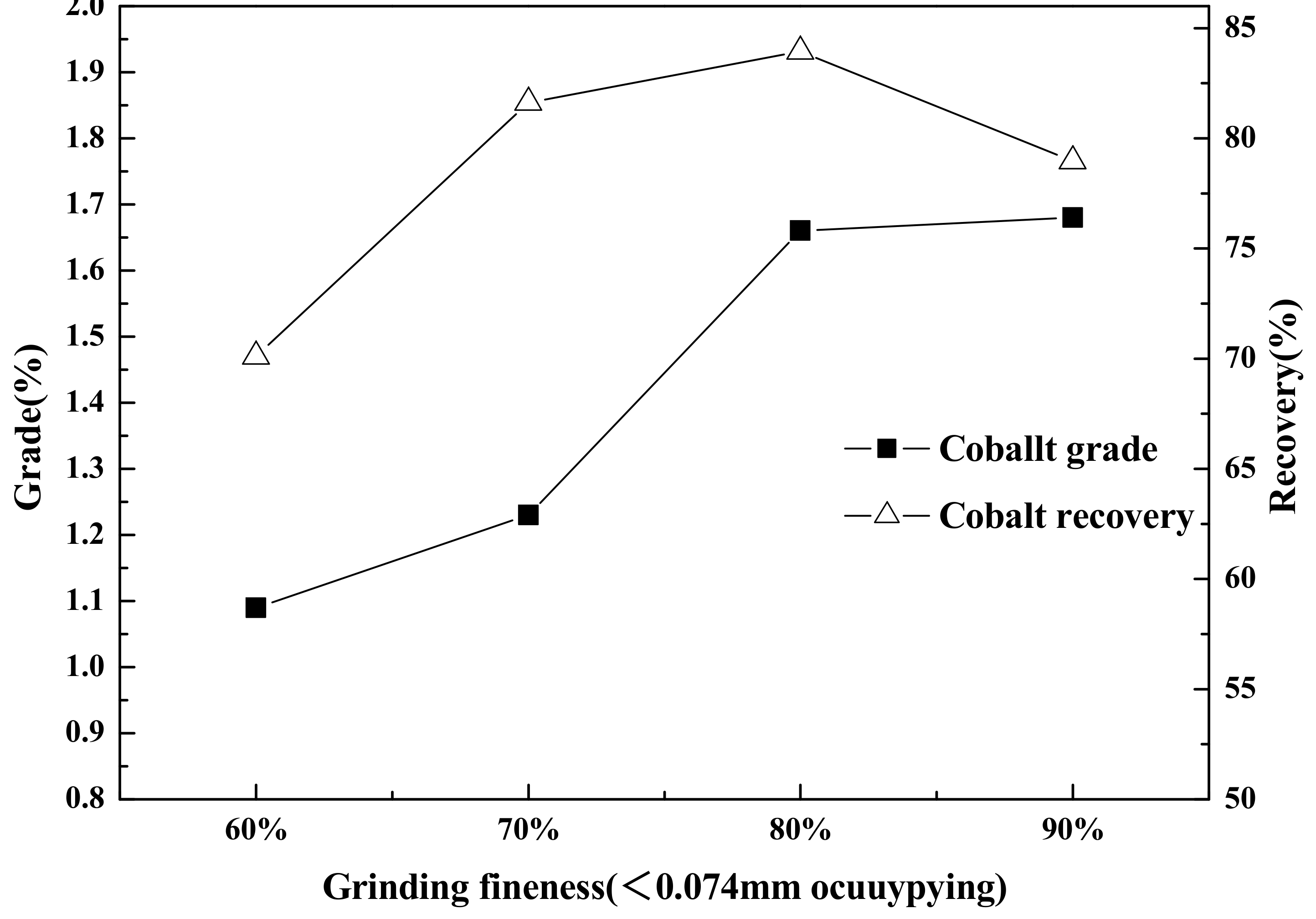
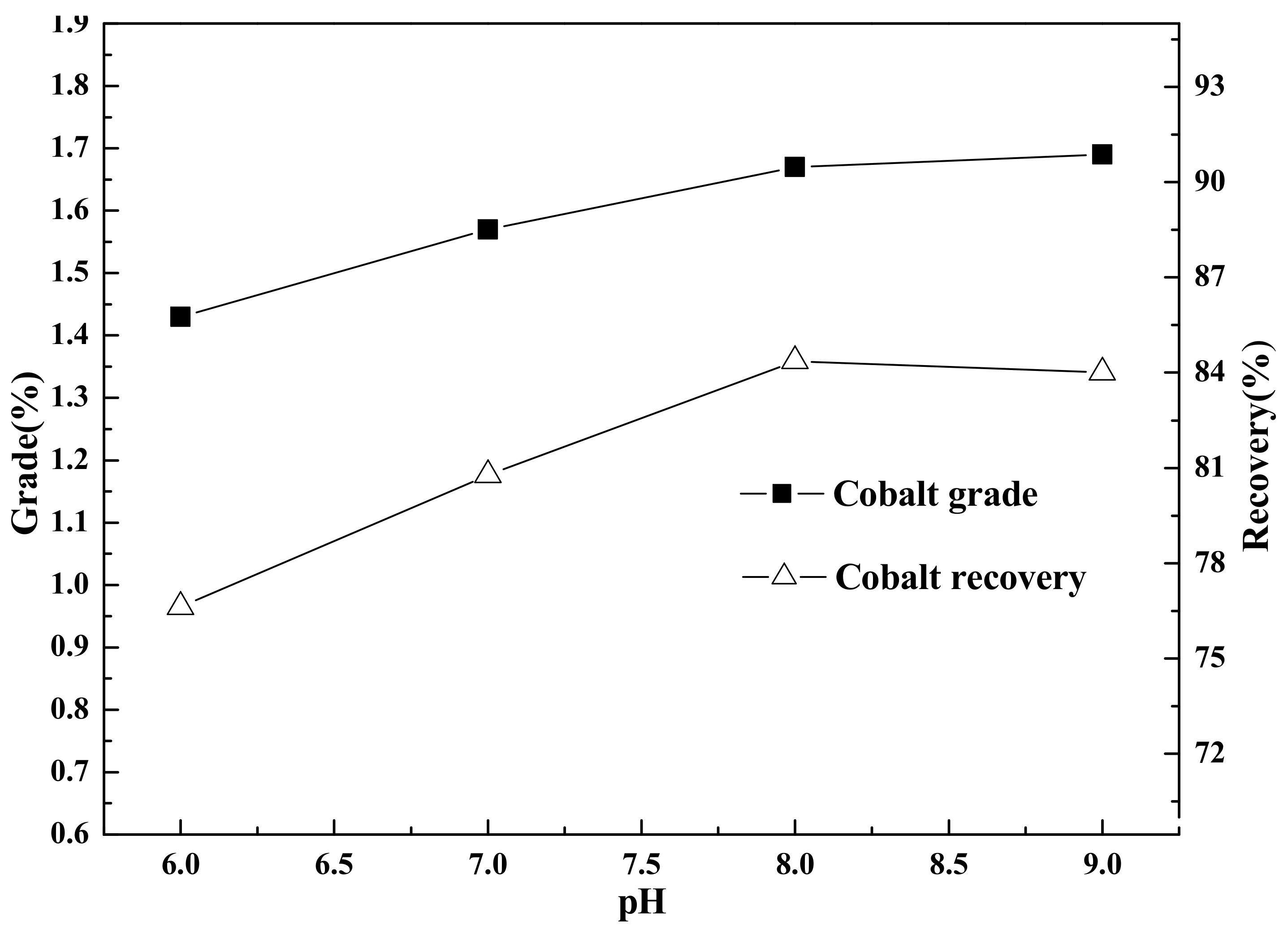
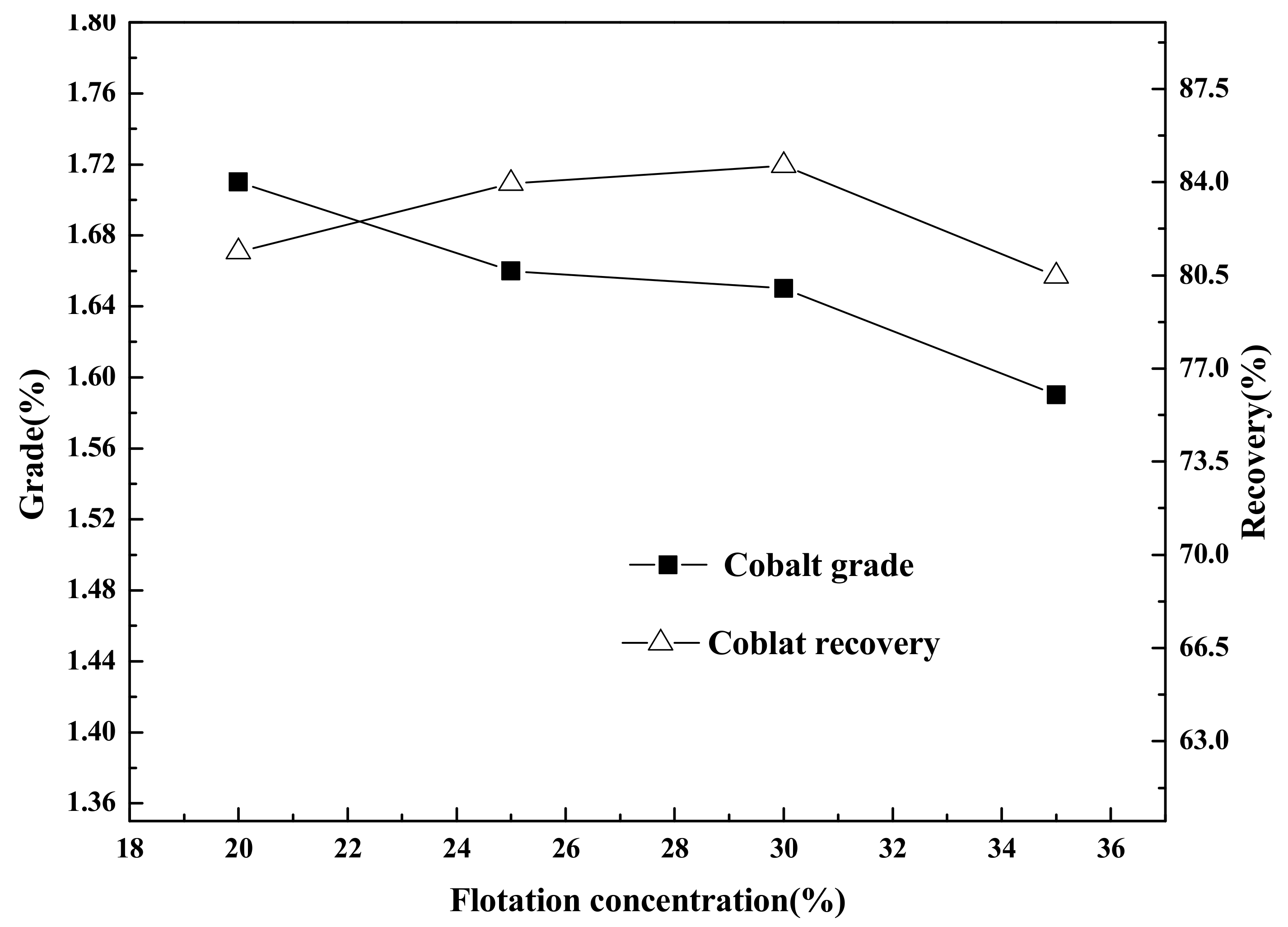
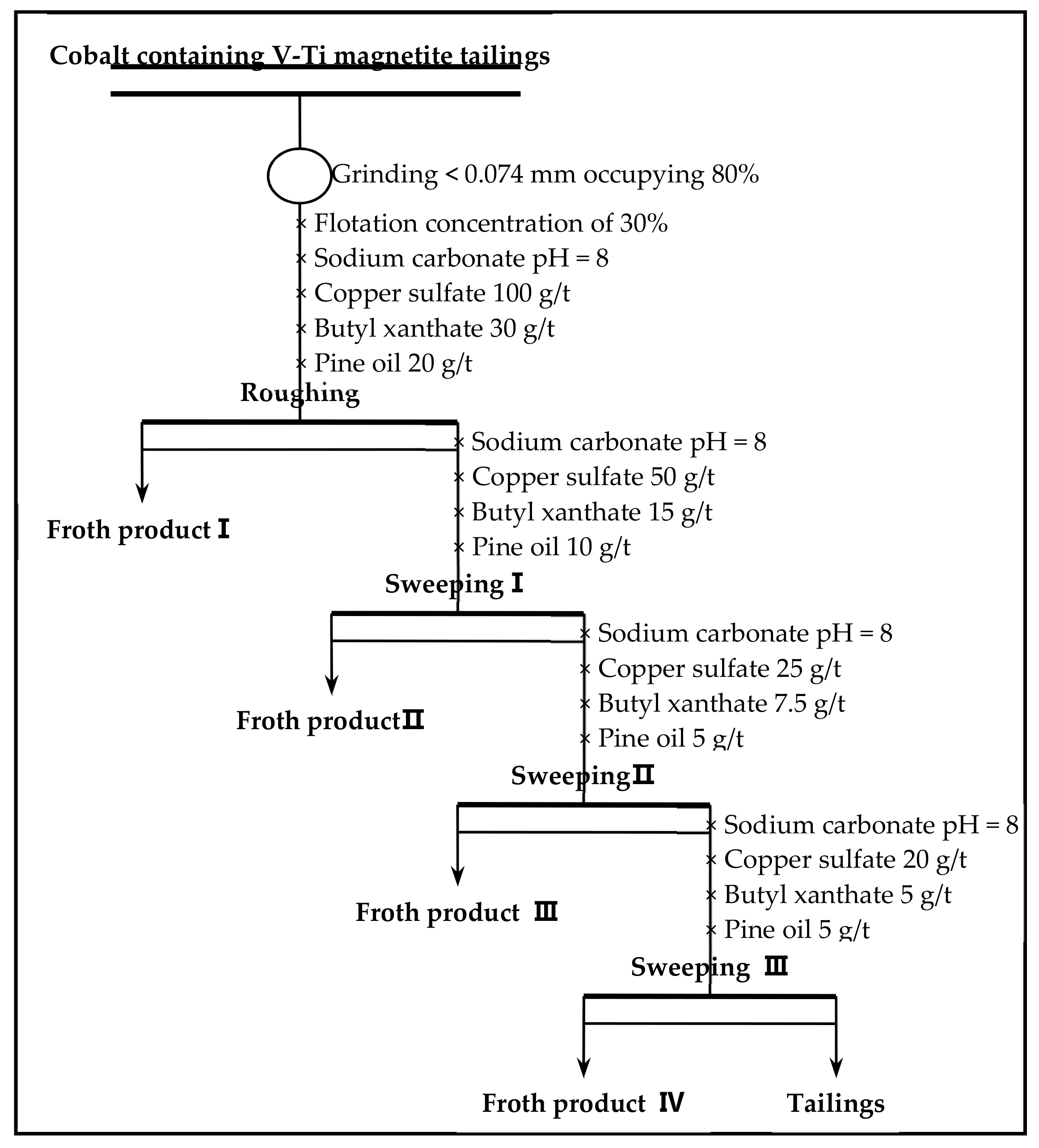
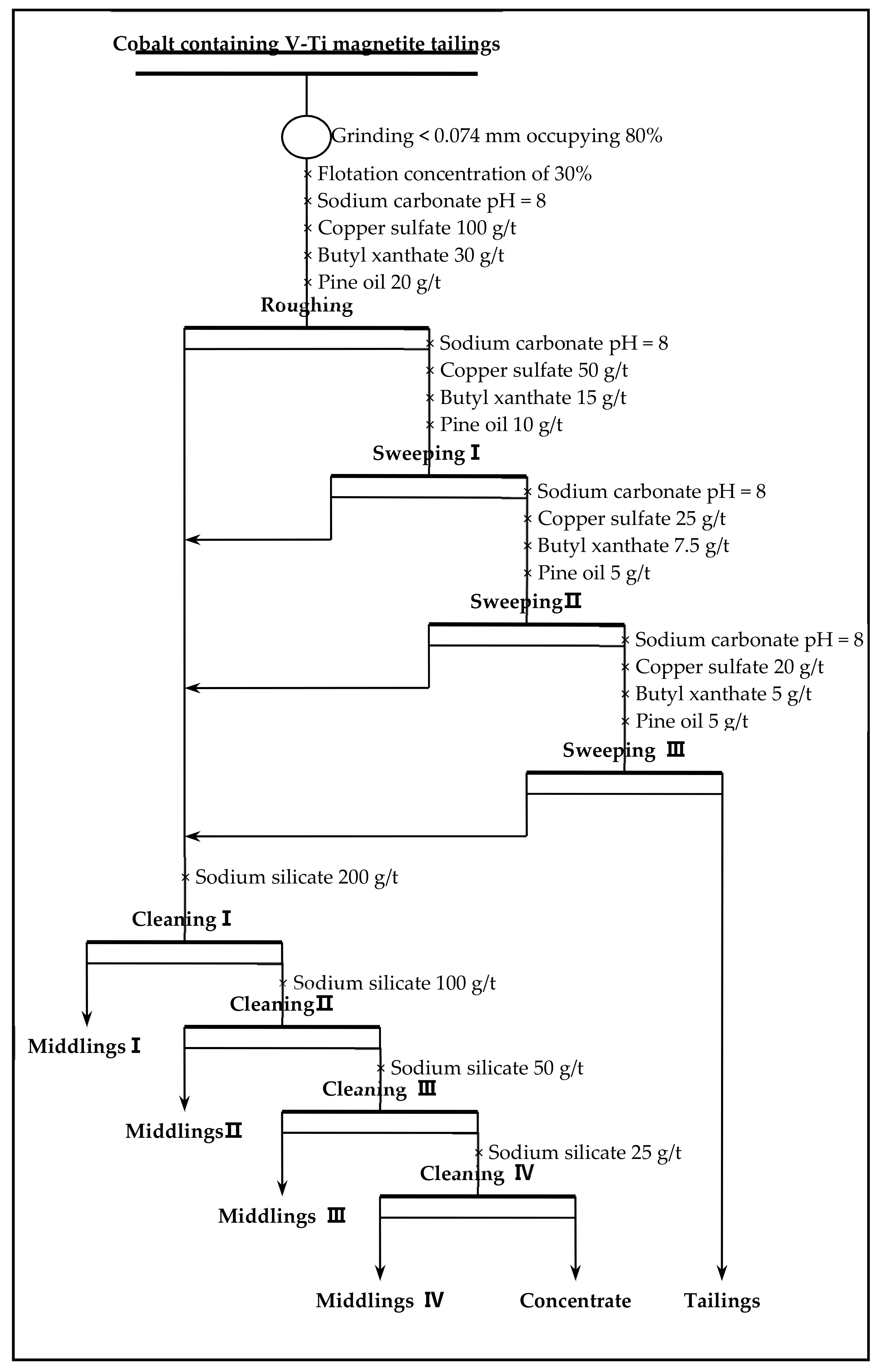
Flotation (roughing, scavenging), aimed at increasing the grade and recovery of cobalt, was carried out using an XFD-1.5L hanging tank flotation machine (Jinlin Exploration Machinery Plant, China) operating at a spindle speed of 1650 r/min. A 500 g mass of cobalt that contained vanadium–titanium magnetite tailings was added to the 1.5 L flotation tank. Flotation (cleaning), aimed at increasing the cobalt grade, was carried out using an XFD-1.0 L hanging tank flotation machine (Jinlin Exploration Machinery Plant, China) operating at a spindle speed of 1650 r/min. A 300 g mass of cobalt containing cobalt–sulfur concentrate was added to the 1.0 L flotation tank. Distilled water (1.0 L) was added and the pulp stirred and mixed for 3 min, followed by adjustment to the required pH using sodium carbonate. After 10 min of pulping, the regulators (depressant or activator) were added to the slurry and conditioned for 3 min. Then, the collectors for improving cobalt grade and recovery were added and agitated for 3 min. Before aeration, the frothers (pine oil) for improving the bubble were added, with another 3 min of stirring. After 3 min of flotation, the froth (cobalt–sulfur concentrate) and in-tank product (flotation tailings) were separately filtered, dried at 80 °C for 4 h, and weighed. Quantitative analyses of cobalt grade were conducted to calculate the cobalt recovery.
2.4. Analysis and Characterization
The chemical composition of solid materials was analyzed by Z-2000 atomic absorption spectrophotometer (Hitachi Co., Ltd.), the diffraction grating was zenier-tana type, 1800 lines /mm, the flash wavelength was 200 nm, the wavelength range was 190~900 nm, the automatic peak seeking setting, and the spectral bandwidth was divided into 4 grades (0.2, 0.4, 1.3, and 2.6 nm) for the analysis of mineral chemical composition.
The phase composition of solid substances (cobalt–sulfur concentrate and cobalt-containing vanadium–titanium magnetite tailings) was analyzed by X-ray diffraction (XRD, X Pert pro, Panaco, The Netherlands).
The microstructure of the solid products was observed by SEM (S440, Leica Cambridge LTD, Germany) equipped with an energy dispersive X-ray spectroscopy (EDS) detector (UItra55, Carl zeissNTS GmbH, Germany).
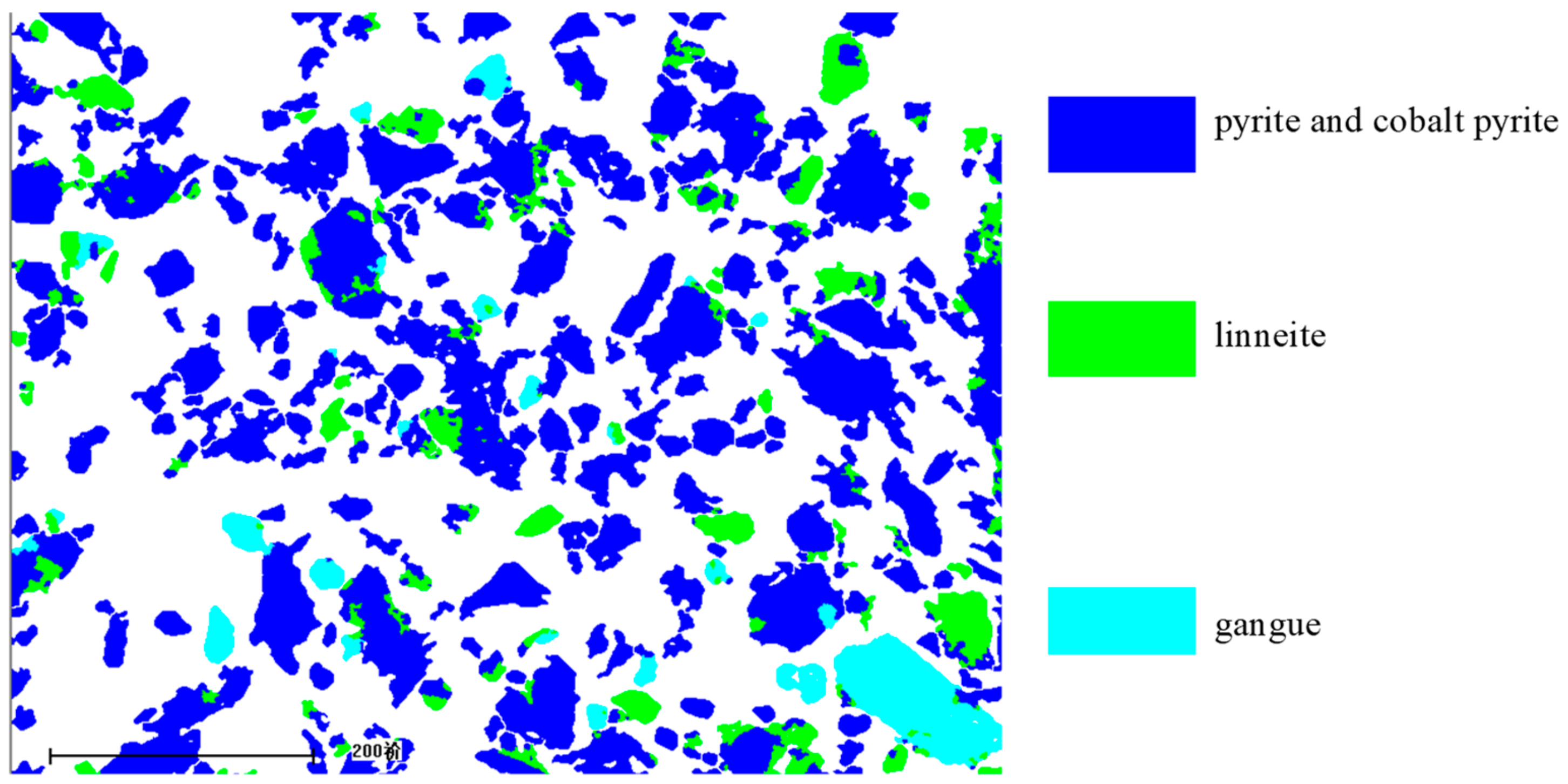
The chemical phase composition of cobalt sulfur concentrate was analyzed by mineral liberation analyzer (MLA) (FEI electronic optics co., LTD, Australia.), which is composed of Quanta 250 environmental scanning electron microscope, EDAX spectrometer, and jktech-mla3.0 process mineralogy automatic test software. Test conditions: Working voltage 25 kV, magnification 300 times, beam Spot 6.8, particle minus minimum size 30 (pixel).
4. Conclusions
(1) The low grade cobalt-bearing vanadium titanomagnetite tailings in the Panxi Region contained a cobalt grade of 0.032% and sulfur grade of 0.56%. The main recovered valuable elements are cobalt and sulfur. The main metal sulfide minerals in the tailings are FeS2, Fe1−xS, Co3S4, and (Fe,Co)S2.
(2) Cobalt and sulfur were recovered in low-grade cobalt-bearing V–Ti tailings by a flotation process of one roughing, three sweepings, and three cleanings. This study obtained the separation indexes of cobalt–sulfur concentrate, with a cobalt grade of 2.08%, sulfur content of 36.12%, cobalt recovery of 84.77%, and sulfur recovery of 85.79%.
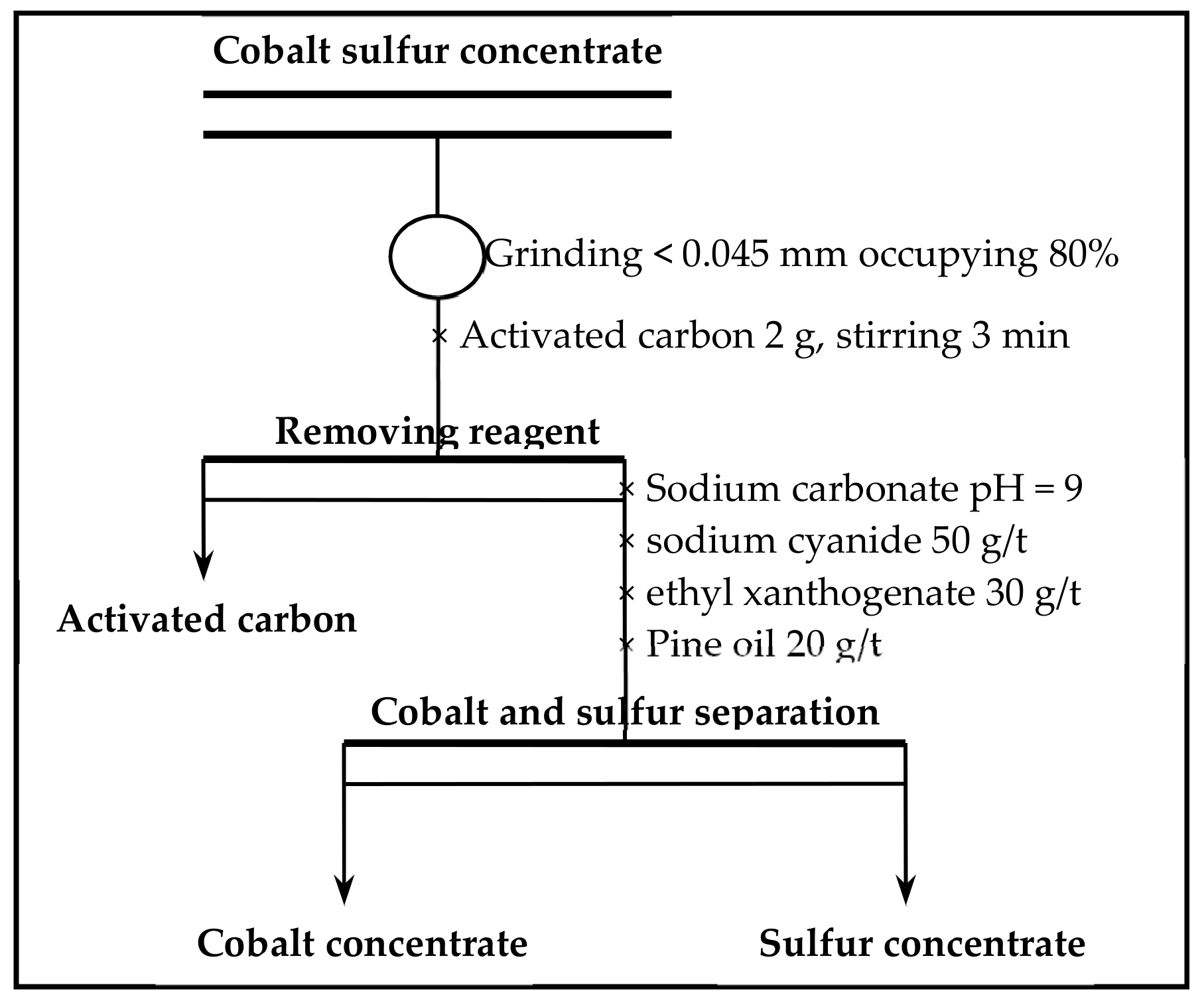
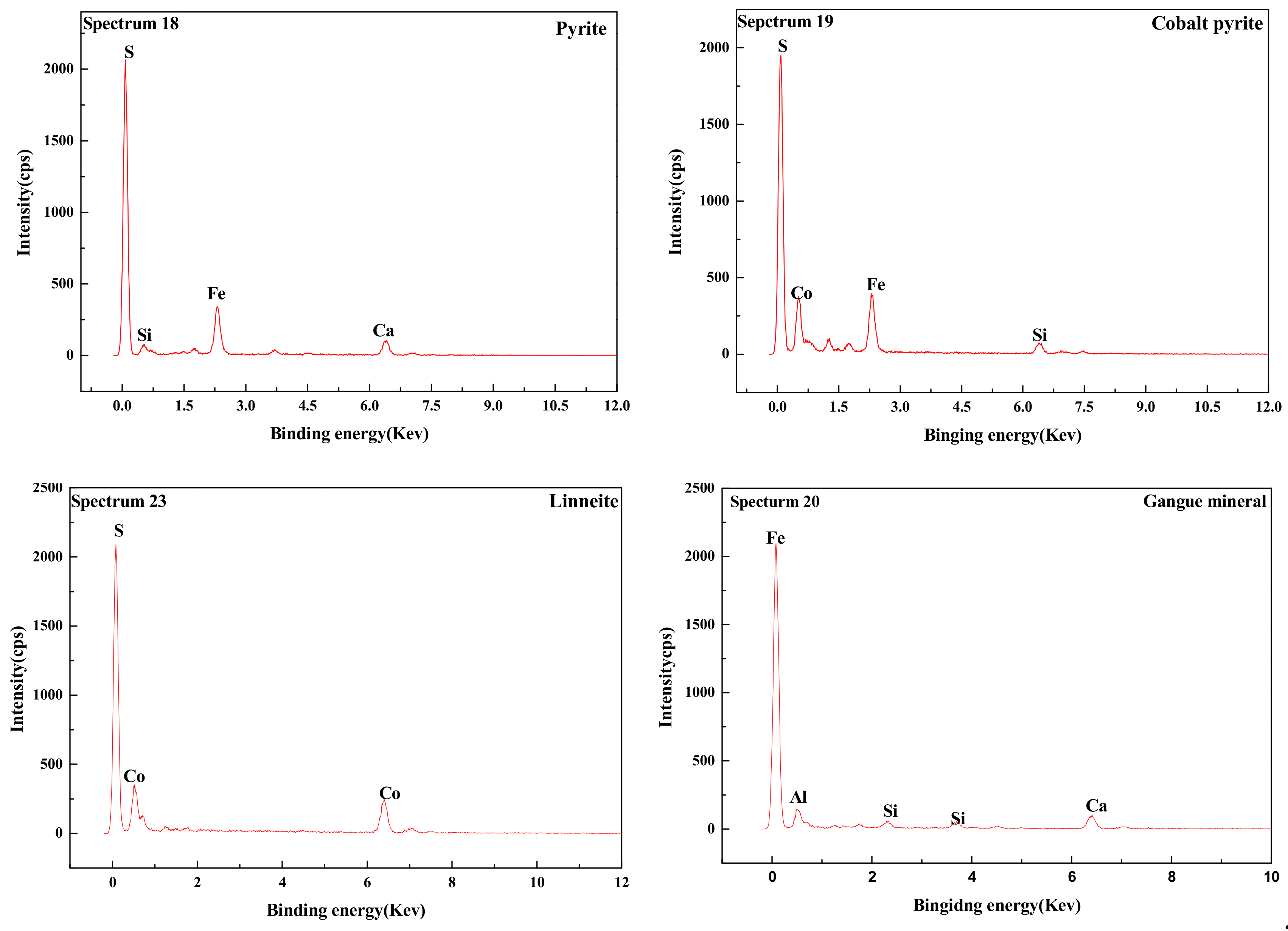
(3) Studies of the characterization and analysis of cobalt–sulfur concentrate by XRD, MLA, SEM, and EDS indicated that the main minerals in cobalt–sulfur concentrate are FeS2, Fe1−xS, Co3S4, and (Fe,Co)S2,, of which FeS2 and (Fe,Co)S2 account for 65.64%, Co3S4 for 22.64%, and gangue mineral for 11.72%. Cobalt in cobalt pyrite is closely related to pyrite in the form of isomorphism, and the flotability difference between cobalt and pyrite is small, which makes it difficult to further separate cobalt and sulfur by flotation. Preconcentration of cobalt and sulfur was appropriate from low-grade cobalt-bearing V–Ti tailings. Cobalt–sulfur concentrate can be processed using pyrometallurgical or hydrometallurgical methods, such as oxidation roasting and pressure leaching, and independent cobalt and sulfur products can be obtained from cobalt–-sulfur concentrate. The efficient utilization of cobalt and sulfur of low-grade cobalt-bearing V–Ti tailings in the Panxi Region can be realized finally.