Abstract
Artemisia species are used as folk medicines in several countries. This work was aimed to shed more light on the effect of methanol, water, ethyl acetate extracts, and essential oil (EO) of A. santonicum on selected enzymes (cholinesterase, tyrosinase α-amylase, and α-glucosidase) as well of their antioxidant and pharmacological effects. The chemical profile of the essential oil was determined using gas chromatography coupled to mass spectrometry (GC-MS) analysis, while the extracts were chemically characterized by high performance liquid chromatography coupled to mass spectrometry (HPLC-MS). Forty-nine constituents were identified and camphor (36.6%), 1,8-cineole (10.2%), α-thujone (10.1%), borneol (4.5%), and β-thujone (3.6%) were the major components. Overall, 45, 74, and 67 components were identified from the ethyl acetate, methanol, and water extracts, respectively. The EO and extracts showed significant antioxidant properties, in a cell-free model; particularly, methanol and water extracts revealed promising sources of antioxidant compounds. Additionally, we evaluated protective effects of EO and extracts in isolated rat colon tissue challenged with lipopolysaccharide (LPS), as an ex vivo model of colon inflammation, and human colon cancer HCT116 cell line. Particularly, we observed that, among all tested samples, A. santonicum ethyl acetate displayed the best pharmacological profile, being able to blunt LPS-induced levels of all tested biomarkers of inflammation and oxidative stress, including colon nitrites, lactate dehydrogenase, prostaglandin E2, and serotonin. Additionally, this extract was also able to reduce HCT116 cell viability, thus suggesting potential antiproliferative effects against colon cancer cells. Based on our results, A. santonicum has great potential for developing novel functional agents including pharmaceuticals, cosmeceuticals, and nutraceuticals.
1. Introduction
The genus Artemisia is one of the most important genera in Asteraceae family, comprising about 500 species [1]. Artemisia members have specific scents or tastes, and are ingredients in the preparation of liqueur in food industry [2,3,4]. Several Artemisia species have been traditionally used in wide plethora of inflammatory and infectious diseases [5,6].
Phytochemical investigations revealed the occurrence of flavonoids [7,8], coumarins [9,10], polysaccharides [11], sterols [12], terpenoids [13,14], and essential oils [15]. The strong and aromatic smell of some species of Artemisia genus is due mainly to the presence of volatile terpenes in high concentrations, as components of their essential oils, especially in leaves and flowers [1,16]. One of the most important drugs derived from this genus is artemisinin, the antimalarial isolated from A. annua [2,15].
In Turkish flora, Artemisia are represented by 26 species. Among them, A. santonicum L., commonly known as Yavşan in Turkey, has been used as a folk remedy in the treatment of diabetes [17]. In literature, there are only a few reports regarding the chemical profile of essential oil and extracts, and biological properties of this species. So far, only the antifungal, antibacterial [18], and anti-diabetic properties of A. santonicum have been reported. Based on aforementioned facts, this study was designed to unveil the chemical composition and pharmacological properties of essential oil and extracts of A. santonicum. Antioxidant effects were assayed by several methods, such as quenching of free radicals, reduction ability, metal chelating, and phosphomolybdenum. Tyrosinase, α-glucosidase, α-amylase, and cholinesterases (AChE and BChE) were considered in the enzyme inhibitory assays. Additionally, considering the promising use of Artemisia species in blunting clinical symptoms related to inflammatory bowel diseases [19,20], we evaluated EO and extract protective effects in isolated rat colon, assaying selected biomarkers of inflammation and oxidative stress, including colon nitrites, lactate dehydrogenase (LDH), prostaglandin (PG)E2, and serotonin (5-HT). Finally, we evaluated potential antiproliferative effect against the selected human colon cancer cell line.
2. Materials and Methods
2.1. Plant Material and Extraction Procedures
Samples of A. santonicum were collected from full bloom wild plants at June 2018 between Ankara and Çankırı (Turkey, GPS: 40°08′49″ N, 33°18′52″ E), and botanical identification was carried out by Dr. Ismail Senkardes from Marmara University (Istanbul, Turkey), where the voucher specimen is deposited (no. MARE-19851). The aerial parts of the plant, previously dried in the dark in ventilated oven (at 35 °C), were further powdered and subjected to extraction processes.
Methanol and ethyl acetate were selected as extraction solvents in the maceration techniques (5 g plant samples were mixed with 100 mL of each solvent for 24 h). After that, the extracts were filtered and evaporated in vacuo at 40 °C. Water extract was prepared as traditional infusion (five grams of plant samples were infused with one hundred mL of boiling water for 20 min). The infusion was filtered and then dried (freeze drying). All extracts were stored at 4 °C and protected from the light until analysis.
2.2. Isolation and Analysis of the Essential Oils
The essential oil was isolated by hydrodistillation method using a Clavenger apparatus (100 g plant materials in 2 L water submitted for 5 h). The obtained oils (yield: 0.25%) were dried with sodium sulphate and then kept at 4 °C until being analyzed.
Agilent 5975 GC-MSD system coupled to an Agilent 7890A GC (Agilent Technologies Inc., Santa Clara, CA, USA) was used to determine chemical profile of the obtained essential oil (in n-hexane). HP-Innowax FSC column (60 m × 0.25 mm, 0.25 μm film thickness) was used as column and all analytical parameters have been reported in our previous paper [21]. We calculated retention index of each components by using a homologous series of n-alkanes (C8–C30), under the same experimental conditions. In order to further provide identifications, we compared unknown compound spectra with libraries such as NIST 05 and Wiley eighth version.
2.3. Liquid Chromatography Analysis
Untargeted high performance liquid chromatography (HPLC) qualitative analysis was performed in order to determine the secondary metabolite fingerprint profile of A. santonicum extracts and essential oil (EO). The accurate description of chromatographic method is reported in our previous published paper [20]. The Thermo Scientific Xcalibur 3.1 (Thermo Scientific, Waltham, MA, USA) and TraceFinder 3.1 (Thermo Scientific, Waltham, MA, USA) softwares were used to record and evaluate data [22]. The concentrations were 5 mg/mL and 2 µL was injected in every run.
Some compounds are marked because they were confirmed by using standards in the tables. Regarding the other compounds, they were identified on the basis of our previous published work [20]. To this end, retention time, the exact molecular mass, characteristic fragment ions, and isotopic pattern were used to identify compounds (Metlin database (https://metlin.scripps.edu)).
Artemisia extracts (5 µg/mL) were analyzed for the phenol quantitative determination using a reversed phase HPLC-fluorimetric in gradient elution mode. Analyses were carried out by using a Waters liquid chromatograph (MOD. 1525) equipped with a fluorimetric detector (MOD. 2475), a C18 reversed-phase column (Dionex AcclaimTM 120, 3 µm, 2.1 × 100 mm), an on-line degasser (Biotech 4-CH degasi compact, LabService, Anzola Emilia, Italy). The gradient elution was achieved by a mobile phase methanol-acetic acid-water (10:2:88, v/v) as solvent A and methanol-acetic acid-water (10:2:88, v/v) as solvent B, in agreement with an already published paper [23]. In accordance with the method of those same authors, we selected λex = 278 nm and λem = 360 nm in order to analyze the following phenolic compounds: gallic acid, catechin, epicatechin, and resveratrol.
2.4. Total Bioactive Components, Antioxidant, and Key Enzymes Inhibitory Effects
The content of two important groups of secondary metabolites, phenols and flavonoids, were measured spectrophotometrically by using well-known methods with Folin-Ciocalteu and AlCl3, respectively [21]. Gallic acid equivalent, as well as rutin equivalent, were used as a measure of their content.
The ability of the extracts to inhibit multiple enzymes—such as α-amylase, α-glucosidase, cholinesterases, and tyrosinase—was carried out through colorimetric assays [24]. Obtained results were expressed in the way prescribed by official methods by using appropriate standards [24].
Antioxidant capacity of the tested extracts was measured through colorimetric ferric reducing ability of plasma (FRAP), cupric reducing antioxidant capacity (CUPRAC), 2,2-diphenyl-1-picrylhydrazyl (DPPH), and 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) assays. All details about the used tests as well as statistical analysis are given in our earlier work [24].
2.5. Artemia Salina Lethality Bioassay
Artemia salina lethality bioassay was performed as previously reported [25]. Briefly, brine shrimp larvae were bred at 25–28 °C for 24 h in presence of Artemisia extracts (0.1–20 mg/mL) and essential oil (0.1–5 µL/mL) dissolved in incubation medium (artificial sea water). After incubation period (24 h) with extracts, the number of surviving shrimps was evaluated and their vitality was compared to untreated control group. Experiments were carried out in triplicate, and percentage mortality was calculated with the following equation: ((T − S)/T) × 100, where T and S are the total number of incubated larvae and surviving napulii, respectively.
2.6. Ex Vivo Studies
Six male adult Sprague Dawley rats (200–250 g) were housed in Plexiglass cages (40 × 25 × 15 cm), two rats per cage, in climatized colony rooms (22 ± 1 °C; 60% humidity), on a 12 h/12 h light/dark cycle (light phase: 7:00 a.m.–7:00 p.m.), with free access to tap water and food, 24 h/day throughout the study, with no fasting periods. Rats were fed a standard laboratory diet (3.5% fat, 63% carbohydrate, 14% protein, 19.5% other components without caloric value; 3.20 kcal/g). Housing conditions and experimentation procedures were strictly in accordance with the European Union ethical regulations on the care of animals for scientific research. The experiments were approved by Local Ethical Committee (University “G. d’Annunzio” of Chieti-Pescara) and Italian Health Ministry (Italian Health Ministry authorization N. F4738.N.XTQ, delivered on 11 November 2018). Rats were sacrificed by CO2 inhalation (100% CO2 at a flow rate of 20% of the chamber volume per min), and explanted colon specimens were stimulated with E. coli lipopolysaccharide (LPS), an ex vivo experimental model of ulcerative colitis [22]. In parallel, colon tissues were treated with Artemisia extracts (100 µg/mL) and essential oil (0.1 µL/mL). After treatment, PGE2 level (ng/mg wet tissue) was measured in the supernatants, as previously reported [26,27,28]. Moreover, nitrites and LDH were spectrophotometrically assayed. The detailed procedures are reported in our previous published paper [29]. Finally, isolated colons were explanted and tissue 5-hydroxyindoleacetic acid (5HIIA) and 5-HT (ng/mg wet tissue) were extracted. Thereafter, neurotransmitter (5-HT) and metabolite (5HIIA) level was determined by HPLC coupled to coulometric detection [30,31]. The results were expressed in terms of 5-HT turnover (5HIIA/5-HT ratio).
2.7. In Vitro Studies
Human colon cancer HCT116 cell line (ATCC® CCL-247™) was cultured as reported in our previous published paper [22]. The effects of A. santonicum ethyl acetate extract (100 μg/mL) on HCT116 viability was assessed through 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) test.
2.8. Statistical Analysis
Data related to pharmacological activity was analyzed by one-way analysis of variance (ANOVA), coupled to Newman-Keuls comparison multiple test (GraphPad Prism version 5.01 for Windows). Data were considered significant for p < 0.05. One-way ANOVA followed by Tukey’s multiple test was also done to investigate significant differences (p < 0.05) between the tested samples in terms of antioxidant and enzyme inhibitory effects.
3. Results
3.1. Chemical Composition of the Essential Oil
The essential oil from the aerial parts of Artemisia santonicum was analyzed by GC–MS and the results are shown in Table 1 and Supplementary Figure S1. Forty-nine components were identified (95.2% of the total components) and the major components were found to be camphor (36.6%), 1,8-cineole (10.2%), α-thujone (10.1%), borneol (4.5%) and β-thujone (3.6%). Kordali et al. [18] has reported camphor (18.2%), 1–8 cineole (7.5%) and borneol (4.0%) as the predominant volatile components in the EO of A. santonicum collected from Turkey. Commercially obtained camphor and 1,8-cineole, which are the major components of the A. santonicum oil, have exhibited antifungal activity. Previous investigations showed that these oxygenated monoterpenes, camphor and 1,8 cineole are major characteristic components of Artemisia species [18,32,33,34,35]. Thujone derivatives are significant agents in the preparation of liqueurs and they have also significant biological effects including anthelmintic, insecticidal, and antinociceptive activity [36]. However, the toxicity of thujone derivatives are also widely documented [37,38,39]. Unlike in the study carried by Kordali et al. [18], in the present study thujone derivatives are major components of the oil of A. santonicum.

Table 1.
Chemical composition of Artemisia santonicum essential oil
3.2. Chemical Composition of the Investigated Extracts
The total phenolic compounds content of the different extracts of A. santonicum was determined using the Folin–Ciocalteu method. The experimental data (Table 2) revealed that water extract (77.45 ± 1.43 mg GAE/g extract), followed by the MeOH extract (70.02 ± 1.87 mg GAE/g extract) displayed the highest total phenol content. The total phenol content in water and MeOH was approximately 3-times higher as compared to EA extract. The total flavonoid content was measured using the aluminium chloride colorimetric assay. The total flavonoid contents ranged from 41.27 ± 1.19 to 23.55 ± 0.25 mgRE/g extract with the highest concentration obtained in the EA extracts and lowest in the water extract. The HPLC-fluorimeter analysis confirmed colorimetric assay. To this regard, water extract displayed highest level of gallic acid (117.86 ± 10.61 mg/g), followed by methanol (31.01 ± 2.79 mg/g) and EA extract (6.37 ± 0.57 mg/g). Finally, HPLC-MS/MS was carried out, in order to characterize the phytochemical composition of the extracts. A total of 45, 74, and 67 components were characterized in EA, methanolic, and water extracts, respectively (Supplementary Tables S1–S3; Supplementary Figures S2–S4). Based on the results, these compounds were classified as phenolic acids, flavonoids, and coumarins.

Table 2.
Total bioactive components of the tested samples
Phenolic acids: Chlorogenic acids belongs to the hydroxycinnamic acid family, a large family of esters condensed by quinic acid moiety (such as shikimic acid or butyl 4-deoxy-quinic acid) and trans-cinnamic acids moiety (such caffeic, ferulic, p-coumaric, sinapic, and dimethoxycinnamic acid) [40,41]. At [M + H]+ m/z 355, two chlorogenic acid isomers were present in the EA extract while three chlorogenic acid isomers were detect in MeOH and water extracts. Two isomers of di-O-caffeoylquinic acid, [M − H]− at m/z 515 were also characterized in investigated extracts. Isomers of caffeoyl-feruloylquinic acid were detected in water (5) and methanol (7) extracts only. 4-O-Feruloylquinic (not in EA extract) acid and 5-O-feruloylquinic acid at [M − H]− at m/z 367 were also identified in the extracts.
Coumarins (2H-1-benzopyran-2-one) are made of fused benzene and α-pyrone rings and are known to possess a number of therapeutic effects including antioxidant, and neuroprotective properties [42]. Several coumarins were observed in the analyzed extracts. Fraxetin, scopoletin, isofraxidin, and α-santonin were present in all tested extracts, while methylcoumarin, hexosyl-2-coumarate and two derivates of dihydroxycoumarin were identified only in water and MeOH extract.
Flavonoids: Flavonoids are another important group of secondary metabolites from Artemisia species [7,43]. In this regard, 30, 35, and 42 flavonoid—mainly belonging to the flavonol and flavone subclasses—were identified in EA, water, and MeOH extracts respectively. In all cases, the following aglycones with sugar or glycoside moieties, and/or their derivatives were observed: quercetin, apigenin, isorhamnetin, and luteolin.
The flavanone homoeriodictyol (3′-methoxy-4′,5,7-trihydroxyflavanone) was characterized in all extracts, while pinocembrin (5,7-dihydroxyflavanone) was detected only in EA and MeOH extract.
Other compounds: Artemisinin and the endoperoxide sesquiterpene lactone were identified in the analyzed extracts at [M + H]+ m/z 263. Artemisinin-based therapies are now generally considered as the best current treatment of malaria, including highly drug-resistant strains [2,44].
3.3. Antioxidant Properties
In the current work, the antioxidant ability of the extracts and EO of A. santonicum was evaluated using multiple in vitro cell-free bioassays. The results are depicted in Table 3. In phosphomolybdenum assay, the EO has shown remarkable antioxidant capacity (61.37 ± 4.17 mmol TE/g); on the other hand, the tested extracts revealed mild effects (2.41 ± 0.02 (water) and 2.12 ± 0.12 (EA) mmol TE/g). Two radicals were used to evaluate the free radical scavenging activity of tested extracts and EO. For both DPPH and ABTS assays, the highest scavenging activity was observed in water extract (DPPH: 298.28 ± 12.75 and ABTS: 278.57 ± 3.77 mg TE/g extract) followed by MeOH extract (DPPH: 277.96 ± 11.73 and ABTS: 217.60 ± 6.31 mg TE/g extract). The tested volatile oil has shown a modest activity (3.76 ± 0.58 TE/g extract) as compared to the extracts. Kordali et al. [18] have studied the antioxidant activities of commercially obtained camphor and 1,8-cineole in order to know the effect of the respective major compounds characterized in EO of A. santonicum in antioxidant and DPPH radical scavenging activities. The results revealed that neither of the compounds have shown scavenging activity at 100 μg/mL with DPPH. In the study carried by Kadri, et al. [45], the EO of A. herba-alba, in which a major amount of α-thujone and β-thujone was present, demonstrated a significant free radical activity, in DPPH assay. In the present work, the EO displayed a modest scavenging activity DPPH, implying that the presence of α-thujone and β-thujone is crucial for its free scavenging activity with DPPH. Intriguingly, the equivalence of the antioxidant potential of EO in the ABTS assay was found to be 14-fold higher as compared with DPPH assay. A similar observation was noted for EA extract, whether the scavenging activity was approximately 2-fold higher for ABTS assay, compared with DPPH assay. The difference in the free radical scavenging capacity (DPPH and ABTS) for one same sample can be explained by different mechanisms substantiating the antiradical interaction, in these two assays. For instance, DPPH has only hydrophobic structure while ABTS has both lipophilic and hydrophilic characters [46,47,48].

Table 3.
Antioxidant activities of the tested samples
The reduction ability is one significant way to determine the electron-donation ability of antioxidants [49]. The best reducing effects were shown by MeOH (515.30 ± 3.19 mg TE/g extract) and water (262.71 ± 3.99 mg TE/g extract) extracts for CUPRAC and FRAP assays, respectively. Chelating properties of the extracts were determined using the metal chelating assay. These findings reflected the significant chelation ability against Fe2+ in EO and all investigated extracts, with the highest value being recorded for EA extract.
3.4. Enzyme Inhibitory Properties
Enzymatic dysregulation is the most common pathological scenario of many chronic diseases like diabetes mellitus (DM), dementia, and hyperpigmentation. Acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) hydrolyze the neurotransmitter acetylcholine (ACh), whose decline is associated with the onset and progression of Alzheimer’s Disease (AD), an age-related neurodegenerative disorder, characterized by cognitive and memory impairment. Therefore, inhibition of cholinesterase is a promising way for managing AD symptoms [50]. Similarly, DM can be managed by inhibiting α-amylase and α-glucosidase, key enzymes that are responsible for the hydrolysis of carbohydrates into glucose and its absorption in the digestive tract, respectively [51,52]. Hyperpigmentation can managed by inhibiting the activity of tyrosinase that synthesized melanin, the pigment that gives color to skin [53].
The experimental results regarding enzyme inhibition effects of the EO and extracts are summarized in Table 4. The best AChE inhibitory effect was provided by ethyl acetate extract (3.87 ± 0.70 mg GALAE/g extract) followed by the MeOH extract (3.21 ± 0.08 70 mg GALAE/g extract). The EO was most potent against BChE (3.52 ± 0.39 mg GALAE/g extract). Yu et al. [54] reported that the anti-AChE activity of flower essential oil of A. annua is mainly attributed to camphor and 1,8-cineole, which were major components of the oil. Interestingly, despite its remarkable antioxidant activities, water extract demonstrated no activity against AChE and BChE. On the other hand, A. santonicum extracts (71.50 ± 1.24–122.43 ± 3.25 mg KAE/g extract) and EO (37.98 ± 1.45 mg KAE/g extract) displayed a noteworthy tyrosinase inhibitory activities. For the tested extracts, the enzyme inhibition can be ranked as MeOH > EA > water. Taherkhani and colleagues [55] reported the anti-tyrosinase activity (IC50 = 6.08 mg/mL) of EO from leaves of A. diffusa, which contains camphor (28.30%), 1,8-cineole (21.03%) and β-thujone (14.20%) as major components. Finally, the extracts and EO displayed moderate inhibitory effects against α-amylase, while EA and MeOH extracts (EA: 24.69 ± 0.10 mmol MeOH: 23.00 ± 2.25 mmol ACAE/g extract) revealed particularly active against α-glucosidase. EO was also potent against α-glucosidase with a mean value of 4.97 ± 0.27 mmol ACAE/g extract.

Table 4.
Enzyme inhibitory properties of the tested extracts
3.5. Toxicological and Pharmacological Studies
The potential toxicity of water, methanol, and ethyl acetate extracts and EO of A. santonicum (0.1–20 mg/mL) was evaluated on brine shrimp (Artemia salina Leach) lethality assay. Experimental procedures were carried out as previously reported [56].
As regards water, MeOH and ethyl acetate extracts, LC50 values were <1 mg/mL. Whereas, LC50 value related to EO was <0.5 µL/mL. The resulting LC50 values were indicatory to identify the biocompatibility range for the subsequent evaluations on rat colon stimulated with LPS. This latter experimental model has been selected in order to evaluate the protective effects exerted by the extracts (100 µg/mL) and EO (0.1 µL/mL) against the burden of oxidative stress and inflammation, in rat colon. To this regard, we evaluated the levels of selected biomarkers of oxidative stress and inflammation, including nitrites, 5-HT, PGE2, and LDH.
Particularly, EO, water, MeOH, and EA extracts were able to reduce LPS-induced LDH (Figure 1) and PGE2 (Figure 2), while only EA revealed effective in blunting LPS-induced nitrite level (Figure 3), in rat colon. As regards 5-HT turnover, measured as 5HIIA/5-HT ratio, EO, water, and MeOH extracts potentiated LPS-induced reduction of 5HIIA/5-HT ratio (Figure 4). Only EA extract was effective in stimulating neurotransmitter turnover. The same extract was also able to reduce HCT116 cell line viability (Figure 5).
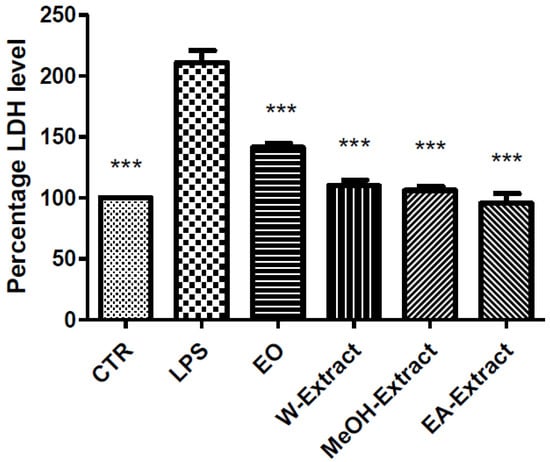
Figure 1.
Effect of essential oil (EO) and water (W), methanol (MeOH) and ethyl acetate (EA) A. santonicum extracts (100 µg/mL) on lipopolysaccharide (LPS)-induced lactate dehydrogenase (LDH) level in rat colon specimens. All extracts were able to restore the level of LDH observed in control (CTR) group. p < 0.001; post-hoc, *** p < 0.001 vs. LPS.
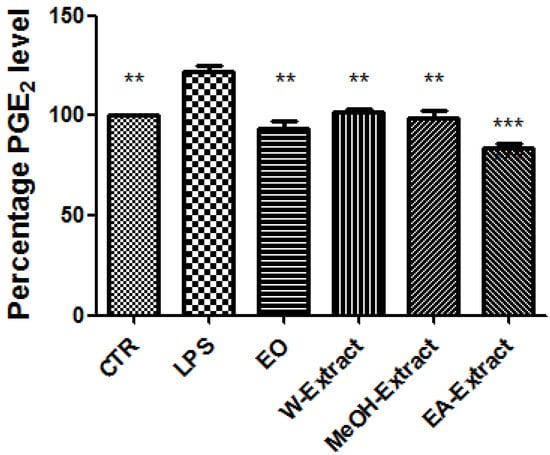
Figure 2.
Effect of essential oil (EO) and water (W), methanol (MeOH) and ethyl acetate (EA) A. santonicum extracts (100 µg/mL) on LPS-induced PGE2 level in rat colon specimens. All extracts and EO were able to restore the level of PGE2 observed in control (CTR) group. p < 0.0001; post-hoc, ** p < 0.01, *** p < 0.001 vs. LPS.
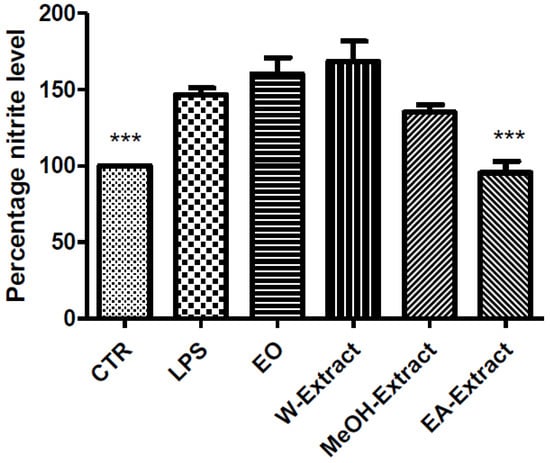
Figure 3.
Effect of essential oil (EO) and water (W), methanol (MeOH) and ethyl acetate (EA) A. santonicum extracts (100 µg/mL) on LPS-induced nitrite level in rat colon specimens. Only EA extract was able to restore the level of nitrites observed in control (CTR) group. p < 0.001; post-hoc, *** p < 0.001 vs. LPS.
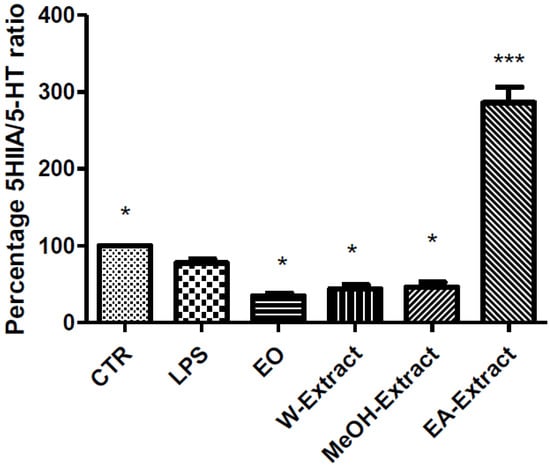
Figure 4.
Effect of essential oil (EO) and water (W), methanol (MeOH) and ethyl acetate (EA) A. santonicum extracts (100 µg/mL) on LPS-induced reduction of 5-HT turnover (5HIIA/5-HT ratio) in rat colon specimens. Only EA extract was able to increase the level of nitrites observed in both control (CTR) and LPS groups. p < 0.0001; post-hoc, * p < 0.05, *** p < 0.001 vs. LPS.
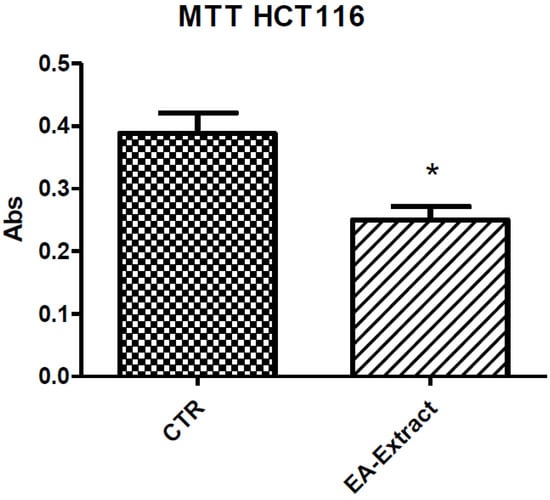
Figure 5.
Effect of ethyl acetate (EA) A. santonicum extracts (100 µg/mL) on tumoral HCT116 cell line viability (MTT test). * p < 0.05 vs. control (CTR) group.
4. Discussion
Increased reactive oxygen/nitrogen species (ROS/RNS) have long been related to chronic inflammatory diseases, including ulcerative colitis [57]. The assessment of nitrite level is a validated tool to evaluate NO synthesis, as an index of disease activity in inflamed colon [58]. While LDH is a well-recognized marker of tissue damage, whose downregulation could represent a protective mechanism, particularly in the colon [59,60]. In the gut, a pro-inflammatory role could also be displayed by 5-HT [61], possibly through 5-HT3 receptor activation [62]. Additionally, 5-HT was previously described as a mitogenic neurotransmitter [61], which stimulates the growth of a wide plethora of tumors, including colorectal carcinoma [63,64,65,66]. We also studied the effects of Artemisia extracts and EO on LPS-induced levels of colon PGE2, a cyclooxygenase (COX)-2-derived pro-inflammatory cytokine, whose upregulation has been long involved in colon inflammation and damage [67].
Regarding the selected markers of oxidative stress and inflammation, the results indicated some discrepancies. EO and all extracts blunted LPS-induced colon LDH level (Figure 1). The most significant inhibitory effect was exerted by ethyl acetate extract. The pattern of inhibition of LPS-induced PGE2 was in agreement with the measurement of LDH levels, being in this case ethyl acetate extract the most promising agent, as well (Figure 2). A different pattern was observed as regards the effects of extracts and EO on nitrite and 5-HT levels. Specifically, only ethyl acetate extract was able to blunt LPS-induced nitrite level (Figure 2). By contrast, MeOH extract and essential oil seem to increase LPS-induced production of nitrites, despite there being no statistical significance (Figure 3). In analogy, the reduction of 5-HT level, confirmed by the stimulated turnover (5HIIA/5-HT ratio), was observed only in the group treated with ethyl acetate extract (Figure 4). On the other, the inhibition of 5-HT turnover induced by MeOH and water extracts and EO could exclude relevant protective effects in colon challenged with LPS. This could be related to more than one speculation. On one side, the paradoxical pro-oxidative effects exerted by antioxidants in the liquids are well known [68]. In this regard, we should consider the recent findings by Abnosi and Yari [69], who highlighted the potential toxicity of gallic acid, in rat bone marrow mesenchymal stem cells, that could be partially related to increased inflammatory and oxidative stress mediators. Additionally, the null effect of EO on LPS-induced nitrites is unclear. Yoon and colleagues [70] found that EO of A. fukudo is able to downregulate nitrite and PGE2 levels, in RAW 264.7 macrophages challenged with LPS. On the other hand, the EO of A. fukudo displayed lower levels of camphor (6.01%) compared to the EO tested in our experiments (36.6%; Table 1). To this regard, we hypothesize that the higher EO camphor level could limit the antioxidant activity of the tested sample [71].
Taken together, these assays indicated ethyl acetate extract as the most promising protective agent, against the burden of inflammatory and oxidative stress, in the colon. Actually, we cannot exclude that the protective effects exerted by this extract on the selected markers of oxidative stress and inflammation could be a direct consequence of multiple factors, such as the showed highest level in total flavonoids (Table 2), alongside with the antiradical and metal chelating activities (Table 3).
Considering these findings, we further evaluated the biological activity of ethyl acetate extract. Particularly, we tested this extract on human colon cancer HCT116 cell line viability (MTT test). We observed that ethyl acetate extract (100 µg/mL) significantly reduced cell viability (Figure 5), thus suggesting potential antiproliferative effects. On the basis of the tested biomarkers, we also hypothesize that the reduction of HCT116 cell viability could be, albeit partially, the result of the downregulation of pro-inflammatory factors involved in HCT116 cell growth and survival, including colon PGE2 and 5-HT [72,73,74].
In summary, our findings proved that A. santonicum EO and extracts exhibited interesting chemical profiles. EO contains camphor, 1,8-cineole, α-thujone, borneol, and β-thujone as major components, while extracts contain important pharmacologically active compounds including chlorogenic acid, caffeic acid, apigenin, and artemisin. Overall, methanol and water extracts have shown superior antiradical potential. EO, EA, and methanol extracts also revealed active against AChE and BChE. A significant enzyme inhibitory activity against tyrosinase and α-glucosidase was observed for all tested extracts. On the other hand, pharmacological tests did not confirm the intrinsic antiradical activity. Conversely, we observed that EO could exert pro-oxidant effects, albeit partially related to the elevated content of camphor. While ethyl acetate extract showed the best pharmacological profile, being able to blunt all tested pro-oxidant/pro-inflammatory markers, in an experimental model of colon inflammation. Additionally, ethyl acetate extract also revealed potential antiproliferative effects against human colon cancer HCT116 cell line.
The results gathered in this study emphasized the biological potential of A. santonicum and further suggest the importance in isolating phytochemical compounds and understanding molecular mechanisms at the basis of the reported pharmacological effects.
Supplementary Materials
The following are available online https://www.mdpi.com/2227-9717/7/8/522/s1. Figure S1: Total ion chromatogram of essential oil from Artemisia santonicum. Figure S2: Chromatogram of the ethyl acetate extract from Artemisia santonicum in positive (a) and negative (b) mode. Figure S3: Chromatogram of the methanol extract from Artemisia santonicum in positive (a) and negative (b) mode. Figure S4: Chromatogram of the water extract from Artemisia santonicum in positive (a) and negative (b) mode. Table S1: Chemical composition of ethyl acetate extract. Table S2: Chemical composition of methanol extract. Table S3: Chemical composition of water extract.
Author Contributions
For research articles with several authors, a short paragraph specifying their individual contributions must be provided. The following statements should be used “conceptualization, C.F., G.Z., G.O., L.M.; methodology, C.F., G.Z., L.M.; software, G.O.; validation, C.F., G.Z., G.O., L.M.; formal analysis, C.F., L.M.; investigation, A.D., J.J., L.R., S.L., A.C., Z.C., D.L., I.S.; resources, C.F., L.M., G.O. data curation, G.O.; writing—original draft preparation, C.F., G.Z, L.M.; writing—review and editing, L.B., M.F.M, G.O.; visualization, L.B., M.F.M; supervision, L.B., M.F.M; project administration, C.F., G.Z.; funding acquisition, C.F., G.O., L.M.
Funding
This research received no external funding.
Acknowledgments
This work was supported by grants from the Italian Ministry of University (FAR 2016 granted to Claudio Ferrante; FAR 2018 granted to Giustino Orlando; FAR 2016 granted to Luigi Menghini). Animal experimental procedures were approved by Italian Ministry of Health (authorization no. F4738.N.XTQ, delivered on 11 November 2018).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Abad, M.J.; Bedoya, L.M.; Apaza, L.; Bermejo, P. The Artemisia L. genus: A review of bioactive essential oils. Molecules 2012, 17, 2542–2566. [Google Scholar] [CrossRef] [PubMed]
- Bora, K.S.; Sharma, A. The genus Artemisia: A comprehensive review. Pharm. Biol. 2011, 49, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Dítě, D.; Eliáš, P.; Melečková, Z. Artemisia santonicum subsp. patens in slovakia: The sad story of obligate halophyte on the northern edge of its distribution range. Hacquetia 2013, 12, 5–16. [Google Scholar] [CrossRef]
- Erel, Ş.B.; Şenol, S.G.; Köse, F.A.; Ballar, P. In vitro cytotoxic properties of six Artemisia L. species. Turk. J. Pharm. Sci. 2011, 8, 247–252. [Google Scholar]
- Sengul, M.; Ercisli, S.; Yildiz, H.; Gungor, N.; Kavaz, A.; Çetin, B. Antioxidant, antimicrobial activity and total phenolic content within the aerial parts of Artemisia absinthum, Artemisia santonicum and Saponaria officinalis. Iran. J. Pharm. Res. IJPR 2011, 10, 49. [Google Scholar] [PubMed]
- Yoon, K.D.; Chin, Y.-W.; Yang, M.H.; Kim, J. Separation of anti-ulcer flavonoids from Artemisia extracts by high-speed countercurrent chromatography. Food Chem. 2011, 129, 679–683. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.F.; Luthria, D.L.; Sasaki, T.; Heyerick, A. Flavonoids from Artemisia annua L. as antioxidants and their potential synergism with artemisinin against malaria and cancer. Molecules 2010, 15, 3135–3170. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Lee, S.H.; Kang, K.H.; Kim, B.K. Flavonol galactosides from Artemisia apiacea. Nat. Prod. Sci. 2005, 11, 10–12. [Google Scholar]
- Kim, K.S.; Lee, S.; Shin, J.S.; Shim, S.H.; Kim, B.-K. Arteminin, a new coumarin from Artemisia apiacea. Fitoterapia 2002, 73, 266–268. [Google Scholar] [CrossRef]
- Kwak, J.H.; Lee, K.B.; Schmitz, F.J. Four New Coumarin Derivatives from Artemisia k eiskeana. J. Nat. Prod. 2001, 64, 1081–1083. [Google Scholar] [CrossRef]
- Chen, J.; Wang, X.; Liu, C. Anti-tumour effects of polysaccharides isolated from Artemisia Annua L by inducing cell apoptosis and immunomodulatory anti-hepatoma effects of polysaccharides. Afr. J. Tradit. Complement. Altern. Med. 2014, 11, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.A.; Jain, D.; Bhakuni, R.; Zaim, M.; Thakur, R. Occurrence of some antiviral sterols in Artemisia annua. Plant Sci. 1991, 75, 161–165. [Google Scholar] [CrossRef]
- Brown, G.D.; Liang, G.Y.; Sy, L.K. Terpenoids from the seeds of Artemisia annua. Phytochemistry 2003, 64, 303–323. [Google Scholar] [CrossRef]
- Tang, H.Q.; Hu, J.; Yang, L.; Tan, R.X. Terpenoids and flavonoids from Artemisia species. Planta Med. 2000, 66, 391–393. [Google Scholar] [CrossRef] [PubMed]
- Bilia, A.R.; Santomauro, F.; Sacco, C.; Bergonzi, M.C.; Donato, R. Essential oil of Artemisia annua L.: An extraordinary component with numerous antimicrobial properties. Evid. Based Complement. Altern. Med. 2014, 2014, 159819. [Google Scholar] [CrossRef] [PubMed]
- Chaieb, I.; Ben Hamouda, A.; Tayeb, W.; Zarrad, K.; Bouslema, T.; Laarif, A. The Tunisian Artemisia essential oil for reducing contamination of stored cereals by Tribolium castaneum. Food Technol. Biotechnol. 2018, 56, 247–256. [Google Scholar] [CrossRef]
- Korkmaz, H.; Gurdal, A. Effect of Artemisia santonicum L. on blood glucose in normal and alloxan-induced diabetic rabbits. Phytother. Res. 2002, 16, 675–676. [Google Scholar] [CrossRef] [PubMed]
- Kordali, S.; Kotan, R.; Mavi, A.; Cakir, A.; Ala, A.; Yildirim, A. Determination of the chemical composition and antioxidant activity of the essential oil of Artemisia dracunculus and of the antifungal and antibacterial activities of Turkish Artemisia absinthium, A. dracunculus, Artemisia santonicum, and Artemisia spicigera essential oils. J. Agric. Food Chem. 2005, 53, 9452–9458. [Google Scholar]
- Algieri, F.; Rodriguez-Nogales, A.; Rodriguez-Cabezas, M.E.; Risco, S.; Ocete, M.; Galvez, J. Botanical drugs as an emerging strategy in inflammatory bowel disease: A review. J. Mediat. Inflamm. 2015, 2015, 179616. [Google Scholar] [CrossRef]
- Langhorst, J.; Wulfert, H.; Lauche, R.; Klose, P.; Cramer, H.; Dobos, G.; Korzenik, J. Systematic review of complementary and alternative medicine treatments in inflammatory bowel diseases. J. Crohns Colitis 2014, 9, 86–106. [Google Scholar] [CrossRef]
- Sarikurkcu, C.; Zengin, G.; Oskay, M.; Uysal, S.; Ceylan, R.; Aktumsek, A. Composition, antioxidant, antimicrobial and enzyme inhibition activities of two Origanum vulgare subspecies (subsp. vulgare and subsp. hirtum) essential oils. Ind. Crop. Prod. 2015, 70, 178–184. [Google Scholar] [CrossRef]
- Zengin, G.; Uysal, A.; Diuzheva, A.; Gunes, E.; Jekő, J.; Cziáky, Z.; Picot-Allain, C.M.N.; Mahomoodally, M.F. Characterization of phytochemical components of Ferula halophila extracts using HPLC-MS/MS and their pharmacological potentials: A multi-functional insight. J. Pharm. Biomed. Anal. 2018, 160, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Rodrıguez-Delgado, M.; Malovana, S.; Perez, J.; Borges, T.; Montelongo, F.G. Separation of phenolic compounds by high-performance liquid chromatography with absorbance and fluorimetric detection. J. Chromatogr. A 2001, 912, 249–257. [Google Scholar] [CrossRef]
- Uysal, S.; Zengin, G.; Locatelli, M.; Bahadori, M.B.; Mocan, A.; Bellagamba, G.; De Luca, E.; Mollica, A.; Aktumsek, A. Cytotoxic and enzyme inhibitory potential of two Potentilla species (P. speciosa L. and P. reptans Wild.) and their chemical composition. Front. Pharmacol. 2017, 8, 290. [Google Scholar] [CrossRef] [PubMed]
- Menghini, L.; Ferrante, C.; Leporini, L.; Recinella, L.; Chiavaroli, A.; Leone, S.; Pintore, G.; Vacca, M.; Orlando, G.; Brunetti, L. An hydroalcoholic chamomile extract modulates inflammatory and immune response in HT29 cells and isolated rat colon. Phytother. Res. 2016, 30, 1513–1518. [Google Scholar] [CrossRef] [PubMed]
- Chiavaroli, A.; Brunetti, L.; Orlando, G.; Recinella, L.; Ferrante, C.; Leone, S.; Di Michele, P.; Di Nisio, C.; Vacca, M. Resveratrol inhibits isoprostane production in young and aged rat brain. J. Biol. Regul. Homeost. Agents 2010, 24, 441. [Google Scholar] [PubMed]
- Locatelli, M.; Macchione, N.; Ferrante, C.; Chiavaroli, A.; Recinella, L.; Carradori, S.; Zengin, G.; Cesa, S.; Leporini, L.; Leone, S. Graminex Pollen: Phenolic pattern, colorimetric analysis and protective effects in immortalized prostate cells (PC3) and rat prostate challenged with LPS. Molecules 2018, 23, 1145. [Google Scholar] [CrossRef]
- Ferrante, C.; Recinella, L.; Ronci, M.; Menghini, L.; Brunetti, L.; Chiavaroli, A.; Leone, S.; Di Iorio, L.; Carradori, S.; Tirillini, B. Multiple pharmacognostic characterization on hemp commercial cultivars: Focus on inflorescence water extract activity. Food Chem. Toxicol. 2019, 125, 452–461. [Google Scholar] [CrossRef]
- Zengin, G.; Locatelli, M.; Stefanucci, A.; Macedonio, G.; Novellino, E.; Mirzaie, S.; Dvorácskó, S.; Carradori, S.; Brunetti, L.; Orlando, G.; et al. Chemical characterization, antioxidant properties, anti-inflammatory activity, and enzyme inhibition of Ipomoea batatas L. leaf extracts. Int. J. Food Prop. 2017, 20, 1907–1919. [Google Scholar] [CrossRef]
- Brunetti, L.; Orlando, G.; Ferrante, C.; Recinella, L.; Leone, S.; Chiavaroli, A.; Di Nisio, C.; Shohreh, R.; Manippa, F.; Ricciuti, A. Peripheral chemerin administration modulates hypothalamic control of feeding. Peptides 2014, 51, 115–121. [Google Scholar] [CrossRef]
- Ferrante, C.; Orlando, G.; Recinella, L.; Leone, S.; Chiavaroli, A.; Di Nisio, C.; Shohreh, R.; Manippa, F.; Ricciuti, A.; Vacca, M. Central inhibitory effects on feeding induced by the adipo-myokine irisin. Eur. J. Pharmacol. 2016, 791, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Çetin, B.; Özer, H.; Çakir, A.; Mete, E.; Tosun, M.; Öztürk, E.; Polat, T.; Kandemir, A. Chemical composition of hydrodistilled essential oil of Artemisia incana (L.) Druce and antimicrobial activity against foodborne microorganisms. Chem. Biodivers. 2009, 6, 2302–2310. [Google Scholar] [PubMed]
- Korolyuk, E.; Tkachev, A. Chemical composition of the essential oil from two wormwood species Artemisia frigida and Artemisia argyrophylla. Russ. J. Bioorg. Chem. 2010, 36, 884–893. [Google Scholar] [CrossRef]
- Padalia, R.C.; Verma, R.S.; Chauhan, A.; Chanotiya, C.S.; Yadav, A. Variation in the volatile constituents of Artemisia annua var. CIM-Arogya during plant ontogeny. Nat. Prod. Commun. 2011, 6, 1934578X1100600221. [Google Scholar] [CrossRef]
- Shafaghat, A.; Sadeghi, H.; Oji, K. Composition and antibacterial activity of essential oils from leaf, stem and root of Chrysanthemum parthenium (L.) Bernh. from Iran. Nat. Prod. Commun. 2009, 4, 1934578X0900400624. [Google Scholar] [CrossRef]
- Höld, K.M.; Sirisoma, N.S.; Ikeda, T.; Narahashi, T.; Casida, J.E. α-Thujone (the active component of absinthe): γ-aminobutyric acid type A receptor modulation and metabolic detoxification. Proc. Natl. Acad. Sci. USA 2000, 97, 3826–3831. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.; Zhang, H.W.; Lin, Z.X. Chapter 48—Treatments used in complementary and alternative medicine. In Side Effects of Drugs Annual; Ray, S.D., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 36, pp. 717–724. [Google Scholar]
- Dzoyem, J.P.; Kuete, V.; Eloff, J.N. Biochemical parameters in toxicological studies in Africa: Significance, principle of methods, data interpretation, and use in plant screenings. In Toxicological Survey of African Medicinal Plants; Elsevier: Amsterdam, The Netherlands, 2014; pp. 659–715. [Google Scholar]
- Lachenmeier, D.W.; Uebelacker, M. Risk assessment of thujone in foods and medicines containing sage and wormwood–evidence for a need of regulatory changes? Regul. Toxicol. Pharmacol. 2010, 58, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Gil, M.; Wianowska, D. Chlorogenic acids—Their properties, occurrence and analysis. Ann. Univ. Mariae Curie Sklodowska Sect. AA Chem. 2017, 72, 61–104. [Google Scholar] [CrossRef]
- Santana-Galvez, J.; Cisneros-Zevallos, L.; Jacobo-Velazquez, D.A. Chlorogenic Acid: Recent advances on its dual role as a food additive and a nutraceutical against metabolic syndrome. Molecules 2017, 22, 358. [Google Scholar] [CrossRef]
- Venugopala, K.N.; Rashmi, V.; Odhav, B. Review on natural coumarin lead compounds for their pharmacological activity. BioMed Res. Int. 2013, 2013, 963248. [Google Scholar] [CrossRef]
- Yuan, H.; Lu, X.; Ma, Q.; Li, D.; Xu, G.; Piao, G. Flavonoids from Artemisia sacrorum Ledeb. and their cytotoxic activities against human cancer cell lines. Exp. Ther. Med. 2016, 12, 1873–1878. [Google Scholar] [CrossRef] [PubMed]
- Krishna, S.; Bustamante, L.; Haynes, R.K.; Staines, H.M. Artemisinins: Their growing importance in medicine. Trends Pharmacol. Sci. 2008, 29, 520–527. [Google Scholar] [CrossRef] [PubMed]
- Kadri, A.; Chobba, I.B.; Zarai, Z.; Békir, A.; Gharsallah, N.; Damak, M.; Gdoura, R. Chemical constituents and antioxidant activity of the essential oil from aerial parts of Artemisia herba-alba grown in Tunisian semi-arid region. Afr. J. Biotechnol. 2011, 10, 2923–2929. [Google Scholar]
- Kim, D.-O.; Lee, K.W.; Lee, H.J.; Lee, C.Y. Vitamin C equivalent antioxidant capacity (VCEAC) of phenolic phytochemicals. J. Agric. Food Chem. 2002, 50, 3713–3717. [Google Scholar] [CrossRef] [PubMed]
- Liang, N.; Kitts, D. Antioxidant property of coffee components: Assessment of methods that define mechanisms of action. Molecules 2014, 19, 19180–19208. [Google Scholar] [CrossRef] [PubMed]
- Mahomoodally, M.F.; Vlaisavljevic, S.; Berezni, S.; Abdallah, H.H.; Zengin, G.; Atanasov, A.G.; Mollica, A.; Lobine, D.; Aktumsek, A. Lotus aegaeus (Gris.) Boiss and Iberis sempervirens L.: Chemical fingerprints, antioxidant potential, and inhibition activities and docking on key enzymes linked to global health problems. Ind. Crop. Prod. 2018, 120, 271–278. [Google Scholar] [CrossRef]
- Uysal, S.; Senkardes, I.; Mollica, A.; Zengin, G.; Bulut, G.; Dogan, A.; Glamočlija, J.; Soković, M.; Lobine, D.; Mahomoodally, F.M. Biologically active compounds from two members of the Asteraceae family: Tragopogon dubius Scop. and Tussilago farfara L. J. Biomol. Struct. Dyn. 2019, 37, 3269–3281. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, A.; Chakraborty, I.; Bhattacharjee, R.; Subhra, T.; Banerjee, D.R.V.; Adapa, D.; Bhardwaj, R. An update on pathological implications of enzymatic dysregulation in Alzheimer’s disease. Biomed. Res. 2018, 29, 2215–2226. [Google Scholar] [CrossRef]
- Telagari, M.; Hullatti, K. In-vitro alpha-amylase and alpha-glucosidase inhibitory activity of Adiantum caudatum Linn. and Celosia argentea Linn. extracts and fractions. Indian J. Pharmacol. 2015, 47, 425–429. [Google Scholar]
- Yilmazer-Musa, M.; Griffith, A.M.; Michels, A.J.; Schneider, E.; Frei, B. Grape seed and tea extracts and catechin 3-gallates are potent inhibitors of α-amylase and α-glucosidase activity. J. Agric. Food Chem. 2012, 60, 8924–8929. [Google Scholar] [CrossRef]
- Chang, T.-S. Natural Melanogenesis Inhibitors acting through the down-regulation of tyrosinase activity. Materials 2012, 5, 1661–1685. [Google Scholar] [CrossRef]
- Yu, Z.; Wang, B.; Yang, F.; Sun, Q.; Yang, Z.; Zhu, L. Chemical composition and anti-acetyl cholinesterase activity of flower essential oils of Artemisia annua at different flowering stage. Iran. J. Pharm. Res. 2011, 10, 265. [Google Scholar] [PubMed]
- Taherkhani, M. Chemical composition, antimicrobial, antioxidant activity, tyrosinase inhibition and chelating ability of the leaf essential oil of Artemisia diffusa. J. Essent. Oil Bear. Plants 2016, 19, 1600–1613. [Google Scholar] [CrossRef]
- Taviano, M.F.; Marino, A.; Trovato, A.; Bellinghieri, V.; Melchini, A.; Dugo, P.; Cacciola, F.; Donato, P.; Mondello, L.; Guvenc, A.; et al. Juniperus oxycedrus L. subsp. oxycedrus and Juniperus oxycedrus L. subsp. macrocarpa (Sibth. and Sm.) Ball. “berries” from Turkey: Comparative evaluation of phenolic profile, antioxidant, cytotoxic and antimicrobial activities. Food Chem. Toxicol. 2013, 58, 22–29. [Google Scholar] [PubMed]
- Achitei, D.; Ciobica, A.; Balan, G.; Gologan, E.; Stanciu, C.; Stefanescu, G. Different profile of peripheral antioxidant enzymes and lipid peroxidation in active and non-active inflammatory bowel disease patients. Dig. Dis. Sci. 2013, 58, 1244–1249. [Google Scholar] [CrossRef] [PubMed]
- Goggins, M.G.; Shah, S.A.; Goh, J.; Cherukuri, A.; Weir, D.G.; Kelleher, D.; Mahmud, N. Increased urinary nitrite, a marker of nitric oxide, in active inflammatory bowel disease. Mediat. Inflamm. 2001, 10, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Kannan, N.; Guruvayoorappan, C. Protective effect of Bauhinia tomentosa on acetic acid induced ulcerative colitis by regulating antioxidant and inflammatory mediators. Int. Immunopharmacol. 2013, 16, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Nagarjun, S.; Dhadde, S.B.; Veerapur, V.P.; Thippeswamy, B.S.; Chandakavathe, B.N. Ameliorative effect of chromium-d-phenylalanine complex on indomethacin-induced inflammatory bowel disease in rats. Biomed. Pharmacother. 2017, 89, 1061–1066. [Google Scholar] [CrossRef]
- Regmi, S.C.; Park, S.Y.; Ku, S.K.; Kim, J.A. Serotonin regulates innate immune responses of colon epithelial cells through Nox2-derived reactive oxygen species. Free Radic. Biol. Med. 2014, 69, 377–389. [Google Scholar] [CrossRef]
- Mousavizadeh, K.; Rahimian, R.; Fakhfouri, G.; Aslani, F.S.; Ghafourifar, P. Anti-inflammatory effects of 5-HT receptor antagonist, tropisetron on experimental colitis in rats. Eur. J. Clin. Investig. 2009, 39, 375–383. [Google Scholar] [CrossRef]
- Seuwen, K.; Pouysségur, J. Serotonin as a growth factor. Biochem. Pharmacol. 1990, 39, 985–990. [Google Scholar] [CrossRef]
- Alpini, G.; Invernizzi, P.; Gaudio, E.; Venter, J.; Kopriva, S.; Bernuzzi, F.; Onori, P.; Franchitto, A.; Coufal, M.; Frampton, G. Serotonin metabolism is dysregulated in cholangiocarcinoma, which has implications for tumor growth. Cancer Res. 2008, 68, 9184–9193. [Google Scholar] [CrossRef] [PubMed]
- Coufal, M.; Invernizzi, P.; Gaudio, E.; Bernuzzi, F.; Frampton, G.A.; Onori, P.; Franchitto, A.; Carpino, G.; Ramirez, J.C.; Alvaro, D.; et al. Increased local dopamine secretion has growth-promoting effects in cholangiocarcinoma. Int. J. Cancer 2010, 126, 2112–2122. [Google Scholar] [CrossRef] [PubMed]
- Nocito, A.; Dahm, F.; Jochum, W.; Jang, J.H.; Georgiev, P.; Bader, M.; Graf, R.; Clavien, P.A. Serotonin regulates macrophage-mediated angiogenesis in a mouse model of colon cancer allografts. Cancer Res. 2008, 68, 5152–5158. [Google Scholar] [CrossRef] [PubMed]
- Soll, C.; Jang, J.H.; Riener, M.O.; Moritz, W.; Wild, P.J.; Graf, R.; Clavien, P.A. Serotonin promotes tumor growth in human hepatocellular cancer. Hepatology 2010, 51, 1244–1254. [Google Scholar] [CrossRef] [PubMed]
- Nagib, M.M.; Tadros, M.G.; ElSayed, M.I.; Khalifa, A.E. Anti-inflammatory and anti-oxidant activities of olmesartan medoxomil ameliorate experimental colitis in rats. Toxicol. Appl. Pharmacol. 2013, 271, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Clement, M.V.; Ramalingam, J.; Long, L.H. Hydrogen peroxide. Ubiquitous in cell culture and in vivo? IUBMB Life 2000, 50, 251–257. [Google Scholar] [CrossRef]
- Abnosi, M.H.; Yari, S. The toxic effect of gallic acid on biochemical factors, viability and proliferation of rat bone marrow mesenchymal stem cells was compensated by boric acid. J. Trace Elem. Med. Biol. 2018, 48, 246–253. [Google Scholar] [CrossRef]
- Yoon, W.J.; Moon, J.Y.; Song, G.; Lee, Y.K.; Han, M.S.; Lee, J.S.; Ihm, B.S.; Lee, W.J.; Lee, N.H.; Hyun, C.G. Artemisia fukudo essential oil attenuates LPS-induced inflammation by suppressing NF-kappaB and MAPK activation in RAW 264.7 macrophages. Food Chem. Toxicol. 2010, 48, 1222–1229. [Google Scholar] [CrossRef]
- Agus, H.H.; Sengoz, C.O.; Yilmaz, S. Oxidative stress-mediated apoptotic cell death induced by camphor in sod1-deficient Schizosaccharomyces Pombe. Toxicol. Res. 2019, 8, 216–226. [Google Scholar] [CrossRef]
- Curtis, J.J.; Seymour, C.B.; Mothersill, C.E. Cell line-specific direct irradiation and bystander responses are influenced by fetal bovine serum serotonin concentrations. Radiat. Res. 2018, 190, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Tsai, F.-M.; Wu, C.-C.; Shyu, R.-Y.; Wang, C.-H.; Jiang, S.-Y. Tazarotene-induced gene 1 inhibits prostaglandin E2-stimulated HCT116 colon cancer cell growth. J. Biomed. Sci. 2011, 18, 88. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).