Selective Cesium Adsorptive Removal on Using Crosslinked Tea Leaves
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
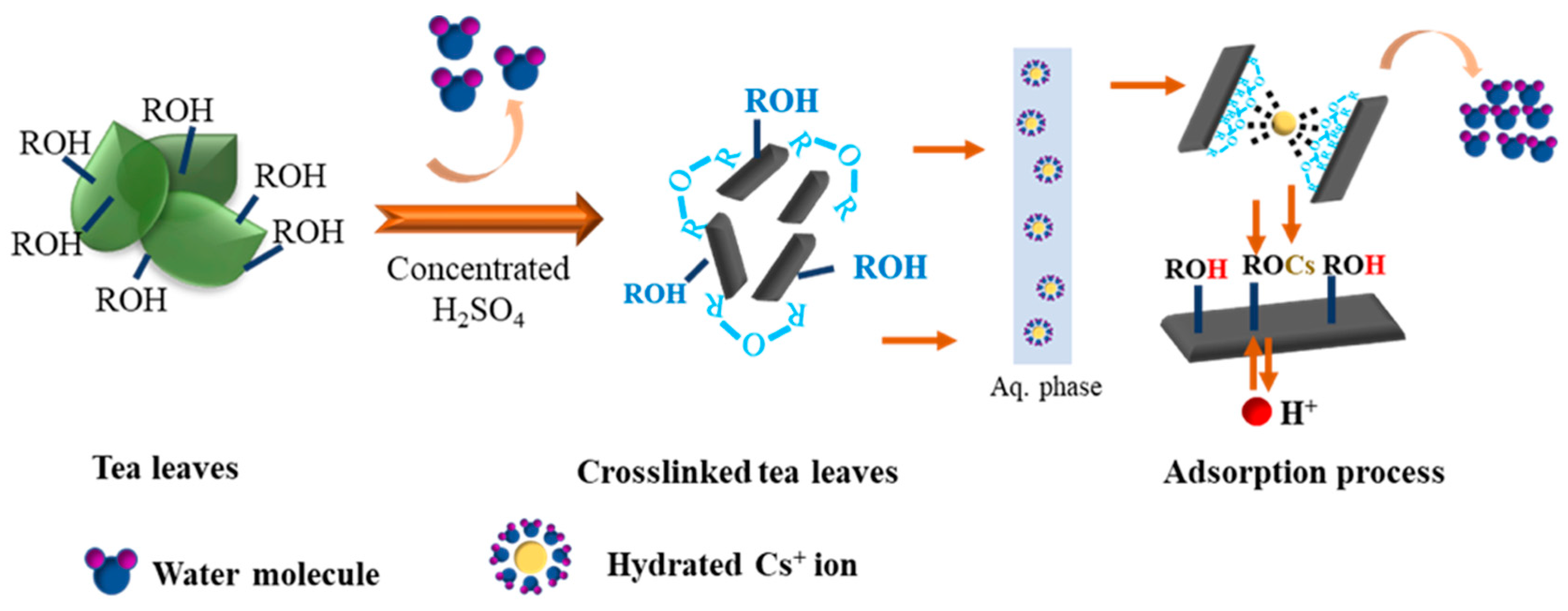
2.2. Preparation Method for Adsorbents
2.3. Batch Adsorption Experiments
2.4. Column Adsorption Experiment
2.5. Evaluation of Adsorption Measurements
3. Results and Discussion
3.1. Adsorption of Cesium Ions on Crude and Crosslinked Tea Leaves
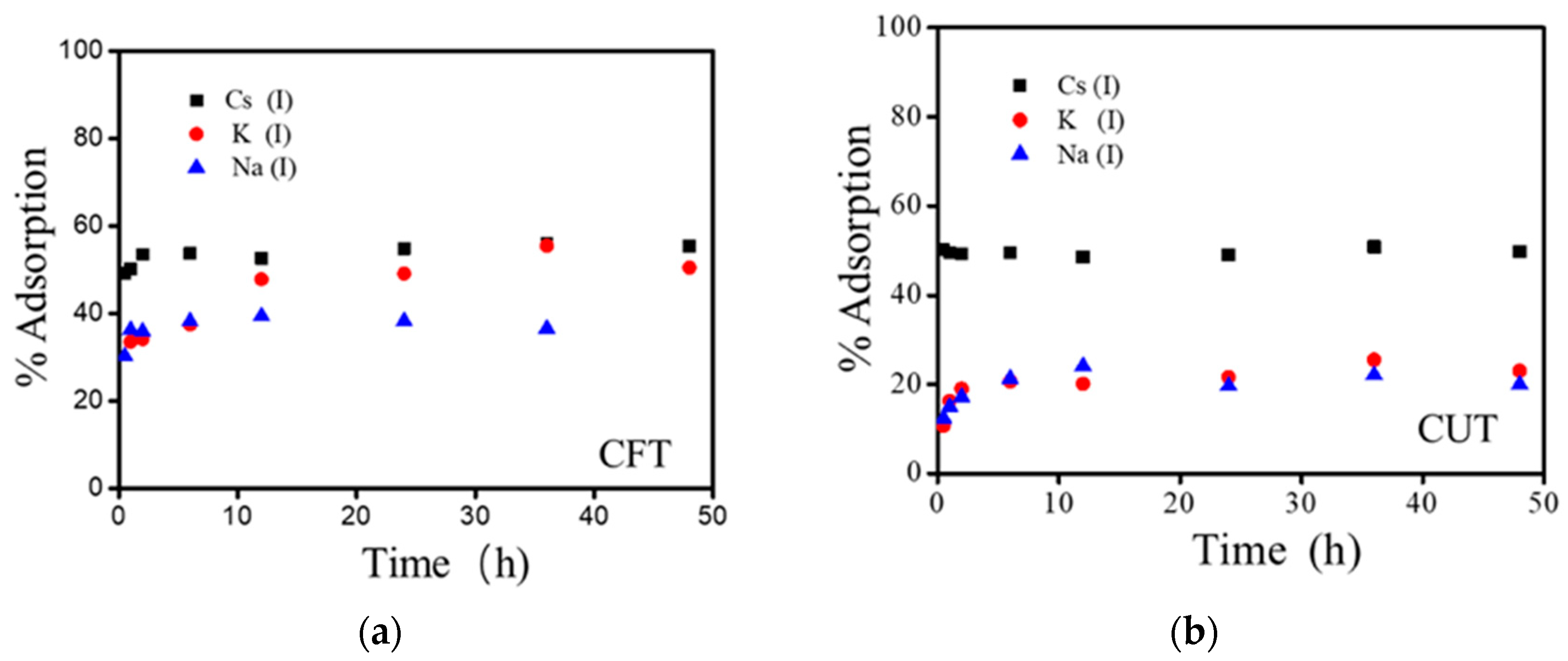
3.2. Effect of Shaking Time on Adsorption of Alkali Metal Ions
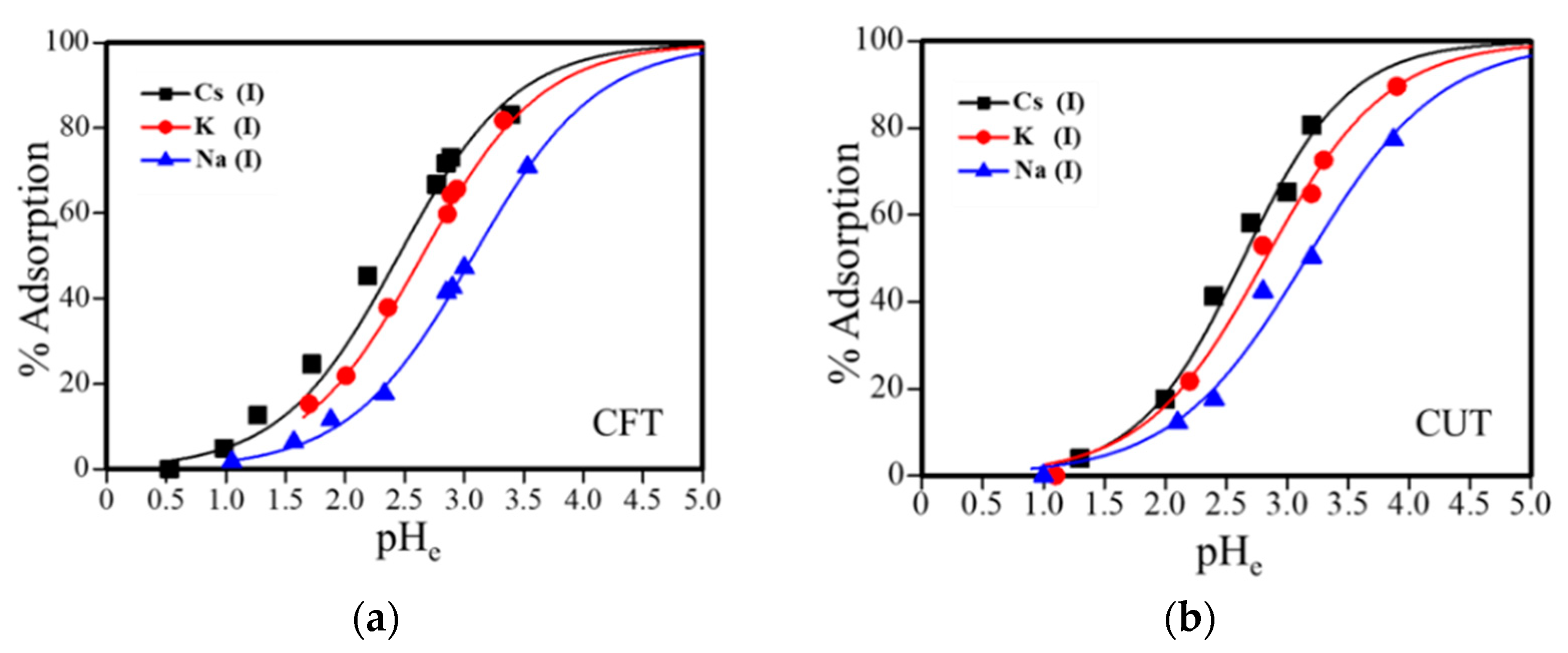
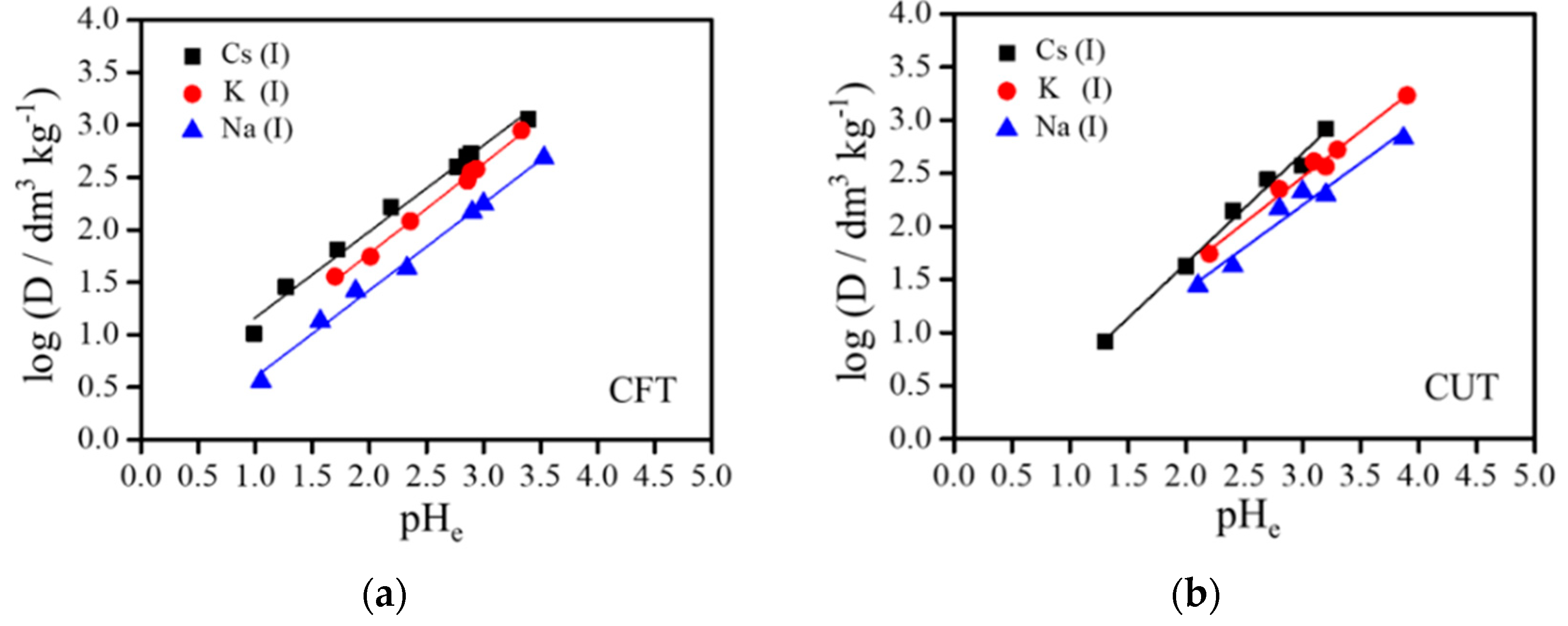
3.3. Effect of pH on Adsorption of Alkali Metal Ions
3.4. Adsorption Isotherms of Na+ and Cs+ Ions on CFT and CUT Adsorbents
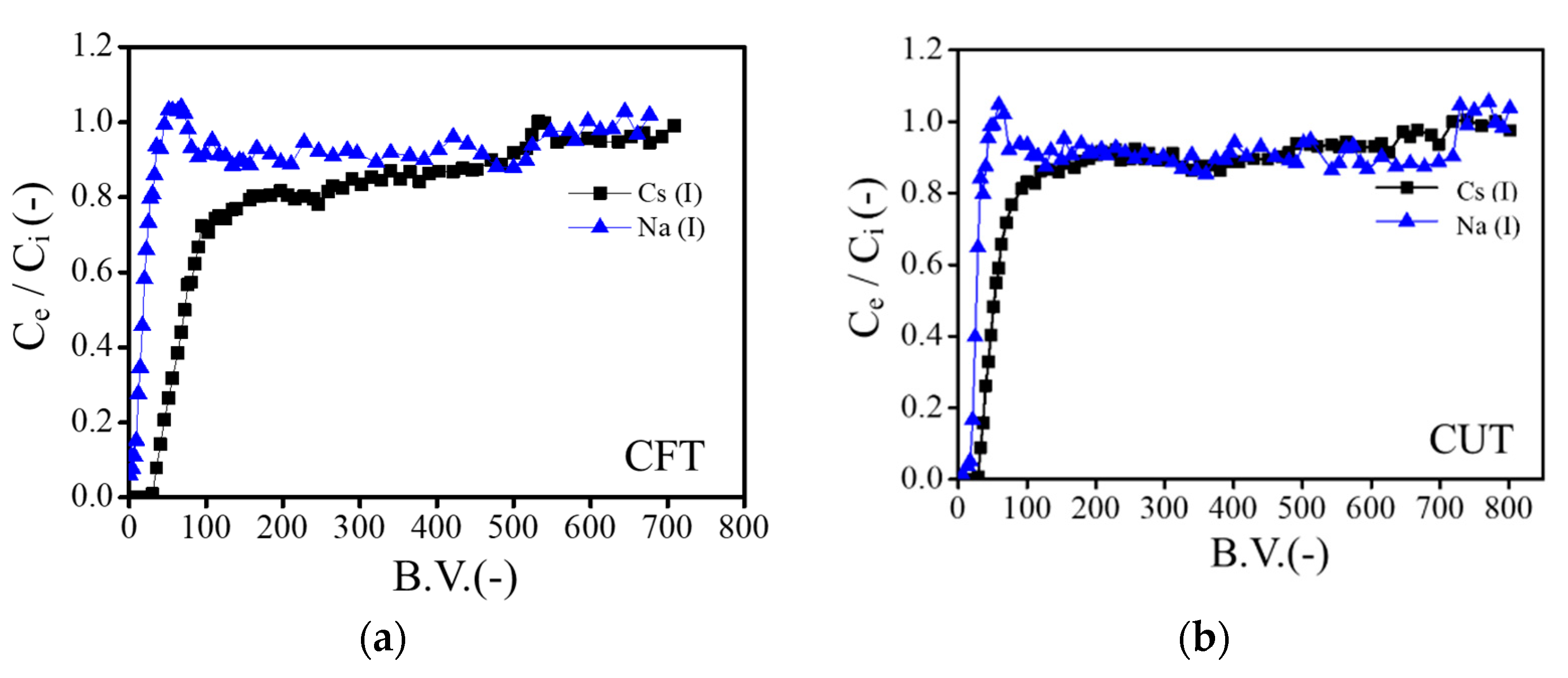
3.5. Chromatography Separation of Cs+ over Na+ Ion Using Crosslinked Tea Leaves
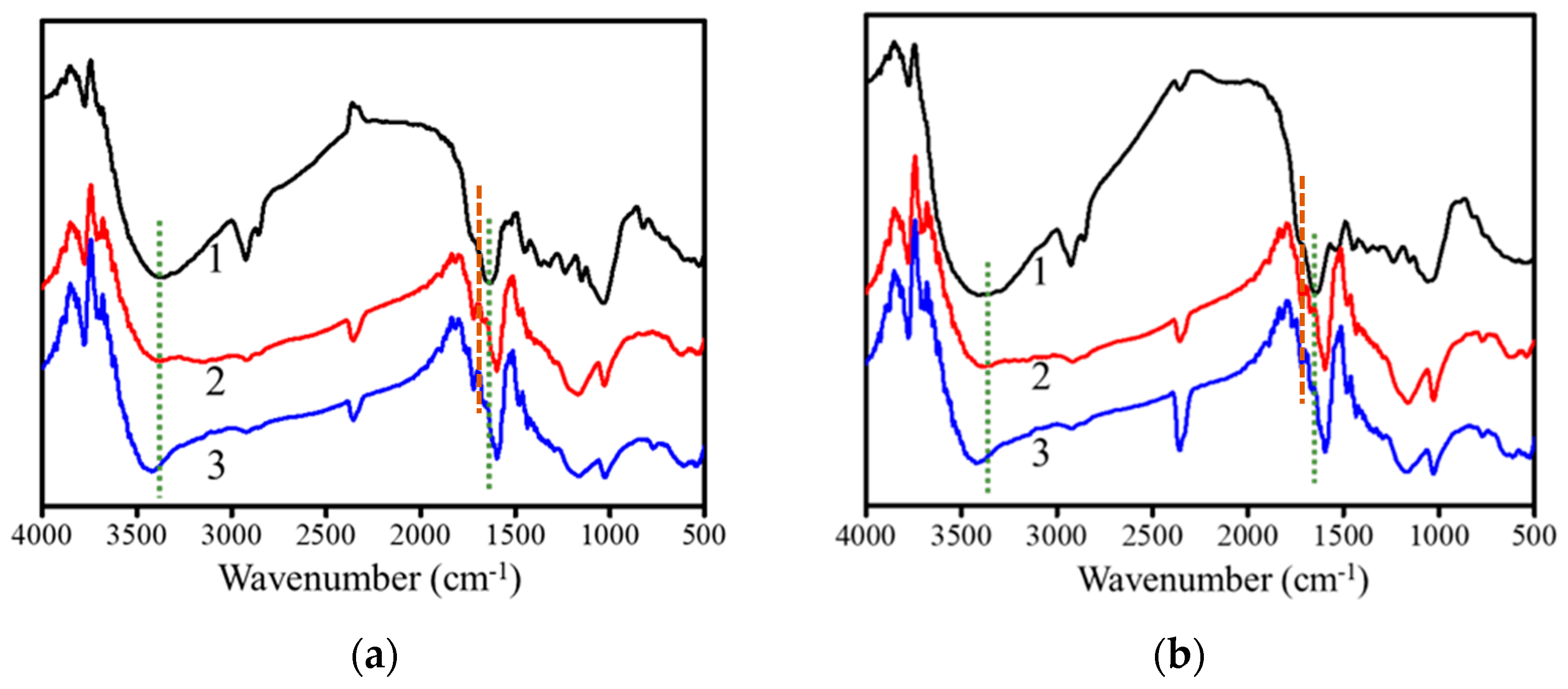
3.6. Fourier-Transfer Infrared Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Peterson, J.; MacDonell, M.; Haroun, L.; Monette, F. Radiological and Chemical Fact Sheets to Support Health Risk Analyses for Contaminated Areas; Argonne National Laboratory Environmental Science Division: Washington, DC, USA, 2007. [Google Scholar]
- Unterweger, M.P.; Fitzgerald, R. Update of NIST half-life results corrected for ionization chamber source-holder instability. Appl. Radiat. Isot. 2014, 87, 92–94. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Wang, J.; Chen, J. Solvent extraction of strontium and cesium: A review of recent progress. Solvent Extr. Ion Exch. 2012, 30, 623–650. [Google Scholar] [CrossRef]
- Shuler, R.G.; Bowers, C.B., Jr.; Smith, J.E., Jr.; van Brunt, V.; Davis, M.W., Jr. The extraction of cesium and strontium from acidic high activity nuclear waste using a purex process compatible organic Extractant. Solvent Extr. Ion Exch. 1985, 3, 567–604. [Google Scholar] [CrossRef]
- Smirnov, I.V.; Stepanova, E.S.; Tyupina, M.Y.; Ivenskaya, N.M.; Zaripov, S.R.; Kleshnina, S.R.; Solov’eva, S.E.; Antipin, I.S. Extraction of cesium and americium with p-alkylcalix[8]arenes from alkaline solutions. Radiochemistry 2016, 58, 329–335. [Google Scholar] [CrossRef]
- Zhang, A.Y.; Chai, Z.F. Adsorption property of cesium onto modified macroporous silica-calix[4]arene-crown based supramolecular recognition materials. Ind. Eng. Chem. Res. 2012, 51, 6196–6204. [Google Scholar] [CrossRef]
- Olatunji, M.A.; Khandaker, M.U.; Mahmud, H.N.M.E. Adsorption kinetics, equilibrium and radiation effect studies of radioactive cesium by polymer-based adsorbent. J. Vinyl Addit. Technol. 2018, 24, 347–357. [Google Scholar] [CrossRef]
- Bostick, B.C.; Vairavamurthy, M.A.; Karthikeyan, K.G.; Chorover, J. Cesium adsorption on clay minerals: An EXAFS spectroscopic investigation. Environ. Sci. Technol. 2002, 36, 2670–2676. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wang, J.L. Removal of Pb2+, Ag+, Cs+ and Sr2+ from aqueous solution by brewery’s waste biomass. J. Hazard. Mater. 2008, 151, 65–70. [Google Scholar] [CrossRef]
- Thirumavalavan, M.; Lai, Y.L.; Lee, J.F. Fourier transform infrared spectroscopic analysis of fruit peels before and after the adsorption of heavy metal ions from aqueous solution. J. Chem. Eng. Data 2011, 56, 2249–2255. [Google Scholar] [CrossRef]
- Hu, X.J.; Wang, J.S.; Liu, Y.G.; Li, X.; Zeng, G.M.; Bao, Z.L.; Zeng, X.X.; Chen, A.W.; Long, F. Adsorption of chromium (VI) by ethylenediamine-modified cross-linked magnetic chitosan resin: Isotherms, kinetics and thermodynamics. J. Hazard. Meter. 2011, 185, 306–314. [Google Scholar] [CrossRef]
- QuYang, X.K.; Jin, R.N.; Yang, L.P.; Wen, Z.S.; Yang, L.Y.; Wang, Y.G.; Wang, C.Y. Partially hydrolyzed bamboo (phyllostachys heterocycla) as a porous bioadsorbent for the removal of Pb (II) from aqueous mixtures. J. Agric. Food Chem. 2014, 62, 6007–6015. [Google Scholar]
- Cabrera, C.; Giménez, R.; López, M.C. Determination of tea components with antioxidant activity. J. Agric. Food Chem. 2003, 51, 4427–4435. [Google Scholar] [CrossRef] [PubMed]
- Reto, M.; Figueira, M.E.; Filipe, H.M.; Almeida, C.M.M. Chemical composition of green tea (Camellia sinensis) infusions commercialized in Portugal. Plant Foods Hum. Nutr. 2007, 62, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Caro, G.; Moreno-Rojas, J.M.; Brindani, N.; Del Rio, D.; Lean, M.E.; Hara, Y.; Crozier, A. Bioavailability of black tea theaflavins: Absorption, metabolism, and colonic catabolism. J. Agric. Food Chem. 2017, 65, 5365–5374. [Google Scholar] [CrossRef]
- Rio, D.D.; Stewart, A.J.; Mullen, W.; Burns, J.; Lean, M.E.J.; Brighenti, F.; Crozier, A. HPLC-MSn analysis of phenolic compounds and purine alkaloids in green and black tea. J. Agric. Food Chem. 2004, 52, 2807–2815. [Google Scholar]
- Fan, S.S.; Wang, Y.; Li, Y.; Tang, J.; Wang, Z.; Tang, J.; Li, X.D.; Hu, K. Facile synthesis of tea waste/Fe3O4 nanoparticle composite for hexavalent chromium removal from aqueous solution. RSC Adv. 2017, 7, 7576–7590. [Google Scholar] [CrossRef]
- Wan, S.L.; Qu, N.; He, F.; Wang, M.K.; Liu, G.B.; He, H. Tea waste-supported hydrated manganese dioxide (HMO) for enhanced removal of typical toxic metal ions from water. RSC Adv. 2015, 5, 88900–88907. [Google Scholar] [CrossRef]
- Taherzadeh, M.J.; Karimi, K. Acid-based hydrolysis processes for ethanol from lignocellulosic materials: A review. Bioresources 2007, 2, 472–499. [Google Scholar]
- Farone, W.A.; Guzens, J.E. Method of Producing Sugars Using Strong Acid Hydrolysis of Cellulosic and Hemicellulosic Materials. U.S. Patent 5,562,777, 13 June 1996. [Google Scholar]
- Mähler, J.; Persson, I. A study of the hydration of the alkali metal ions in aqueous solution. Inorg. Chem. 2012, 51, 425–438. [Google Scholar] [CrossRef]
- Smith, D.W. Ionic hydration enthalpies. J. Chem. Educ. 1977, 54, 540–542. [Google Scholar] [CrossRef]
- Langmiur, I. The constitution and fundamental properties of solids and liquids. II Liquids. J. Am. Chem. Soc. 1917, 39, 1848–1906. [Google Scholar] [CrossRef]
- Manos, M.J.; Kanatzidis, M.G. Highly efficient and rapid Cs+ uptake by the layered metal sulfide K2xMnxSn3-xS6 (KMS-1). J. Am. Chem. Soc. 2009, 131, 6599–6607. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.O.; Lee, S.M.; Jeon, C. Adsorption characteristics of sericite for cesium ions from an aqueous solution. Chem. Eng. Res. Des. 2014, 92, 368–374. [Google Scholar] [CrossRef]
- Lee, J.; Park, S.M.; Jeon, E.K.; Baek, K. Selective and irreversible adsorption mechanism of cesium on illite. Appl. Geochem. 2017, 85, 188–193. [Google Scholar] [CrossRef]
- Hu, B.; Fugetsu, B.; Yu, H.; Abe, Y. Prussian blue caged in spongiform adsorbents using diatomite and carbon nanotubes for elimination of cesium. J. Hazard. Mater. 2012, 217–218, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Rad, R.J.; Ghafourian, H.; Asef, Y.; Dalir, S.T.; Sahafipour, M.H.; Gharanjik, B.M. Biosorption of cesium by native and chemically modified biomass of marine algae: Introduce the new biosorbents for biotechnology applications. J. Hazard. Mater. 2004, B116, 125–134. [Google Scholar]
- Dahiya, S.; Tripathi, R.M.; Hegde, A.G. Biosorption of heavy metals and radionuclide from aqueous solutions by pre-treated arca shell biomass. J. Hazard. Mater. 2008, 150, 376–386. [Google Scholar] [CrossRef]
- Inczedy, J. Analytical Application of Ions Exchangers; Pergamon Press: New York, NY, USA, 1966; pp. 348–352. [Google Scholar]
- Hayashita, T.; Bartsch, R.A. Competitive sorption of alkali-metal and alkaline-earth-metal cations by carboxylic acid resins containing acyclic or cyclic polyether units. Anal. Chem. 1991, 63, 1847–1850. [Google Scholar] [CrossRef]
- Subramonian, S.; Clifford, D. Monovalent/divalent selectivity and the charge separation concept. React. Polym. 1988, 9, 195–209. [Google Scholar] [CrossRef]
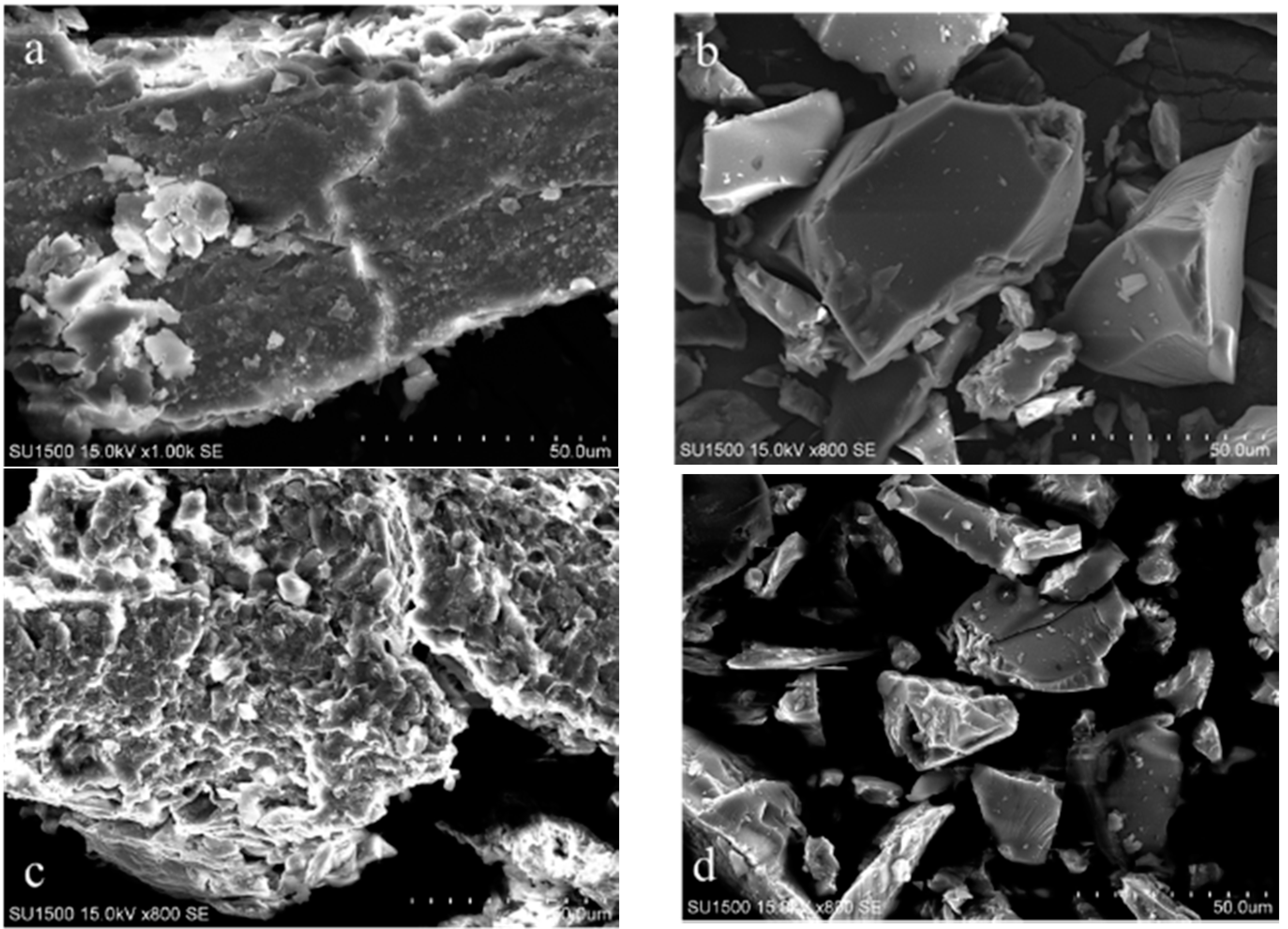




| Adsorbent | Cs+ | K+ | Na+ | |||
|---|---|---|---|---|---|---|
| Slope | R2 | Slope | R2 | Slope | R2 | |
| CFT | 0.822 | 0.9863 | 0.858 | 0.9953 | 0.831 | 0.9919 |
| CUT | 0.997 | 0.9795 | 0.861 | 0.9860 | 0.780 | 0.9510 |
| CUT Adsorbent | CFT Adsorbent | |||||
|---|---|---|---|---|---|---|
| Qm (mmol g−1) | B (dm3 mmol−1) | R2 | Qm (mmol g−1) | B (dm3 mmol−1) | R2 | |
| Na+ | 2.40 | 0.6431 | 0.999 | 2.25 | 0.3182 | 0.999 |
| Cs+ | 2.48 | 0.2802 | 0.997 | 2.56 | 0.9950 | 0.996 |
| Adsorbent | Adsorption Capacity (mmol g−1) | pH | Temperature(K) | Reference |
|---|---|---|---|---|
| Layered metal sulfide (KMS−1) | 1.70 | ≈7.0 (pHe) | 298 | [24] |
| Sericite | 0.050 | 5.0 (pHi) | 298 | [25] |
| RIP of clay minerals | 0.250 | 7.0 (pHi) | * | [26] |
| Prussian blue | 1.254 | Water | 298 | [27] |
| Biomass of marine algae | 0.248 | 5.5 (pHi) | 303 | [28] |
| Arca shell biomass | 0.036 | 5.5 (pHi) | 298 | [29] |
| CUT | 2.48 | 7.0 ± 1.0 (pHe) | 303 | Present work |
| CFT | 2.56 | 7.0 ± 1.0 (pHe) | 303 | Present work |
| CUT Adsorbent | CFT Adsorbent | |||||
|---|---|---|---|---|---|---|
| Adsorbed (mmol g−1) | Eluted (mmol g−1) | % Recovery | Adsorbed (mmol g−1) | Eluted (mmol g−1) | % Recovery | |
| Na+ | 0.491 | 0.484 | 98.57 | 0.528 | 0.497 | 94.13 |
| Cs+ | 0.804 | 0.716 | 89.05 | 0.992 | 0.951 | 95.78 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, D.; Morisada, S.; Kawakita, H.; Ohto, K.; Inoue, K.; Song, X.; Zhang, G. Selective Cesium Adsorptive Removal on Using Crosslinked Tea Leaves. Processes 2019, 7, 412. https://doi.org/10.3390/pr7070412
Yu D, Morisada S, Kawakita H, Ohto K, Inoue K, Song X, Zhang G. Selective Cesium Adsorptive Removal on Using Crosslinked Tea Leaves. Processes. 2019; 7(7):412. https://doi.org/10.3390/pr7070412
Chicago/Turabian StyleYu, Dan, Shintaro Morisada, Hidetaka Kawakita, Keisuke Ohto, Katsutoshi Inoue, Ximing Song, and Guolin Zhang. 2019. "Selective Cesium Adsorptive Removal on Using Crosslinked Tea Leaves" Processes 7, no. 7: 412. https://doi.org/10.3390/pr7070412
APA StyleYu, D., Morisada, S., Kawakita, H., Ohto, K., Inoue, K., Song, X., & Zhang, G. (2019). Selective Cesium Adsorptive Removal on Using Crosslinked Tea Leaves. Processes, 7(7), 412. https://doi.org/10.3390/pr7070412







