Improvement of 1,3-Butadiene Separation in 2,3-Butanediol Dehydration Using Extractive Distillation
Abstract
:1. Introduction
- -
- Provides steady-state simulation results for conventional and extractive distillation processes for the recovery of 1,3-BD from bio-fermentation products.
- -
- Calculates an advantageous price of 1,3-BD for the selection of the extractive distillation.
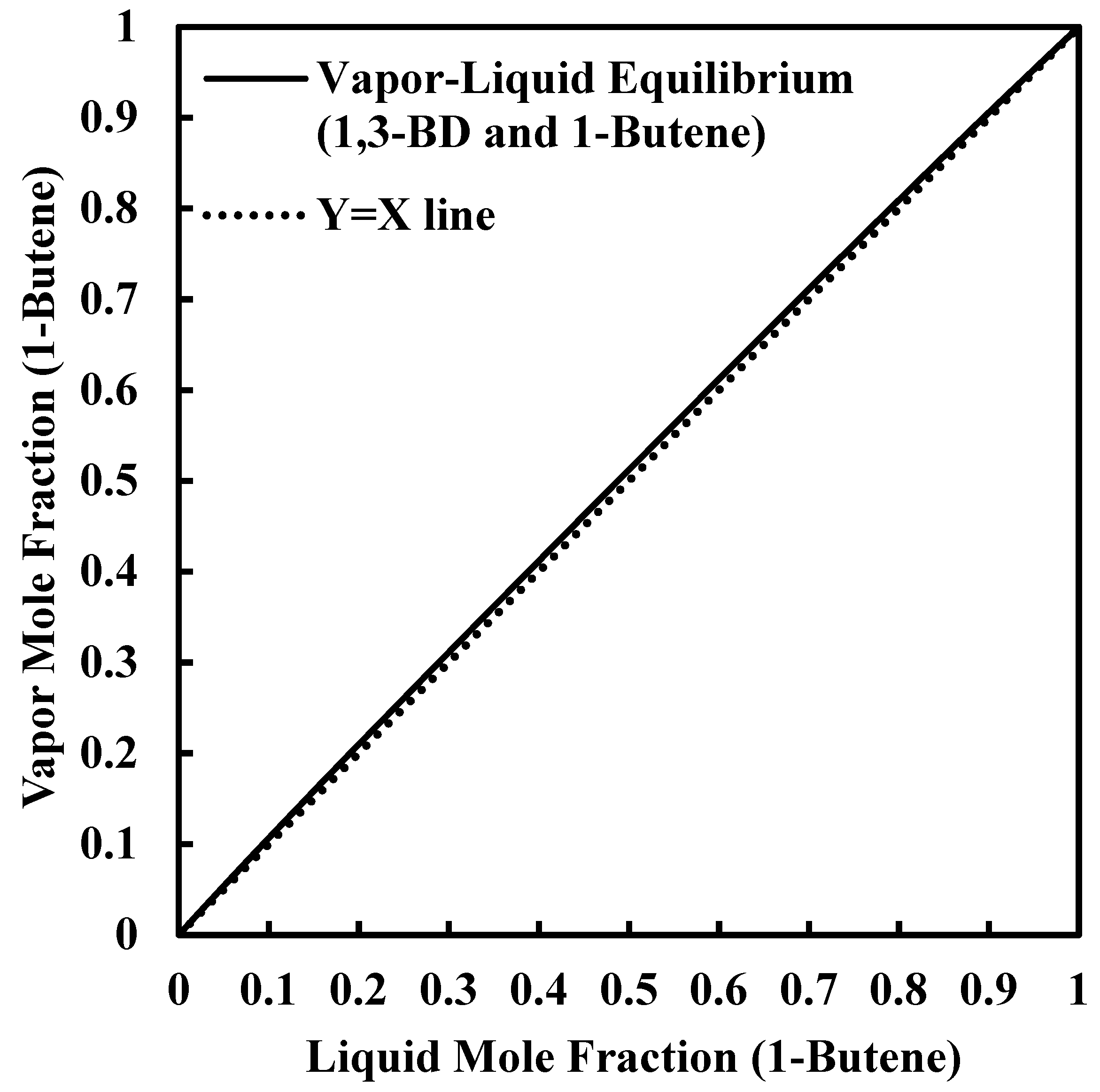
2. Theoretical Background
The 2,3-Butanediol (2,3-BDO) Dehydration Process
3. Process Design
3.1. The Thermodynamic Property Model
3.2. The Conventional Distillation Process
3.3. The Extractive Distillation Process
4. Results and Discussion
4.1. Column Specifications and Performance
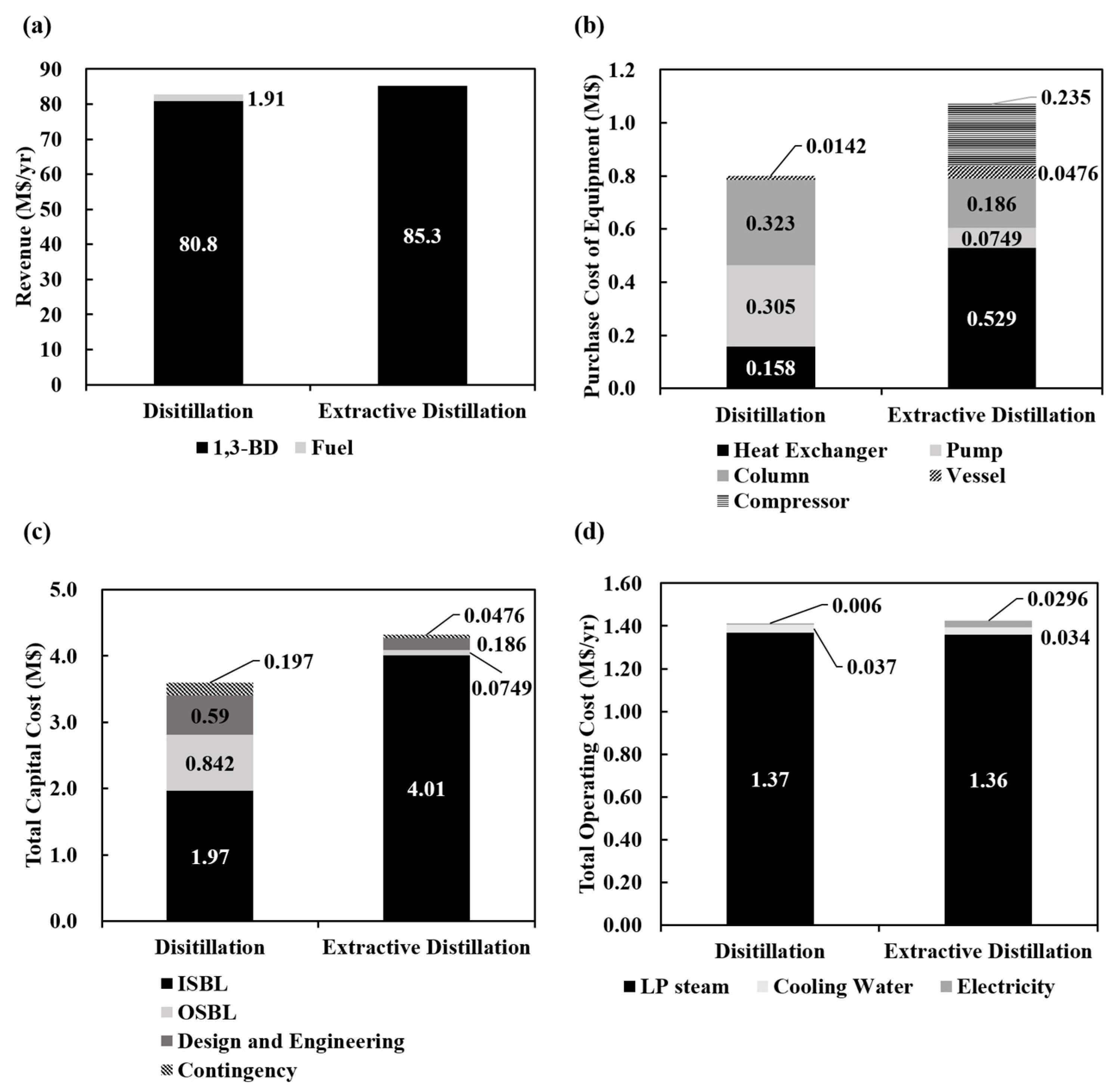
4.2. Economic Assessment
- (1)
- The plant is located on the west coast of the USA.
- (2)
- The plant operates for 8000 h annually.
- (3)
- The plant is constructed using stainless steel (SS304 or SS316) because 1,3-BD is corrosive to carbon steel.
4.3. Economic Feasibility with a Changing 1,3-BD Price
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| 1,3-BD | 1,3-Butadiene |
| 2,3-BDO | 2,3-Butanediol |
| DMF | Dimethylformamide |
| ED-BD | 1,3-BD product stream of the extractive distillation process |
| ISBL | Inside battery limits |
| NMP | N-methyl-2-pyrrolidone |
| NRTL | Non-random two-liquid model |
| NRTL-RK | Non-random two-liquid Redlich–Kwong model |
| OSBL | Outside battery limits |
| D-BD | 1,3-BD product stream of the distillation process |
| UNIFAC | UNIQUAC functional group activity coefficient |
| UNIQUAC | Universal quasi-chemical model |
| VLE | Vapor-liquid equilibrium |
References
- White, W.C. Butadiene production process overview. Chem. Biol. Interact. 2007, 166, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Celińska, E.; Grajek, W. Biotechnological production of 2,3-butanediol-Current state and prospects. Biotechnol. Adv. 2009, 27, 715–725. [Google Scholar] [CrossRef] [PubMed]
- Köpke, M.; Mihalcea, C.; Liew, F.; Tizard, J.H.; Ali, M.S.; Conolly, J.J.; Al-Sinawi, B.; Simpson, S.D. 2,3-Butanediol Production by Acetogenic Bacteria, an Alternative Route to Chemical Synthesis, Using Industrial Waste Gas. Appl. Environ. Microbiol. 2011, 77, 5467–5475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duan, H.; Yamada, Y.; Sato, S. Efficient production of 1,3-butadiene in the catalytic dehydration of 2,3-butanediol. Appl. Catal. A Gen. 2015, 491, 163–169. [Google Scholar] [CrossRef]
- Nguyen, N.T.T.; Matei-Rutkovska, F.; Huchede, M.; Jaillardon, K.; Qingyi, G.; Michel, C.; Millet, J.M.M. Production of 1,3-butadiene in one step catalytic dehydration of 2,3-butanediol. Catal. Today 2019, 323, 62–68. [Google Scholar] [CrossRef]
- Kim, W.; Shin, W.; Lee, K.J.; Cho, Y.S.; Kim, H.S.; Filimonov, I.N. 2,3-Butanediol dehydration catalyzed by silica-supported alkali phosphates. Appl. Catal. A Gen. 2019, 570, 148–163. [Google Scholar] [CrossRef]
- Song, D. Modeling of a Pilot-Scale Fixed-Bed Reactor for Dehydration of 2,3-Butanediol to 1,3-Butadiene and Methyl Ethyl Ketone. Catalysts 2018, 8, 72. [Google Scholar] [CrossRef]
- Haider, J.; Qyyum, M.A.; Hussain, A.; Yasin, M.; Lee, M. Techno-economic analysis of various process schemes for the production of fuel grade 2,3-butanediol from fermentation broth. Biochem. Eng. J. 2018, 140, 93–107. [Google Scholar] [CrossRef]
- Hong, J.; Van Duc Long, N.; Harvianto, G.R.; Haider, J.; Lee, M. Design and optimization of multi-effect-evaporation-assisted distillation configuration for recovery of 2,3-butanediol from fermentation broth. Chem. Eng. Process. Process Intensif. 2019, 136, 107–115. [Google Scholar] [CrossRef]
- Penner, D.; Redepenning, C.; Mitsos, A.; Viell, J. Conceptual Design of Methyl Ethyl Ketone Production via 2,3-Butanediol for Fuels and Chemicals. Ind. Eng. Chem. Res. 2017, 56, 3947–3957. [Google Scholar] [CrossRef]
- Song, D.; Yoon, Y.G.; Lee, C.J. Conceptual design for the recovery of 1,3-Butadiene and methyl ethyl ketone via a 2,3-Butanediol-dehydration process. Chem. Eng. Res. Des. 2017, 123, 268–276. [Google Scholar] [CrossRef]
- Harvianto, G.R.; Haider, J.; Hong, J.; Van Duc Long, N.; Shim, J.J.; Cho, M.H.; Kim, W.K.; Lee, M. Purification of 2,3-butanediol from fermentation broth: Process development and techno-economic analysis. Biotechnol. Biofuels 2018, 11, 18. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yin, X.; Ouyang, P. Simulation of 1,3-Butadiene Production Process by Dimethylfomamide Extractive Distillation. Chin. J. Chem. Eng. 2009, 17, 27–35. [Google Scholar] [CrossRef]
- Farzad, S.; Mandegari, M.A.; Görgens, J.F. Integrated techno-economic and environmental analysis of butadiene production from biomass. Bioresour. Technol. 2017, 239, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Yoon, Y.G.; Lee, C.J. Techno-economic evaluation of the 2,3-butanediol dehydration process using a hydroxyapatite-alumina catalyst. Korean J. Chem. Eng. 2018, 35, 2348–2354. [Google Scholar] [CrossRef]
- Carlson, E.C. Don’t gamble with physical properties for simulations. Chem. Eng. Prog. 1996, 92, 35–46. [Google Scholar]
- Kim, Y.H.; Kim, S.Y.; Lee, B. Simulation of 1,3-butadiene extractive distillation process using N-methyl-2-pyrrolidone solvent. Korean J. Chem. Eng. 2012, 29, 1493–1499. [Google Scholar] [CrossRef]
- Henglein, F.A. Chemical Technology, 1st ed.; Pergamon Press: Oxford, UK, 1969; ISBN 978-14-8316-025-2. [Google Scholar]
- Sinnott, R.K.; Towler, G. Chemical Engineering Design, 2nd ed.; Butterworth-Heinemann: Oxford, UK, 2013; ISBN 9780080966595. [Google Scholar]
- Douglas, J.M. Conceptual Design of Chemical Processes; McGraw-Hill Science: New York, NY, USA, 1988; ISBN 0-07-017762-7. [Google Scholar]
- Lim, Y.; Lee, C.J.; Jeong, Y.S.; Song, I.H.; Lee, C.J.; Han, C. Optimal design and decision for combined steam reforming process with dry methane reforming to reuse CO2 as a raw material. Ind. Eng. Chem. Res. 2012, 51, 4982–4989. [Google Scholar] [CrossRef]
- Hyde, B.; Koster, R.; Chen, W.; Song, A.; Maselli, J.; Salamanca, S. Global C4 Olefins & Elastomers: Appendix; IHS Markit: London, UK, 2017. [Google Scholar]


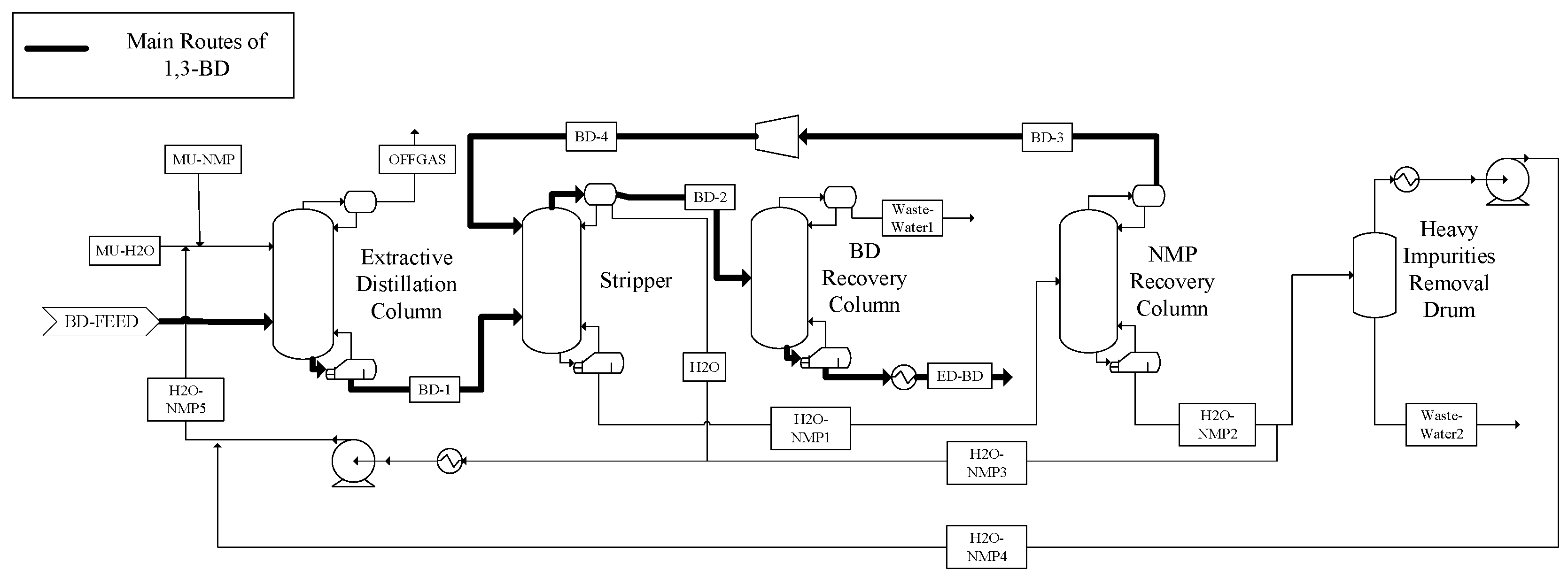



| Conventional Distillation Process | Extractive Distillation Process | |||||
|---|---|---|---|---|---|---|
| Column | Name | Number of stages | Heat duty (MW) | Name | Number of stages | Heat duty (MW) |
| 1st distillation column | 80 | 2.51 | Extractive distillation column | 36 | 1.43 | |
| 2nd distillation column | 56 | 1.08 | Stripper | 15 | 1.05 | |
| - | - | - | NMP recovery column | 5 | 0.675 | |
| - | - | - | BD recovery column | 15 | 0.0165 | |
| Compressor | None | Electricity [kW] | 51.3 | |||
| Stream | BD-FEED | D-BD [11] | ED-BD | |
|---|---|---|---|---|
| Property | ||||
| Mass flow rate (kg/h) | 3628 | 3367 | 32 | |
| Mass fraction | ||||
| 1,3-BD | 0.977 | 0.99 | 0.993 | |
| 1,3-BD recovery rate (%) | 94 | 99 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, D.; Yoon, Y.-G.; Seo, S.-K.; Lee, C.-J. Improvement of 1,3-Butadiene Separation in 2,3-Butanediol Dehydration Using Extractive Distillation. Processes 2019, 7, 410. https://doi.org/10.3390/pr7070410
Song D, Yoon Y-G, Seo S-K, Lee C-J. Improvement of 1,3-Butadiene Separation in 2,3-Butanediol Dehydration Using Extractive Distillation. Processes. 2019; 7(7):410. https://doi.org/10.3390/pr7070410
Chicago/Turabian StyleSong, Daesung, Young-Gak Yoon, Seung-Kwon Seo, and Chul-Jin Lee. 2019. "Improvement of 1,3-Butadiene Separation in 2,3-Butanediol Dehydration Using Extractive Distillation" Processes 7, no. 7: 410. https://doi.org/10.3390/pr7070410





