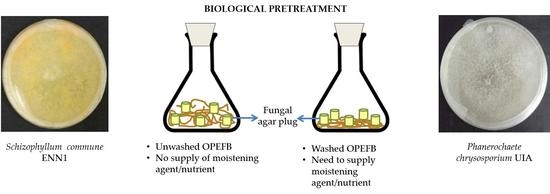
Biological Pretreatment of Oil Palm Empty Fruit Bunch by Schizophyllum commune ENN1 without Washing and Nutrient Addition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Oil Palm Empty Fruit Bunch
2.2. Microorganisms
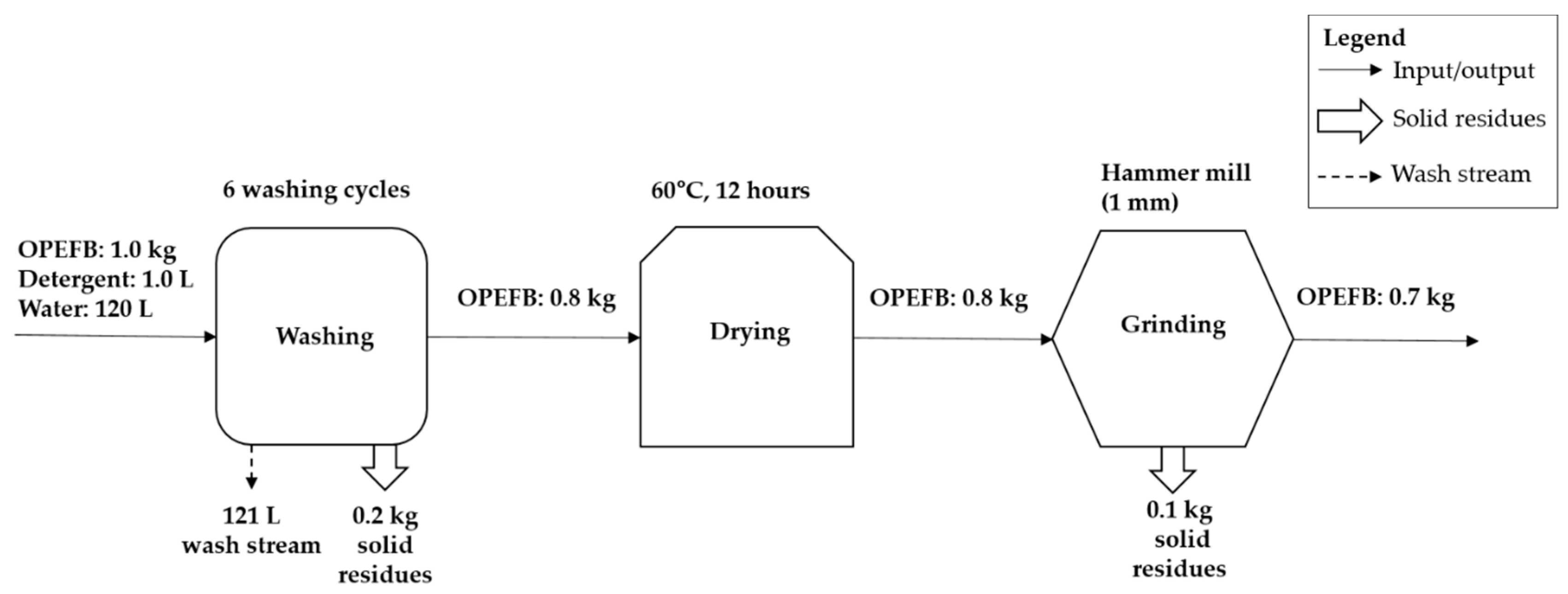
2.3. Mass Balance of OPEFB Preparation Process
2.4. Biological Pretreatment
2.5. Analytical Methods
2.6. Enzymatic Saccharification of OPEFB
3. Results and Discussion
3.1. OPEFB Preparation Process
3.2. Composition of Pretreated OPEFB
3.3. Moisture and Oil Residue Contents of Pretreated OPEFB
3.4. Enzymatic Saccharification
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Malaysian Palm Oil Board. Overview of the Malaysian oil palm industry 2017. Malays. Palm Oil Board MPOB 2018, 1, 1–6. [Google Scholar]
- Begum, S.; Kumaran, P.; Jayakumar, M. Use of oil palm waste as a renewable energy source and its impact on reduction of air pollution in context of Malaysia. IOP Conf. Ser. Earth Env. Sci. 2013, 16, 012026. [Google Scholar] [CrossRef]
- Law, K.-N.; Daud, W.R.W.; Ghazali, A. Morphological and chemical nature of fiber strands of oil palm empty-fruit-bunch (OPEFB). BioResources 2007, 2, 351–362. [Google Scholar]
- Simarani, K.; Hassan, M.A.; Abd-Aziz, S.; Wakisaka, M.; Shirai, Y. Effect of palm oil mill sterilization process on the physicochemical characteristics and enzymatic hydrolysis of empty fruit bunch. J. Am. Med. Assoc. 2009, 1, 57–66. [Google Scholar] [CrossRef]
- Abdullah, N.; Sulaiman, F. The oil palm wastes in Malaysia. In Biomass Now—Sustainable Growth and Use; Intech Open: London, UK, 2013; pp. 75–100. ISBN 978-953-51-1105-4. [Google Scholar]
- Mathew, G.M.; Sukumaran, R.K.; Singhania, R.R.; Pandey, A. Progress in research on fungal cellulases for lignocellulose degradation. J. Sci. Ind. Res. (India) 2008, 67, 898–907. [Google Scholar]
- Ishola, M.; Millati, R.; Syamsiah, S.; Cahyanto, M.; Niklasson, C.; Taherzadeh, M. Structural changes of oil palm empty fruit bunch (OPEFB) after fungal and phosphoric acid pretreatment. Molecules 2012, 17, 14995–15012. [Google Scholar]
- Asgher, M.; Wahab, A.; Bilal, M.; Nasir Iqbal, H.M. Lignocellulose degradation and production of lignin modifying enzymes by Schizophyllum commune IBL-06 in solid-state fermentation. Biocatal. Agric. Biotechnol. 2016, 6, 195–201. [Google Scholar] [CrossRef]
- Afzanizam, N.; Nazri, M.; Jaafar, M.; Tung, C.; Jo-han, N. A review of palm oil biomass as a feedstock for syngas fuel technology. J. Teknol. Sci. Eng. 2015, 5, 13–18. [Google Scholar]
- NCIM. National collection of industrial micro-organisms. NCIM Cat. Strains 2014, 4, 1–14. [Google Scholar]
- Hamisan, A.F.; Abd-Aziz, S.; Kamaruddin, K.; Shah, U.K.M.; Shahab, N.; Hassan, M.A. Delignification of oil palm empty fruit bunch using chemical and microbial pretreatment methods. Int. J. Agric. Res. 2009, 4, 1–7. [Google Scholar] [CrossRef]
- Iwamoto, S.; Abe, K.; Yano, H. The effect of hemicelluloses on wood pulp nanofibrillation and nanofiber network characteristics. Biomacromolecules 2008, 9, 1022–1026. [Google Scholar] [CrossRef] [PubMed]
- Yunos, N.S.H.M.; Baharuddin, A.S.; Yunos, K.F.M.; Hafid, H.S.; Busu, Z.; Mokhtar, M.N.; Sulaiman, A.; Som, A.M. The physicochemical characteristics of residual oil and fibers from oil palm empty fruit bunches. BioResources 2015, 10, 14–29. [Google Scholar]
- Sluiter, A.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of extractives in biomass. Natl. Renew. Energy Lab. 2008, 1, 1–9. [Google Scholar]
- Xu, F.; Sun, J.X.; Geng, Z.C.; Liu, C.F.; Ren, J.L.; Sun, R.C.; Fowler, P.; Baird, M.S. Comparative study of water-soluble and alkali-soluble hemicelluloses from perennial ryegrass leaves (Lolium peree). Carbohydr. Polym. 2007, 67, 56–65. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Akanksha, K.; Sukumaran, R.K.; Pandey, A.; Rao, S.S.; Binod, P. Material balance studies for the conversion of sorghum stover to bioethanol. Biomass Bioenergy 2016, 85, 48–52. [Google Scholar] [CrossRef]
- Zahari, M.A.K.M.; Ariffin, H.; Mokhtar, M.N.; Salihon, J.; Shirai, Y.; Hassan, M.A. Case study for a palm biomass biorefinery utilizing renewable non-food sugars from oil palm frond for the production of poly(3-hydroxybutyrate) bioplastic. J. Clean. Prod. 2015, 87, 284–290. [Google Scholar] [CrossRef]
- Tan, J.P.; Jahim, J.M.; Harun, S.; Wu, T.Y.; Mumtaz, T. Utilization of oil palm fronds as a sustainable carbon source in biorefineries. Int. J. Hydrog. Energy 2016, 41, 4896–4906. [Google Scholar] [CrossRef]
- Abdul, P.M.; Jahim, J.M.; Harun, S.; Markom, M.; Lutpi, N.A.; Hassan, O.; Balan, V.; Dale, B.E.; Mohd Nor, M.T. Effects of changes in chemical and structural characteristic of ammonia fibre expansion (AFEX) pretreated oil palm empty fruit bunch fibre on enzymatic saccharification and fermentability for biohydrogen. Bioresour. Technol. 2016, 211, 200–208. [Google Scholar] [CrossRef]
- Kumar, N.S.; Min, K. Phenolic compounds biosorption onto Schizophyllum commune fungus: FTIR analysis, kinetics and adsorption isotherms modeling. Chem. Eng. J. 2011, 168, 562–571. [Google Scholar] [CrossRef]
- Meehnian, H.; Jana, A.K.; Jana, M.M. Effect of particle size, moisture content, and supplements on selective pretreatment of cotton stalks by Daedalea flavida and enzymatic saccharification. 3 Biotech. 2016, 6, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kam, Y.C.; Hii, S.L.; Sim, C.Y.Y.; Ong, L.G.A. Schizophyllum commune lipase production on pretreated sugarcane bagasse and its effectiveness. Int. J. Polym. Sci. 2016, 2016, 6. [Google Scholar] [CrossRef]
- Hermiati, E.; Anita, S.H.; Risanto, L.; Styarini, D.; Sudiyani, Y.; Hanafi, A.; Abimanyu, H. Biological pretreatment of oil palm frond fiber using white-rot fungi for enzymatic saccharification. Makara Seri. Teknol. 2013, 17, 39–43. [Google Scholar]
- Saha, B.C.; Qureshi, N.; Kennedy, G.J.; Cotta, M.A. Biological pretreatment of corn stover with white-rot fungus for improved enzymatic hydrolysis. Int. Biodeterior. Biodegrad. 2016, 109, 29–35. [Google Scholar] [CrossRef] [Green Version]
- Baharuddin, A.S.; Asma, N.; Razak, A.; Aini, N.; Rahman, A. Bioconversion of oil palm empty fruit bunch by Aspergillus niger EB4 under solid-state fermentation. Pertanika J. Trop. Agric. Sci. 2009, 32, 143–151. [Google Scholar]


| OPEFB Samples | Composition 1 (%) | |||||
|---|---|---|---|---|---|---|
| Lignin | Hemicellulose | Cellulose | Extractives | Ash | ||
| Water | Solvent | |||||
| Untreated OPEFB | 21.0 ± 0.1 c | 39.0 ± 0.4 b | 32.8 ± 0.5 d | 2.3 ± 0.4 a | 5.9 ± 0.4 b | 1.16 ± 0.2 b |
| Schizophyllum commune ENN1 (this study) | 9.7 ± 1.0 b | 43.8 ± 4.6 b | 38.4 ± 4.4 c | 15. 3 ± 1.1 c | 5.7 ± 0.4 b | 2.57 ± 0.4 c |
| Phanerochaete chrysosporium2 | 12.9 ± 2.5 b | 37.0 ± 0.5 b | 44.2 ± 0.0 b | 7.5 ± 0.2 b | 3.7 ± 0.1 b | 1.45 ± 0.3 b |
| Time (hour) | Untreated | Biological Pretreatment | |||||
|---|---|---|---|---|---|---|---|
| S. commune ENN1 | P. chrysosporium (control) 2 | ||||||
| Sugar Yield 1 (mg/g) | Hydrolysis Yield (%) | Sugar Yield 1 (mg/g) | Hydrolysis Yield (%) | Sugar Yield 1 (mg/g) | Hydrolysis Yield (%) | ||
| 24 | 96.4 ± 0.05 b | 26.5 | 151.9 ± 0.16 a | 35.6 | 78.6 ± 0.08 c | 15.8 | |
| 48 | 106.8 ± 0.08 b | 29.3 | 187.1 ± 0.04 a | 43.9 | 45.0 ± 0.13 c | 9.4 | |
| 72 | 123.8 ± 0.06 b | 34.0 | 200.4 ± 0.00 a | 47.0 | 58.7 ± 0.02 c | 11.8 | |
| 96 | 128.2 ± 0.00 b | 35.2 | 230.4 ± 0.19 a | 54.0 | 101.2 ±0.04 c | 20.3 | |
| 120 | 147.2 ± 0.04 b | 40.4 | 224.2 ± 0.05 a | 52.5 | 102.4 ± 0.06 c | 20.6 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arbaain, E.N.N.; Bahrin, E.K.; Ibrahim, M.F.; Ando, Y.; Abd-Aziz, S. Biological Pretreatment of Oil Palm Empty Fruit Bunch by Schizophyllum commune ENN1 without Washing and Nutrient Addition. Processes 2019, 7, 402. https://doi.org/10.3390/pr7070402
Arbaain ENN, Bahrin EK, Ibrahim MF, Ando Y, Abd-Aziz S. Biological Pretreatment of Oil Palm Empty Fruit Bunch by Schizophyllum commune ENN1 without Washing and Nutrient Addition. Processes. 2019; 7(7):402. https://doi.org/10.3390/pr7070402
Chicago/Turabian StyleArbaain, Enis Natasha Noor, Ezyana Kamal Bahrin, Mohamad Faizal Ibrahim, Yoshito Ando, and Suraini Abd-Aziz. 2019. "Biological Pretreatment of Oil Palm Empty Fruit Bunch by Schizophyllum commune ENN1 without Washing and Nutrient Addition" Processes 7, no. 7: 402. https://doi.org/10.3390/pr7070402