The Impact of Erythrocytes Injury on Blood Flow in Bionic Arteriole with Stenosis Segment
Abstract
1. Introduction
2. Materials and Methods
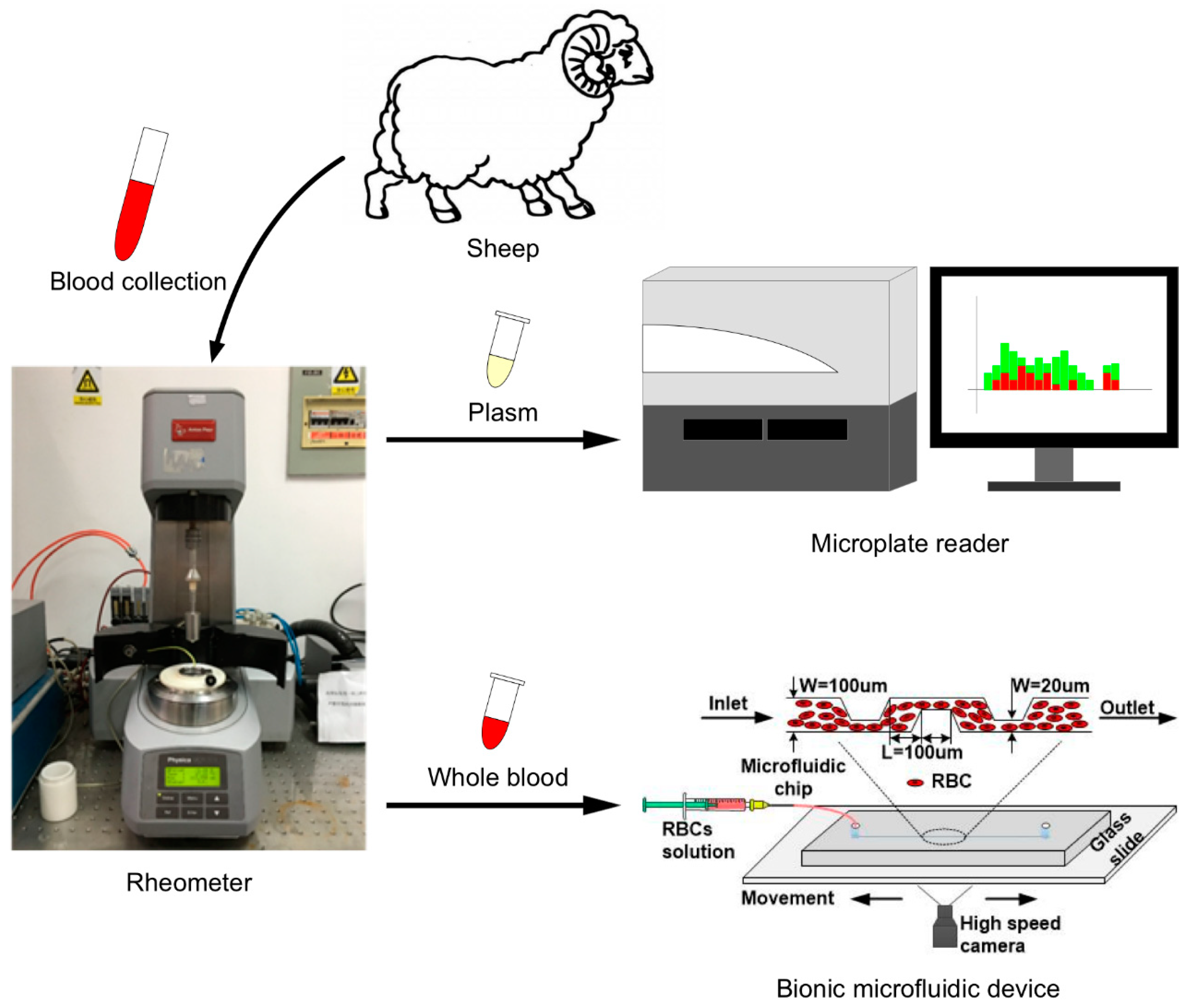
2.1. Blood Collection
2.2. Hemolysis Evaluation under Fixed Shear Stress
2.3. Bionic Microfluidic Device Design and Visualization of Injected Erythrocyte Trajectories
2.4. Statistical Analysis
3. Results
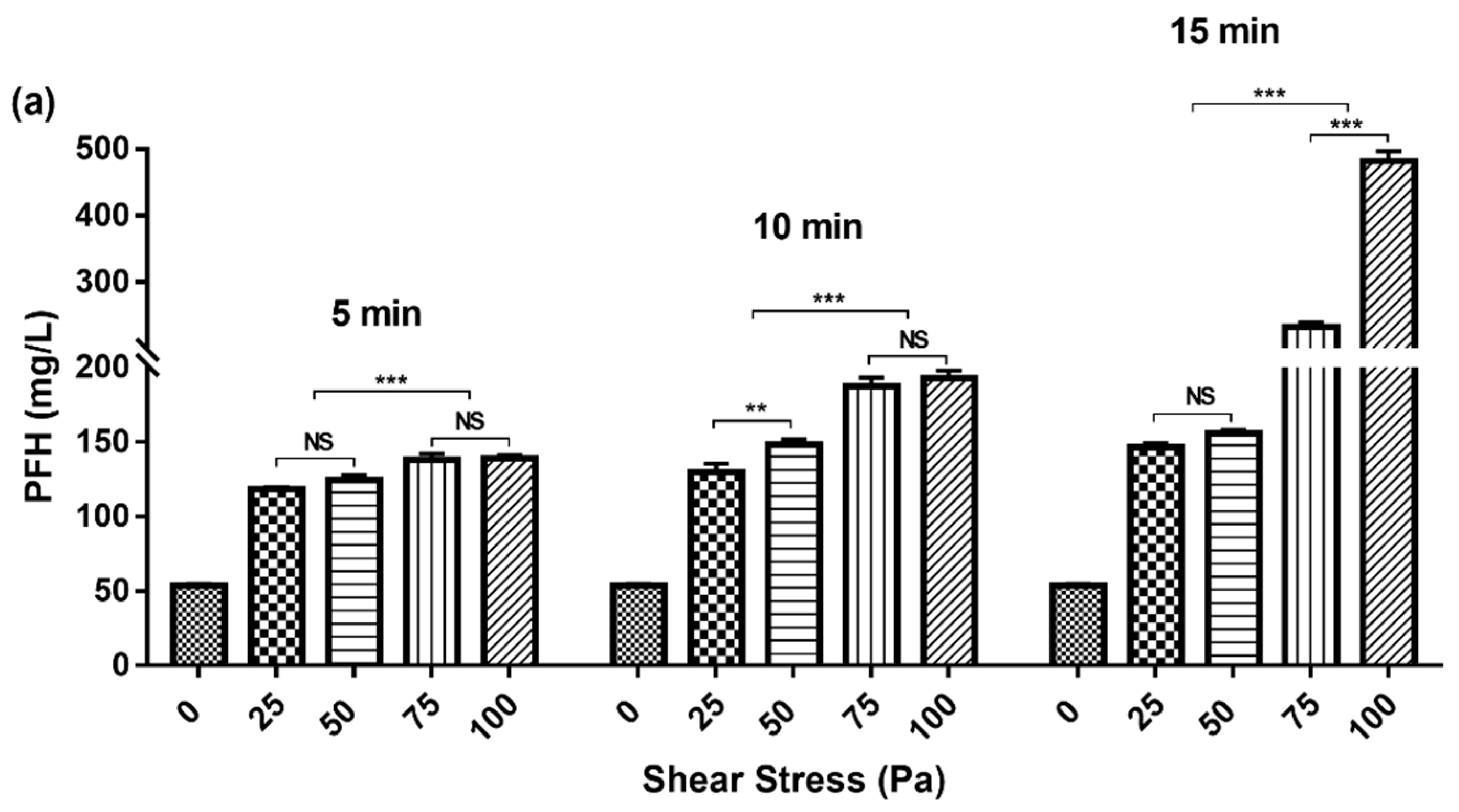
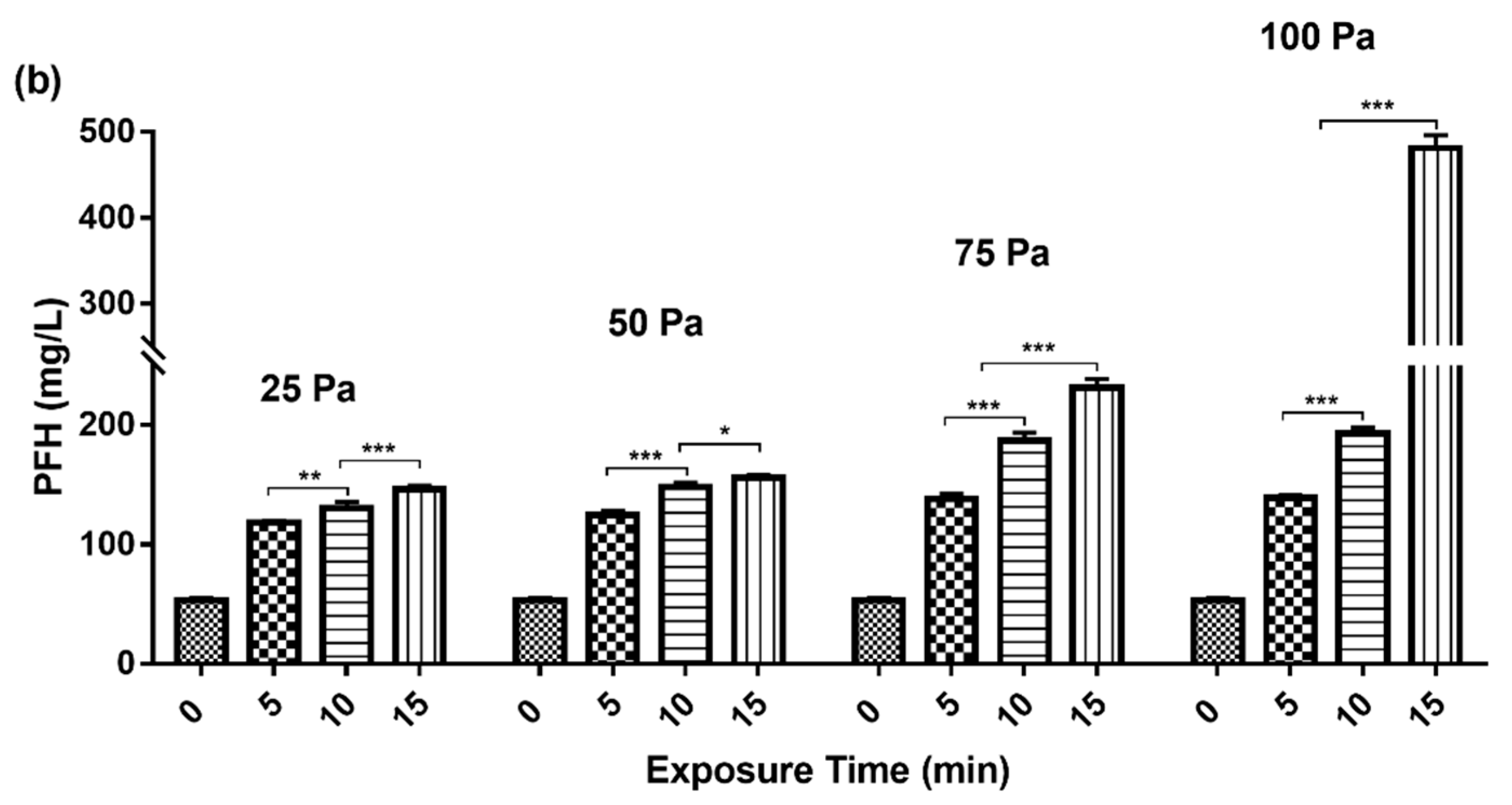
3.1. Variation of PFH Concentration with Gradient Change of Shear Stress and Exposure Time
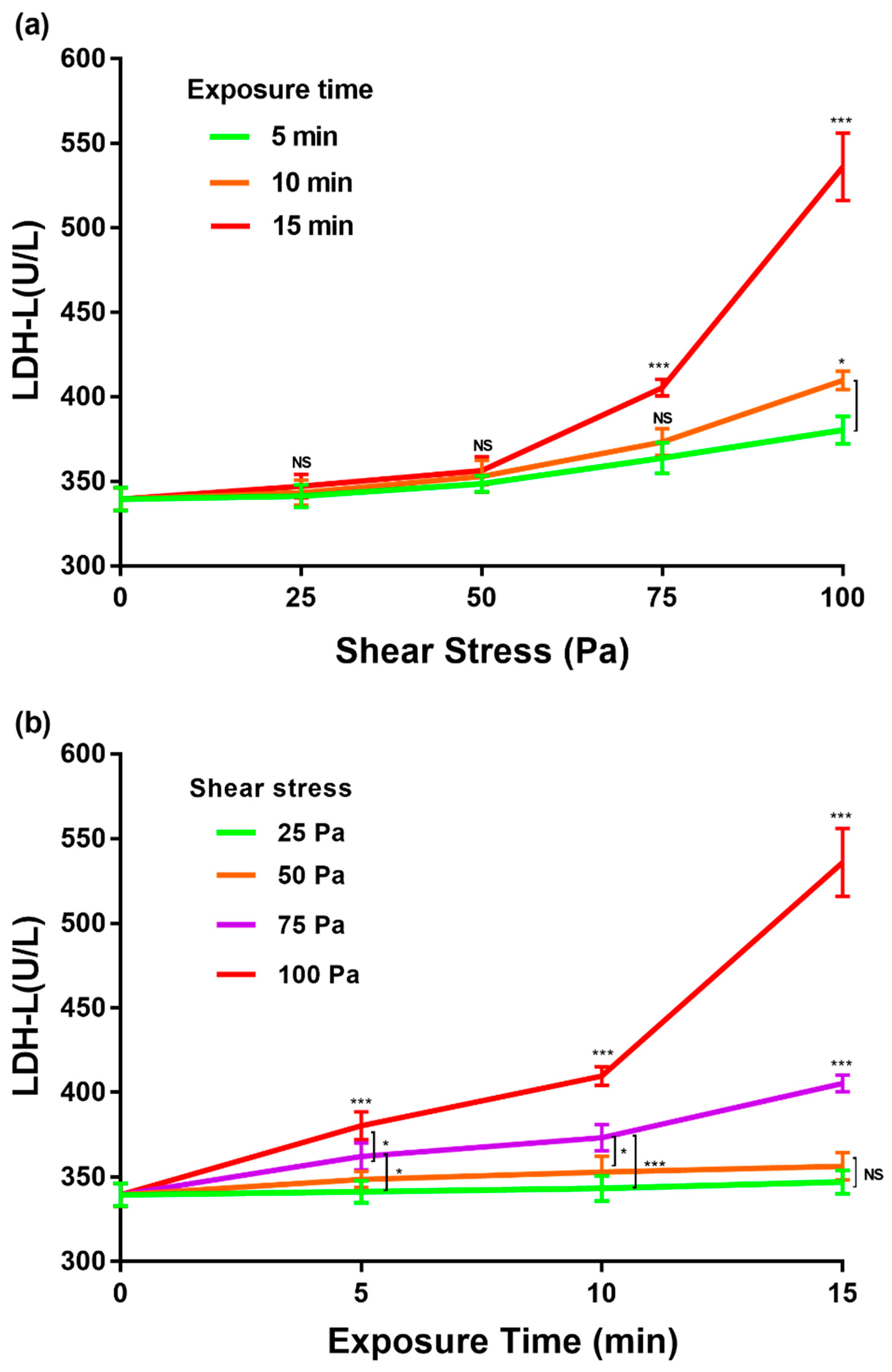
3.2. LDH Release with Gradient Change of Shear Stress and Exposure Time
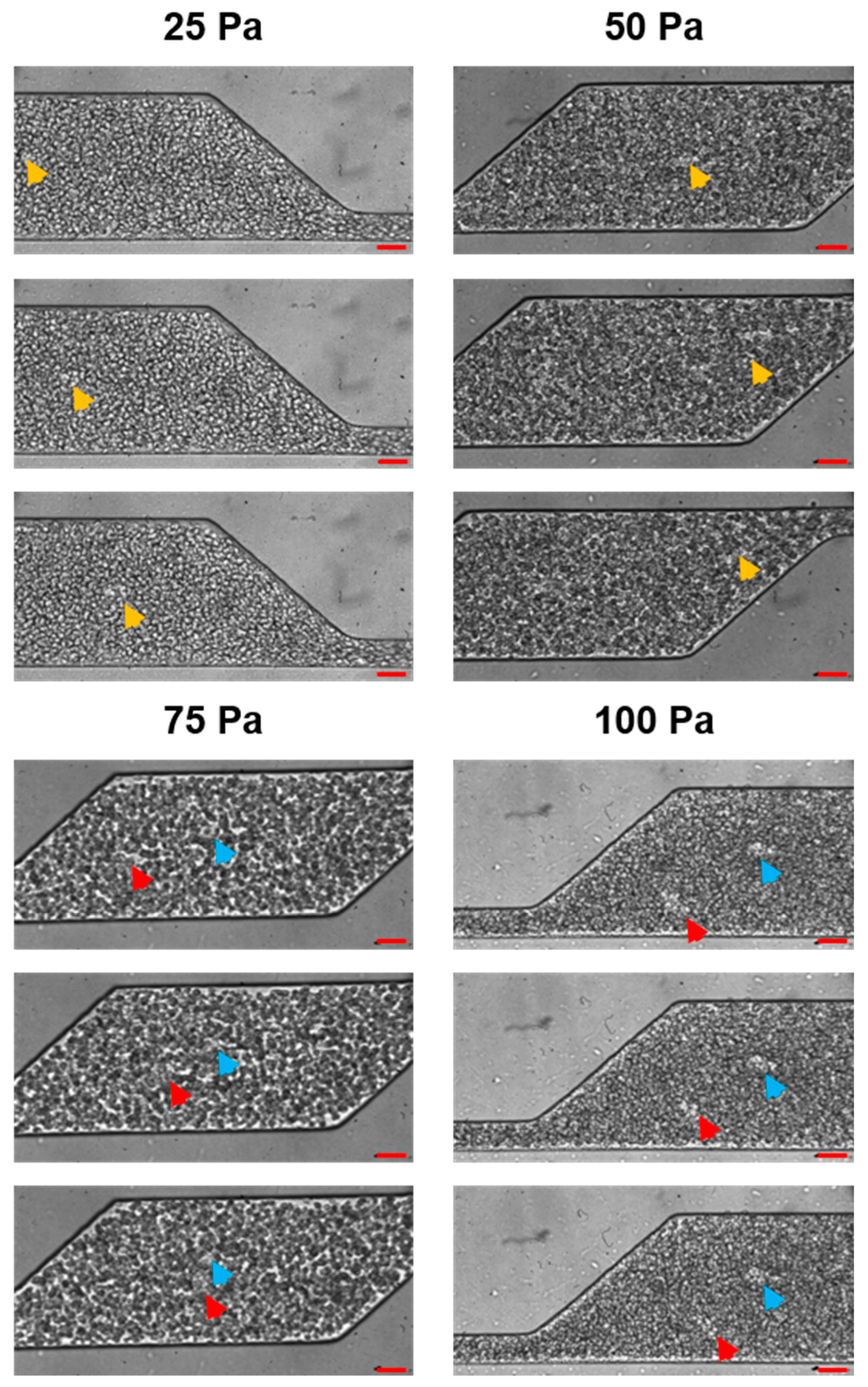
3.3. Flow Patterns Monitoring with Bionic Microfluidic Devices
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Slaughter, M.S.; Rogers, J.G.; Milano, C.A.; Russell, S.D.; Conte, J.V.; Feldman, D.; Sun, B.; Tatooles, A.J.; Delgado, R.M., 3rd; Long, J.W.; et al. Advanced heart failure treated with continuous-flow left ventricular assist device. N. Engl. J. Med. 2009, 361, 2241–2251. [Google Scholar] [CrossRef] [PubMed]
- Kirklin, J.K.; Pagani, F.D.; Kormos, R.L.; Stevenson, L.W.; Blume, E.D.; Myers, S.L.; Miller, M.A.; Baldwin, J.T.; Young, J.B.; Naftel, D.C. Eighth annual INTERMACS report: Special focus on framing the impact of adverse events. J. Heart Lung Transplant. 2017, 36, 1080–1086. [Google Scholar] [CrossRef] [PubMed]
- Kapur, N.K.; Esposito, M.L.; Bader, Y.; Morine, K.J.; Kiernan, M.S.; Pham, D.T.; Burkhoff, D. Mechanical Circulatory Support Devices for Acute Right Ventricular Failure. Circulation 2017, 136, 314–326. [Google Scholar] [CrossRef] [PubMed]
- Kirklin, J.K.; Naftel, D.C.; Pagani, F.D.; Kormos, R.L.; Myers, S.; Acker, M.A.; Rogers, J.; Slaughter, M.S.; Stevenson, L.W. Pump thrombosis in the Thoratec HeartMate II device: An update analysis of the INTERMACS Registry. J. Heart Lung Transpl. 2015, 34, 1515–1526. [Google Scholar] [CrossRef] [PubMed]
- Najjar, S.S.; Slaughter, M.S.; Pagani, F.D.; Starling, R.C.; McGee, E.C.; Eckman, P.; Tatooles, A.J.; Moazami, N.; Kormos, R.L.; Hathaway, D.R.; et al. An analysis of pump thrombus events in patients in the HeartWare ADVANCE bridge to transplant and continued access protocol trial. J. Heart Lung Transplant. 2014, 33, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, D.J.; Aaronson, K.D.; Tatooles, A.J.; Silvestry, S.C.; Jeevanandam, V.; Gordon, R.; Hathaway, D.R.; Najarian, K.B.; Slaughter, M.S.; Investigators, A. Gastrointestinal bleeding in recipients of the HeartWare Ventricular Assist System. JACC Heart Fail. 2015, 3, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Joy, P.S.; Kumar, G.; Guddati, A.K.; Bhama, J.K.; Cadaret, L.M. Risk Factors and Outcomes of Gastrointestinal Bleeding in Left Ventricular Assist Device Recipients. Am. J. Cardiol. 2016, 117, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Willey, J.Z.; Gavalas, M.V.; Trinh, P.N.; Yuzefpolskaya, M.; Reshad Garan, A.; Levin, A.P.; Takeda, K.; Takayama, H.; Fried, J.; Naka, Y.; et al. Outcomes after stroke complicating left ventricular assist device. J. Heart Lung Transplant. 2016, 35, 1003–1009. [Google Scholar] [CrossRef]
- Cowger, J.; Rao, V.; Massey, T.; Sun, B.; May-Newman, K.; Jorde, U.; Estep, J.D. Comprehensive review and suggested strategies for the detection and management of aortic insufficiency in patients with a continuous-flow left ventricular assist device. J. Heart Lung Transpl. 2015, 34, 149–157. [Google Scholar] [CrossRef]
- Jorde, U.P.; Uriel, N.; Nahumi, N.; Bejar, D.; Gonzalez-Costello, J.; Thomas, S.S.; Han, J.; Morrison, K.A.; Jones, S.; Kodali, S.; et al. Prevalence, Significance, and Management of Aortic Insufficiency in Continuous Flow Left Ventricular Assist Device Recipients. Circ Heart Fail. 2014, 7, 310–319. [Google Scholar] [CrossRef]
- Uriel, N.; Han, J.; Morrison, K.A.; Nahumi, N.; Yuzefpolskaya, M.; Garan, A.R.; Duong, J.; Colombo, P.C.; Takayama, H.; Thomas, S.; et al. Device thrombosis in HeartMate II continuous-flow Left ventricular assist devices: A multifactorial phenomenon. J. Heart Lung Transplant. 2014, 33, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Graveline, A.; Waterhouse, A.; Vernet, A.; Flaumenhaft, R.; Ingber, D.E. A shear gradient-activated microfluidic device for automated monitoring of whole blood haemostasis and platelet function. Nat. Commun. 2016, 7, 10176. [Google Scholar] [CrossRef] [PubMed]
- Westein, E.; van der Meer, A.D.; Kuijpers, M.J.; Frimat, J.P.; van den Berg, A.; Heemskerk, J.W. Atherosclerotic geometries exacerbate pathological thrombus formation poststenosis in a von Willebrand factor-dependent manner. Proc. Natl. Acad. Sci. USA 2013, 110, 1357–1362. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Hotaling, N.A.; Ku, D.N.; Forest, C.R. Microfluidic thrombosis under multiple shear rates and antiplatelet therapy doses. PLoS ONE 2014, 9, e82493. [Google Scholar] [CrossRef]
- Para, A.N.; Ku, D.N. A low-volume, single pass in-vitro system of high shear thrombosis in a stenosis. Thromb. Res. 2013, 131, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wu, Q.; Liu, S.; Chen, Y.; Chen, H.; Ruan, Y.; Zhang, Y. Lactic Dehydrogenase in the In Vitro Evaluation of Hemolytic Properties of Ventricular Assist Device. Artif. Organs 2017, 41, E274–E284. [Google Scholar] [CrossRef] [PubMed]
- McDonald, J.C.; Duffy, D.C.; Anderson, J.R.; Chiu, D.T.; Wu, H.K.; Schueller, O.J.A.; Whitesides, G.M. Fabrication of microfluidic systems in poly(dimethylsiloxane). Electrophoresis 2000, 21, 27–40. [Google Scholar] [CrossRef]
- Baskurt, O.u.K. Handbook of Hemorheology and Hemodynamics; IOS Press: Amsterdam, The Netherlands; Washington, DC, USA, 2007; 455p. [Google Scholar]
- Li, D.H.; Wu, Q.Y.; Ji, J.J.; Liu, S.H.; Zhang, M.K.; Zhang, Y. Hemolysis in a continuous-flow ventricular assist device with/without chamfer. Adv. Mech. Eng. 2017, 9. [Google Scholar] [CrossRef]
- Shimono, T.; Makinouchi, K.; Nose, Y. Total Erythrocyte Destruction Time - the New Index for the Hemolytic Performance of Rotary Blood Pumps. Artif. Organs 1995, 19, 571–575. [Google Scholar] [CrossRef] [PubMed]
- Byrnes, J.R.; Wolberg, A.S. Red blood cells in thrombosis. Blood 2017. [Google Scholar] [CrossRef] [PubMed]
- Walton, B.L.; Byrnes, J.R.; Wolberg, A.S. Fibrinogen, red blood cells, and factor XIII in venous thrombosis. J. Thromb. Haemost. 2015, 13 (Suppl. 1), S208–S215. [Google Scholar] [CrossRef] [PubMed]
- Lowe, G.D.; Lee, A.J.; Rumley, A.; Price, J.F.; Fowkes, F.G. Blood viscosity and risk of cardiovascular events: The Edinburgh Artery Study. Br. J. Haematol. 1997, 96, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Holley, L.; Woodland, N.; Hung, W.T.; Cordatos, K.; Reuben, A. Influence of fibrinogen and haematocrit on erythrocyte sedimentation kinetics. Biorheology 1999, 36, 287–297. [Google Scholar] [PubMed]
- Rampling, M.W. The binding of fibrinogen and fibrinogen degradation products to the erythrocyte membrane and its relationship to haemorheology. Acta Biol. Med. Ger. 1981, 40, 373–378. [Google Scholar] [PubMed]
- Neeves, K.B.; Onasoga, A.A.; Hansen, R.R.; Lilly, J.J.; Venckunaite, D.; Sumner, M.B.; Irish, A.T.; Brodsky, G.; Manco-Johnson, M.J.; Di Paola, J.A. Sources of variability in platelet accumulation on type 1 fibrillar collagen in microfluidic flow assays. PLoS ONE 2013, 8, e54680. [Google Scholar] [CrossRef] [PubMed]
- Hosokawa, K.; Ohnishi, T.; Kondo, T.; Fukasawa, M.; Koide, T.; Maruyama, I.; Tanaka, K.A. A novel automated microchip flow-chamber system to quantitatively evaluate thrombus formation and antithrombotic agents under blood flow conditions. J. Thromb. Haemost. 2011, 9, 2029–2037. [Google Scholar] [CrossRef] [PubMed]
- Westein, E.; de Witt, S.; Lamers, M.; Cosemans, J.M.; Heemskerk, J.W. Monitoring in vitro thrombus formation with novel microfluidic devices. Platelets 2012, 23, 501–509. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.; Li, G.; Chen, Y.; Man, J.; Wu, Q.; Zhang, M.; Chen, H.; Zhang, Y. The Impact of Erythrocytes Injury on Blood Flow in Bionic Arteriole with Stenosis Segment. Processes 2019, 7, 372. https://doi.org/10.3390/pr7060372
Li D, Li G, Chen Y, Man J, Wu Q, Zhang M, Chen H, Zhang Y. The Impact of Erythrocytes Injury on Blood Flow in Bionic Arteriole with Stenosis Segment. Processes. 2019; 7(6):372. https://doi.org/10.3390/pr7060372
Chicago/Turabian StyleLi, Donghai, Guiling Li, Yuanyuan Chen, Jia Man, Qingyu Wu, Mingkui Zhang, Haosheng Chen, and Yu Zhang. 2019. "The Impact of Erythrocytes Injury on Blood Flow in Bionic Arteriole with Stenosis Segment" Processes 7, no. 6: 372. https://doi.org/10.3390/pr7060372
APA StyleLi, D., Li, G., Chen, Y., Man, J., Wu, Q., Zhang, M., Chen, H., & Zhang, Y. (2019). The Impact of Erythrocytes Injury on Blood Flow in Bionic Arteriole with Stenosis Segment. Processes, 7(6), 372. https://doi.org/10.3390/pr7060372




