Screening and Optimization of Demulsifiers and Flocculants Based on ASP Flooding-Produced Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Simulated Produced Water
2.3. Oil Concentration Determination
2.4. Screening Tests of Demulsifiers
2.5. Screening Tests of Flocculants
3. Results and Discussion
3.1. Demulsifier
3.1.1. Screening of Demulsifier Single Agent
3.1.2. Compound Demulsifiers
3.1.3. Optimization of Demulsifying Conditions
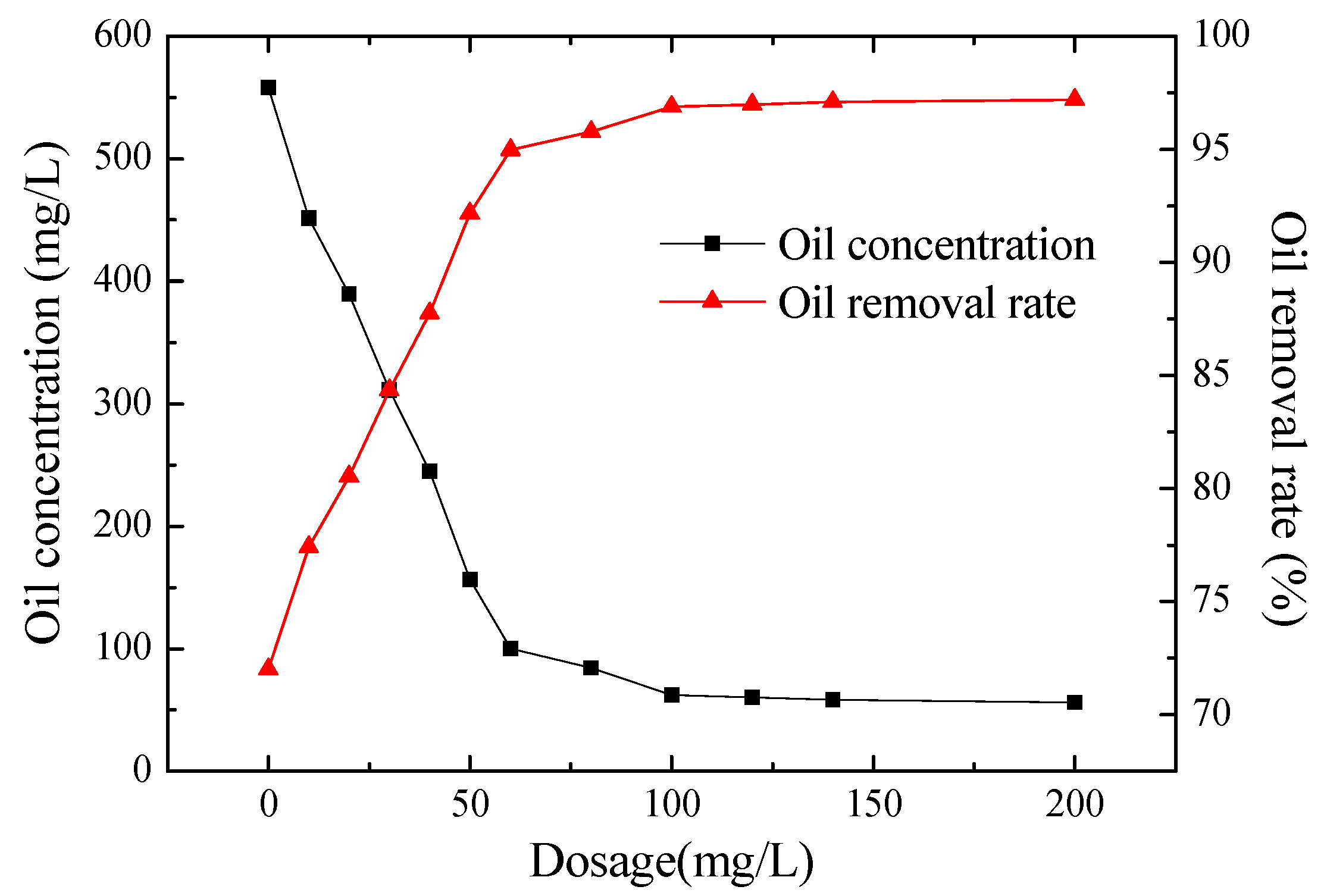
Effect of the Dosage
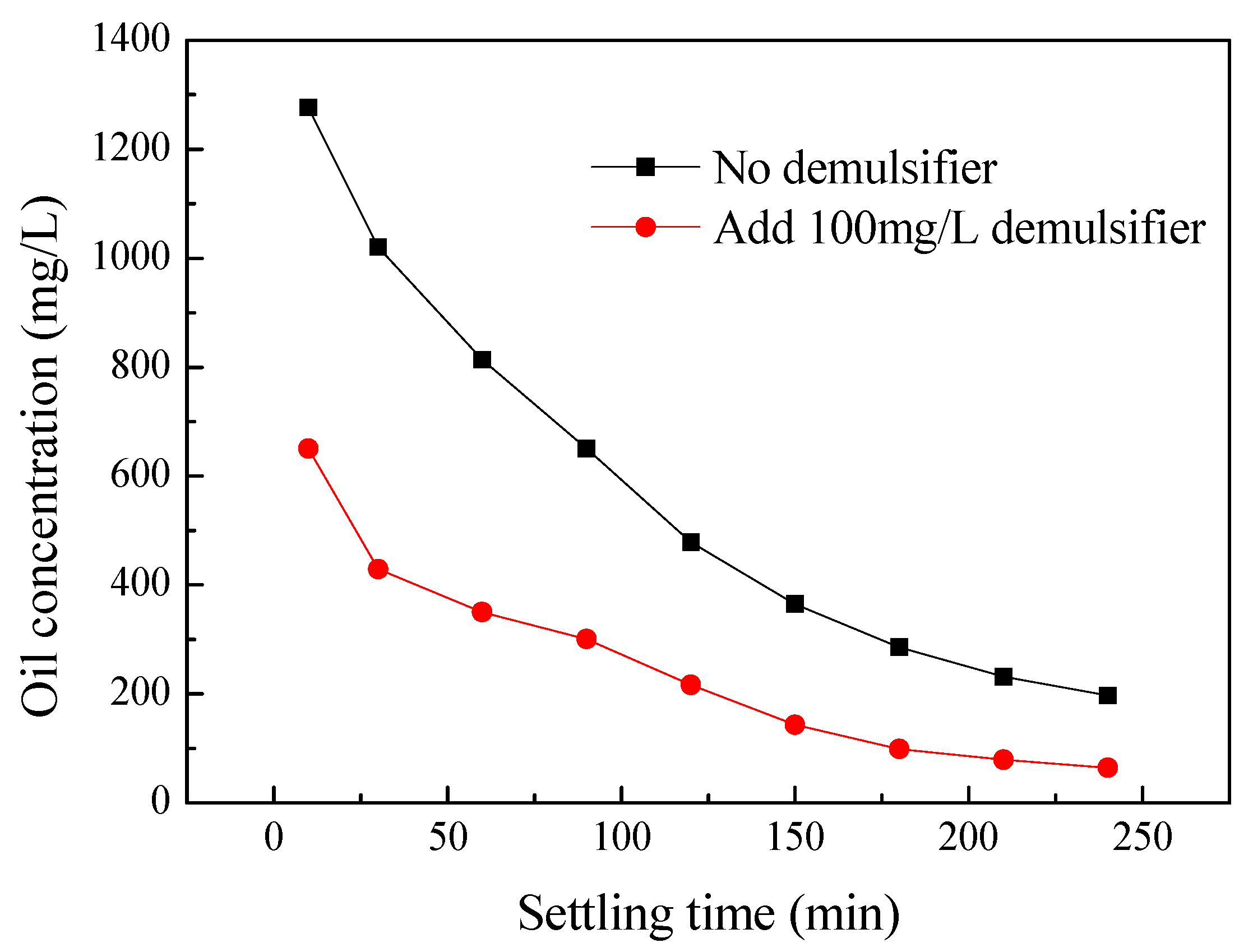
Effect of the Settling Time
Effect of the Settling Temperature
3.2. Flocculants
3.2.1. Screening of Flocculant Single Agents
3.2.2. Compound Flocculants
3.2.3. Optimization of Flocculation Conditions
Effect of the Dosage
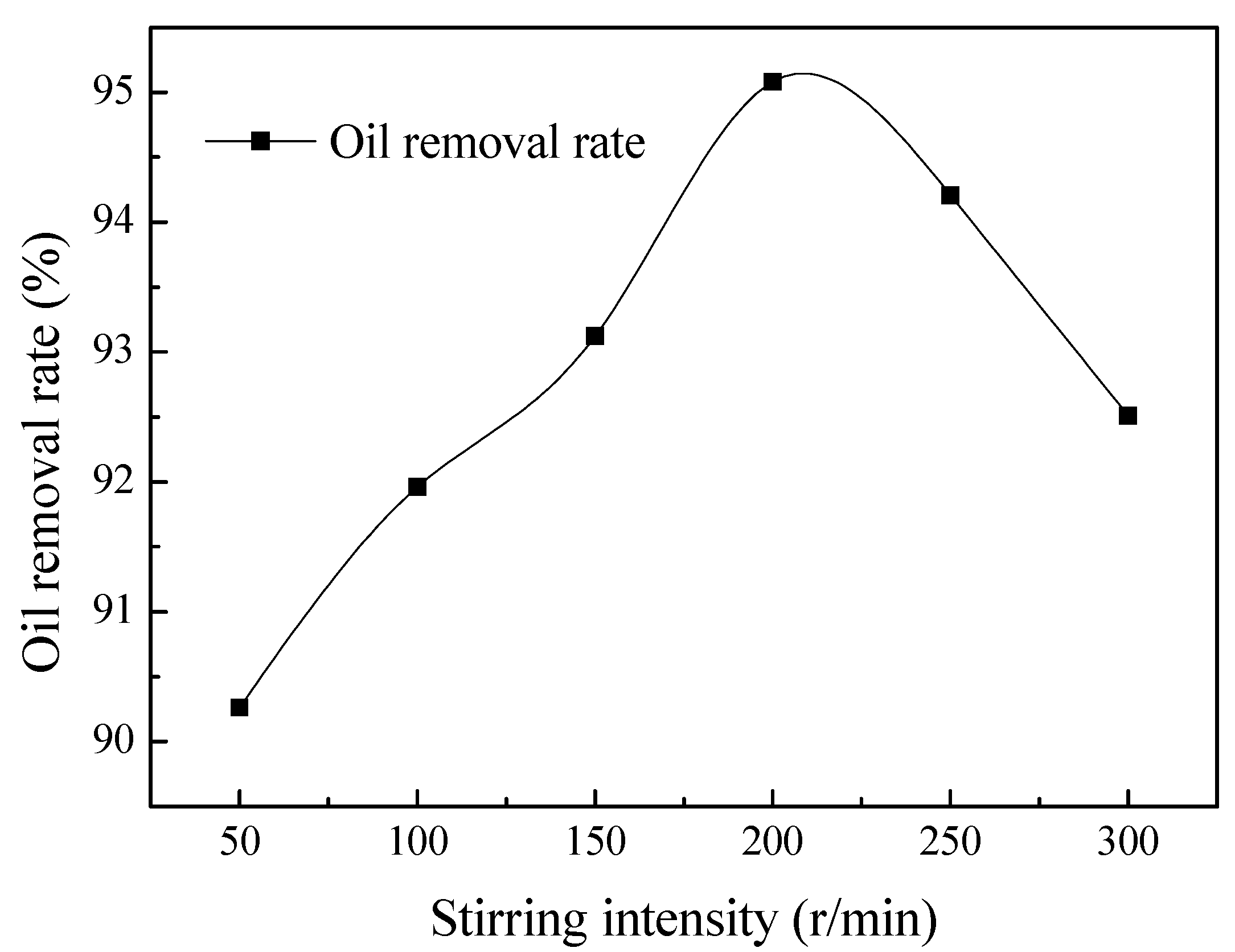
Effect of the Stirring Intensity
Effect of the Stirring Time
3.3. Experimental Study on Demulsification-Flocculation
3.3.1. Optimization of Demulsification-Flocculation Conditions
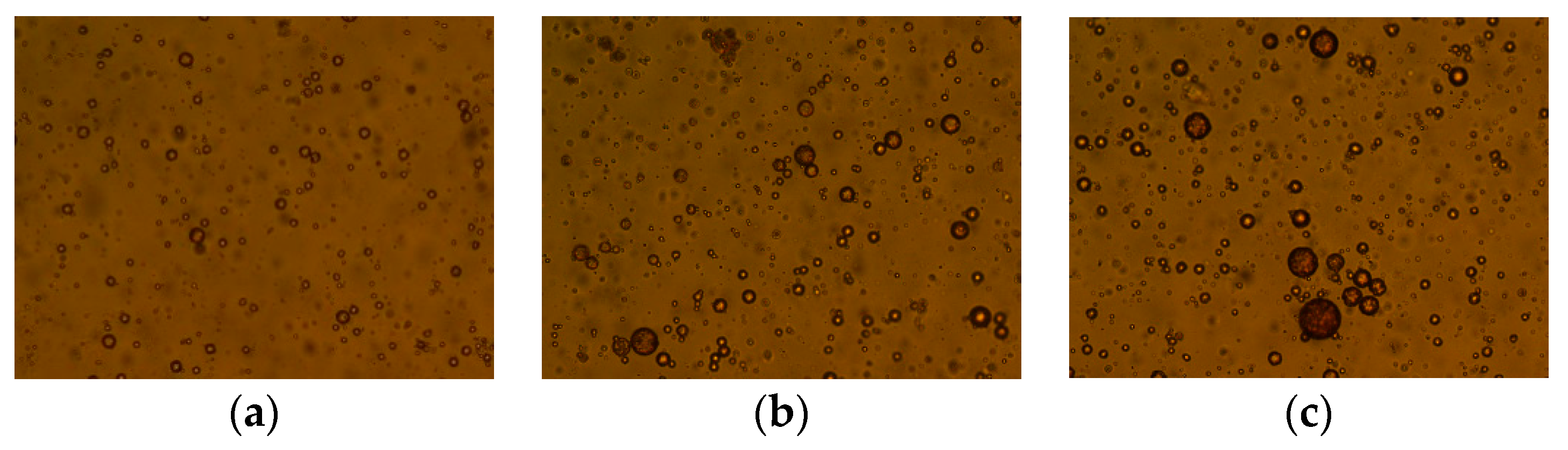
3.3.2. Analysis of the Results of Demulsification-Flocculation
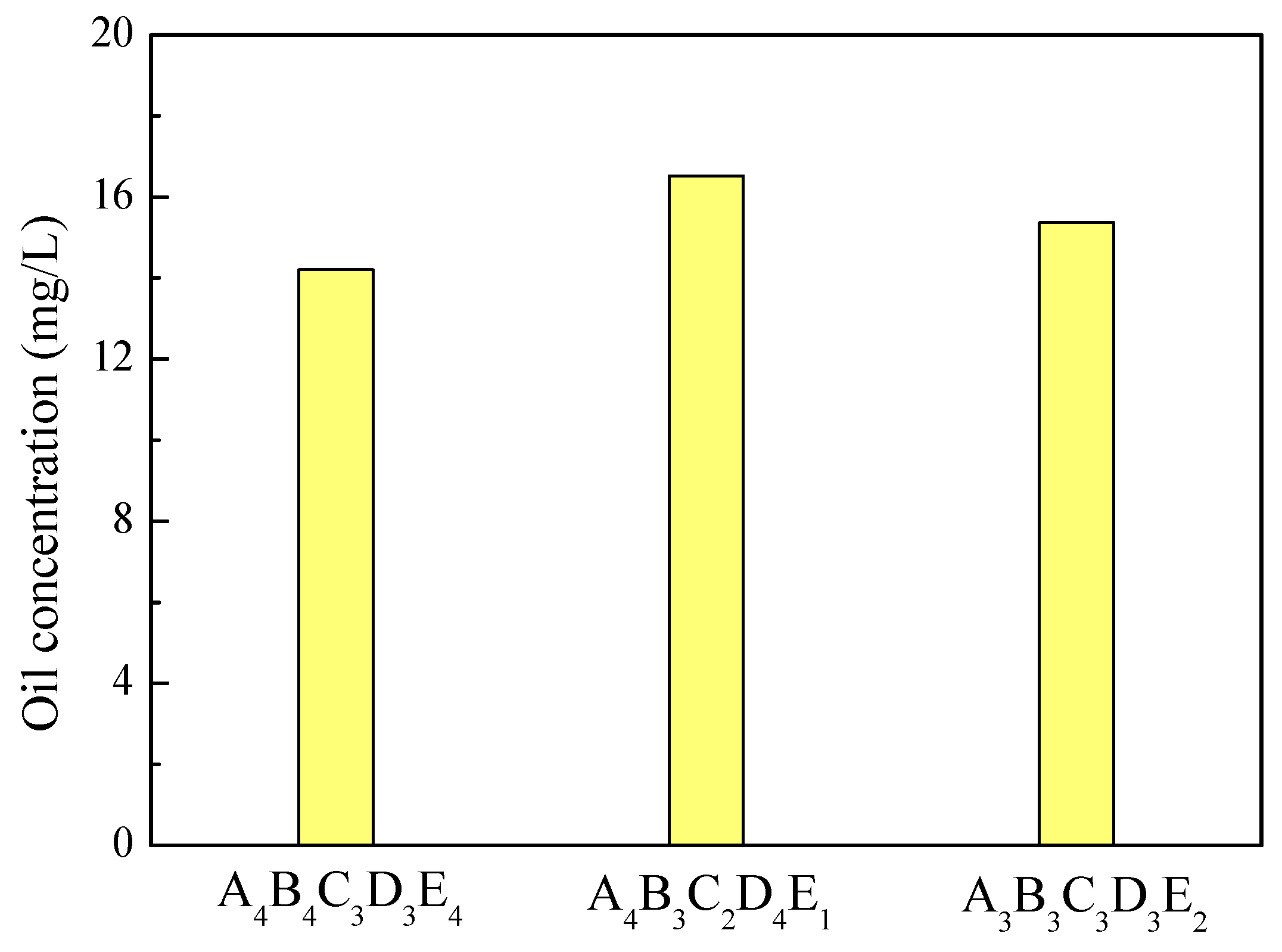
3.3.3. Verification Analysis of Optimal Treatment Conditions
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cheng, J.; Liao, G.; Yang, Z. Pilot test of ASP flooding in daqing oilfield. Pet. Geol. Oilfield Dev. Daqing 2001, 2, 46–49. [Google Scholar]
- Deng, S.; Zhou, F.; Yu, G. Speciality of oil-field produced water and its treatment technology. Ind. Water Treat. 2000, 20, 10–12. [Google Scholar]
- Wu, D.; Wang, C.; Zhao, F.; Zhang, H.; Meng, X.; Sun, J.; Zhao, M. Microscopic structures and oil/water separation behaviors of produced water by ASP flooding using Na2CO3, petroleum sulfonate and partially hydrolyzed polyacrylamide. Fine Spec. Chem. 2000, 18, 45–47. [Google Scholar]
- Ye, Q. Research on Oil/Water Separation Characteristic and Treatment in ASP Produced Water; Shanghai Jiaotong University: Shanghai, China, 2008. [Google Scholar]
- Deng, S.; Bai, R.; Chen, J.P.; Yu, G.; Jiang, Z.; Zhou, F. Effects of alkaline/surfactant/polymer on stability of oil droplets in produced water from ASP flooding. Coll. Surf. A Physicochem. Eng. Asp. 2002, 2002, 275–284. [Google Scholar] [CrossRef]
- Gu, W.; Chen, Z.; Zhao, Q.; Xu, D.; Du, D. Technology of ASP flooding produced water treatment in Daqing oilfield. Ind. Water Wastewater 2018, 49, 48–53. [Google Scholar]
- Li, C.; Wei, L.; Chen, Z.; Yu, G. ASP flooding produced fluid characteristic and treatment process. In Proceedings of the International Conference on Energy Equipment Science and Engineering (ICEESE 2015), Guangzhou, China, 30–31 May 2015; pp. 147–150. [Google Scholar]
- Chen, H.; Deng, Z.; Gong, Z.; Yu, G.; Chen, W.; Chen, D.; Gao, Y. Progress of treatment technologies for oilfield produced water. Environ. Sci. Technol. 2017, 40, 95–101. [Google Scholar]
- Zhao, Q.; Xu, D.; Shi, H. Adaptability study on the sequencing batch sedimentation and filtration process for treating strong alkali alkaline surfactant polymer produced water. Oil Gasfield Surf. Eng. 2018, 37, 23–26. [Google Scholar]
- Di, M.; Xu, C.; Zhao, S.; Wang, F.; Pang, H.; LI, W. Air floating/hydrolysis acidification/biological contact oxidation/filtration process for treatment of ASP flooding produced water. China Water Wastewater 2016, 32, 69–70. [Google Scholar]
- Etchepare, R.; Oliveira, H.; Azevedo, A.; Rubio, J. Separation of emulsified crude oil in saline water by dissolved air flotation with micro and nanobubbles. Sep. Purif. Technol. 2017, 186, 326–332. [Google Scholar] [CrossRef]
- Aliff Radzuan, M.; Abia-Biteo Belope, M.; Thorpe, R.B. Removal of fine oil droplets from oil-in-water mixtures by dissolved air flotation. Chem. Eng. Res. Des. 2016, 115, 19–33. [Google Scholar] [CrossRef]
- Al-Maamari, R.S.; Sueyoshi, M.; Tasaki, M.; Okamura, K.; Al-Lawati, Y.; Nabulsi, R.; Al-Battashi, M. Flotation, filtration, and adsorption: Pilot trials for oilfield produced-water treatment. Oil Gas Facil. 2014, 4, 56–66. [Google Scholar] [CrossRef]
- Jiang, L. Carbon Dioxide Gas Dissolving Device for Ternary Compound Flooding Produced Water and Treatment Method. China Patent ZL201510386836.4, 7 October 2015. [Google Scholar]
- Li, J.X.; Liu, Y.; Wu, D.; Meng, X.C.; Zhao, F.L. The synergistic effects of alkaline, surfactant, and polymer on the emulsification and destabilization of oil-in-water crude oil emulsion produced by alkaline-surfactant-polymer flooding. Petrol. Sci. Technol. 2013, 31, 399–407. [Google Scholar] [CrossRef]
- Ortiz, D.; Baydak, E.; Yarranton, H. Effect of surfactants on interfacial films and stability of water-in-oil emulsions stabilized by asphaltenes. J. Coll. Interface Sci. 2010, 351, 542–555. [Google Scholar] [CrossRef] [PubMed]
- Márquez, A.L.; Medrano, A.; Panizzolo, L.A.; Wagner, J.R. Effect of calcium salts and surfactant concentration on the stability of water-in-oil (w/o) emulsions prepared with polyglycerol polyricinoleate. J. Coll. Interface Sci. 2010, 341, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Sun, N.; Wang, J.; Tong, J. Demulsification efficiency of heavy oil-in-water emulsion stabilized by organic alkali and compound surfactants. Oilfield Chem. 2016, 33, 338–344. [Google Scholar]





| Composition | NaCl | NaHCO3 | Na2CO3 | Na2SO4 | CaCl2 | MgCl2·6H2O |
|---|---|---|---|---|---|---|
| Concentration (mg/L) | 1523 | 2820 | 168.7 | 10.5 | 56.9 | 35.5 |
| Number | Demulsifier | Demulsifier Concentration (mg/L) | Oil Concentration (mg/L) | Demulsifier Concentration (mg/L) | Oil Concentration (mg/L) |
|---|---|---|---|---|---|
| 0 | Blank sample | 0 | 450.24 | 0 | 450.24 |
| 1 | DPR-1193 | 100 | 396.72 | 200 | 309.32 |
| 2 | DPR-1612 | 100 | 366.29 | 200 | 278.18 |
| 3 | DPR-1814 | 100 | 406.55 | 200 | 321.78 |
| 4 | DPR-1815 | 100 | 372.08 | 200 | 284.25 |
| 5 | DPR-1884 | 100 | 296.75 | 200 | 201.64 |
| 6 | DPR-1885 | 100 | 280.26 | 200 | 189.31 |
| 7 | DYRPR-1625 | 100 | 61.91 | 200 | 77.62 |
| 8 | DYRPR-1712 | 100 | 126.75 | 200 | 114.82 |
| 9 | DYRPR-1881 | 100 | 243.52 | 200 | 174.99 |
| 10 | DYRPR-1882 | 100 | 102.65 | 200 | 63.48 |
| 11 | DYRPR-1869 | 100 | 95.49 | 200 | 57.88 |
| 12 | DYRPR-1870 | 100 | 81.08 | 200 | 55.37 |
| DYRPR-1625 (mg/L) | DYRPR-1870 (mg/L) | Ratio | Total (mg/L) | Oil Concentration (mg/L) |
|---|---|---|---|---|
| 60 | 60 | 1:1 | 120 | 55.23 |
| 40 | 80 | 1:2 | 120 | 58.46 |
| 30 | 90 | 1:3 | 120 | 60.17 |
| 80 | 40 | 2:1 | 120 | 50.18 |
| 48 | 72 | 2:3 | 120 | 53.68 |
| 90 | 30 | 3:1 | 120 | 48.21 |
| 72 | 48 | 3:2 | 120 | 51.84 |
| 120 | 0 | 1:0 | 120 | 55.67 |
| 0 | 120 | 0:1 | 120 | 64.59 |
| Number | Flocculants | Flocculant Dosage (mg/L) | Oil Concentration (mg/L) |
|---|---|---|---|
| 0 | Blank sample | 0 | 450.24 |
| 1 | PAC | 200 | 250.53 |
| 2 | PFS | 200 | 274.03 |
| 3 | PAFC | 200 | 220.41 |
| 4 | D2N-1647 | 40 | 210.63 |
| 5 | D2N-1651 | 40 | 208.79 |
| 6 | D2N-1650 | 40 | 192.23 |
| 7 | D2N-1648 | 40 | 196.31 |
| 8 | CPAM | 40 | 156.32 |
| 9 | APAM | 40 | 214.36 |
| 10 | PAM | 40 | 205.24 |
| CPAM (mg/L) | D2N-1650 (mg/L) | Ratio | Total (mg/L) | Oil concentration (mg/L) |
|---|---|---|---|---|
| 30 | 30 | 1:1 | 60 | 55.23 |
| 20 | 40 | 1:2 | 60 | 58.46 |
| 15 | 45 | 1:3 | 60 | 60.17 |
| 40 | 20 | 2:1 | 60 | 50.18 |
| 24 | 36 | 2:3 | 60 | 53.68 |
| 45 | 15 | 3:1 | 60 | 48.21 |
| 36 | 24 | 3:2 | 60 | 51.84 |
| 48 | 0 | 1:0 | 60 | 55.67 |
| 0 | 48 | 0:1 | 60 | 64.59 |
| Level | Experimental Factor | ||||
|---|---|---|---|---|---|
| Demulsifier Dosage (mg/L) A | Flocculant Dosage (mg/L) B | Settling Time (min) C | Stirring Intensity (r/min) D | Stirring Time (min) E | |
| 1 | 80 | 40 | 180 | 100 | 2 |
| 2 | 90 | 50 | 210 | 150 | 3 |
| 3 | 100 | 60 | 240 | 200 | 4 |
| 4 | 110 | 70 | 270 | 250 | 5 |
| Trial No. | Experimental Factor | Trial No. | Experimental Factor | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | A | B | C | D | E | ||
| 1 | 1 | 1 | 1 | 1 | 1 | 9 | 3 | 1 | 3 | 4 | 2 |
| 2 | 1 | 2 | 2 | 2 | 2 | 10 | 3 | 2 | 4 | 3 | 1 |
| 3 | 1 | 3 | 3 | 3 | 3 | 11 | 3 | 3 | 1 | 2 | 4 |
| 4 | 1 | 4 | 4 | 4 | 4 | 12 | 3 | 4 | 2 | 1 | 3 |
| 5 | 2 | 1 | 2 | 3 | 4 | 13 | 4 | 1 | 4 | 2 | 3 |
| 6 | 2 | 2 | 1 | 4 | 3 | 14 | 4 | 2 | 3 | 1 | 4 |
| 7 | 2 | 3 | 4 | 1 | 2 | 15 | 4 | 3 | 2 | 4 | 1 |
| 8 | 2 | 4 | 3 | 2 | 1 | 16 | 4 | 4 | 1 | 3 | 2 |
| Number | Experimental Factor | Oil Concentration (mg/L) | ||||
|---|---|---|---|---|---|---|
| A | B | C | D | E | ||
| 1 | 1 | 1 | 1 | 1 | 1 | 38.45 |
| 2 | 1 | 2 | 2 | 2 | 2 | 36.57 |
| 3 | 1 | 3 | 3 | 3 | 3 | 32.62 |
| 4 | 1 | 4 | 4 | 4 | 4 | 34.21 |
| 5 | 2 | 1 | 2 | 3 | 4 | 28.63 |
| 6 | 2 | 2 | 1 | 4 | 3 | 31.63 |
| 7 | 2 | 3 | 4 | 1 | 2 | 28.15 |
| 8 | 2 | 4 | 3 | 2 | 1 | 27.59 |
| 9 | 3 | 1 | 3 | 4 | 2 | 22.19 |
| 10 | 3 | 2 | 4 | 3 | 1 | 21.38 |
| 11 | 3 | 3 | 1 | 2 | 4 | 20.53 |
| 12 | 3 | 4 | 2 | 1 | 3 | 18.96 |
| 13 | 4 | 1 | 4 | 2 | 3 | 20.61 |
| 14 | 4 | 2 | 3 | 1 | 4 | 18.27 |
| 15 | 4 | 3 | 2 | 4 | 1 | 16.59 |
| 16 | 4 | 4 | 1 | 3 | 2 | 19.19 |
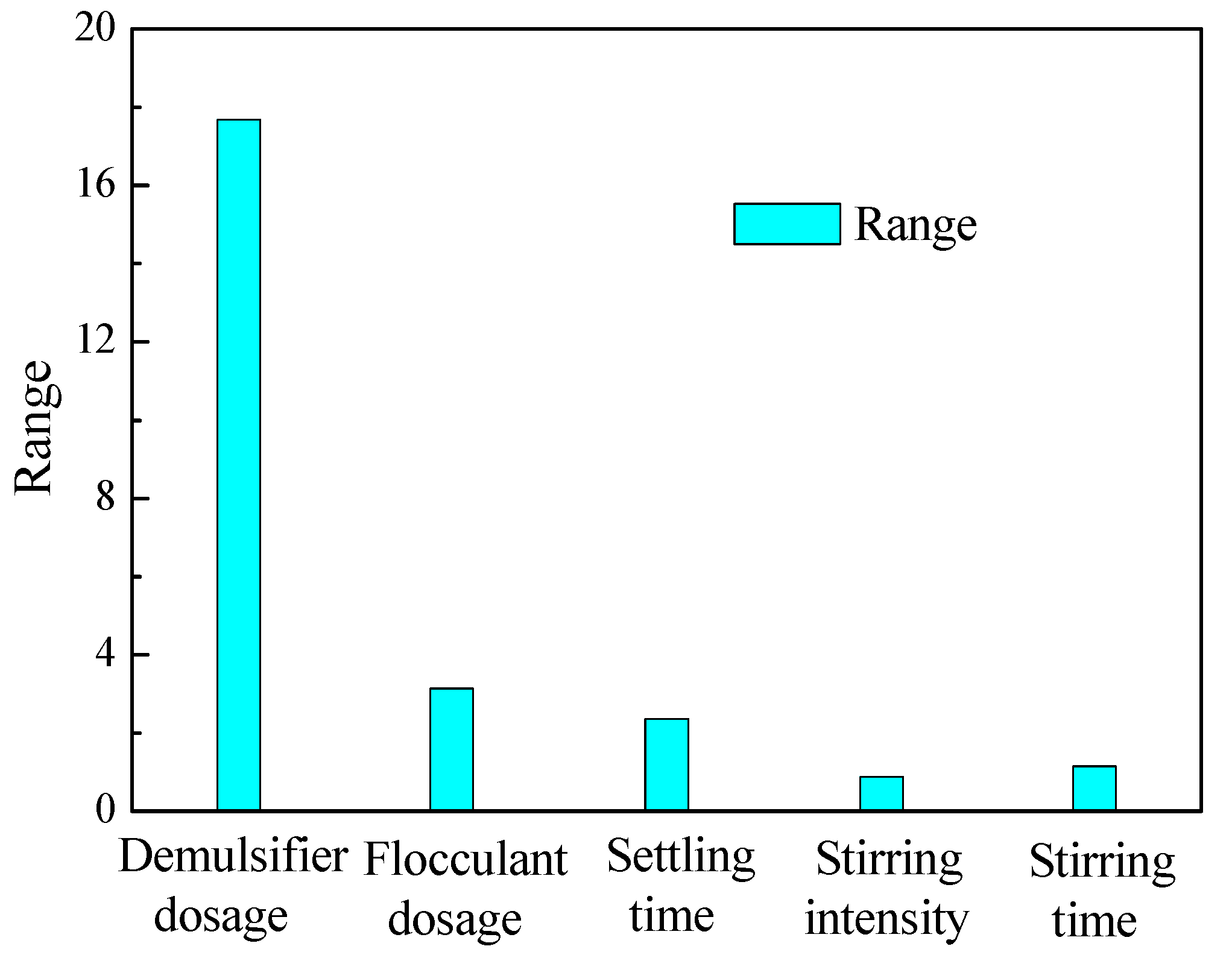
| 35.463 | 27.470 | 27.450 | 25.957 | 26.003 | ||
| 29.000 | 26.962 | 25.188 | 26.324 | 26.525 | ||
| 20.765 | 27.473 | 25.167 | 25.455 | 25.955 | ||
| 18.665 | 24.987 | 26.087 | 26.155 | 25.410 | ||
| Optimal levels | A4 | B4 | C3 | D3 | E4 | |
| 16.798 | 2.997 | 2.283 | 0.870 | 1.115 | ||
| Primary and secondary order | ABCED | |||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, B.; Wang, J.; Zhang, W.; Fu, C.; Wang, Y.; Liu, X. Screening and Optimization of Demulsifiers and Flocculants Based on ASP Flooding-Produced Water. Processes 2019, 7, 239. https://doi.org/10.3390/pr7040239
Huang B, Wang J, Zhang W, Fu C, Wang Y, Liu X. Screening and Optimization of Demulsifiers and Flocculants Based on ASP Flooding-Produced Water. Processes. 2019; 7(4):239. https://doi.org/10.3390/pr7040239
Chicago/Turabian StyleHuang, Bin, Jie Wang, Wei Zhang, Cheng Fu, Ying Wang, and Xiangbin Liu. 2019. "Screening and Optimization of Demulsifiers and Flocculants Based on ASP Flooding-Produced Water" Processes 7, no. 4: 239. https://doi.org/10.3390/pr7040239
APA StyleHuang, B., Wang, J., Zhang, W., Fu, C., Wang, Y., & Liu, X. (2019). Screening and Optimization of Demulsifiers and Flocculants Based on ASP Flooding-Produced Water. Processes, 7(4), 239. https://doi.org/10.3390/pr7040239




